Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.109685
Revised: June 18, 2025
Accepted: July 18, 2025
Published online: August 27, 2025
Processing time: 101 Days and 1.1 Hours
T helper 17 (Th17) cell infiltration and interleukin (IL)-17 secretion in intrahepatic small bile ducts is a critical driver of immune-mediated injury in primary biliary cholangitis (PBC). IL-6 is an essential upstream activator of Th17 cells. Basophil-derived IL-6 promotes the differentiation of CD4+ T cells and Th1 cells into Th17 cells, thereby regulating their immunological functions.
To investigate the activation status and cytokine expression of basophils in PBC, elucidating potential mechanisms through which basophils contribute to its pathogenesis.
This single-center retrospective case-control study conducted at Guangdong Medical University Affiliated Hospital (China) between September 2019 and August 2024 enrolled 65 consecutive treatment-naïve patients with PBC (PBC group), 65 age- and sex-matched patients with chronic hepatitis B (CHB group), and 65 healthy controls (Normal group). Fourteen participants per group (subgroup) were randomly selected for flow cytometry analysis of basophil proportion, activation markers (CD203c and CD62 L mean fluorescence intensity), IL-6-positive basophils (IL-6+ basophils as a percentage of total basophils), and IL-17-positive T lymphocytes (CD3+CD4+IL-17+ cells) proportion among T cells. Data were analyzed using Kruskal-Wallis and χ2 tests as appropriate.
Routine blood tests revealed significantly higher basophil counts and proportions in the PBC group compared to the CHB and Normal groups (P < 0.001 for both comparisons), with no significant differences between the CHB and Normal groups (P = 0.201). Flow cytometry revealed a higher basophil proportion in the PBC subgroup compared to the CHB (P = 0.011) and Normal subgroups (P < 0.001). The mean fluorescence intensity of CD203c on basophil surfaces was elevated in the PBC subgroup compared to the CHB (P = 0.032) and Normal subgroups (P = 0.039). The proportion of IL-6+ basophils was significantly higher in the PBC subgroup than in the CHB (P < 0.01) and Normal subgroups (P < 0.001). Similarly, the Th17 cell proportion was markedly elevated in the PBC compared to the CHB (P < 0.001) and Normal subgroups (P < 0.001).
Patients with PBC have increased peripheral basophil counts with enhanced activation. Activated basophils have increased IL-6 expression, which may indirectly induce Th17 cell proliferation and contribute to PBC pathogenesis.
Core Tip: Peripheral basophil counts and proportions were significantly elevated in patients with primary biliary cholangitis (PBC), with increased expression of the activation marker CD203c mean fluorescence intensity and intracellular interleukin (IL)-6. Concurrently, CD4+ T lymphocytes in patients with PBC showed markedly increased IL-17 expression. These findings suggest that activated basophils, through IL-6 upregulation, may promote T helper 17 cell proliferation and promote PBC pathogenesis.
- Citation: Han HQ, Bao JM, Deng W, Guo WF, Li YF, Zheng WQ, Liu HF. Peripheral basophil activation: A hidden player in the immunopathogenesis of primary biliary cholangitis. World J Hepatol 2025; 17(8): 109685
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/109685.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.109685
Primary biliary cholangitis (PBC) is an autoimmune liver disease characterized by progressive inflammation of intrahepatic small bile ducts, which may advance to cirrhosis and liver failure[1]. PBC is gaining increasing recognition among clinicians, with a notable rise in diagnosed cases[2,3].
Current evidence suggests that PBC arises from aberrant autoimmune responses triggered by genetic predisposition, infections, or environmental factors[4,5]. Although adaptive and innate immune cells are known to contribute to bile duct destruction[6,7], the immunological network underlying PBC is highly complex, and existing theories do not fully account for its pathophysiological processes. In fact, the pathogenesis of PBC remains unresolved, necessitating further research to clarify its underlying mechanisms[8]. Additionally, frontline treatments, such as ursodeoxycholic acid, and second-line therapies like obeticholic acid have not fully addressed the therapeutic challenges of PBC[9]. Thus, a deeper understanding of the disease’s immunological characteristics is essential for identifying novel therapeutic targets.
While multiple pathways may explain the immunological pathogenesis of PBC, T helper 17 (Th17) cells play a particularly critical role[5,10]. A study even suggested that Th17 cells exert a more significant pathogenic effect than Th1 cells in PBC[11]. During PBC progression, a shift from Th1 to Th17 cells is accompanied by increased Th17 infiltration in the portal vein[12], driving disease progression. Elevated serum levels of inflammatory cytokines, including interleukin (IL)-17, IL-6, and IL-23, are observed in PBC, with IL-17 Levels positively correlating with liver and bile duct inflammation markers such as alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT)[13,14].
IL-6, a key upstream activator of Th17 cells, is indispensable for upregulating Th17 effector functions, enhancing their pathogenicity, and promoting IL-17 secretion[15-18]. The differentiation and generation of Th17 cells are induced and driven by IL-6, which also amplifies IL-17 secretion, further contributing to pathogenesis[19,20]. The next critical question is identifying the cellular source of IL-6 that drives PBC progression.
Our previous studies have demonstrated that basophils are closely associated with the onset and progression of immune-mediated diseases, including systemic lupus erythematosus, minimal change disease, and rheumatoid arthritis[21-24]. Preliminary findings revealed significantly elevated peripheral basophil counts and proportions in patients with PBC, suggesting a potential role of basophils in PBC pathogenesis.
IL-6 derived from basophils is known to promote the differentiation of CD4+ T cells and Th1 cells into Th17 cells, thereby modulating their immune functions[23,25]. However, whether basophils in PBC exhibit heightened IL-6 expression remains unclear. Despite advances in understanding Th17-mediated bile duct injury in PBC, further exploration of the upstream triggers driving Th17 cell activation is warranted. Basophils may promote Th17 differentiation by producing IL-6. However, this contribution to PBC pathogenesis has not been investigated. Thus, this study aimed to evaluate basophil counts, activation status, and IL-6 expression in patients with PBC in order to determine the potential basophil contribution to PBC pathogenesis.
This single-center retrospective case-control study included 65 treatment-naïve patients with PBC (PBC group, defined as never having received therapy for PBC, including ursodeoxycholic acid, obeticholic acid, fibrates, or immunosuppressants) who were diagnosed at the Affiliated Hospital of Guangdong Medical University between September 2019 and August 2024. Given that cirrhosis is a primary adverse outcome of PBC, and chronic hepatitis B (CHB) is a leading cause of cirrhosis in China, 65 age- and sex-matched patients with CHB (CHB group) and 65 healthy controls (Normal group) were enrolled as comparators during the same period. Clinical data and peripheral basophil profiles were retrospectively analyzed across the three groups.
Peripheral blood was collected from 14 patients with PBC (PBC subgroup), 14 patients with CHB (CHB subgroup), and 14 healthy controls (Normal subgroup). Flow cytometry was employed to measure basophil counts, activation markers [CD203c and CD62 L mean fluorescence intensity (MFI)], proportion of IL-6-positive basophils, and proportion of Th17 cells (IL-17+ CD4+ T lymphocytes).
PBC: Diagnosis was based on the 2022 Asia-Pacific Association for the Study of the Liver clinical practice guidelines[26], requiring two or more of the following: (1) Biochemical evidence of cholestasis, primarily elevated ALP and GGT, with exclusion of extrahepatic biliary obstruction via imaging; (2) Presence of antimitochondrial antibodies, anti-sp100, or anti-gp210; and (3) Histological evidence of non-suppurative destructive cholangitis primarily affecting interlobular bile ducts.
CHB: Diagnosis adhered to the 2022 Chinese Medical Association guidelines for chronic hepatitis B[27], requiring positive serum hepatitis B surface antigen, positive hepatitis B virus DNA, persistent or recurrent ALT abnormalities, or histological evidence of significant inflammation/necrosis or fibrosis (≥ F2).
Patients with (1) Concomitant autoimmune liver diseases (e.g., autoimmune hepatitis, primary sclerosing cholangitis, IgG4-related cholangitis); (2) Other viral hepatitis (e.g., chronic hepatitis C); (3) Malignant tumors or hematologic malignancies; and (4) Pregnancy were excluded.
Peripheral venous blood samples from patients with PBC and CHB and healthy controls were collected and processed within 2 hours. Each blood sample was divided into three tubes: An extracellular basophil tube (Ba extracellular), an intracellular basophil tube (Ba intracellular), and a lymphocyte tube (Ly). The Ba extracellular tube assessed basophil proportions and extracellular CD203c and CD62 L MFI. The Ba intracellular tube measured the proportion of IL-6-positive basophils (IL-6+ Ba). The Ly tube was used to evaluate the proportion of Th17 cells.
For the Ba extracellular tube, red blood cells (RBCs) were lysed using RBC lysis buffer, followed by staining with CD45, CD203c, CD123, and CD62 L antibodies. Samples were washed and prepared for analysis.
For the Ba intracellular tube, RBCs were lysed, and samples were stained with CD45, CD203c, and CD123 antibodies. After washing, leukocytes were fixed with a Fixation Buffer, incubated for 40 min at room temperature in the dark, and permeabilized with an Intracellular Staining Permeabilization Wash Buffer. IL-6 antibodies were added, followed by washing and preparation for analysis.
For the Ly tube, whole blood was diluted with 1640 medium and aliquoted into 12-well plates. A stimulation and blocking cocktail was added, and samples were incubated at 37 °C for 6 hours. RBCs were lysed, and samples were washed and treated with Human TruStain FcX blocking reagent. CD3 and CD4 antibodies were added to label T lymphocytes. After incubation in the dark, leukocytes were fixed with the Fixation Buffer, permeabilized with the Intracellular Staining Perm Wash Buffer, and stained with IL-17A antibodies. Samples were washed and prepared for analysis.
In flow cytometry, basophils were gated as CD203c-PE and CD123-FITC double-positive leukocytes in the Ba extracellular tube, with proportions relative to total leukocytes and MFI of CD203c-PE and CD62 L-APC/Cyanine7 measured. In the Ba intracellular tube, IL-6-PerCP/Cyanine5.5-positive basophils were quantified. In the Ly tube, CD3-APC/Cyanine7 and CD4-FITC double-positive leukocytes were gated as CD4+ T lymphocytes, and IL-17-APC-positive cells were measured. All the aforementioned antibodies were purchased from BioLegend, United States.
Data were acquired and analyzed using a FACScantoTM II flow cytometer (Becton Dickinson, United States) and FlowJo 10.0 software.
This study was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University (Approval No.: YS2020-115). Informed consent was obtained from the participants in accordance with the guidelines of the Declaration of Helsinki.
Data were analyzed using SPSS 27.0 software. Normally distributed quantitative data were expressed as mean ± SD and analyzed using one-way analysis of variance with Bonferroni correction for pairwise comparisons. Non-normally distributed quantitative data were presented as median (M) and interquartile range (IQR) and analyzed using the Kruskal−Wallis test with Dunn’s correction for pairwise comparisons.
Categorical data were compared using χ2 tests or Fisher’s exact test. P < 0.05 was considered statistically significant.
Among the 65 treatment-naïve patients with PBC, 20 had compensated cirrhosis, and two had decompensated cirrhosis. Among the 65 patients with CHB, 18 had compensated cirrhosis, and one had decompensated cirrhosis. The three groups were comparable in sex (P = 0.192) and age (P = 0.603).
Biochemical analysis showed that the PBC group had higher ALP levels compared to the CHB (P < 0.001) and the Normal groups (P < 0.001). Similarly, GGT levels were elevated in the PBC group compared to the CHB (P < 0.001) and Normal groups (P < 0.001), with the CHB group also showing higher GGT than the Normal group (P = 0.009).
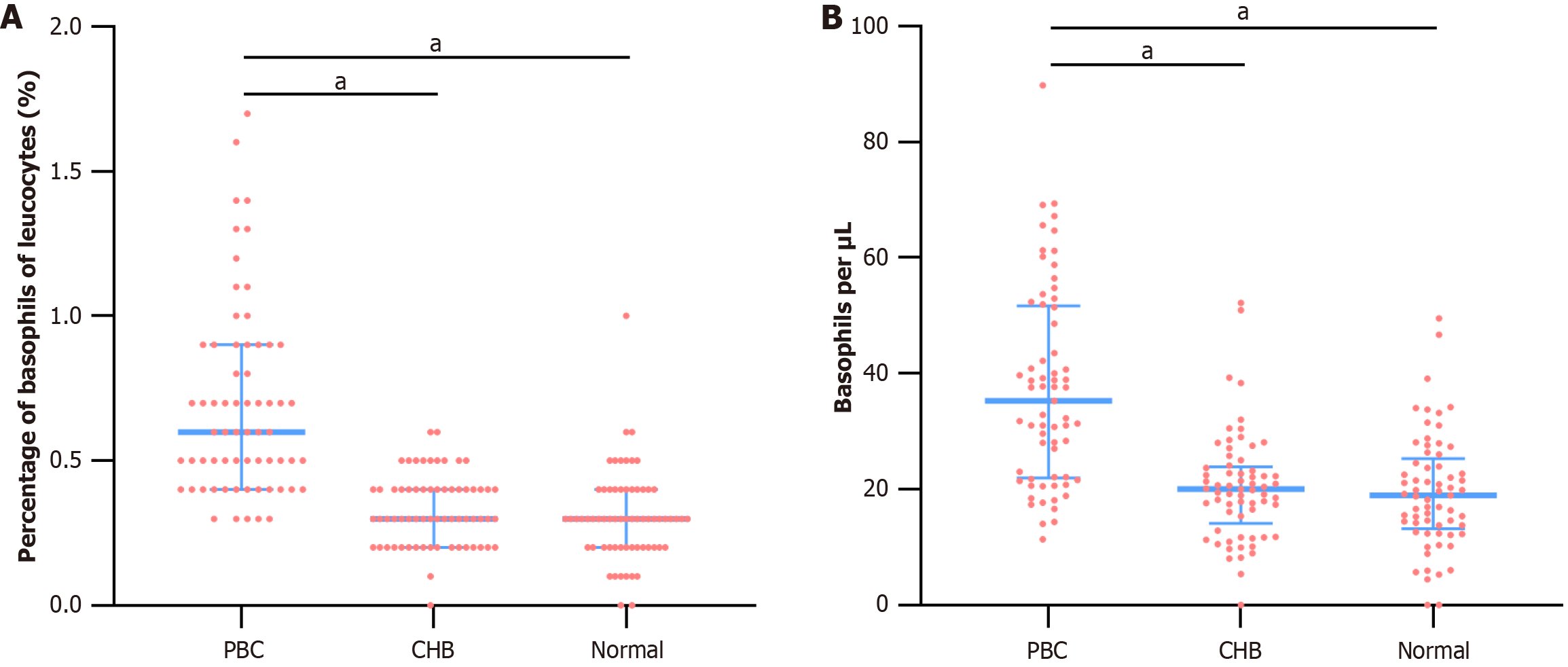
Routine blood tests indicated that the PBC group had a higher proportion of basophils (0.6%, median) compared to the CHB (0.3%, median) (P < 0.001) and Normal groups (0.3%, median) (P < 0.001). Basophil counts were also elevated in the PBC group compared to the CHB and Normal groups (P < 0.001) (Table 1 and Figure 1).
| Parameter | PBC (n = 65) | CHB (n = 65) | Normal (n = 65) | χ2/F/H | P value |
| Sex (female,%) | 48 (73.8) | 49 (75.4) | 40 (61.5) | 3.297 | 0.192 |
| Age (years) | 58.22 ± 13.63 | 57.03 ± 11.07 | 59.18 ± 11.80 | 0.507 | 0.603 |
| White blood cell count (×109/L) | 5.69 ± 1.80 | 6.19 ± 1.60 | 6.41 ± 1.59 | 3.190 | 0.043 |
| ALT (IQR, U/L) | 48.70 (28.25, 102.90) | 23.00 (15.80, 41.45) | 16.80 (11.15, 22.95) | 55.948 | < 0.001 |
| AST (IQR, U/L) | 58.70 (33.60, 107.20) | 24.50 (19.35, 33.88) | 18.70 (16.10, 22.30) | 88.404 | < 0.001 |
| ALP (IQR, U/L) | 204.90 (113.50, 318.55) | 79.40 (63.45, 101.20) | 71.00 (59.80, 85.15) | 81.054 | < 0.001 |
| GGT (IQR, U/L) | 188.60 (67.00, 387.75) | 32.70 (17.60, 53.00) | 19.20 (15.10, 28.60) | 85.797 | < 0.001 |
| Basophil proportion (%) | 0.6 (0.4, 0.9) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 81.478 | < 0.001 |
| Absolute basophil count (counts/μL) | 35.32 (21.98, 51.71) | 20.00 (14.15, 23.92) | 18.96 (13.19, 25.31) | 51.511 | < 0.001 |
Serum immunoglobulin E (IgE) levels were measured in a subset of patients with PBC and healthy controls. The median IgE level in patients with PBC [58.50 (16.90, 137.00) IU/mL] was significantly higher than in healthy controls [15.80 (7.69, 42.80) IU/mL; Z = 305.500, P = 0.006].
Peripheral blood samples from 14 patients with PBC (PBC subgroup), 14 patients with CHB (CHB subgroup), and 14 healthy controls (Normal subgroup) were analyzed using flow cytometry. Among the 14 patients with PBC, 7 had cirrhosis. Among the 14 patients with CHB, 8 had cirrhosis. The three subgroups were comparable in age (P = 1.000) and sex (P = 0.759) (Table 2).
| Parameter | PBC subgroup (n = 14) | CHB subgroup (n = 14) | Normal subgroup (n = 14) | χ2/F | P value |
| Female (%) | 13 (92.9) | 12 (85.7) | 13 (92.9) | 0.721 | 1.000 |
| Age (years) | 56.79 ± 10.95 | 58.43 ± 6.61 | 59.07 ± 6.79 | 0.278 | 0.759 |
| White blood cell count (× 109/L) | 6.25 ± 1.02 | 6.07 ± 1.60 | 6.45 ± 1.30 | 0.314 | 0.732 |
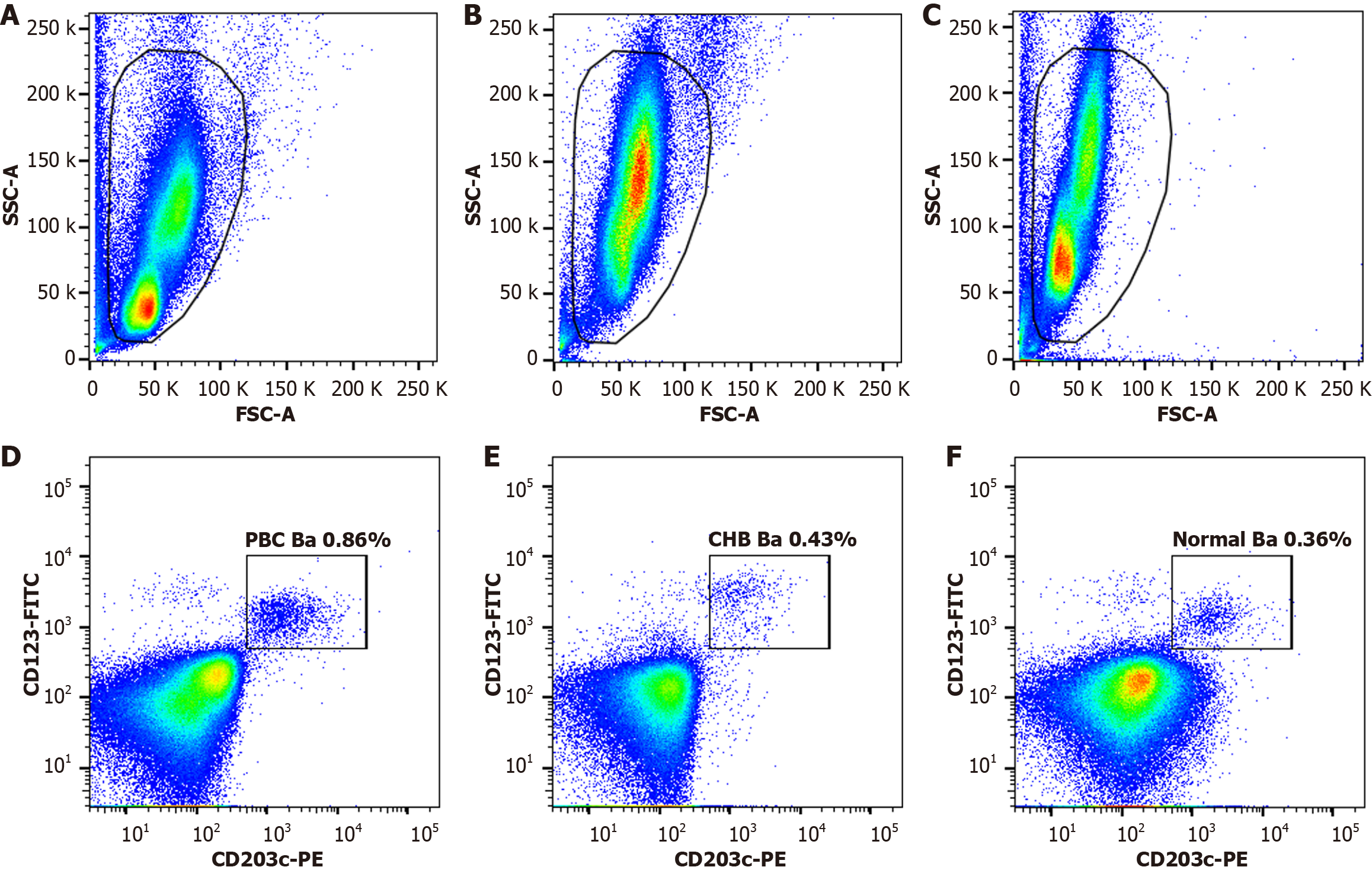
Flow cytometry revealed that the basophil proportion in the PBC subgroup [0.86% (0.59%, 1.20%)] was significantly higher than in the CHB [0.43% (0.29%, 0.70%); P = 0.011] and Normal subgroup [0.36% (0.24%, 0.51%); P < 0.001]. No significant difference was observed between the CHB and Normal subgroups (P = 0.260) (Figure 2).
The MFI of CD203c on basophil surfaces was higher in the PBC subgroup compared to the CHB (P = 0.032) and Normal subgroups (P = 0.039), with no significant difference between the CHB and Normal subgroups (P = 0.999). The MFI of CD62 L was higher in the PBC subgroup than in the CHB and Normal subgroups, but the differences were not statistically significant (P = 0.644) (Table 3).
| Activation marker | PBC subgroup (n = 14) | CHB subgroup (n = 14) | Normal subgroup (n = 14) | H | P value |
| CD203c MFI | 1792.00 (1301.50, 29410) | 1575.00 (871.0, 2079.00) | 1014.50 (652.25, 1784.75) | 8.465 | 0.015 |
| CD62 L MFI | 3130.00 (2290.0, 4760.75) | 2956.00 (1933.75, 4118.75) | 2963.00 (2386.75, 4946.50) | 0.880 | 0.644 |
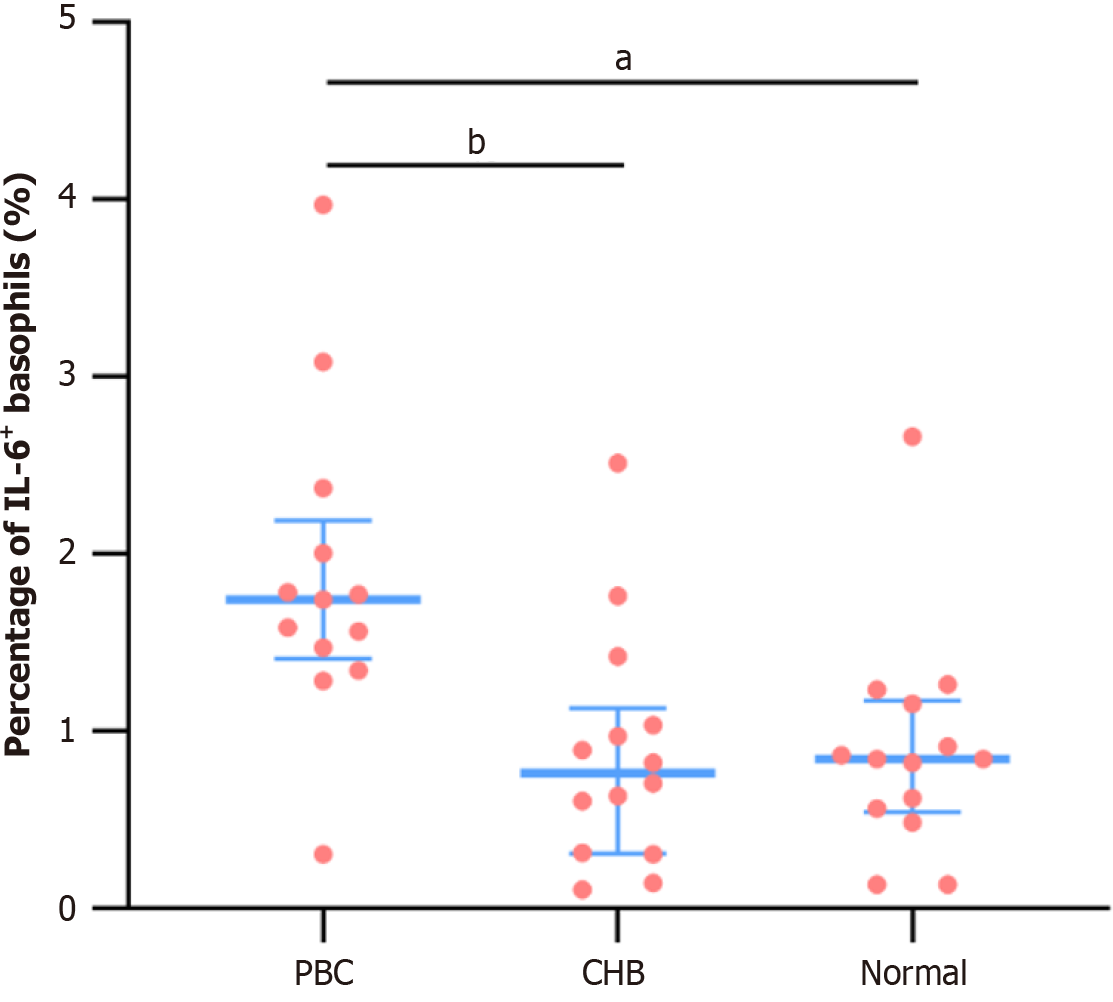
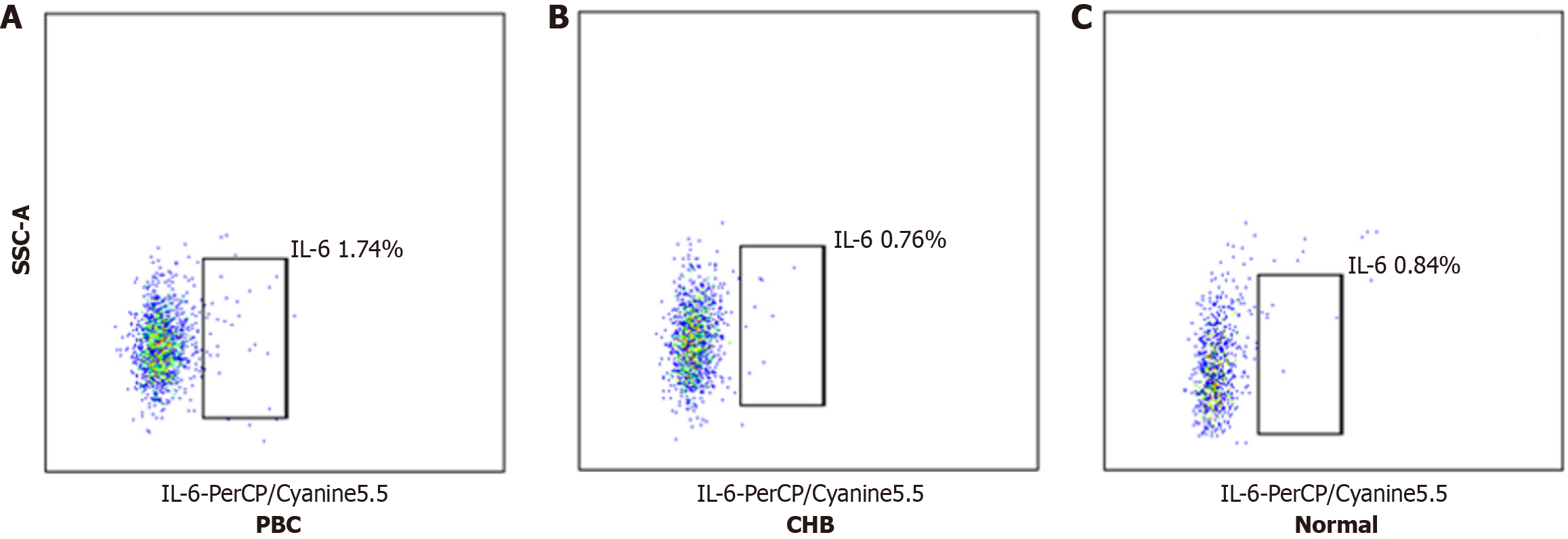
The proportion of IL-6-positive basophils (IL-6+ Ba%) was significantly higher in the PBC subgroup compared to the CHB and Normal subgroups (P < 0.001), with no significant difference between the CHB and Normal subgroups (P = 0.999) (Table 4, Figure 3 and 4).
| PBC subgroup (n = 13) | CHB subgroup (n = 14) | Normal subgroup (n = 14) | H | P value | |
| IL-6+Ba, M (IQR),% | 1.74 (1.41, 2.19) | 0.76 (0.31, 1.13) | 0.84 (0.54, 1.17) | 13.373 | < 0.001 |
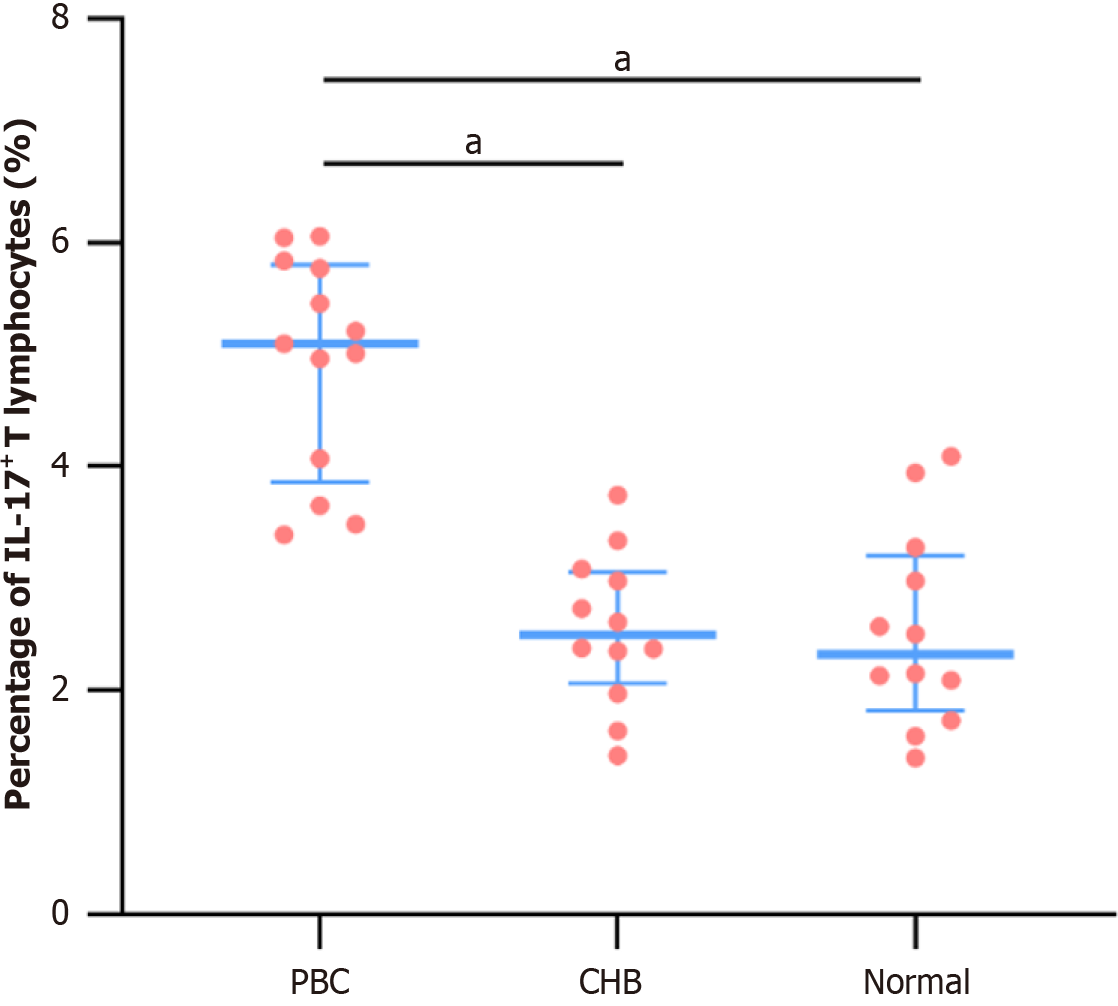
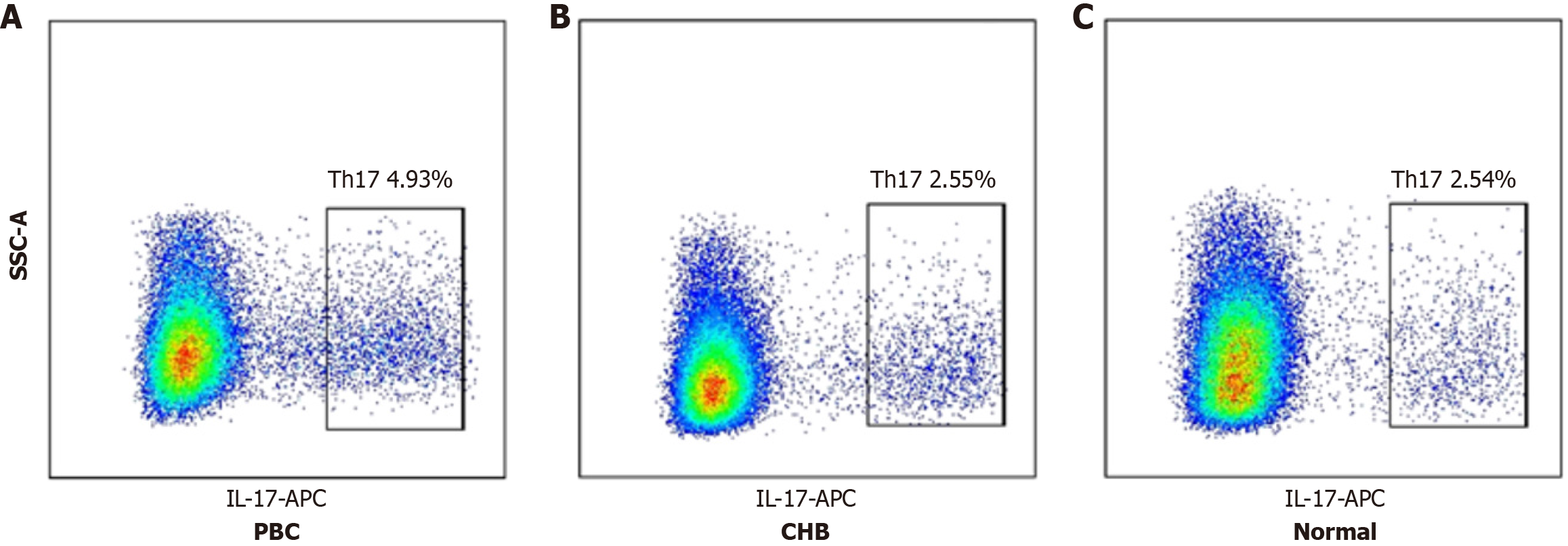
Flow cytometry analysis showed Th17 proportions of 4.93% ± 0.97% in the PBC subgroup, 2.55% ± 0.68% in the CHB subgroup, and 2.54% ± 0.88% in the Normal subgroup. The differences were statistically significant (P < 0.001). Spe
Recently, researchers have explored PBC pathogenesis from the perspective of environmental factors, genetics, and immunology. Although the precise mechanisms remain elusive, immune-mediated injury is a well-established cause of cholangitis and cirrhosis, driven by various immune cells, cytokine release, and infiltration, which collectively contribute to bile duct damage[3].
This study provides novel evidence whereby both routine blood tests and flow cytometry demonstrate significantly elevated basophil proportions and counts in patients with PBC compared to healthy controls. This suggests that basophils may play a role in PBC pathogenesis. To determine whether this is a general feature of hepatitis or cholangitis or specific to PBC, we analyzed basophils in patients with CHB and found no significant increase compared to healthy controls. This strongly indicates that elevated basophil levels are associated with the onset and progression of PBC rather than being a consequence of hepatitis or cholangitis. Nevertheless, future studies should directly compare the basophils in other cholestatic conditions (e.g., primary sclerosing cholangitis, IgG4-related cholangitis) to confirm this.
Given that PBC is a rare disease and basophils constitute a small fraction of leukocytes, their role in PBC pathogenesis has historically been overlooked. We believe this finding is significant and offers a novel avenue for investigating the immunological mechanisms of PBC.
CD123 and CD203c are specific antigens for basophils, enabling precise identification via flow cytometry. Additionally, CD203c and CD62 L serve as sensitive markers of basophil activation[28-30]. In our cellular experiments, we observed an increased basophil proportion in patients with PBC as well as increased expression of the activation marker CD203c. IL-6, a primary cytokine secreted by basophils, exhibited higher intracellular expression in patients with PBC. Compared to CD203c, increased IL-6 secretion reflects the functional consequence of basophil activation, indicating that activated basophils are capable of secreting active mediators. These results suggest that basophils in PBC are in an activated state, with the capacity to secrete cytokines, such as IL-6, which are closely linked to PBC pathogenesis.
Previous studies have established the pathogenic roles of Th17 cells and IL-17 in PBC[10]. To corroborate these findings, we used flow cytometry to measure the proportion of IL-17-positive CD4+ T lymphocytes (Th17 cells) in patients with PBC. We observed a significant increase compared to patients with CHB and healthy controls. This finding indicates that activation of CD4+ T lymphocytes and increased IL-17 secretion promote cholangitis and fibrosis in PBC.
The biological activity of basophils depends on their activation, which can be triggered by various stimuli, including IgE and environmental factors. IgE crosslinks with the FcεRI receptor on basophil surfaces, mediating activation[31]. We found elevated serum IgE levels in patients with PBC, suggesting that IgE may contribute to PBC pathogenesis, po
Multiple studies have reported elevated serum IL-17, IL-6, and IL-23 Levels in patients with PBC[13,14], with increased Th17 infiltration in the portal vein area accompanying a Th1-to-Th17 shift[11,12]. Building on these findings and our results, we propose the following hypothesis: In PBC, peripheral basophils are activated by environmental factors or IgE, leading to the upregulation of surface molecules, such as CD203c, and rapid secretion of cytokines like IL-6. Elevated IL-6, in concert with IL-23 and other cytokines, promotes the differentiation of naïve CD4+ T cells (Th cells) into Th17 cells, facilitating the conversion of Th1 to Th17 cells. Th17 cells secrete large amounts of IL-17, which acts on hepatocytes, while Th17 cells infiltrate bile duct cells, collectively contributing to PBC onset and progression, ultimately driving liver fibrosis and cirrhosis. Although this hypothesis requires further validation, it proposes a plausible pathway for basophil involvement in PBC pathogenesis.
This study has several limitations. First, the study design of clinical components was retrospective, potentially introducing selection bias, though the significant increase in basophil counts in patients with PBC is robust. Second, CHB was used as a control group, but many patients with CHB lack pronounced cholangitis. Third, the sample size for cellular experiments was small, and the study spanned a long period, which may have affected the accuracy of the results. Although this allowed for statistical comparisons, it may lack sensitivity for more subtle immunological interactions. Replication in larger cohorts is warranted to assess the robustness of these cellular findings. Finally, as prior studies have confirmed elevated serum IL-17, IL-6, and IL-23 as well as hepatic IL-17 expression in PBC, we did not measure these. Thus, further investigation is warranted. Future prospective longitudinal studies should track basophil and IL-6/Th17 Levels in PBC patients over time, particularly before and after treatment with ursodeoxycholic acid. Meanwhile, the causal role of basophil-derived IL-6 in Th17 differentiation requires validation through functional studies.
In conclusion, patients with PBC exhibit increased peripheral basophil counts and enhanced activation, with activated basophils expressing elevated IL-6 Levels and indirectly inducing Th17 cell proliferation, contributing to PBC pa
| 1. | Lleo A, Wang GQ, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet. 2020;396:1915-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 2. | Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Tanaka A, Ma X, Takahashi A, Vierling JM. Primary biliary cholangitis. Lancet. 2024;404:1053-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 4. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology. 2022;75:1012-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Jallouli I, Doulberis M, Kountouras J. Primary biliary cholangitis: a summary of pathogenesis and therapies. Ann Gastroenterol. 2025;38:121-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Tanaka A, Leung PSC, Gershwin ME. Evolution of our understanding of PBC. Best Pract Res Clin Gastroenterol. 2018;34-35:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 8. | Ronca V, Davies SP, Oo YH, Lleo A. The immunological landscape of primary biliary cholangitis: Mechanisms and therapeutic prospects. Hepatology. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Abreu ES, Reginato PH, Pitanga JFJ, Borges PN, Soret PA, Corpechot C, Cançado GGL. Obeticholic Acid vs Fibrates as Second-Line Therapy for Primary Biliary Cholangitis: Systematic Review and Meta-Analysis. Dig Dis Sci. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 909] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 11. | Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP, Bowlus CL, Yang GX, Leung PS, Ansari AA, Wu L, Coppel RL, Gershwin ME. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology. 2014;59:1944-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Lan RY, Salunga TL, Tsuneyama K, Lian ZX, Yang GX, Hsu W, Moritoki Y, Ansari AA, Kemper C, Price J, Atkinson JP, Coppel RL, Gershwin ME. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun. 2009;32:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Sun Q, Wang Q, Feng N, Meng Y, Li B, Luo D, Shang X, Lv J, Monsaf AM, Wang C, Ma X. The expression and clinical significance of serum IL-17 in patients with primary biliary cirrhosis. Ann Transl Med. 2019;7:389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Qian C, Jiang T, Zhang W, Ren C, Wang Q, Qin Q, Chen J, Deng A, Zhong R. Increased IL-23 and IL-17 expression by peripheral blood cells of patients with primary biliary cirrhosis. Cytokine. 2013;64:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Guggino G, Giardina AR, Raimondo S, Giardina G, Sireci G, Dieli F, Peralta M, Alessandro R, Triolo G, Ciccia F. Targeting IL-6 signalling in early rheumatoid arthritis is followed by Th1 and Th17 suppression and Th2 expansion. Clin Exp Rheumatol. 2014;32:77-81. [PubMed] |
| 16. | Raveney BJ, Oki S, Yamamura T. Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS One. 2013;8:e56595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5475] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 18. | Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1001] [Cited by in RCA: 984] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 19. | Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, Murakami M, Hirano T. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol. 2007;19:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1399] [Cited by in RCA: 1480] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 21. | Han H, Xu YZ, Liao S, Xiao H, Chen X, Lu X, Wang S, Yang C, Liu HF, Pan Q. Increased number and activation of peripheral basophils in adult-onset minimal change disease. J Cell Mol Med. 2020;24:7841-7849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Tang P, Chen Q, Lan Q, Chen Y, Yang H, An N, Xiao H, Liu H, Wu P, Xie T, Pan Q. Role of basophils in rheumatoid arthritis (Review). Exp Ther Med. 2015;9:1567-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Chen J, Pan Q, Lu L, Huang X, Wang S, Liu X, Lun J, Xu X, Su H, Guo F, Yang L, You L, Xiao H, Luo W, Liu HF, Pan Q. Atg5 deficiency in basophils improves metabolism in lupus mice by regulating gut microbiota dysbiosis. Cell Commun Signal. 2025;23:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Pan Q, Ye L, Deng Z, Li L, Liu H. Effects of red blood cell lysing solutions on the detection of peripheral basophils of healthy normals and SLE patients by flow cytometry. J Immunoassay Immunochem. 2014;35:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Yuk CM, Park HJ, Kwon BI, Lah SJ, Chang J, Kim JY, Lee KM, Park SH, Hong S, Lee SH. Basophil-derived IL-6 regulates T(H)17 cell differentiation and CD4 T cell immunity. Sci Rep. 2017;7:41744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | You H, Ma X, Efe C, Wang G, Jeong SH, Abe K, Duan W, Chen S, Kong Y, Zhang D, Wei L, Wang FS, Lin HC, Yang JM, Tanwandee T, Gani RA, Payawal DA, Sharma BC, Hou J, Yokosuka O, Dokmeci AK, Crawford D, Kao JH, Piratvisuth T, Suh DJ, Lesmana LA, Sollano J, Lau G, Sarin SK, Omata M, Tanaka A, Jia J. APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis. Hepatol Int. 2022;16:1-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 27. | You H, Wang F, Li T, Xu X, Sun Y, Nan Y, Wang G, Hou J, Duan Z, Wei L, Jia J, Zhuang H; Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022). J Clin Transl Hepatol. 2023;11:1425-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 28. | Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandené L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007;320:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Bühring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Sturm EM, Kranzelbinder B, Heinemann A, Groselj-Strele A, Aberer W, Sturm GJ. CD203c-based basophil activation test in allergy diagnosis: characteristics and differences to CD63 upregulation. Cytometry B Clin Cytom. 2010;78:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Charles N, Blank U. IgE-Mediated Activation of Mast Cells and Basophils in Health and Disease. Immunol Rev. 2025;331:e70024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |