INTRODUCTION
The metabolic syndrome (MS) is a multicomponent clinical entity with a prevalence of about 20%-25%. Several definitions of MS have been suggested, including those of the World Health Organization[1,2], the European group for the study of insulin resistance[3], the National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III)[4] and the International Diabetes Federation[5,6]. Although these definitions share many common features, some criteria are different. According to the most widely accepted diagnostic definition of NCEP ATP III, metabolic syndrome is diagnosed if three or more of the following parameters are present: waist circumference greater than 102 cm in men and 88 cm in women, serum triglyceride level higher than 150 mg/dL (1.7 mmol/L), HDL-C level lower than 40 mg/dL (1.04 mmol/L) in men and lower than 50 mg/dL in women, blood pressure higher than 130/85 mmHg and fasting glucose level at least 110 mg/dL (6.1 mmol/L). The most important complication of metabolic syndrome is the increased (approximately doubled) risk of the development of ischemic heart disease.
The metabolic syndrome is much more than the simple sum of the symptoms. It is a multifactorial disease, which is not due to a single genetic defect and lacks a unique pathomechanism. Its complex etiology likely includes inherited predisposition, intrauterine effects, lifestyle factors and excessive calorie intake. Central (omental) adiposity and the pro-inflammatory conditions in the adipose tissue have emerged as crossing points of these etiological factors.
The phenotype and symptoms (e.g. serum lipid pattern and derangements of carbohydrate metabolism) of the metabolic syndrome are remarkably similar to those of Cushing syndrome, which is caused by excessive glucocorticoid production or medication. This clinical observation leads to the hypothesis that glucocorticoids might play a role in the pathogenesis of the metabolic syndrome. Although plasma cortisol levels are normal, both cortisol excretion and total body cortisol production were found to be increased in patients with abdominal obesity[7]. Preclinical data on rodent models proved the role of glucocorticoids in obesity[8]. The absence of elevated serum cortisol levels both in human and rodent metabolic syndrome suggests the existence of a local glucocorticoid effect in the background of this phenomenon. The activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) has appeared in the focus of the pathogenesis recently.
BIOCHEMISTRY OF THE GLUCOSE-6-PHOSPHATE TRANSPORTER- HEXOSE-6-PHOSPHATE DEHYDROGENASE-11βHSD1 SYSTEM
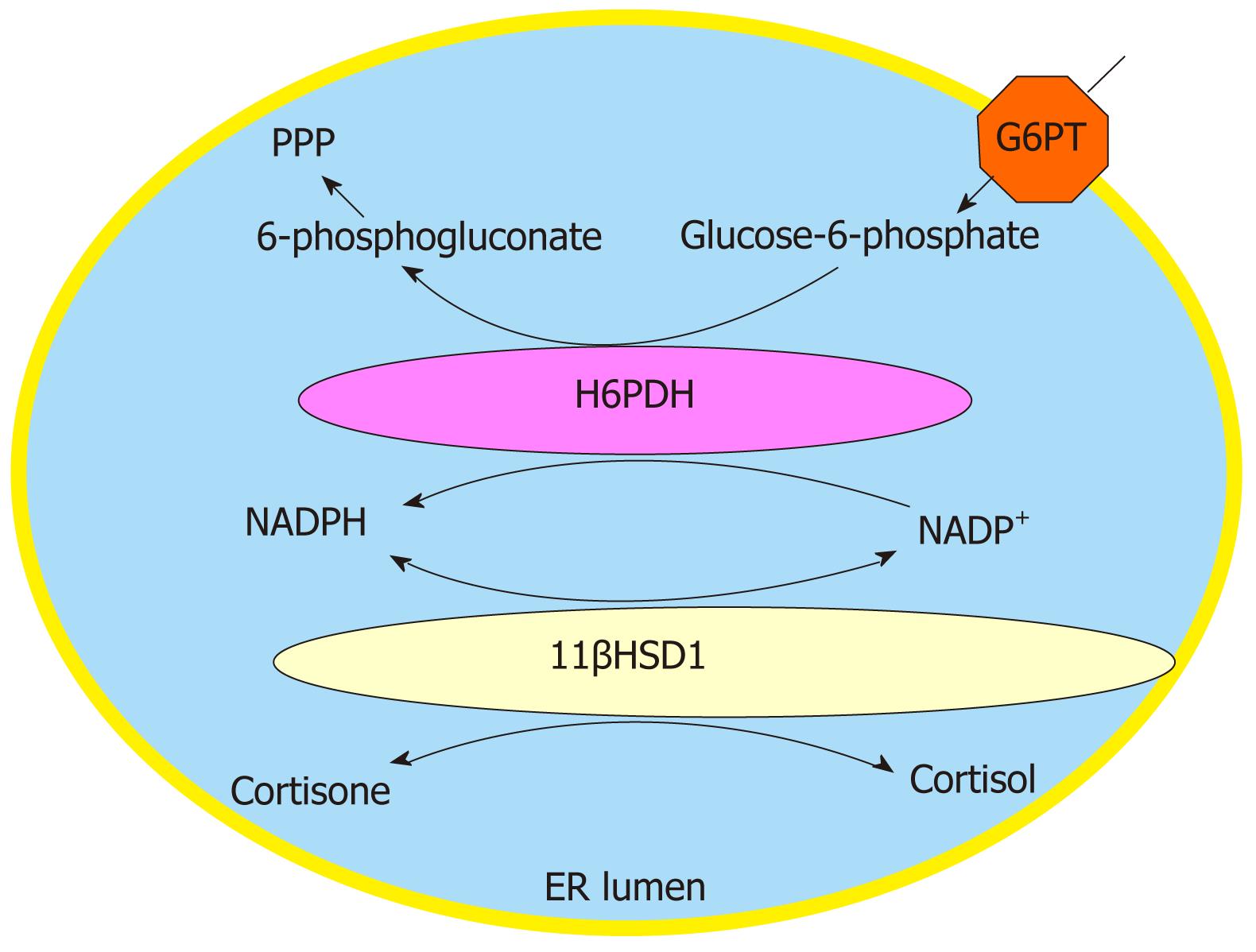
11βHSD1 is a luminal enzyme of the endoplasmic reticulum (ER), which is expressed in many organs and tissues. The enzyme expressed in the liver and adipose tissue plays presumably the most important role in the pathogenesis of the metabolic syndrome. Its main function is the regulation and enhancement of local glucocorticoid effect at tissue level. 11βHSD1 catalyzes the reversible interconversion of cortisone and cortisol in vitro, by using nicotinamide adenine dinucleotide phosphate (NADP)+ or NADPH as a cofactor, which makes the activity sensitive to modifications in cofactor supply. The fact that 11βHSD1 acts exclusively as a reductase in vivo suggests a high luminal NADPH/NADP+ ratio in the ER. This ratio is generated by hexose-6-phosphate dehydrogenase (H6PDH), another luminal enzyme. H6PDH seems to be the major, if not the only, enzyme responsible for NADP+ reduction in the ER lumen[9]. This tandem enzyme catalyzes the first two steps of the pentose-phosphate pathway, i.e. the formation of 6-phosphogluconate from glucose-6-phosphate. Besides their colocalization and direct physical interaction[10], cooperativity between 11βHSD1 and H6PDH was proved by biochemical[11], as well as by genetic[12] approaches. The activities of the two enzymes are linked by cofactor sharing, i.e. they mutually generate cofactors for each other. Their physical interaction and functional cooperation allow cortisone reduction despite the otherwise oxidative environment in the ER lumen. In agreement with in vivo observations, the existence of a dominantly reduced intraluminal pyridine nucleotide pool was reported in the ER[13,14].
The substrate supply for and the specificity of H6PDH are ensured by glucose-6-phosphate transporter (G6PT), an ER membrane protein. In the ER of hepatocytes, adipocytes and neutrophil granulocytes (and possibly a number of other cells), 11βHSD1 can be considered as a component of a complex system, which also includes H6PDH and G6PT (Figure 1).
Figure 1 The glucose-6-phosphate transporter - hexose-6-phosphate dehydrogenase - 11β-hydroxysteroid dehydrogenase type 1 triad.
Local glucocorticoid activation is catalyzed by a triad of the endoplasmic reticulum, composed by glucose-6-phosphate-transporter, hexose-6-phosphate dehydrogenase and 11β-hydroxysteroid dehydrogenase type 1. The role of their cooperation is the enhancement of local glucocorticoid effect. PPP: pentose phosphate pathway; G6PT: Glucose-6-phosphate transporter; H6PDH: Hexose-6-phosphate dehydrogenase; 11βHSD1: 11β-hydroxysteroid dehydrogenase type 1; NADP: Nicotinamide adenine dinucleotide phosphate; ER: Endoplasmic reticulum.
The stringent cooperation of the members of the G6PT-H6PDH-11βHSD1 system can convert metabolic effects to an endocrine response; thus, the triad can act as a nutrient sensor[15,16]. Intracellular glucose-6-phosphate accumulation can accelerate the concerted action of the G6PT-H6PDH-11βHSD1 triad, which promotes intracellular glucocorticoid activation. Beyond its physiological sensor role, the triad also detects overnutrition. It can participate in the pathomechanism of gluco-, lipo-, and glucolipotoxicity[15,17-20]. Excessive glucose and fatty supply activates the unfolded protein response and induces ER stress by an unknown mechanism; local glucocorticoid activation might represent an alternative signaling pathway[15,16].
As it can be supposed from the variety of symptoms of human metabolic syndrome, the G6PT-H6PDH-11βHSD1 triad present in different cell types and tissues can contribute to the development of this complex disease in various ways and to different extents. The existence of the triad has been proved in hepatocytes[11], neutrophil granulocytes[19] and adipocytes[21] and the system is presumably present also in other cell types. Its exact role in the pathogenesis of the metabolic syndrome has been best clarified in adipose tissue.
TISSUE SPECIFIC EXPRESSION AND COOPERATION OF THE G6PT-H6PDH-11βHSD1 TRIAD
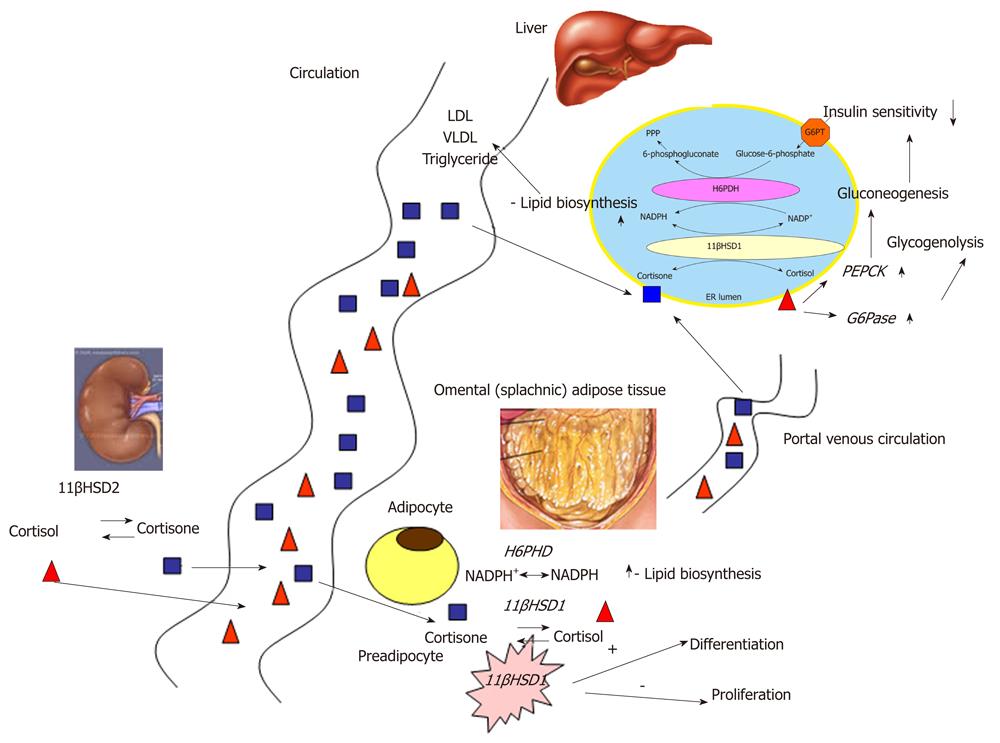
The hepatic and adipose G6PT-H6PDH-11βHSD1 triad plays a crucial role in the pathogenesis of metabolic syndrome (Figure 2). Blood circulation, especially the portal venous system, keeps the triads of different localization connected by transporting glucocorticoid metabolites. Glucocorticoid supply is an important determinant of the activity. Glucocorticoids are synthesized de novo in the adrenal gland; however, glucocorticoid precursors can also derive from type 2 isoform of 11β-hydroxysteroid dehydrogenase (11βHSD2) activity. 11βHSD2 is present primarily in the kidney[22] and other mineralocorticoid target tissues, such as colon and salivary gland[23,24]. Its physiological role is to prevent the action of glucocorticoids through mineralocorticoid receptor by the conversion of cortisol to inactive cortisone (Figure 2).
Figure 2 The participation of the glucose-6-phosphate transporter-hexose-6-phosphate dehydrogenase -11β-hydroxysteroid dehydrogenase type 1 system in the pathogenesis of the metabolic syndrome.
The different, tissue specific localization of G6PT-H6PDH-11βHSD1 triad contributes to the pathogenesis of the metabolic syndrome both by its effect on local, tissue specific lipid and carbohydrate metabolism and by the systemic connection between the liver and the omental adipose tissue. Renal 11βHSD2 activity plays a crucial role in the maintenance of systemic connection. PPP: pentose phosphate pathway; G6PT: Glucose-6-phosphate transporter; H6PDH: Hexose-6-phosphate dehydrogenase; 11βHSD1: 11β-hydroxysteroid dehydrogenase type 1; NADP: Nicotinamide adenine dinucleotide phosphate; ER: Endoplasmic reticulum; VLDL: Very low density lipoprotein; PEPCK: Phosphoenolpyruvate carboxykinase.
It has been recently proved that omental 11βHSD2 activity is also an important substrate supply for hepatic 11βHSD1 activity[25]. Another possible mechanism for the omental cortisone production has been forwarded: 11βHSD1 activity changes during the differentiation of preadipocytes. The existence of 11βHSD1 in adipose tissue was proved more than ten years ago with the conversion of radioactively labeled cortisone to cortisol in abdominal adipose tissue. Both the activity and expression of the enzyme were higher in comparison with subcutaneous adipocytes[26]. Abdominal obesity is known as the Cushing’s disease of the omentum[27]. The existence of the G6PT-H6PDH-11βHSD1 system was found in adipocytes as well[21].
However, the enzyme was suggested to function in a bidirectional manner in adipose tissue; the direction is determined by the developing stage of the preadipocyte or adipocyte. While cortisol oxidation dominates in preadipocytes, cortisone reduction is predominant in matured adipocytes. Inactive glucocorticoid metabolites play an important role in the formation and hence in the localization of adipose tissue: they inhibit the development of adipocytes from preadipocytes. If 11βHSD1 acts as a dehydrogenase, it inactivates cortisol and corticosterone, which leads to the inhibition of preadipocyte proliferation[28,29]. When preadipocytes start to differentiate, the reductase activity of the enzyme is progressively increased, which leads to an accelerating cortisol production that drives adipocyte differentiation[30,31,27]. This mechanism leads to the special localization of adipose tissue in Cushing syndrome and in the metabolic syndrome. The omentum contains preadipocytes that only start to differentiate under excessive cortisol effects leading to central or visceral adiposity. Increase in H6PDH expression and its association with the increased 11βHSD1 activity was suggested to be present in the background of adipocyte differentiation[32].
Adipocyte differentiation is enhanced in 11βHSD1 overexpressing mice as well[33]. Direction of enzyme activity depends on the developmental stage, as it was proved in rodent adipocyte cell lines[34,35]. However, recent findings showed that adipose H6PDH activity is constantly high in human adipose-derived mesenchymal stem cells during differentiation, which suggests that other factors can be responsible for the dehydrogenase-to-reductase switch in 11βHSD1 activity[36].
HEPATIC G6PT-H6PDH-11βHSD1 SYSTEM AND THE PATHOGENESIS OF THE METABOLIC SYNDROME
As the main site of lipid and carbohydrate metabolism, the liver has a crucial role in the pathogenesis of the metabolic syndrome. Metabolic effects of glucocorticoids, as well as the alterations caused by excessive blood glucocorticoid concentrations are widely known. However, the local enhancement of glucocorticoid effect in liver and its role in the metabolic syndrome has been recently investigated.
Glucocorticoid effect in the liver
The presence of glucocorticoid receptor and of the above detailed components of the prereceptorial glucocorticoid activating system has been reported in the liver. Besides the adrenal gland, other organs also contribute significantly to cortisol production. It has been recently proved that the main site of splanchnic cortisol production in obese nondiabetic human is the liver[25]. Receptorial glucocorticoid effects are well known in the liver: their main outcome is mobilization of nutrients from the depots supporting the maintenance of normoglycemia in stress and starvation[37]. Stimulation of gluconeogenesis via increasing the expression of phosphoenolpyruvate carboxykinase, the rate-limiting enzyme, is one of the most important elements of this action. It also activates glucose production from glycogen via induction of glucose-6-phosphatase.
11βHSD1 and its functioning in the liver
11βHSD1 expression and enzyme activity have been described both in rodent and human liver. The first description of 11βHSD1 in human liver showed the highest enzyme activity around the vena centralis[38]. Direction, rather than localization, of the enzyme activity has a major importance in pathophysiology. Although in isolated and perfused rat liver, both reductase and dehydrogenase activities were described[38,39], in human and rat liver cell cultures, the enzyme was found to be acting exclusively as a reductase. Reductase activity of 11βHSD1 that can be detected in intact cells is responsible for its main pathophysiological effects: decrease in insulin sensitivity and stimulation of hepatic gluconeogenesis via enhancement of local glucocorticoid effect. Both transgene 11βHSD1 deficient mice and the selective inhibition of the enzyme[40] proved that decreased glucocorticoid effect caused by the impaired enzyme activity leads to increased insulin sensitivity in hepatocytes.
Hepatic 11βHSD1 in the metabolic syndrome –11βHSD1 transgenic animals
Growing data suggest the role of hepatic 11βHSD1 in the development of abnormalities in carbohydrate and lipid metabolism that occur in the metabolic syndrome. Therefore, the expression and activity of this enzyme are promising therapeutic targets for the future.
The impact of hepatic 11βHSD1 on enhancing local glucocorticoid effect and in the pathogenesis of the metabolic syndrome was proved by using 11βHSD1 knockout mice[41]. 11βHSD1-nul mice develop normally, become fertile, and their blood pressure is in the normal range. After adrenalectomy, the 11βHSD1 knockout mice are unable to convert 11-dehydrocorticosterone to corticosterone. Despite the elevated serum 11-dehydrocorticosterone levels, the intracellular glucocorticoid regeneration is decreased so its antagonism on insulin effect is impaired as well. This is manifested in the impairment of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase activities, which leads to a decrease in stress-induced hyperglycemia. The observation that 11βHSD1 knockout mice are resistant to the development of the metabolic syndrome, despite high-fat feeding, is another evidence for the role of the enzyme in the pathogenesis. Although the serum corticosterone level is mildly elevated in these animals, the intracellular level is significantly decreased. Their serum lipid profile shows a cardioprotective pattern: serum triglyceride level is decreased and the serum HDL level is increased[42]. This pattern could be influenced neither by high-fat feeding nor by permanent hyperglycemia induced by chronic stress. Expression of the enzymes of gluconeogenesis is decreased, while expression of the enzymes of lipid peroxidation is highly increased. These data show that 11βHSD1 inactivation protects against the development of the pathophysiological alterations that are responsible for the metabolic syndrome.
The role of 11βHSD1 in the regulation of gluconeogenesis was proved by the selective inhibition of the enzyme as well. The seven-day-long carbenoxolone (non-selective 11βHSD inhibitor) treatment of rodents significantly decreased phosphoenolpyruvate carboxykinase and glucose-6-phosphatase expression at mRNA level in hyperglycemic KKAγ mice strain, which leads to a decrease in the circulating serum insulin level[43]. Selective inhibition of the enzyme leads to increased insulin sensitivity in type 2 diabetes mellitus model mice[44].
In contrast, transgenic mice overexpressing 11βHSD1 selectively in the liver show all symptoms of the metabolic syndrome except for obesity. Transgenic mice overexpressing 11βHSD1 selectively in the adipose tissue show all symptoms of the metabolic syndrome: these animals are obese, have hypertension, insulin resistance and dyslipidemia[33,45]. The corticosterone, serum leptin, tumor necrosis factor-alpha (TNFα) concentrations and lipoprotein lipase mRNA level in their adipose tissue are elevated. Hypertension is due to elevated angiotensinogen level. These data show that overexpression of 11βHSD1 in adipocytes is mainly responsible for the development of the typical obesity in metabolic syndrome, while hepatic 11βHSD1 contributes to the establishment of the metabolic alterations of the disease.
These data suggest, independently from the exact mechanism, that decreased or abolished activity of 11βHSD1 protects against the development of the metabolic syndrome. On the other hand, overexpression of 11βHSD1 is an underlying condition in the pathomechanism of the disease.
Hepatic 11βHSD1 in animal models of type 2 diabetes and hereditary hyperlipidemia
Studies on expression and activity in hereditary hyperlipidemic and diabetic animal models provided important results strongly supporting the contribution of the enzyme in the pathogenesis of the metabolic syndrome. Investigations on other model animals of type 2 diabetes and the hereditary hyperlipidemic model revealed the participation of 11βHSD1 in the pathogenesis, as also observed in transgenic animals.
Experiments on type 2 diabetic Goto-Kakizaki rats helped to clarify the role of 11βHSD1 in the development of the metabolic syndrome. Goto-Kakizaki rats show all of the metabolic, hormonal and vascular abnormalities of human type 2 diabetes. The animals, unlike many other type 2 diabetes models, have a lean phenotype. Hepatic 11βHSD1 expression and enzyme activity are elevated, but expression and activity of the adipose enzyme are decreased[14]. These data suggest that this combination of expression and enzyme activity of 11βHSD1 can lead to and is responsible for the lean phenotype. To the contrary, Zucker fat rats, the obese model of human metabolic syndrome, show an altered pattern of 11βHSD1 activity and expression compared to the control Zucker lean animals and to Goto-Kakizaki rats. In Zucker fat rats, expression of 11βHSD1 is increased in the adipose tissue but decreased in the liver. The reason for this difference in enzyme activities remains unclear: the role of insulin, growth factors and cytokines were proposed but none of them could be proved[46,22].
Besides the liver and adipose tissue, the hippocampus also shows decreased 11βHSD1 activity in obese Zucker rats: this phenomenon correlates to the abnormalities in hypothalamus-hypophysis-adrenal axis that is known to have a role in human obesity[47].
Several data from different hyperglycemic and hyperinsulinemic mice strains suggest a possible protective effect of the downregulation of 11βHSD1 against the metabolic syndrome. The expression of hepatic 11βHSD1 is decreased in the leptin deficiency model ob/ob mice[48]. However, in type 2 diabetes model db/db mice, hepatic 11βHSD1 activity is elevated. This is followed by enhanced glucocorticoid receptor expression, elevated phosphoenolpyruvate carboxykinase level and increased insulin sensitivity[49]. In the KKAγ polygenic type 2 diabetes model, adult animals have hyperglycemia, hyperinsulinemia and glucose intolerance. Its hepatic 11βHSD1 expression and activity are decreased compared to the non-diabetic control. In a polygenic metabolic syndrome animal model[50], the body fat content of the “fat” animals was 21%, while in the lean animals it was 4%. The fat animals developed the metabolic syndrome with insulin resistance, fatty degeneration of the liver and hypertension. In fat animals, the expression of adipose 11βHSD1 and serum glucocorticoid levels are impaired, but the expression of the hepatic enzyme is increased. Despite a high-fat diet, the metabolic syndrome does not develop in lean animals. Hereditary hypertriglyceridemic Prague rats show elevated 11βHSD1 activity. Lipid content of the liver is highly elevated compared to normal control. 11βHSD1 inhibition seems to have no effect on hepatic lipid content[51]. Inhibition of 11βHSD1 in diet-induced obesity model rat leads to reduced hepatic very low density lipoprotein secretion, which improves hypertriglyceridemia[52]. Carbenoxolone treatment of hereditary hypertriglyceridemic rat strain shows the same alteration in lipid metabolism[53].
These data show that the changes of hepatic 11βHSD1 activity in obese animals may be a protective mechanism against the development of metabolic abnormalities that lead to type 2 diabetes. Downregulation of hepatic 11βHSD1 in obese rats is due to elevated serum glucocorticoid levels rather than to insulin resistance alone; therefore, it cannot be prevented by oral antidiabetic drugs (thiazolidinediones or metformin) in Zucker rats[46]. Nevertheless, the exact underlying mechanisms remain to be elucidated. It is also clarified in type 2 diabetes and metabolic syndrome model animals that hepatic 11βHSD1 enzyme is mainly responsible for the metabolic abnormalities (insulin resistance, serum lipid profile alterations etc), while the adipose enzyme is responsible for the phenotypical alterations (abdominal obesity) characteristic to the disease.
Human hepatic 11βHSD1 in the metabolic syndrome
The role of 11βHSD1 in human metabolic syndrome is uncertain in many points and controversial data appear in the literature[54]. Serum glucocorticoid levels in patients with metabolic syndrome are in normal range or only mildly elevated, which supports the role of local enhancement of glucocorticoid action by 11βHSD1. In human individuals, the whole body 11βHSD1 activity can only be monitored indirectly and noninvasively, by the measurement of 24 h urine tetrahydrocortisol and tetrahydrocortisone levels and cortisone/cortisol ratio. Many authors reported correlation between body mass index and 11βHSD1 activity: some of them found a positive[55-57], while others reported a negative correlation[58]. Some authors did not find correlation between BMI and enzyme activity[59-61]. However, increased urinary cortisone/cortisol ratio was found in women with increased abdominal fat compared to peripheral distribution of adipose tissue[62].
The role of different tissue specific 11βHSD1 enzymes was investigated in the metabolic syndrome: just like the non-human models, the hepatic and adipose tissue enzymes are the most important. Ethical burdens limit the possibilities of measuring different tissue-specific 11βHSD1 enzymes in human individuals: adipose enzyme can be examined via fat biopsies of the subcutaneous fat depot, but human studies based on sample taking from the omental tissue, as well as liver biopsies, are rarely available and do not provide sufficient data.
The activity of hepatic 11βHSD1 negatively correlates to body mass index in human individuals. Impairment of enzyme activity leads to decreased hepatic glucose production and increased insulin sensitivity[55]. It has been recently proved that hepatic 11βHSD1 activity negatively correlates with abdominal adipose tissue area, and the expression positively correlates with phosphoenolpyruvate carboxykinase expression[63]. The lack of impairment of hepatic 11βHSD1 activity in type 2 diabetic obese individuals[64] is an intriguing observation. The absence of hepatic 11βHSD1 downregulation in obese diabetics emphasizes its possible role in pathogenesis. This raises the hypothesis that inhibition of 11βHSD1 in obese people who develop impaired glucose tolerance could protect from progression to type 2 diabetes.
Regulation of 11βHSD1 in liver
Many factors can influence hepatic 11βHSD1 activity. In rodent liver, estrogen and insulin decrease the activity of the enzyme[65,66]. Growth factors (TGFβ, bFGF, EGF, HGF) seem to be ineffective[38]. Reductase activity of the enzyme was inhibited by insulin and IGF-1, while increased by dexamethasone in a rat hepatoma cell line[67]. The stimulating effect of interleukin (IL)-1, IL-2, IL-5, IL-6, IL-13, leptin, estradiol and gonadotropins on 11βHSD1 activity was proved as well[54].
In the human hepatoma cell line, TNFα, IL-1β increases and the clinically used oral antidiabetic PPARγ agonist significantly decreases the transcriptional activity of hepatic 11βHSD1 gene[68]. This phenomenon was further supported by studies on transgenic mice overexpressing TNFα: 11βHSD1 mRNA level and activity are elevated in their livers[69].
CEBPα transcription factor regulates hepatic 11βHSD1[70]. The exact mechanism of this regulatory process was recently revealed: TNFα-induced transcription of 11βHSD1 gene acts via the p38 MAPK pathway in HepG2 cell line[69]. Besides CEBPα, CEBPβ has an important role in the control of basal 11βHSD1 transcription too. Thyroxin influences both the transcription and the activity of the enzyme[71,72]. Metyrapone inhibits 11βHSD1 activity in sheep liver[73]. The mechanism underlying the increase of 11βHSD1 activity in liver cirrhosis remains unclear [74].
The exact roles of these numerous factors having impact on 11βHSD1 activity in cell lines and different animal models have not been totally clarified in the pathogenesis of metabolic syndrome. Some of them provide a possible new therapeutic option in the future or help to understand the exact mechanism of clinically used antidiabetic and antihyperlipidemic drugs.
Options for therapeutic intervention at hepatic 11βHSD1
Carbenoxolone is the most widely examined non-selective inhibitor of 11βHSD. Results on animal models and human individuals proved its positive effect on metabolic alterations: it increases insulin sensitivity, decreases hepatic gluconeogenesis, serum cholesterol and improves triglyceride profile[75-78].
Although human data are not available yet, inhibition of hepatic 11βHSD1 with newly developed targeted drugs (compound 544 Merck, BVT2733 biovitrum) seems to be effective in animal models: both compounds increase hepatic insulin sensitivity and decrease serum triglyceride and cholesterol levels. Besides this effect, compound 544 Merck decreases fasting glucose level, food intake and adipose tissue mass[79]. BVT2733 seems to be effective in lowering hepatic gluconeogenesis and plasma glucose and insulin levels[80,43,44,54]. These compounds have been applied mainly in the preclinical phase or human phase 1 drug development studies, so little data are available about their efficacy and safety. Their possible clinical application is now a future goal in the pharmaceutical industry.
H6PDH
The exact role of H6PDH in obesity and metabolic syndrome is not fully elucidated. It is known that diet and macronutrient composition can influence glucocorticoid metabolism, which might be due to the impact of carbohydrate intake on the activity of the pentose phosphate pathway. Sucrose ingestion increases both 11βHSD1 and H6PDH mRNA levels in mesenteric fat, while it decreases 11βHSD1 and increases H6PDH mRNA levels in liver. These observations support the hypothesis that increased 11βHSD1 activity in the adipose tissue contributes to sucrose-induced obesity[81].
The symptoms observed in H6PDH knockout mice and a decreased negative feedback on the hypothalamic–pituitary–adrenal axis show the importance of H6PDH in the regulation of 11βHSD1 as well as in the pathogenesis of the disease[82,83]. As a consequence of diminished H6PDH activity, 11βHSD1 enzyme activity switches from a reductase to a dehydrogenase in the livers of H6PDH knockout mice, which leads to glucocorticoid inactivation. Since 11βHSD1 activity greatly influences hepatic glucose output, this switch causes many alterations in glucose homeostasis of H6PDH knockout mice. Compared to wild-type mice, H6PDH knockout animals have a reduced weight gain, a peripheral fasting hypoglycemia, an improved glucose tolerance, and elevated plasma corticosterone concentrations. Both fed and fasted H6PDH knockout mice have normal plasma insulin levels; however, insulin and plasma glucose levels are reduced 4 h after fasted animals are refed, indicating an improved insulin sensitivity. There is a preserved induction and activity of the glucocorticoid-responsive gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in the livers of fasted H6PDH knockout mice. Glycogen storage is elevated in the liver of fed H6PDH knockout mice, with enhanced glycogenesis. These data suggest a partial retention of glucocorticoid sensitivity in the liver[84].
It can be hypothesized that the switch in 11βHSD1 activity from reductase to dehydrogenase caused by the lack of H6PDH activity leads to a protection against type 2 diabetes at high-fat feeding. This mechanism might be responsible for the above-mentioned metabolic alterations in H6PDH knockout animal.