Published online Mar 27, 2013. doi: 10.4254/wjh.v5.i3.109
Revised: September 24, 2012
Accepted: November 14, 2012
Published online: March 27, 2013
AIM: This study was undertaken to evaluate the hepatic effects of silybum marianum on non alcoholic fatty liver disease (NAFLD).
METHODS: In 72 patients affected by NAFLD, main metabolic, hepatic and anti-inflammatory parameters were assayed after 3 mo of a restricted diet and before silymarin treatment (twice a day orally). The brightness of liver echography texture (hepatorenal ratio brightness) was also defined at same time. These evaluations were repeated after 6 mo of treatment.
RESULTS: Serum levels of some metabolic and anti-inflammatory data nonsignificantly lowered after 6 mo of silymarin. On the contrary, Steato test, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transpeptidase were significantly (P < 0.001) reduced. Instead, the AST/ALT ratio unchanged. Finally, the hepatorenal brightness ratio, as an index of hepatic steatosis, significantly (P < 0.05) dropped.
CONCLUSION: The obtained results indicate that silymarin appears to be effective to reduce the biochemical, inflammatory and ultrasonic indices of hepatic steatosis. Some parameters indicative of early stage of atherosclerosis were also lowered.
- Citation: Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol 2013; 5(3): 109-113
- URL: https://www.wjgnet.com/1948-5182/full/v5/i3/109.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i3.109
Non alcoholic fatty liver disease (NAFLD) consists of a spectrum of conditions ranging from a simple fatty infiltration to steatohepatitis, fibrosis and cirrhosis. NAFLD is histologically similar to alcoholic liver disease, but without a history of ingesting significant amounts of ethanol. It produces few symptoms but can have evidence of fat in the liver on ultrasound[1-3] and hepatocellular fibrosis and/or inflammation evidenced by some biochemical tests. Obesity, type 2 diabetes mellitus and hyperlipidemia are often associated with NALFD[4-7]. These aspects may coexist with insulin resistance[8-10]. In turn, insulin resistance may lead to endothelial dysfunction and systemic hypertension and may induce an abnormal lipid profile. All these changes may promote the development of atherosclerotic vascular disease, even if the increased values of insulin resistance may be responsible for metabolic syndrome (MS) only.
Several medications have been proposed as NAFLD therapies, such as Metformin[11], Peroxisome proliferator-activated receptor-γ agonists[12] and Ezetimibe[13]. Silymarin (a mixture of at least 4 closely related flavonolignans, 60% to 70% of which is a mixture of 2 diastereomers of silybin) also demonstrated antioxidant properties, through stimulation of polymerase and RNA transcription, by protecting the cell membrane from radical-induced damage and blocking the uptake of toxins. Studies performed in patients with liver disease have shown that silymarin increased superoxide dismutase activity of lymphocytes and erythrocytes. In addition, it has been shown to increase patient serum levels of glutathione and glutathione peroxidase[14]. In a previous report, silymarin treatment was associated with a reduction of insulin resistance and a significant decrease in fasting insulin levels, suggesting an improvement of the activity of endogenous and exogenous insulin[15]. In another study, silymarin extract caused an improvement in serum alanine aminotransferase (ALT) levels. Hajaghamohammadi et al[16] demonstrated that silymarin decreased serum aspartate aminotransferase (AST) levels. Federico et al[17] showed that a new silybin vitamin E complex improves insulin resistance and liver damage in patients with NAFLD. More recently, Hasjiani et al[18] found that in two patient groups receiving silymarin and Vitamin E, respectively, the AST and ALT serum levels significantly decreased.
In this study, we evaluated the effects of a mixture of silybum marianum (silymarin), vitamin B12, vitamin E (Epaclin) and a restricted diet on NAFLD patients.
From January 2010 to June 2011, 72 subjects (40 male and 32 female) with a mean age of 44 ± 3.2 years were selected. Exclusion criteria were ethanol intake > 20 g/d and the customary ingestion of drugs known to produce fatty liver disease, such as steroids, estrogens, amiodarone, tamoxifen or other chemotherapeutic agents. Viral hepatitis was excluded by the negative results of hepatitis B surface antigen and anti-hepatitis C virus tests. Hemochromatosis, autoimmune hepatitis, Wilson’s disease and other hepatic diseases were also ruled out. After three months of a restricted diet, a food supplement containing Vitamin E, L-gluthatione, L-cysteine, L-methionine and silybum marianum (Epaclin 3.5 g) was given twice a day (as packet) in all selected subjects. Homeostatic model assessment insulin resistance (HOMA-IR)[19], total cholesterol (in mg/dL), high density lipoprotein (HDL)-C, low density lipoprotein (LDL)-C, triglycerides and fasting plasma glucose were evaluated at baseline and after six months of treatment. Steato test, ALT, AST with their ratio serum levels (ALT/AST) and γ-glutamyl peptidase (γ-GT) were also measured, both in basal conditions and after 6 mo. In addition, serum levels of tumor necrosis factor-α (TNF-α) were defined both at baseline and after silymarin treatment (6 mo). Finally, the hepatorenal brightness ratio was defined at the entry of the study and after 6 mo, using the sonographic brightness difference of the hepatorenal index[20].
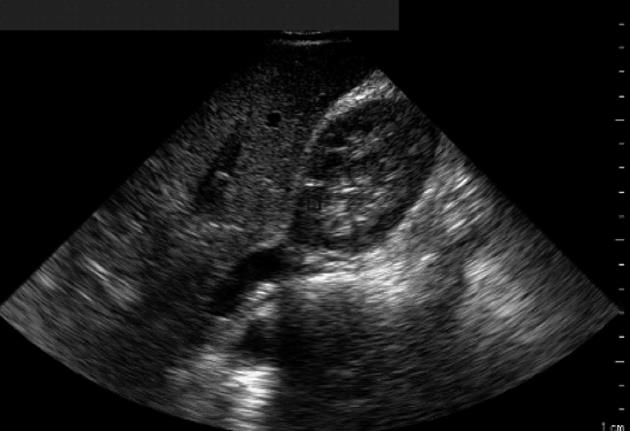
An iE-33 ultrasonographic machine (Philips, Amsterdam) with a 3.5 MHz phased array convex transducer was used to verify the possible presence and the degree of hepatic steatosis. All subjects were evaluated in the left lateral recumbent position of 15°-20° to see the liver parenchyma and the right kidney cortex was seen contemporaneously. The brightness of both zones was examined. The liver was recorded from the intercostal space, posing the region of interest (ROI) (of 1.5 cm × 1.5 cm) in the mid or anterior axillary line (seventh or eighth intercostal space). The right kidney was evaluated, posing the ROI (0.5 cm × 0.5 cm) in the cortical zone[20]. Normal liver echo texture was considered as the absence of steatosis, as shown in Figure 1. In this case, the hepatorenal brightness ratio was 1. Mild steatosis was diagnosed for hyperechogenic liver tissue (compared with the kidney cortex) when the sonographic index results were between 1 and 2. Values between 2 and 2.5 were indicative of moderate liver steatosis. Finally, hepatic steatosis was judged as severe when the hepatorenal ratio was > 2.5. In each case, the calculation of the hepatorenal index was repeated at least twice. The main demographic, metabolic, serum liver indices and ultrasonographic data recorded at baseline and after 6 mo of treatment are reported in Table 1.
| Variables | Before | After | P value |
| Age (yr) | 44 ± 3.2 | ||
| Sex (M/F) | 40/32 | ||
| BMI (kg/m2) | 26.7 ± 1.67 | 26.4 ± 1.34 | NS |
| Fasting plasma glucose (mg/mL) | 105.7 ± 0.8 | 101 ± 0.5 | NS |
| HOMA-IR | 6.42 ± 0.4 | 5.27 ± 1.2 | NS |
| Total Cholesterol (mg/dL) | 205.7 ± 9.3 | 200.6 ± 8.1 | NS |
| LDL-C (mg/mL) | 157.4 ± 4.3 | 136 ± 1.8 | NS |
| HDL-C (mg/mL) | 43.6 ± 2.1 | 45.8 ± 1.1 | NS |
| Triglycerides (mg/mL) | 178.4 ± 4.1 | 155.7 ± 3.4 | NS |
| Steato test | 0.71 ± 0.07 | 0.40 ± 0.05 | < 0.001 |
| ALT (U/L) | 109.48 ± 4.4 | 75.12 ± 3.3 | < 0.01 |
| AST (U/L) | 72.39 ± 8.4 | 48.65 ± 3.2 | < 0.01 |
| AST/ALT ratio | 0.66 ± 0.4 | 0.64 ± 0.9 | NS |
| γ-GT (IU/L) | 45.51 ± 1.2 | 29.33 ± 1.1 | < 0.001 |
| TNF-α (pg/mL) | 16.2 ± 0.9 | 9.7 ± 0.7 | < 0.001 |
| Hepatorenal ratio | 2.5 ± 0.3 | 1.8 ± 0.6 | < 0.05 |
Calculations were performed as paired data, by comparing the biochemical (metabolic and serum liver-indices) values recorded at baseline and after 6 mo. Continuous variables are presented as mean ± SD. P < 0.05 was considered statistically significant for analysis. The degree of the liver echogenicity and the hepatorenal index recorded in basal conditions and at the end of treatment (6 mo) were also compared. All calculations were made using SPSS Version 13.0 for Microsoft Windows.
BMI was not significantly different before and after treatment in all subjects. Mean glucose fasting levels measured at baseline were 105 ± 0.7 mg/mL. This value fell to 101 ± 0.5 mg/mL after a restricted diet and silymarin treatment. HOMA-IR results were 6.42 ± 0.4 vs 5.27 ± 1.2, without significant differences. Total cholesterol was 205.7 ± 9.3 mg/dL before and 200.6 ± 8.1 mg/dL after treatment. In agreement, both LDL-C and HDL-C were slightly and not significantly reduced after silymarin and a restricted diet (157.4 ± 4.3 and 43.6 ± 2.1 mg/mL in basal conditions and 136 ± 1.8 and 45.8 ± 1.1 mg/mL, respectively). Triglycerides levels were not significantly lower, going from 178.4 ± 4.1 to 155.7 ± 3.4 mg/mL. On the contrary, Steato test significantly (P < 0.001) reduced from baseline (0.71 ± 0.07) to the end of treatment (0.40 ± 0.05). ALT serum levels (P < 0.01) failed from a mean level of 109.48 ± 4.4 to 75.12 ± 3.3 U/L. AST recorded at baseline (72.39 ± 8.4 U/L) also significantly reduced (P < 0.05) after silymarin and diet (48.65 ± 3.2 U/L). Instead, AST/ALT ratio was not significantly decreased from basal conditions (0.66 ± 0.4) to the end of silymarin treatment (0.64 ± 0.9). γ-GT decreased from 45.51 ± 1.2 to 29.33 ± 1.1, with a significant difference (P < 0.001). TNF-α significantly fell (P < 0.001) from basal conditions (16.2 pg/mL) to post treatment phase (6 mo). Finally, the hepatorenal ratio dropped from 2.5 ± 0.3 to 1.8 ± 0.6. The reduction was also significant (P < 0.05) (Table 1).
NAFLD is the most common silent liver disease worldwide, marked by fat accumulation in the liver (steatosis)[21] and alterations in liver biochemical tests occurring in those who do not consume high amounts of alcohol. The prevalence of NAFLD in western countries is estimated to be 20%-30%[22,23]. Current guidelines recommended liver biopsy for diagnosis, that is the “gold standard” for quantification of hepatic steatosis associated with NAFLD[24]. However, it is hard to be accepted due to its invasiveness and a significant degree of sampling error. In addition, it is invasive, costly and prone to complications[25,26].
In our experience, NAFLD is associated with insulin resistance (HOMA-IR). Previous studies demonstrated that insulin resistance almost universally induces NAFLD[27,28]. It is known that this condition may precede the development of cardiovascular disease[29,30]. To confirm the connection between NAFLD and atherosclerosis, carotid atherosclerosis has recently been detected in patients with NAFLD[31]. Pathogenetic mechanisms responsible for that include an increased lipolysis and increased delivery of free fatty acids to the liver[32]. Other abnormalities that can contribute to fat accumulation in the liver include decreased synthesis of apolipoproteins and microsomal transfer protein gene polymorphism, both conditions that lead to decreased export of triglycerides out of the liver[33]. We also found an increased value of Steato test at baseline that significantly reduced after 6 mo of silymarin and a restricted diet. It is known that the test (score from 0 to 1) gives a quantitative estimation of steatosis of different origins. The behavior of the Steato test in patients with NAFLD is more important than ultrasonography for noninvasive diagnosis of steatosis and may reduce the need of liver biopsy, particularly in patients with other risk factors[34]. Mild to moderate elevation of serum aminotransferases (ALT and AST) found in our subjects at baseline represents the most common abnormality found in patients with NAFLD[1]. Their serum levels significantly reduced after diet and silymarin treatment. Unlike those with alcohol-induced steatohepatitis (who typically manifest disproportionate increases in the ALT level), patients with NAFLD usually have an AST/ALT ratio < 1 because the ALT level is higher than AST in NAFLD[35]. On the contrary, the AST/ALT ratio tends to increase with the development of cirrhosis, thus losing its diagnostic accuracy[36]. The reduction both of AST and ALT (with AST/ALT < 1) after silymarin treatment seems due to the antioxidant effect of silybum marianum that is also able to protect the liver against toxins. Previous studies also demonstrated that silymarin acts as a cytoprotectant, anticarcinogenic and supportive agent for liver damage from Amanita phalloides poisoning[37,38]. Mean serum levels of γ-GT were above the normal limits in selected subjects with NAFLD. That is due to obesity, hyperinsulinemia, oxidative inflammation and changes in hepatocyte membrane permeability[39]. In our study, silymarin was demonstrated to reduce its high serum levels, probably for stabilization of the hepatocyte membrane structure, thereby preventing toxins from entering the cells. This happens through enterohepatic recirculation and by promoting liver regeneration. A previous study demonstrated that this happens by stimulating nucleolar polymerase A and increasing ribosomal protein synthesis[40]. TNF-α was also reduced after silymarin therapy, as a consequence of the anti-inflammatory action of the drug. This clearly demonstrated a reduction of the hepatic inflammation. Finally, the changes of hepatorenal brightness index clearly demonstrated the reduction of hepatic fat accumulation after silymarin therapy. From this point of view, although several techniques can be used to assess liver steatosis, ultrasonography represents the most commonly used method for this[20]. It can be assessed by hyperechogenicity of liver tissue (“bright liver”) compared to the echogenicity of the kidney cortex (hepatorenal contrast). The ultrasound index is the main noninvasive imaging modality for the evaluation of hepatic steatosis[41-43]. The sensitivity of ultrasonography in detecting steatosis is between 60% and 90%[44], even if it is difficult to identify the inflammatory liver findings. It is also difficult to differentiate steatosis from steatohepatitis[45].
In conclusion, the results obtained indicate that silymarin seems to be effective in reducing the biochemical and ultrasonographic changes induced by NAFLD. These results are in agreement those obtained by other authors[46]. A pilot study performed in patients with NAFLD confirmed an improvement in liver enzymes and insulin resistance when a complex of silybin, vitamin E and phospholipid was given[47]. The effects of silybum marianum and vitamin E on some biochemical indices of atherosclerotic progression must be confirmed by other experiences performed on a wide range, even though these metabolic data may be reported, not only for atherosclerosis, but also for MS alone.
Studies performed in patients with liver disease have shown that silymarin increased superoxide dismutase activity of lymphocytes and erythrocytes. In addition, it has been shown to increase patient serum levels of glutathione and glutathione peroxidase.
The results obtained indicate that silymarin seems to be effective in reducing the biochemical and ultrasonographic changes induced by non alcoholic fatty liver disease (NAFLD). The effects of silybum marianum and vitamin E on some biochemical indices of atherosclerotic progression must be confirmed by other experiences performed on a wide range, even though these metabolic data may be reported, not only for atherosclerosis, but also for metabolic syndrome alone.
In this study, the authors evaluated the effects of a mixture of silybum marianum (silymarin), vitamin B12, vitamin E (Epaclin) and a restricted diet on NAFLD patients.
This is a nice piece of work that addresses the effects of a mixture of vitamin B12, vitamin E and silybum marianum in improving the syndrome related to NAFLD and/or atherosclerosis. This manuscript is acceptable for publication.
P- Reviewer Zhang KZ S- Editor Xiong L L- Editor Roemmele A E- Editor Li JY
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3718] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 2. | Vehmas T, Kaukiainen A, Luoma K, Lohman M, Nurminen M, Taskinen H. Liver echogenicity: measurement or visual grading? Comput Med Imaging Graph. 2004;28:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Gaiani S, Avogaro A, Bombonato GC, Bolognesi M, Amor F, Vigili de Kreutzenberg S, Guarneri G, Sacerdoti DF. Nonalcoholic fatty liver disease (NAFLD) in nonobese patients with diabetes: prevalence and relationships with hemodynamic alterations detected with doppler sonography. J Ultrasound. 2009;12:1-5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 395] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 6. | Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, Patel N, Madan A, Amarapurkar A. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Cacciapuoti F. Visceral adiposity as a cause of some cardiovascular disorders. Old and new adipocytokines. Obes Metab. 2010;6:35-46. |
| 8. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. [PubMed] |
| 9. | Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 10. | Noguchi H, Tazawa Y, Nishinomiya F, Takada G. The relationship between serum transaminase activities and fatty liver in children with simple obesity. Acta Paediatr Jpn. 1995;37:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Park H, Shima T, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, Itoh Y, Yoshikawa T, Fukui M, Hasegawa G. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 289] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 134] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Hajaghamohammadi AA, Ziaee A, Raflei R. The efficacy of silymarin in decreasing transaminase activities in nonalcoholic fatty liver disease. A randomized controlled clinical trial. Hepat Mon. 2008;8:191-195. |
| 17. | Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Del Vecchio Blanco C, Loguercio C. A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: preliminary observations. Gut. 2006;55:901-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Hasjiani E, Hasahemi SJ. Comparison of therapeutic effects of Silymarin and Vitamin E in nonalcoholic fatty liver disease: results of an open-label, propective, randomized study. JJNPP. 2009;4:8-14. |
| 19. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24510] [Article Influence: 612.8] [Reference Citation Analysis (0)] |
| 20. | Webb M, Yeshua H, Zelber-Sagi S, Santo E, Brazowski E, Halpern Z, Oren R. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol. 2009;192:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 652] [Article Influence: 28.3] [Reference Citation Analysis (2)] |
| 22. | Williams R. Global challenges in liver disease. Hepatology. 2006;44:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 546] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 23. | Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 892] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 24. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1736] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 25. | Labayle D, Chaput JC, Albuisson F, Buffet C, Martin E, Etienne JP. Comparison of the histological lesions in tissue specimens taken from the right and left lobe of the liver in alcoholic liver disease (author’s transl). Gastroenterol Clin Biol. 1979;3:235-240. [PubMed] |
| 26. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1549] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 27. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 781] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 28. | Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1100] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 30. | Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579-1584. [PubMed] |
| 31. | Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 822] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 32. | Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Bernard S, Touzet S, Personne I, Lapras V, Bondon PJ, Berthezène F, Moulin P. Association between microsomal triglyceride transfer protein gene polymorphism and the biological features of liver steatosis in patients with type II diabetes. Diabetologia. 2000;43:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, Abella A, Massard J, Ngo Y. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 36. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1090] [Article Influence: 41.9] [Reference Citation Analysis (1)] |
| 37. | Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med. 2002;113:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 39. | Stranges S, Trevisan M, Dorn JM, Dmochowski J, Donahue RP. Body fat distribution, liver enzymes, and risk of hypertension: evidence from the Western New York Study. Hypertension. 2005;46:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Blumenthal M, Goldberg A, Brinckmann J. Herbal Medicine: Expanded Commission E Monographs. Newton, MA: Integrative Medicine Communications 2000; . |
| 41. | Xia MF, Yan HM, He WY, Li XM, Li CL, Yao XZ, Li RK, Zeng MS, Gao X. Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: an improvement method. Obesity (Silver Spring). 2012;20:444-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996;24:25-29. [PubMed] |
| 43. | Webb M, Hanny Yeshua H, Zelber-Sagie S, Santo M, Barawoski E, Katz R, Halpern Z, Oren R. A pratical index for ultrasonographic quantification of liver steatosis. Boston, MA: The 58th Annual Meeting of the American Association for the study of liver disease 2007; . |
| 44. | Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114-1122. [PubMed] |
| 45. | Fierbinteanu-Braticevici C, Dina I, Petrisor A, Tribus L, Negreanu L, Carstoiu C. Noninvasive investigations for non alcoholic fatty liver disease and liver fibrosis. World J Gastroenterol. 2010;16:4784-4791. [PubMed] |
| 46. | Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 47. | Loguercio C, Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Niosi M, D’Auria MV, Capasso R, Del Vecchio Blanco C. The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:2387-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (1)] |