Published online Jun 27, 2011. doi: 10.4254/wjh.v3.i6.137
Revised: May 10, 2011
Accepted: May 17, 2011
Published online: June 27, 2011
Primary sclerosing cholangitis (PSC) is a rare cholestatic liver disease with major morbidity and mortality. Therapeutic management is difficult, due to lack of conclusive data and individual disease progression. High-dose UDCA was used for years as a pharmacotherapeutic agent to prevent disease progression, based on a positive trend in pilot studies, but has recently been proven to have a negative effect in advanced disease. Immunosuppressants might be useful in patients with overlap syndromes. Dominant bile duct stenoses should be treated endoscopically, and cholangiocellular carcinoma (CCC) still remains a therapeutic challenge in PSC patients. Early diagnosis of CCC must be improved and new strategies such as neoadjuvant radiochemotherapy with subsequent liver transplantation in selected patients are further options to be considered.
- Citation: Lutz HH, Tischendorf JJ. Management of primary sclerosing cholangitis. World J Hepatol 2011; 3(6): 137-141
- URL: https://www.wjgnet.com/1948-5182/full/v3/i6/137.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i6.137
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease, characterized by intra- and extrahepatic bile duct inflammation and consecutive fibrosis[1]. PSC is generally a rare disease typically affecting middle aged men with ulcerative colitis. It presents with an increased risk of disease in relatives[2], showing a genetic susceptibility, combined with an autoimmune effect and toxicity of bile acids. Despite its low incidence, it is one of the main reasons for liver transplantation in northern European countries. The risk of developing cholangiocellular carcinoma (CCC) is high and associated with a dismal outcome. Optimizing treatment is difficult, and conflicting data on pharmacotherapy prevail in the literature. Endoscopic therapy of dominant stenoses, and liver transplantation in advanced disease, are effective treatment options. Small-duct PSC rarely progresses to large-duct PSC and has a more benign course, with slower progression and lower rates of cholangiocarcinoma[3,4]. In this editorial, therapeutic management of PSC to date is presented.
Although many drugs have been tested, effective medical treatment of PSC remains a problem. Promising results from pilot studies have raised hopes of slowing down or even reversing disease progression, but these results could not be proven in larger prospective randomized studies. In the following, the most important pharmacotherapeutic therapies evaluated in PSC are discussed.
Since the 1990s, Ursodeoxycholic acid (UDCA), a hydrophilic bile acid, has been used for patients with PSC, initially in a dosage of 10-15 mg/kg bodyweight per day. Multiple possible effects of UDCA have been discussed since then: increased bile flow, direct and indirect cytopretective mechanisms and immunomodulation. In 2005, Olsson et al published a prospective randomized trial, which showed a trend towards increased survival for patients treated with high-dose UDCA, though statistical significance was not reached due to lack of power[5]. Although UDCA used to be the standard therapy relating to this and two further promising pilot studies, it has recently come back into the limelight[6]: having been proven to be effective in other cholestatic liver diseases, UDCA does not seem to improve the outcome of patients with advanced PSC. On the contrary, high dosages could be toxic. It is clear that further studies are needed to assess the risk of UDCA in patients with advanced PSC, and to reevaluate the use of UDCA in the prevention of disease progression. Based on the available data to date, routine use of high-dose ursodeoxycholic acid in patients with advanced PSC can no longer be recommended[7,8].
With UDCA having lost its pivotal role in PSC medication, 24-norursodeoxycholic acid (NorUDCA) has recently been further evaluated as a future therapy, since it is possible that it does not have the same toxic effect recently discovered for UDCA. As this hydrophobic C23-homolog of UDCA is poorly conjugated, different physiological and therapeutical mechanisms (presumably cholehepatic shunting) in comparison to UDCA have been proposed[9]. So far, promising results could be shown primarily in MDR2 (-/-) mice, which are an established model for sclerosing cholangitis[10,11]: NorUDCA treatment in mice reduced periductal fibrosis, hydroxyproline content, proliferating hepato- and cholangiocytes, infiltrating immune cells and improved biochemical markers of cholestatic hepatopathy.
In addition, in a NEMO/NF-κB knockout mouse model for NASH, NorUDCA has shown anti-inflammatory effects through downregulation of TNF, IL-12, IFN-γ and CCL5 expression as well as anticholestatic and strikingly antifibrotic potency through normalization of expression of key bile transporters and genes involved in hepatic fibrosis (e.g. FXR)[12]. Despite all these results, reliable data for NorUDCA-treatment in PSC patients is still missing. Positive effects of NorUDCA have to be proven in prospective studies with a large number of patients and an adequate period of observation, long enough to reach primary endpoints, and to finally provide statistical significance.
The role of antibiotics today is limited to the treatment of cholangitis in the case of significant stenosis with cholestasis[13]. Trying to prevent recurrent cholangitis by continuous antibiotic therapy, a small prospective study could show lower levels of alkaline phosphatase, however, liver histology was not significantly improved[14]. Evidence favoring a continuous antibiotic therapy is not available.
Multiple immunosuppressants, such as methotrexate, steroids, cyclosporin A, azathioprine or tacrolimus, have been evaluated, with rather disappointing results. When addressing steroid therapy, it is important to consider possible IgG4-associated cholangitis or an overlap with features of autoimmune hepatitis, especially in younger patients. Current AASLD guidelines favor the use of steroids and other immunosuppressants in these cases. Anti-inflammatory substances like infliximab or etanercept have been tested, but have failed to show any beneficial effect[15]. Penicillamine, with copper-chelating and immunomodulatory functions, had no positive effects in one prospective study[16], neither did cholestyramine, which failed to change prognosis through reduction of the enterohepatic circulation of bile acids.
Various antifibrotic substances have been evaluated in the past for all sorts of chronic liver disease, in which treatment of the underlying disease is difficult or impossible. The results so far have been disappointing. Interferon gamma, as one of the most promising substances, failed to reverse fibrosis in patients with advanced liver disease caused by chronic hepatitis C[17]. Furthermore, pathomechanisms of progressive fibrosis in cholestatic liver diseases are not well understood, and cannot easily be adopted from other chronic liver diseases. Blocking activation of Kupffer cells and consecutive cytokine release with pentoxifylline could not improve liver tests or symptoms in PSC patients[18]. Colchicine, though promising in various studies of chronic liver diseases with improvement of biochemical parameters, has not been shown to have positive effects on fibrosis progression in PSC patients.
On the other hand, as already mentioned earlier, NorUDCA might have a significant anti-fibrotic potency itself (see above). Bezafibrate is not only known to reduce alkaline phosphatase levels[19], but can also prevent fibrogenesis through prevention of stellate cell activation in a murine model[20]. As silymarin is able to reduce fibrosis in bile duct ligated rats[21], a first clinical trial raised hope for a positive effect in humans[22]. In summary though, a significant clinical effect still has to be proven for all of these substances in prospective trials.
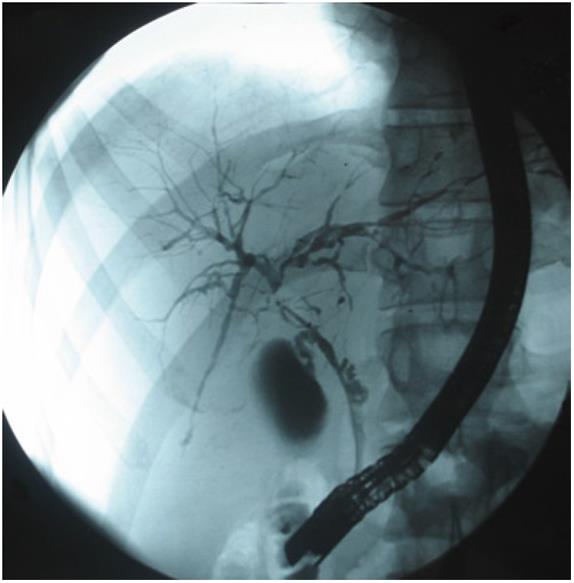
During the natural course of the disease, worsening of symptoms such as pruritus, abdominal pain, fever with chills, and jaundice due to insufficient biliary drainage can be observed. Cholestasis with consecutive cholangitis is often caused by dominant bile duct stenoses, which develop in up to 50% of PSC patients (see also Figure 1). Endoscopic treatment of these stenoses is recommended, especially because of bad outcomes in patients with these stenoses[13,23]. Repeated endoscopic balloon dilatation has been shown to be a useful technique to preserve common bile duct function[24] and has been established as a standard therapy over the past years.
A regular follow-up using ultrasound and measurement of blood parameters is recommended at least annually, due to the risk of cholangiocellular carcinoma and because of the higher risk of malignant gall polyps in patients with PSC[25]. Cholecystectomy should be performed even when polyps do not reach 1 cm in diameter.
Cholangiocellular carcinoma is the most lethal complication in patients with PSC, and has a frequency of up to 20% in end-stage PSC. Diagnosis of CCC can be difficult, due to negative or inconclusive sampling. This is especially problematic in PSC, because of a five times higher risk of developing a desmoplastic reaction in PSC-associated CCC, compared to idiopathic CCC. Polysomy shown in a FISH(fluorescent in situ hybridization)-Test could be a diagnostic sign of CCC in suspected malignancy, but can´t be used as a screening parameter in more highly progressed PSC[26]. Furthermore, lack of highly sensitive imaging methods or blood parameters make reliable early CCC diagnosis extremely difficult. Intraductal ultrasound is a promising method to distinguish benign from malignant stenoses[27]. Due to frequent multifocal tumor growth in PSC, local R0 resection is usually not possible. Photodynamic therapy, with or without surgery, is capable of improving outcome[28], as well as a multimodal approach including chemoembolization[29] or a combination of local and systemic chemotherapy[30]. In selected patients, transplantation might be a therapeutic option (see below).
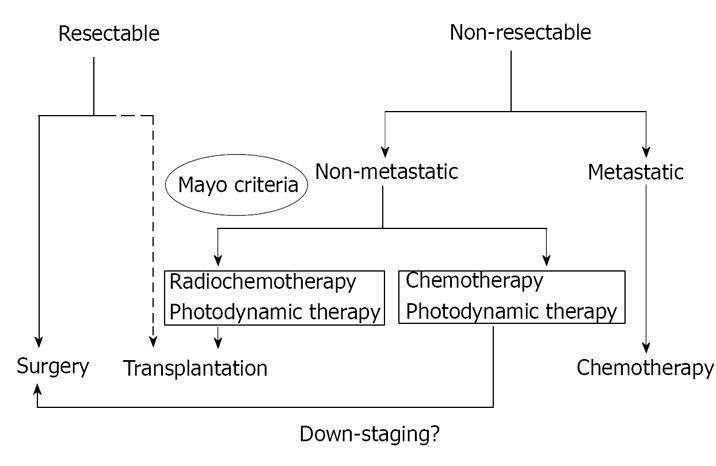
Transplanted end-stage PSC patients show an excellent outcome, based on the Mayo Score[31,32]. However, choosing the optimal time for transplantation remains a major problem. Listing for transplantation is based on the MELD-Score and the appearance of refractory bacterial cholangitis. In addition to that, sufficient screening for malignancy (cholangiocellular carcinoma and colonic cancer) must have taken place. In the case of non-resectable CCC, newer therapeutic options, with neoadjuvant radiochemotherapy and liver transplantation can be considered, though only a small number of patients qualify for this regimen (see also Figure 2). These patients, following a special protocol with explorative laparotomy and lymphadenectomy to exclude lymphatic metastasis, have a one year survival of 91 % and a five year survival of 76%[33].
Preventing disease progression remains the major problem in PSC patients. Based on new studies, the therapeutic use of UDCA might not be beneficial for all patients. Especially in advanced stages of the disease, UDCA therapy at high doses could possibly bring with it substantial risk - the positive effect at lower doses has not been proven, and still needs to be validated. NorUDCA, with fewer toxic effects could be a possible option for the future, but further prospective studies still have to prove a positive effect in humans. The existence of overlap syndromes should be evaluated in each patient to discover possible immunosuppressive therapeutic options. In case of advanced disease, liver transplantation is the best therapeutic option, and is known to have an excellent outcome.
Peer reviewer: Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, via A. Manzoni 113, Rozzano 20089, Milan, Italy
S- Editor Zhang HN L- Editor Herholdt A E- Editor Zhang L
| 1. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Bergquist A, Montgomery SM, Bahmanyar S, Olsson R, Danielsson A, Lindgren S, Prytz H, Hultcrantz R, Lööf LA, Sandberg-Gertzén H. Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2008;6:939-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Björnsson E, Olsson R, Bergquist A, Lindgren S, Braden B, Chapman RW, Boberg KM, Angulo P. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology. 2008;134:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Broomé U, Glaumann H, Lindstöm E, Lööf L, Almer S, Prytz H, Sandberg-Gertzén H, Lindgren S, Fork FT, Järnerot G. Natural history and outcome in 32 Swedish patients with small duct primary sclerosing cholangitis (PSC). J Hepatol. 2002;36:586-589. [PubMed] |
| 5. | Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, Bell H, Gangsøy-Kristiansen M, Matre J, Rydning A. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 7. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 8. | Chapman RW. High-dose ursodeoxycholic acid in the treatment of primary sclerosing cholangitis: throwing the urso out with the bathwater? Hepatology. 2009;50:671-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Denk GU, Maitz S, Wimmer R, Rust C, Invernizzi P, Ferdinandusse S, Kulik W, Fuchsbichler A, Fickert P, Trauner M. Conjugation is essential for the anticholestatic effect of NorUrsodeoxycholic acid in taurolithocholic acid-induced cholestasis in rat liver. Hepatology. 2010;52:1758-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Halilbasic E, Fiorotto R, Fickert P, Marschall HU, Moustafa T, Spirli C, Fuchsbichler A, Gumhold J, Silbert D, Zatloukal K. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2-/- mice. Hepatology. 2009;49:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Beraza N, Ofner-Ziegenfuss L, Ehedego H, Boekschoten M, Bischoff SC, Mueller M, Trauner M, Trautwein C. Nor-ursodeoxycholic acid reverses hepatocyte-specific nemo-dependent steatohepatitis. Gut. 2011;60:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Tischendorf JJ, Schirin-Sokhan R. Primary sclerosing cholangitis: The importance of treating stenoses and infections. Nat Rev Gastroenterol Hepatol. 2009;6:691-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Färkkilä M, Karvonen AL, Nurmi H, Nuutinen H, Taavitsainen M, Pikkarainen P, Kärkkäinen P. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology. 2004;40:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Hommes DW, Erkelens W, Ponsioen C, Stokkers P, Rauws E, van der Spek M, ten Kate F, van Deventer SJ. A double-blind, placebo-controlled, randomized study of infliximab in primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | LaRusso NF, Wiesner RH, Ludwig J, MacCarty RL, Beaver SJ, Zinsmeister AR. Prospective trial of penicillamine in primary sclerosing cholangitis. Gastroenterology. 1988;95:1036-1042. [PubMed] |
| 17. | Pockros PJ, Jeffers L, Afdhal N, Goodman ZD, Nelson D, Gish RG, Reddy KR, Reindollar R, Rodriguez-Torres M, Sullivan S. Final results of a double-blind, placebo-controlled trial of the antifibrotic efficacy of interferon-gamma1b in chronic hepatitis C patients with advanced fibrosis or cirrhosis. Hepatology. 2007;45:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Bharucha AE, Jorgensen R, Lichtman SN, LaRusso NF, Lindor KD. A pilot study of pentoxifylline for the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2338-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Mizuno S, Hirano K, Tada M, Yamamoto K, Yashima Y, Yagioka H, Kawakubo K, Ito Y, Kogure H, Sasaki T. Bezafibrate for the treatment of primary sclerosing cholangitis. J Gastroenterol. 2010;45:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Nakano S, Nagasawa T, Ijiro T, Inada Y, Tamura T, Maruyama K, Kuroda J, Yamazaki Y, Kusama H, Shibata N. Bezafibrate prevents hepatic stellate cell activation and fibrogenesis in a murine steatohepatitis model, and suppresses fibrogenic response induced by transforming growth factor-beta1 in a cultured stellate cell line. Hepatol Res. 2008;38:1026-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Angulo P, Jorgensen RA, Kowdley KV, Lindor KD. Silymarin in the treatment of patients with primary sclerosing cholangitis: an open-label pilot study. Dig Dis Sci. 2008;53:1716-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Rudolph G, Gotthardt D, Klöters-Plachky P, Kulaksiz H, Rost D, Stiehl A. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J Hepatol. 2009;51:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Gotthardt DN, Rudolph G, Klöters-Plachky P, Kulaksiz H, Stiehl A. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Buckles DC, Lindor KD, Larusso NF, Petrovic LM, Gores GJ. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol. 2002;97:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Bangarulingam SY, Bjornsson E, Enders F, Barr Fritcher EG, Gores G, Halling KC, Lindor KD. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Tischendorf JJ, Meier PN, Schneider A, Manns MP, Krüger M. Transpapillary intraductal ultrasound in the evaluation of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2007;42:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Quyn AJ, Ziyaie D, Polignano FM, Tait IS. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB (Oxford). 2009;11:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, Olthoff K, Christians K, Pappas S, Rilling W. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2010;117:1498-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Andrašina T, Válek V, Pánek J, Kala Z, Kiss I, Tuček S, Slampa P. Multimodal oncological therapy comprising stents, brachytherapy, and regional chemotherapy for cholangiocarcinoma. Gut Liver. 2010;4 Suppl 1:S82-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Brandsaeter B, Friman S, Broomé U, Isoniemi H, Olausson M, Bäckman L, Hansen B, Schrumpf E, Oksanen A, Ericzon BG. Outcome following liver transplantation for primary sclerosing cholangitis in the Nordic countries. Scand J Gastroenterol. 2003;38:1176-1183. [PubMed] |
| 32. | Kornasiewicz O, Lewandowski Z, Dudek K, Stankiewicz R, Nyckowski P, Krawczyk M. Prediction of graft loss and death in patients with primary sclerosing cholangitis. Transplant Proc. 2009;41:3110-3113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Petrowsky H, Hong JC. Current surgical management of hilar and intrahepatic cholangiocarcinoma: the role of resection and orthotopic liver transplantation. Transplant Proc. 2009;41:4023-4035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Tischendorf JJ, Geier A, Trautwein C. Current diagnosis and management of primary sclerosing cholangitis. Liver Transpl. 2008;14:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |