Published online Oct 27, 2013. doi: 10.4254/wjh.v5.i10.568
Revised: August 10, 2013
Accepted: September 13, 2013
Published online: October 27, 2013
Processing time: 165 Days and 9.5 Hours
AIM: To investigate the presence of autoantibodies directed against liver sinusoidal cells in primary biliary cirrhosis (PBC).
METHODS: Liver biopsies from 21 PBC patients were studied and compared with 12 liver biopsies from disease controls [3 patients with hepatitis B (HBV) virus, 3 patients with hepatitis C virus (HCV), 3 patients with non-alcoholic steatohepatitis and 3 patients with acute alcoholic hepatitis (AAH)]. As healthy controls, we used tissue specimens adjacent to metastatic liver adenocarcinoma. Normal serum was taken from staff members of the unit. The determination of the cell type targeted by autoantibodies present in the patients sera was performed by indirect immunofluorescence (IIF) analysis using paraffin-embedded liver sections as a substrate. Sera from homologous or heterologous PBC patients or sera from the disease control group were used as primary antibodies. The presence of autoantibodies was identified using confocal microscopy.
RESULTS: In total, 18/21 (85.7%) PBC patients exhibited positive staining in the sinusoidal cells, 10/21 (47.6%) in lymphocytes, 8/21 (38%) in cholangiocytes and 7/21 (33.3%) in hepatocytes, when homologous serum and fluorescein isothiocyanate-conjugated immunoglobulin type G (IgG) secondary antibody were used. PBC sections incubated with heterologous PBC serum showed reduced staining (20% for sinusoidal cells, 20% for lymphocytes, 20% for cholangiocytes and 13.3% for hepatocytes). When IgM immunoglobulin, instead of IgG, was used as secondary antibody, positive staining was observed in 75% of lymphocytes, 62.5% of cholangiocytes, 37.5% of hepatocytes and 50% of the sinusoidal cells with a much stronger staining intensity. No staining was observed when either normal or PBC sera were used as a primary antibody on liver sections from the disease control group. When PBC sera were incubated with healthy control sections, weak positive staining of cholangiocytes was observed in 3/21 (14.3%) PBC serum samples. Steatohepatitis serum on PBC sections gave a positive staining of some hepatocytes and lymphocytes but no staining on viral hepatitis sections. Incubation with HBV sera stained some hepatocytes, cholangiocytes and intra-sinusoidal or portal lymphocytes of PBC, HBV and AAH patients but not HCV patients.
CONCLUSION: In this study, for the first time in diseased liver tissue, we have demonstrated that a large proportion of PBC patients have disease specific autoantibodies against liver sinusoidal cells.
Core tip: In this study, indirect immunofluorescence staining was performed on paraffin-embedded human liver sections of various chronic liver diseases to demonstrate the presence of disease specific autoantibodies targeting sinusoidal cells in patient sera. Liver sections from normal and disease controls were used as the substrate, and sera were the source of primary antibodies. Our findings indicate that disease specific antibodies against liver sinusoidal cells circulate in primary biliary cirrhosis (PBC). Various non disease specific antibodies were also found in PBC and chronic hepatitis B but not in other chronic liver diseases.
- Citation: Sfakianaki O, Tzardi M, Voumvouraki A, Afgoustaki A, Koulentaki M, Kouroumalis E. Presence of disease specific autoantibodies against liver sinusoidal cells in primary biliary cirrhosis. World J Hepatol 2013; 5(10): 568-576
- URL: https://www.wjgnet.com/1948-5182/full/v5/i10/568.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i10.568
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease of unknown etiology that leads to a progressive nonsuppurative destruction of small- and medium-sized intrahepatic bile ducts and eventually to cirrhosis and liver failure[1]. The diagnosis of PBC is based on three criteria: the presence of detectable anti-mitochondrial antibodies (AMAs) in the serum, the elevation of cholestatic enzymes (most commonly, alkaline phosphatase (ALP) for > 6 mo and histological findings in the liver that are compatible with the presence of the disease. A probable diagnosis requires the presence of two criteria, and a definite diagnosis requires all three[2].
The major mitochondrial autoantigens recognized by AMAs are members of the 2-oxoacid dehydrogenase complex (ODC) family[3-5]. Studies based on immunohistochemistry and affinity mass spectrometry have suggested that either PDC-E2 (a member of the ODC family) or a cross-reactive molecule is present in greatly increased amounts at the apical surface of biliary epithelial cells (BECs) from patients with AMA-positive or AMA-negative PBC, but not from normal individuals or patients with other liver diseases[4,5]. Additionally, in other studies, in addition to the intense staining of the apical surface of BECs, positive staining was observed in a subset of macrophages in portal lymph nodes[6] and in hepatocytes[7].
In addition to AMAs, which are the hallmark of PBC, antinuclear antibodies (ANAs) have also been detected in 30% of PBC patients[8-10].
Two PBC-specific ANA immunofluorescence patterns have been identified[11,12]: “multiple nuclear dots”, corresponding to the antigens Sp100 and Sp140, promyelocytic leukemia (PML) nuclear body proteins and small ubiquitin-like modifiers (SUMOs)[13,14], and “nuclear membrane” (rim), caused by anti-nuclear envelope antibodies (ANEAs), such as gp210 and nucleoporin p62[15,16]. The anti-gp210 antibodies are highly specific for PBC and are associated with disease activity and severity[17,18].
Nakamura et al[19] found that the expression of gp210 is markedly increased on the nuclear envelope of small bile ducts and sometimes at infiltrating mononuclear cells in the portal area and/or periportal hepatocytes in PBC and this expression was positively correlated to disease activity. We also reported that 46.9% of patients with PBC have detectable ANEAs and 21% of them had detectable anti-gp210 antibodies. The presence of these antibodies identifies a subgroup of PBC patients with advanced disease and poor prognosis[20]. So far, no antibodies against liver sinusoidal cells have been reported in diseased liver tissue.
In our previous studies, we hypothesized that the primary initiating event in PBC may be the overproduction of endothelins by liver sinusoidal cells[21,22].
The aim of the present study was therefore to identify the presence of circulating antibodies against liver sinusoidal cells in PBC patients.
Liver biopsies from 21 PBC patients were studied and compared with 12 liver biopsies from disease controls [3 patients with hepatitis B virus (HBV), 3 patients with hepatitis C virus (HCV), 3 patients with non-alcoholic steatohepatitis and 3 patients with acute alcoholic hepatitis (AAH)]. As healthy controls, we used tissue specimens adjacent to metastatic liver adenocarcinoma. Normal serum was taken from staff members of the unit. The mean age of the 21 PBC patients was 52.9 ±13.4 years (range: 31-76 years), and the mean Mayo risk score was 4.2 ± 1.0 (range: 2.8-6.5) at diagnosis. Seventeen (81%) patients were stage I-II, and 4 (19%) were stage III-IV, according to Ludwig[23]. Fifteen patients were positive for ANEAs, and 7 were anti-gp210 positive.
No patients or disease controls had any associated autoimmune diseases.
The study was approved by the Ethics Committee of the University Hospital of Heraklion, and written informed consent was collected from all patients and controls participating in the study.
The determination of cell types targeted by autoantibodies present in the patients sera was performed by indirect immunofluorescence (IIF) staining using paraffin-embedded sections as a substrate. As a primary antibody, serum from each patient (homologous), serum from another PBC patient (heterologous) or serum from normal or disease control was used. Paraffin-embedded liver biopsy sections of 3 μm thickness were deparaffinized in xylene and rehydrated in graded ethanol solutions (100%, 95%, 80% and 70%).
IIF analysis was performed as previously described[24]. Briefly, antigen retrieval was achieved by incubation with citrate buffer (1.8 mmol/L citric acid and 8.2 mmol/L sodium citrate) for 2 h at 37 °C. After blocking with phosphate-buffered saline (PBS) containing 2 mL/L Triton X-100, 2 mmol/L MgCl2 and 10 mL/L gelatin from cold-water fish skin (Sigma-Aldrich, Germany) for 10 min, the sections were incubated with patient serum for 2 h (dilution 1:50). The sections were washed with blocking buffer and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-human immunoglobulin type G (IgG) (dilution 1:500, H + L secondary antibody, Chemicon, Millipore, Germany) or immunoglobulin type M (IgM) (dilution 1:100, DAKO, Carpinteria, CA) for 45 min at room temperature. Afterward, nuclei were counterstained by incubating the sections for 5 min with TO-PRO-3 iodide (TO-PRO) (dilution 1:1000 in blocking buffer, Molecular Probes, Inc). Lastly, the sections were rinsed in PBS and mounted with mounting medium containing 4',6-diamidino-2-phenylindole (DAPI) (Santa Cruz Biotechnology, Inc, Germany). Negative controls were generated by omitting the primary antibody. Fluorescence was observed under a Leica SP confocal microscope. In preliminary experiments, the sections were incubated with dilutions of sera up to 1:200. The results were similar, but the staining intensity was weaker, so a dilution of 1:50 was used throughout.
We have observed five types of staining: apical staining of BECs; perinuclear and nuclear staining of lymphocytes, hepatocytes, cholangiocytes and sinusoidal cells, punctuated staining of hepatocytes and cytoplasmic staining of lymphocytes, hepatocytes, cholangiocytes and sinusoidal cells.
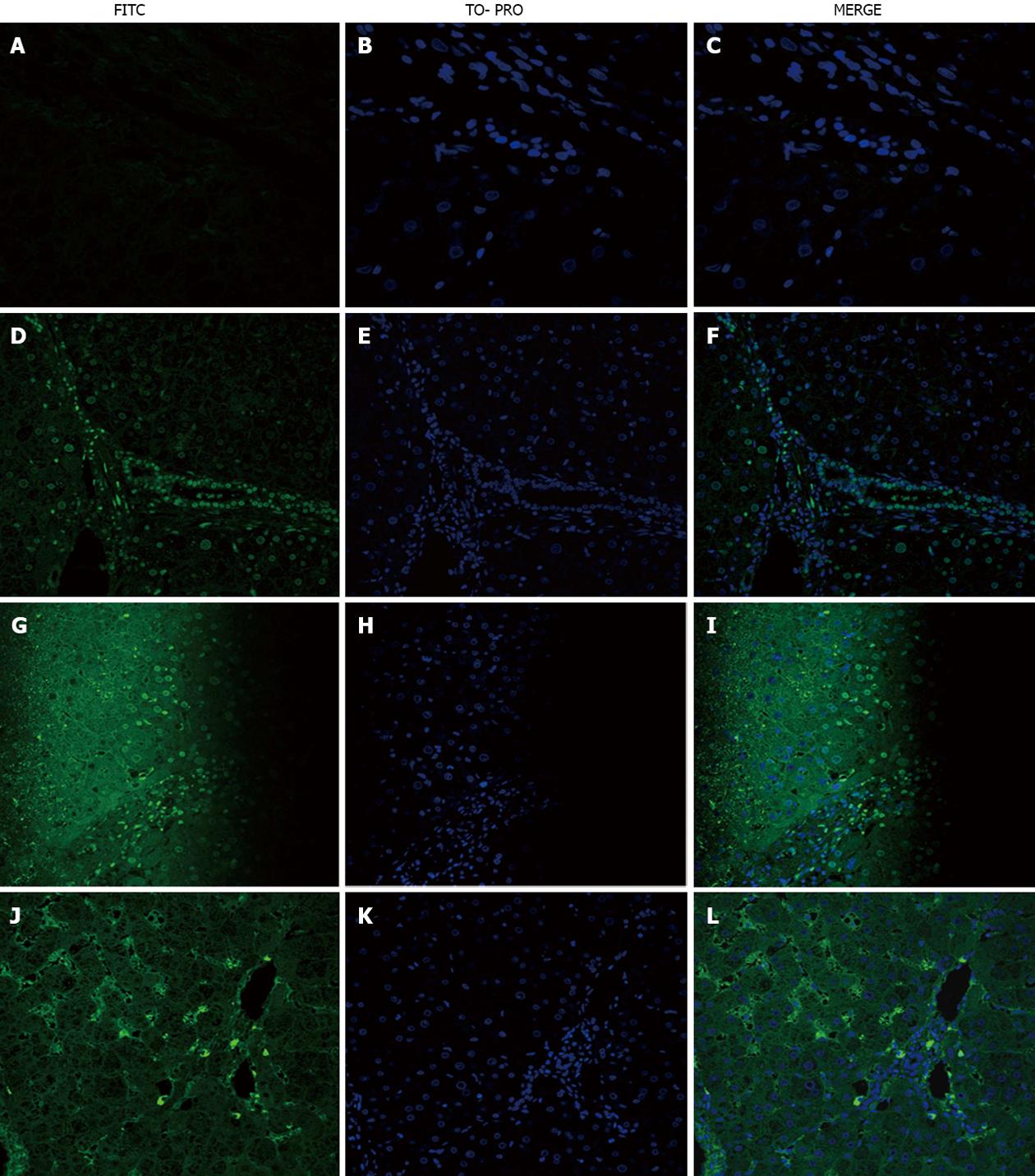
Homologous serum: Of the 21 PBC patients 10 (47.6%) yielded positive staining in lymphocytes, 8 (38%) in cholangiocytes, 7 (33.3%), in hepatocytes; and 18 (85.7%) in the sinusoidal cells, (Table 1, Figure 1D-F), although the sinusoidal staining was weak.
| Patient | Target cells | |||
| Lymphocytes | Cholangiocytes | Hepatocytes | Sinusoidal cells | |
| 1 | P | N | N | P |
| 2 | N | N | N | P |
| 3 | P | P | N | P |
| 4 | P | N | N | P |
| 5 | N | P | N | P |
| 6 | P | N | N | N |
| 7 | P | N | P | P |
| 8 | N | N | P | P |
| 9 | P | P | N | P |
| 10 | N | N | N | P |
| 11 | P | P | P | P |
| 12 | N | P | P | N |
| 13 | N | N | N | N |
| 14 | N | P | N | P |
| 15 | N | N | N | P |
| 16 | N | P | P | P |
| 17 | N | N | N | P |
| 18 | N | N | N | P |
| 19 | P | N | N | P |
| 20 | P | P | P | P |
| 21 | P | N | P | P |
| Total positive n (%) | 10 (47.6) | 8 (38.0) | 7 (33.3) | 18 (85.7) |
Heterologous serum: PBC sections with heterologous PBC serum showed reduced staining [(lymphocytes: 20% of patients, cholangiocytes: 20%, hepatocytes: 13% and sinusoidal cells: 20% (Figure 1G-I)].
Staining with homologous serum was different when IgM was used as a secondary antibody rather than IgG. Positive staining was observed in lymphocytes (75% of patients), cholangiocytes (62%), hepatocytes (38%) and sinusoidal cells (50%), but the staining was very strong (Figure 1J-L).
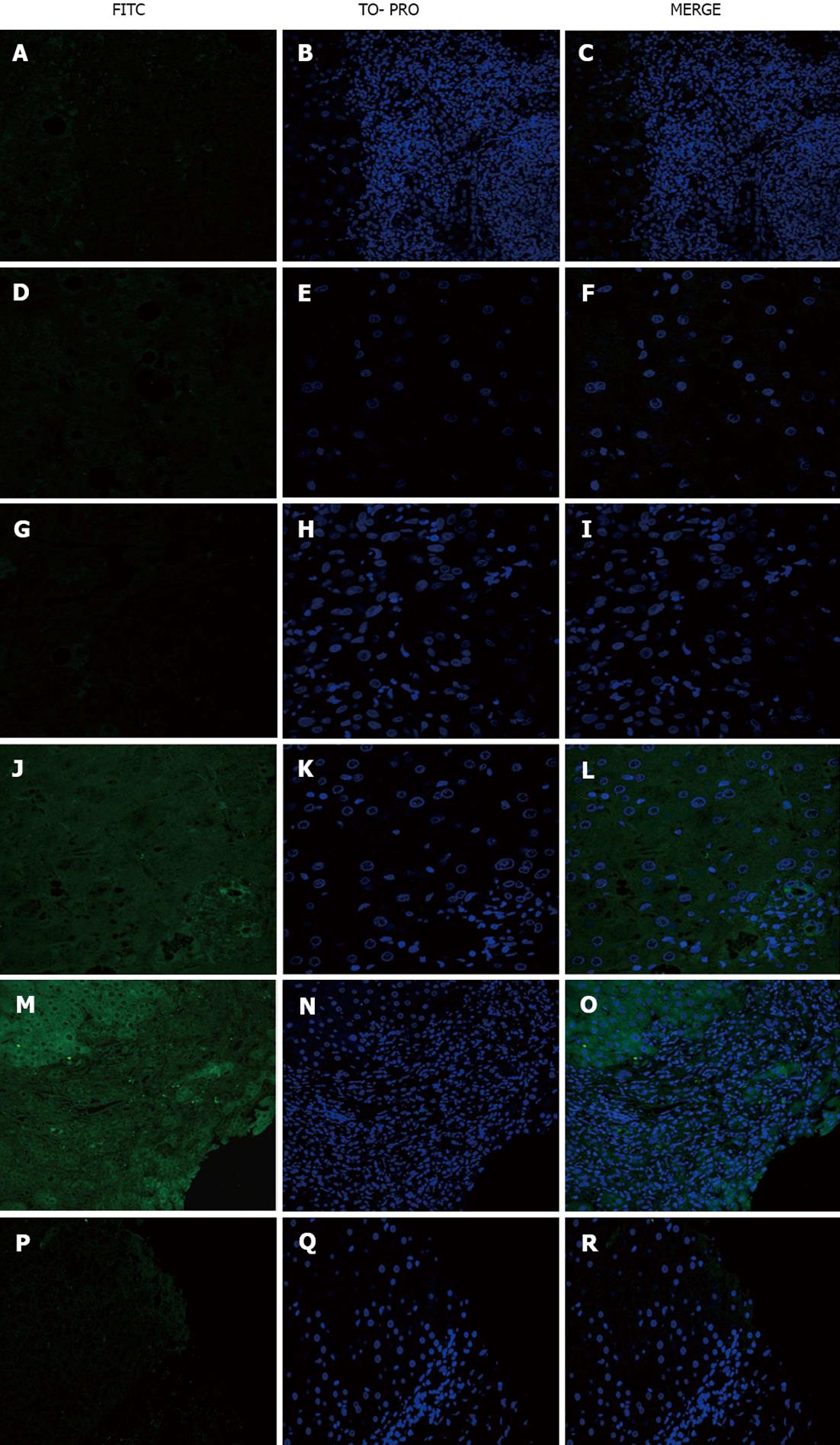
No staining was observed (Figure 2A-F).
No staining was observed when PBC sera were used on sections from the disease control group (Figure 2G-I). When PBC sera were used as a primary antibody on healthy control sections, weak positive staining of cholangiocytes in 3/21 (14.3%) PBC sera was observed (Figure 2J-L).
Steatohepatitis serum on PBC sections yielded positive staining of some hepatocytes and lymphocytes (Figure 2M-O) and no staining of viral hepatitis sections (Figure 2P-R).
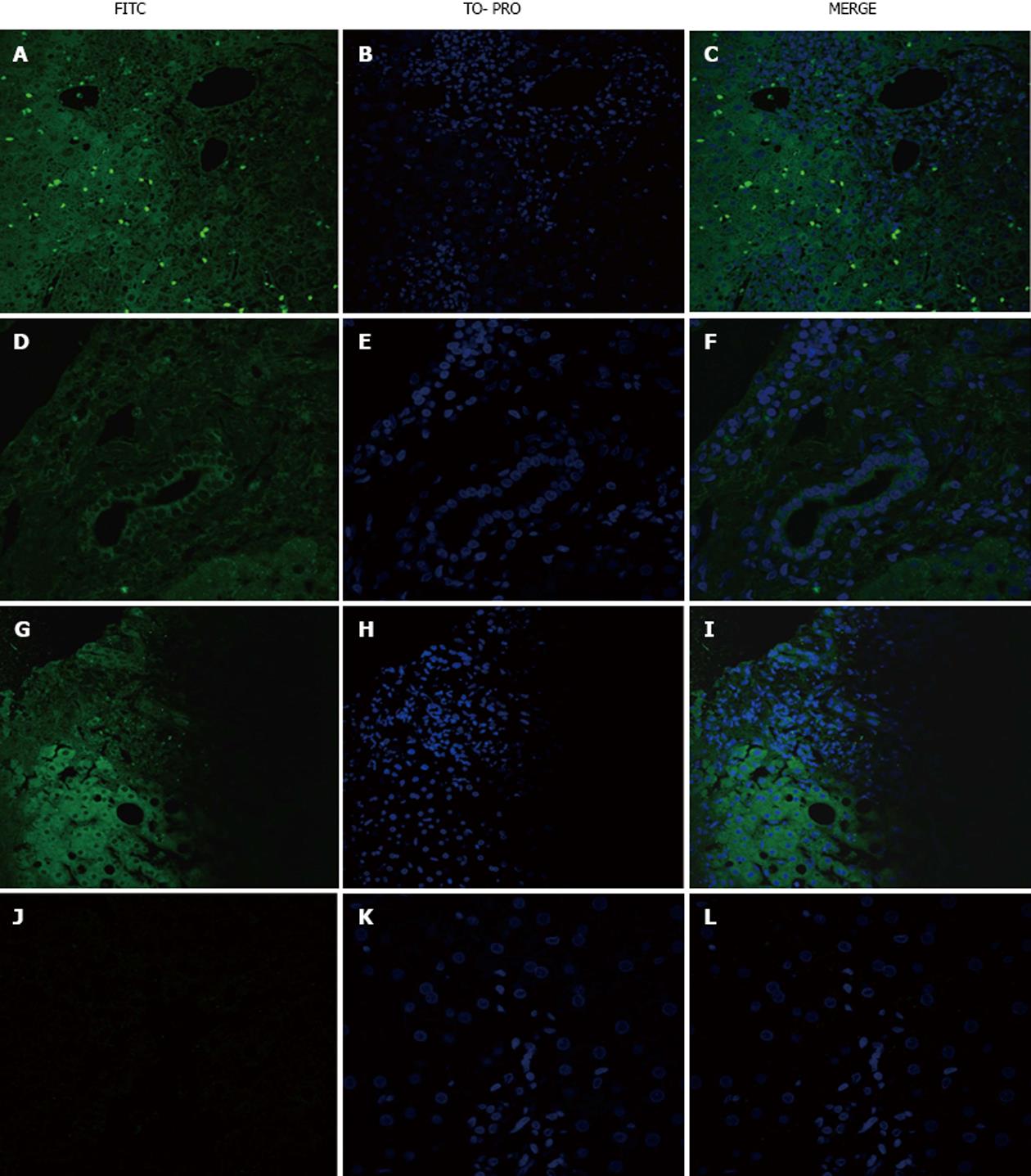
Incubation with HBV sera yielded positive staining of the hepatocytes, intra-sinusoidal and portal lymphocytes and cholangiocytes of some PBC (Figure 3A-C), HBV (Figure 3D-F) and AAH (Figure 3G-I) patients but not HCV patients (Figure 3J-L).
In the present study, for the first time in diseased liver tissue, we have identified sinusoidal cells as liver cells targeted by circulating autoantibodies in PBC patients. We previously hypothesized that the primary event in PBC is the excessive production of endothelin 2, possibly by sinusoidal cells[21,22].
The role of certain autoantibodies in PBC is well established from previous studies. Panels of mouse monoclonal antibodies and human combinatorial autoantibodies against specific antigens, such as members of the ODC family[4,6,25-27] or the gp210 antigen[19,28], have been used in paraffin-embedded liver sections, and their significance in diagnosis and prognosis has been verified. Tsuneyama et al[4] have demonstrated that both AMA-positive and AMA-negative PBC patients, but not controls, have abnormal expression of either PDC-E2 or a cross-reacting molecule in the apical region of the biliary epithelium.
Using in situ nucleic acid hybridization, Harada et al[29]. found that there were no increased levels of PDC-E2 mRNA in PBC livers. The researchers suggested that the increased levels of immunoreactive material either did not arise in BECs or were not derived from material encoded by the PDC-E2 gene sequence[29]. These data and the recurrence of such abnormal apical staining in liver allografts from PBC but not controls are most easily explained by the suggestion that the molecule at the apical surface of bile ducts in PBC tissue is not PDC-E2, but rather a molecule that bears a cross-reactive epitope. One possible source of such a molecular mimic may be infecting microorganisms, although no specific molecule from such organisms has been identified[7,27].
However, in PBC, as in many other so-called autoimmune diseases, there are many other autoantibodies whose significance is usually unknown[11].
In the present study, we used paraffin-embedded liver sections and homologous and heterologous sera from PBC patients to detect liver cells that are the targets of circulating antibodies. When homologous serum was used along with an IgG secondary antibody, 48% of PBC patients exhibited positive staining of lymphocytes, 38% in bile ducts, 33% in hepatocytes and 86% in sinusoidal cells of the liver. A difference in staining was found when IgM was used rather than IgG to detect autoantibodies in patients with PBC. The staining of sinusoidal cells was weak with IgG but very strong when an IgM secondary antibody was used.
This difference between IgM and IgG antibodies may have a dual explanation. First, patients with PBC express IgM more frequently[30,31] than do patients with other autoimmune hepatic diseases. Alternatively, as we have shown in our previous study, these differences between IgG and IgM depend on the cell type that is being used as a substrate[32]. When heterologous PBC sera were used, similar staining patterns were found, but the percentage was lower, and approximately 20% of the sinusoidal cells of PBC patients were positive. No staining was observed when PBC serum was incubated with liver biopsies from the disease controls.
The staining of the sinusoidal cells seemed to be specific for PBC patients because staining of lymphocytes, biliary epithelial cells and hepatocytes, but not sinusoidal cells, was observed in all other serum-tissue combinations that we used. Thus, sera from the disease control group incubated with either PBC liver or disease control liver showed that in chronic HBV, positive staining was observed in the lymphocytes of 17% of PBC patients, but not in the lymphocytes of patients with other liver diseases. In addition, the hepatocytes of 17% of patients with PBC and 20% of patients with other liver diseases were positive when incubated with chronic hepatitis B serum. The serum of a patient with steatohepatitis yielded very weak positive staining in lymphocytes and hepatocytes from certain patients with PBC but resulted no positive staining in the tissues of patients with other liver diseases.
In two earlier reports, antibodies against liver sinusoidal endothelial cells were described in both autoimmune hepatitis (AIH) and PBC. In the first report[33], sera from patients were incubated with isolated rat liver endothelial cells, and IgG bound to endothelial cells was found in 13% of patients with PBC. In a similar study, patient sera were incubated with isolated human liver sinusoidal endothelial cells, and 59% of PBC patients had reactive antibodies. Moreover, cells incubated with the F(ab)2 fragments of antibodies from either AIH or PBC patients were transformed into a vascular cell phenotype[34]. Since we used sections of liver tissue rather than isolated cells, our findings are not directly comparable with the results of these studies. However, a higher proportion of our patients showed circulating antibodies against sinusoidal cells compared with the proportion in both previous studies. This finding might indicate that these antibodies may recognize other sinusoidal cells, such as Kupffer cells, in addition to endothelial cells.
Our findings indicate that both patient specific and disease specific antibodies circulate in PBC. At the same time, non disease specific antibodies were found in PBC and chronic HBV but not in other chronic liver diseases. However, the findings of the present study do not answer the question of whether antibodies circulating in the serum of patients with chronic hepatitis B recognize the same epitopes as circulating antibodies in PBC; this question needs to be further examined. More detailed studies are also required to further elucidate the specific sinusoidal cell type(s) against which these antibodies are directed.
In conclusion, the present study shows that a large proportion of PBC patients have disease specific autoantibodies against liver sinusoidal cells. To our knowledge, this result has not been previously reported for diseased liver tissue. The target antigens of the sinusoidal cells and the specificity of these antibodies need further study. Moreover, the antibodies clinical significance requires clarification, along with clinical follow-up of a large cohort of patients.
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease of unknown etiology. The authors previously hypothesized that the primary initiating event in PBC may be the overproduction of endothelins by liver sinusoidal cells. The presence of autoantibodies against isolated rat and human liver endothelial cells have been described in several cases of PBC, but so far, such autoantibodies have not been described in diseased human liver tissue.
The research objective was to investigate the presence of disease specific autoantibodies against liver sinusoidal cells in PBC serum using human liver tissue as a substrate.
In two previous studies, isolated rat or human liver endothelial cells were used as substrates to detect the presence of specific serum autoantibodies in PBC. In this study, for the first time, indirect immunofluorescence staining was performed using human diseased liver tissues as the substrate and sera from PBC and other chronic liver disease patients as the source of primary antibody.
The findings of this study indicate the presence of disease specific autoantibodies directed against liver endothelial cells and possibly Kupffer cells in a large proportion of PBC patients. The exact specificity and functional significance of these antibodies remains to be further elucidated and might be related to the pathogenesis of this disease.
The authors explore the presence of autoantibodies directed against liver sinusoidal cells in PBC. For this, they evaluate the liver biopsies of patients with PBC, and from patients with other liver diseases.
P- Reviewers Montano-Loza AJ, Shimoda S S- Editor Zhai HH L- Editor A E- Editor Wu HL
| 1. | Selmi C, Invernizzi P, Zuin M, Podda M, Seldin MF, Gershwin ME. Genes and (auto)immunity in primary biliary cirrhosis. Genes Immun. 2005;6:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 922] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 3. | Surh CD, Ahmed-Ansari A, Gershwin ME. Comparative epitope mapping of murine monoclonal and human autoantibodies to human PDH-E2, the major mitochondrial autoantigen of primary biliary cirrhosis. J Immunol. 1990;144:2647-2652. [PubMed] |
| 4. | Tsuneyama K, Van De Water J, Van Thiel D, Coppel R, Ruebner B, Nakanuma Y, Dickson ER, Gershwin ME. Abnormal expression of PDC-E2 on the apical surface of biliary epithelial cells in patients with antimitochondrial antibody-negative primary biliary cirrhosis. Hepatology. 1995;22:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Yip TT, Van de Water J, Gershwin ME, Coppel RL, Hutchens TW. Cryptic antigenic determinants on the extracellular pyruvate dehydrogenase complex/mimeotope found in primary biliary cirrhosis. A probe by affinity mass spectrometry. J Biol Chem. 1996;271:32825-32833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Van de Water J, Turchany J, Leung PS, Lake J, Munoz S, Surh CD, Coppel R, Ansari A, Nakanuma Y, Gershwin ME. Molecular mimicry in primary biliary cirrhosis. Evidence for biliary epithelial expression of a molecule cross-reactive with pyruvate dehydrogenase complex-E2. J Clin Invest. 1993;91:2653-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Migliaccio C, Nishio A, Van de Water J, Ansari AA, Leung PS, Nakanuma Y, Coppel RL, Gershwin ME. Monoclonal antibodies to mitochondrial E2 components define autoepitopes in primary biliary cirrhosis. J Immunol. 1998;161:5157-5163. [PubMed] |
| 8. | Courvalin JC, Worman HJ. Nuclear envelope protein autoantibodies in primary biliary cirrhosis. Semin Liver Dis. 1997;17:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Drebber U, Mueller JJ, Klein E, Kasper HU, Schulze F, Schardt K, Quasdorff M, Schulte S, Odenthal M, Dienes HP. Liver biopsy in primary biliary cirrhosis: clinicopathological data and stage. Pathol Int. 2009;59:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Szostecki C, Guldner HH, Will H. Autoantibodies against “nuclear dots” in primary biliary cirrhosis. Semin Liver Dis. 1997;17:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Bogdanos DP, Komorowski L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta. 2011;412:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Granito A, Yang WH, Muratori L, Lim MJ, Nakajima A, Ferri S, Pappas G, Quarneti C, Bianchi FB, Bloch DB. PML nuclear body component Sp140 is a novel autoantigen in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Sternsdorf T, Guldner HH, Szostecki C, Grötzinger T, Will H. Two nuclear dot-associated proteins, PML and Sp100, are often co-autoimmunogenic in patients with primary biliary cirrhosis. Scand J Immunol. 1995;42:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Courvalin JC, Lassoued K, Bartnik E, Blobel G, Wozniak RW. The 210-kD nuclear envelope polypeptide recognized by human autoantibodies in primary biliary cirrhosis is the major glycoprotein of the nuclear pore. J Clin Invest. 1990;86:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Wesierska-Gadek J, Hohenuer H, Hitchman E, Penner E. Autoantibodies against nucleoporin p62 constitute a novel marker of primary biliary cirrhosis. Gastroenterology. 1996;110:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, Maggioni M, Meroni PL, Penner E, Wesierska-Gadek J. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol. 2001;34:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Muratori P, Muratori L, Ferrari R, Cassani F, Bianchi G, Lenzi M, Rodrigo L, Linares A, Fuentes D, Bianchi FB. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Nakamura M, Takii Y, Ito M, Komori A, Yokoyama T, Shimizu-Yoshida Y, Koyabu M, Matsuyama M, Mori T, Kamihira T. Increased expression of nuclear envelope gp210 antigen in small bile ducts in primary biliary cirrhosis. J Autoimmun. 2006;26:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Sfakianaki O, Koulentaki M, Tzardi M, Tsangaridou E, Theodoropoulos PA, Castanas E, Kouroumalis EA. Peri-nuclear antibodies correlate with survival in Greek primary biliary cirrhosis patients. World J Gastroenterol. 2010;16:4938-4943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Dimoulios P, Kolios G, Notas G, Matrella E, Xidakis C, Koulentaki M, Tsagarakis N, Kouroumalis A, Kouroumalis E. Ursodeoxycholic acid reduces increased circulating endothelin 2 in primary biliary cirrhosis. Aliment Pharmacol Ther. 2005;21:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Kouroumalis E, Notas G. Pathogenesis of primary biliary cirrhosis: a unifying model. World J Gastroenterol. 2006;12:2320-2327. [PubMed] |
| 23. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 528] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Voumvouraki A, Koulentaki M, Tzardi M, Sfakianaki O, Manousou P, Notas G, Kouroumalis E. Increased ΤGF-β3 in primary biliary cirrhosis: an abnormality related to pathogenesis? World J Gastroenterol. 2010;16:5057-5064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Tsuneyama K, Van de Water J, Leung PS, Cha S, Nakanuma Y, Kaplan M, De Lellis R, Coppel R, Ansari A, Gershwin ME. Abnormal expression of the E2 component of the pyruvate dehydrogenase complex on the luminal surface of biliary epithelium occurs before major histocompatibility complex class II and BB1/B7 expression. Hepatology. 1995;21:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Tsuneyama K, Van de Water J, Nakanuma Y, Cha S, Ansari A, Coppel R, Gershwin ME. Human combinatorial autoantibodies and mouse monoclonal antibodies to PDC-E2 produce abnormal apical staining of salivary glands in patients with coexistent primary biliary cirrhosis and Sjögren’s syndrome. Hepatology. 1994;20:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Van de Water J, Gerson LB, Ferrell LD, Lake JR, Coppel RL, Batts KP, Wiesner RH, Gershwin ME. Immunohistochemical evidence of disease recurrence after liver transplantation for primary biliary cirrhosis. Hepatology. 1996;24:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Rong G, Zhong R, Lleo A, Leung PS, Bowlus CL, Yang GX, Yang CY, Coppel RL, Ansari AA, Cuebas DA. Epithelial cell specificity and apotope recognition by serum autoantibodies in primary biliary cirrhosis. Hepatology. 2011;54:196-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Harada K, Sudo Y, Kono N, Ozaki S, Tsuneyama K, Gershwin ME, Nakanuma Y. In situ nucleic acid detection of PDC-E2, BCOADC-E2, OGDC-E2, PDC-E1alpha, BCOADC-E1alpha, OGDC-E1, and the E3 binding protein (protein X) in primary biliary cirrhosis. Hepatology. 1999;30:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Daniels JA, Torbenson M, Anders RA, Boitnott JK. Immunostaining of plasma cells in primary biliary cirrhosis. Am J Clin Pathol. 2009;131:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Moreira RK, Revetta F, Koehler E, Washington MK. Diagnostic utility of IgG and IgM immunohistochemistry in autoimmune liver disease. World J Gastroenterol. 2010;16:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Tsangaridou E, Polioudaki H, Sfakianaki R, Samiotaki M, Tzardi M, Koulentaki M, Panayotou G, Kouroumalis E, Castanas E, Theodoropoulos PA. Differential detection of nuclear envelope autoantibodies in primary biliary cirrhosis using routine and alternative methods. BMC Gastroenterol. 2010;10:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Han K, Hashimoto N, Ikeda Y, Shiratori Y, Kato H, Toda G, Komatsu Y, Yamada H, Tanaka A, Kurokawa K. Occurrence of antibody against rat hepatic sinusoidal endothelial cells in sera of patients with autoimmune hepatitis. Dig Dis Sci. 1995;40:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, Hultenby K, Christensson B, Ericzon BG, Holgersson J. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 2003;163:1275-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |