Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.108678
Revised: June 5, 2025
Accepted: July 9, 2025
Published online: August 27, 2025
Processing time: 129 Days and 9.1 Hours
Hepatitis C virus (HCV) infection has been increasingly associated with cardio
Core Tip: Hepatitis C virus (HCV) infection has been shown to contribute to cardiovascular complications, extending beyond its liver-related effects. This review highlights the link between HCV and various examples of these complications, such as atherosclerosis and cardiomyopathy, emphasizing the roles of chronic inflammation, oxidative stress, immune dysregulation, and coagulation imbalance. Understanding these mechanisms may help guide future research and improve cardiovascular risk assessment and management in patients with chronic HCV infection.
- Citation: Elkhattib I, Raafat KW, Elsayed B, Elnaggar M. Chronic hepatitis C and the risk for atherosclerotic and cardiomyopathic heart disease. World J Hepatol 2025; 17(8): 108678
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/108678.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.108678
Chronic hepatitis C (CHC) is a significant global health burden, affecting millions of people worldwide. In the United States, an estimated 2.7 to 3.9 million people have CHC, with approximately 17000 new cases reported each year. Alarmingly, up to 75% of individuals with CHC are unaware of their infection[1].
CHC is primarily known for its devastating effects on the liver, with a 2019 report estimating 540000 deaths and 15.3 million disability-adjusted life years (DALYs)[2]. However, emerging evidence highlights a concerning association between CHC and cardiovascular disease (CVD)[3,4]. This connection is multifaceted, involving both direct and indirect mechanisms that contribute to increased cardiovascular risk in individuals with CHC.
Atherosclerosis, characterized by the buildup of plaques in arterial walls, poses a critical threat to cardiovascular health and is linked to increased morbidity and mortality in hepatitis C virus (HCV)-infected populations. Studies have de
The effect of HCV on the heart remains a controversial topic, with strong opinions on both sides[7]. Two major car
Atherosclerosis has been found to occur more frequently in patients with HCV infection. Adinolfi et al[8] explored the relationship between HCV infection and carotid atherosclerosis (CA) in a cohort of 803 individuals. The study included 326 treatment-naïve CHC patients and 477 matched controls. The study found a significantly higher prevalence of CA in CHC patients compared to controls (53.7% vs 34.3%). Younger CHC patients (< 50 years) also showed a greater risk.
The study provides novel insights into the role of serum HCV RNA levels and steatosis in the development of atherosclerosis, identifying associations between HCV RNA and both early (intima-media thickness) and advanced atherosclerotic plaques. It also provides a valuable comparison between CHC patients with HCV-related steatosis and patients with non-alcoholic fatty liver disease (NAFLD). The finding that HCV-related steatosis poses a higher risk for atherosclerosis compared to NAFLD patients contributes to the ongoing debate about the relative risks posed by these liver conditions.
However, the study design, which focuses on treatment-naïve patients, prevents it from establishing causality and limits generalizability. Without follow-up, it is unclear whether the observed atherosclerosis leads to clinical car
Another case-control study investigated the prevalence of CA in 174 biopsy-proven CHC patients compared with 174 control patients. The study found a significantly higher prevalence of carotid plaques in cases vs controls (41.9% vs 22.9%). Additionally, CHC patients had greater intima-media thickness measurements. Severe hepatic fibrosis was identified as an independent risk factor for atherosclerosis, particularly in younger patients[9].
The development of atherosclerosis in HCV-infected patients involves several stages: (1) Endothelial damage: This occurs due to factors like systemic inflammation; (2) Inflammation and oxidative stress: Immune cells are attracted to injury sites, leading to fatty streak formation and plaque buildup; and (3) Plaque formation: Plaques can rupture, leading to obstructed blood flow.
The process of plaque formation and subsequent rupture, depending on the site, can cause obstructed blood flow to the tissue or organ upstream[10,11].
Several clinical studies have identified the most common cytokines reported in HCV-affected patients. Th1 cytokines and Th2, which are responsible for the activation of cell-mediated and humoral immune systems respectively, suggesting a mixed immune reaction, through tumor necrosis factor-alpha (TNF-α), interferon-gamma, interleukin (IL)-2, IL-4, IL-5, IL-6, IL-8, IL-10. These cytokines modulate the inflammatory response either in a protective or causative role[12,13].
HCV disrupts endothelial function, particularly nitric oxide production, leading to vasoconstriction and turbulent blood flow, which promotes lipoprotein infiltration into the arterial walls. Pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8 are elevated in HCV patients, promoting plaque formation[13-16]. In contrast, antiatherogenic cytokines like IL-4, IL-5, and IL-10 are downregulated, reducing protection against plaque formation. Table 1 summarizes these cytokines and effects[17-20].
| Cytokine | T helper cell type | Target cell | Action | Net effect on plague formation |
| TNF-α | Th1 | Macrophages, endothelial cells | Promotes atherogenesis by increasing the expression of adhesion molecules on endothelial cells, leading to the recruitment of inflammatory cells into the arterial wall | Proatherogenic |
| IFN-γ | Th1 | Macrophages, CD8, T cells, NK cells | Despite its antiviral effects, it increases adhesion molecules expression | Proatherogenic |
| IL-2 | Th1 | T cell | B cell clonal expansion and T cell development | Proatherogenic (less well-established) |
| IL-4 | Th2 | T and B cells | Promotes T cell development, upregulate pro-inflammatory mediators | Antiatherogenic (less well-established) |
| IL-5 | Th2 | B cell | B cell activation and production of protective natural immunoglobulin M antibodies against oxidation-specific antigens | Antiatherogenic (less well-established) |
| IL-6 | Macrophages | Macrophages, Th1 | Increase adhesion molecule expression | Proatherogenic |
| IL-8 | Th2 | Neutrophils | Releasing reactive oxygen species and promoting inflammation | Proatherogenic |
| IL-10 | T reg cell | Macrophages, B cells | Suppresses activation of Th1 cells and macrophages | Antiatherogenic |
Another key to pathogenesis is oxidative stress, an important factor in changing the accumulated lipoproteins which attracts the attention of the macrophages. The source of the reactive oxygen species is mitochondria and rough endo
Many markers were investigated to better understand the pathological process and limit it pharmacologically. Biomarkers like malondialdehyde and oxidative stress index are elevated in HCV patients, supporting the link between HCV and oxidative stress[22].
HCV proteins are responsible for inducing oxidative damage in the tissues, namely core, E1, E2, NS3, NS4B, and NS5A. These proteins overwhelm the glutathione system’s ability to protect cells from damage, leading to endothelial dys
Furthermore, oxidative stress is also responsible for carcinomas and other extra-hepatic disorders such as diabetes, lymphoproliferative disorders, neuropathy, and embolic strokes[23,24].
The adhesion of circulating leukocytes is an essential step in vasculitis and the migration of these immune cells into the tissues in the inflammation process. HCV infection increases the expression of adhesion molecules on endothelial cells, promotes local vascular inflammation, and enhances the production of proinflammatory cytokines and free radicals by macrophages, all of which accelerate atherosclerosis. A family of surface molecules is responsible for this exact step, mainly intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and integrins on lymphocytes such as lymphocyte function-associated antigen-1 (LFA-1, CD11a/CD18) and very late antigen-4 (VLA-4, CD49/CD29). LFA-1 and VLA-4 are found mainly on monocytes and T cells but not neutrophils. These surface molecules interact with each other to bind, arrest, and promote the migration of monocytes and T cells. Some adhesion molecules are essential in the process of atherosclerosis and the subsequent myocardial infarction[25,26].
Analyzing HCV core antigen isolated from liver, kidney, heart, and bone marrow samples by immunohistochemistry found CD68-positive macrophages. HCV core and NS4 antibodies stained mostly infiltrating cells in the heart. These results suggest that macrophages play a role in directly attacking cardiac myocytes[27].
The relationship between HCV infection and endothelial surface markers is evident especially after eradication of disease, with a notable improvement in endothelial function. For example, liver transplant (LT) recipients with history of HCV infection showed significantly higher levels of endothelial activation markers such as soluble E-selectin, soluble ICAM-1, and soluble VCAM-1 compared to LT recipients without HCV infection. Furthermore, these markers are crucial indicators of endothelial dysfunction, which is associated with an increased risk of CVD. E-selectin, ICAM-1, and VCAM-1 play a role in recruiting white blood cells to endothelial cells, promoting inflammation and atherogenesis. The higher levels of these markers in LT+/HCV+ patients suggest that HCV infection contributes to endothelial activation, likely exacerbating the risk of atherosclerosis and subsequent cardiovascular complications post-transplant[28].
Another important marker is MMP-9 which is involved in extracellular matrix remodeling and is linked to the progression of atherosclerosis and plaque destabilization. Interestingly, LT+/HCV+ patients have lower levels of MMP-9 compared to the non-HCV group. These findings suggest that HCV infection plays a key role in endothelial dysfunction and could heighten cardiovascular risk in LT recipients. The fact that inflammatory and endothelial activation markers were significantly elevated in the HCV-infected group (while insulin resistance remained similar) points to HCV’s direct involvement in vascular damage, rather than through metabolic pathways alone. The lower MMP-9 Levels in HCV-infected patients add complexity to the understanding of how HCV impacts vascular health, potentially indicating differences in how the disease affects the stability of arterial plaques. It is worth noting that the study suffers from a small sample size and lack of pre-intervention assessment for atherosclerosis[28,29].
Another key mechanism of HCV infection is triggering an immune response characterized by elevated levels of GM-CSF and IgG, particularly in cirrhotic patients. This suggests an overactive yet ineffective immune response to the virus, contributing to cardiovascular complications[22].
HCV is lymphotropic and can infect immune cells such as B cells and monocytes, in addition to hepatocytes, via receptors including CD81, scavenger receptor class B type I, claudin 1 and occludin. This direct infection of immune cells leads to altered immune function, chronic immune activation, and the production of autoantibodies and immune complexes, which are implicated in extrahepatic manifestations such as mixed cryoglobulinemia and vasculitis. These immune-mediated processes contribute to vascular inflammation and endothelial dysfunction, key steps in the path
The literature reviewed in the New England Journal of Medicine by Cacoub and Saadoun[33] highlights that HCV-induced chronic antigenic stimulation reduces the threshold for B-cell activation, promotes clonal B-cell expansion and leads to immune complex deposition in small vessels.
HCV-infected patients (without cirrhosis) demonstrate a significant procoagulant imbalance characterized by elevated FVIII levels, higher FVIII/protein C ratios and increased thrombin potential. This imbalance contributes to an increased risk of cardiovascular damage and atherosclerosis, as indicated by the association between elevated FVIII/PC ratio and increased carotid intima-media thickness (CIMT), a marker of early atherosclerosis and cardiovascular risk[34].
Atherosclerosis reduces oxygen-rich blood supply leading to a variety of symptoms that affect quality of life. These symptoms depend on which arteries are affected and the extent of blood flow obstruction. When the coronary arteries are affected, it can result in chest pain (angina), cold sweats, dizziness, extreme fatigue, heart palpitations, shortness of breath, nausea, and general weakness, all of which are common signs of coronary artery disease.
When the peripheral arteries are involved, particularly in the legs, individuals may experience pain, aching, heaviness, or cramping when walking or climbing stairs. These symptoms typically subside after a period of rest and are indicative of peripheral artery disease. Furthermore, when the vertebral arteries are compromised, early symptoms may include problems with thinking and memory, weakness or numbness on one side of the body or face, and vision difficulties. If the condition progresses, it can lead to more serious outcomes, such as a transient ischemic attack.
Mesenteric artery ischemia, affecting the intestines, causes severe pain after eating, unintended weight loss and diarrhea. Additionally, erectile dysfunction (ED) can serve as an early warning sign of atherosclerosis and its complications, as it may indicate plaque buildup in the arteries. Men experiencing ED are advised to consult their healthcare providers to assess their risk of CVD[10].
HCV-associated heart disease and cardiovascular complications typically develop after a period of chronic infection, not concurrently with acute HCV infection. The literature consistently shows that the increased risk of atherosclerosis, coronary artery disease, stroke, and other cardiovascular events is associated with chronic HCV infection, which is usually defined as infection persisting beyond six months after exposure. Most extrahepatic manifestations, including CVD, are linked to the chronic inflammatory state, metabolic disturbances, and direct vascular effects that accumulate over years of persistent infection rather than during the acute phase.
There is evidence that subclinical atherosclerosis, such as increased CIMT and coronary artery plaque, can be detected in HCV-infected individuals before overt cardiovascular events occur, suggesting a gradual process rather than an immediate complication[30,35-38]. The risk of cardiovascular events is higher in those with active viremia (detectable HCV RNA), and the risk increases with age and the presence of comorbidities such as diabetes and hypertension[30,39].
The risk of cardiovascular events is higher in those with active viremia (detectable HCV RNA), and increases with age and the presence of comorbidities such as diabetes and hypertension[33,36,40].
Accurately diagnosing atherosclerosis in patients with HCV is essential, given their increased cardiovascular risk. Both invasive and noninvasive diagnostic techniques play crucial roles in detecting the disease and assessing its severity. There are no specific guideline-recommended screening protocols for early detection of heart disease unique to HCV-infected individuals. However, the medical literature supports vigilant assessment of traditional cardiovascular risk factors and consideration of subclinical atherosclerosis screening (e.g., carotid ultrasound, coronary calcium scoring) in patients with chronic HCV infection, especially those with additional risk factors. Early antiviral treatment with direct-acting antivirals is associated with improvement in cardiovascular risk markers and may reduce the incidence of cardiovascular events. Standard cardiovascular risk reduction strategies (blood pressure, lipid, and glucose control; smoking cessation) remain essential[33,37-42].
Coronary Arteriography remains the gold standard for assessing coronary atherosclerosis. It provides detailed images of the arterial lumen, allowing direct visualization of blockages[43,44].
Angiography offers a comprehensive view of the coronary tree; angiography is helpful in identifying stenosis or blockages[43,44].
Ultrasound and magnetic resonance imaging are methods that allow visualization of atherosclerotic plaques, particularly by assessing fibrous cap integrity and necrotic core size, key indicators of plaque stability. However, these techniques may struggle to detect smaller or less pronounced plaques[44,45].
CIMT: CIMT is an ultrasound-based method used to measure the thickness of the intima and media layers of the carotid artery. This technique is useful for detecting early atherosclerosis, especially in high-risk populations such as HCV patients. CIMT has been shown to correlate with the presence of coronary atherosclerosis and is widely used for risk stratification in CVD[45].
CIMT is often elevated in HCV-infected patients, particularly those with cirrhosis. CIMT values exceeding 0.9 mm or falling above the 75th percentile are considered diagnostic for atherosclerosis. CHC is associated with significantly increased CIMT compared to non-cirrhotic HCV patients, indicating an intensified risk of vascular disease progression in those with liver damage[46,47].
Electron-beam computed tomography and Ankle-Brachial index: Electron-beam computed tomography is used to measure coronary calcium, which is an indicator of atherosclerotic burden, while Ankle-Brachial index is useful for detecting peripheral artery disease. Both methods help identify early atherosclerosis but have limitations in terms of sensitivity and specificity[48].
HCV’s contribution to atherosclerosis is further supported by the detection of HCV RNA in carotid plaques of anti-HCV-positive patients, establishing a direct viral presence in atherosclerotic lesions. This local viral infection may drive plaque formation and instability, contributing to vascular dysfunction. Additionally, patients with CHC exhibit increased aortic stiffness, a marker of early arterial changes that precede overt atherosclerosis. This stiffness occurs independent of traditional cardiovascular risk factors like lipid profile or HCV-RNA levels, suggesting alternative mechanisms-likely mediated by chronic inflammation and immune activation-that contribute to vascular remodeling and stiffness[49].
Emerging diagnostic biomarkers are playing an increasingly important role in understanding the complex mechanisms behind atherosclerosis, particularly in high-risk groups like HCV patients. These biomarkers offer new avenues for early detection, risk stratification, and therapeutic monitoring.
Inflammation plays a central role in atherosclerosis, particularly in the context of chronic infections such as HCV. Biomarkers like VCAM-1 and P-selectin have gained attention for their involvement in plaque vulnerability. These markers are indicators of endothelial activation and leukocyte adhesion, which are critical in the development and pro
A study investigated the relationship between HCV infection, its markers, and the risk of atherosclerosis in patients with CHC. The results demonstrate that both HCV infected patients with steatosis have a higher prevalence of CA. Additionally, higher HCV viral load is linked to increased levels of inflammatory markers, mainly serum CRP and fibrinogen levels, and a greater risk of atherosclerosis[8].
Advanced biological tracers are being developed to evaluate the metabolic state of atherosclerotic plaques, further refining risk prediction and treatment strategies. These tracers offer real-time insights into plaque activity by highlighting metabolic changes, such as increased glucose uptake, that often precede clinical events. This enhanced understanding allows for better risk stratification and more tailored therapeutic interventions, particularly in patients with HCV, who are at increased risk of accelerated atherosclerosis[51].
The management of atherosclerosis in patients with HCV, particularly those with advanced fibrosis or cirrhosis, poses unique challenges. Historically, patients with advanced liver disease were considered “difficult-to-treat” due to the limited efficacy and high toxicity of peginterferon and ribavirin combination therapies. However, the introduction of direct-acting antiviral agents (DAAs) has revolutionized the treatment landscape, offering higher efficacy and fewer adverse effects[52].
First-generation DAAs like boceprevir and telaprevir, used in combination with peginterferon and ribavirin, sig
Next-generation DAAs, including second-generation HCV NS3/4A protease inhibitors, HCV NS5A inhibitors, and HCV NS5B inhibitors, offer more promising options for HCV patients with atherosclerosis. These agents are highly effective and have a lower toxicity profile, allowing for their use in interferon-free regimens, with or without ribavirin. Interferon-free regimens are especially beneficial for patients with cirrhosis or those intolerant to interferon. Clinical trials have demonstrated that these regimens can achieve similar efficacy and safety in both cirrhotic and non-cirrhotic patients[52].
For HCV patients with atherosclerosis and advanced liver disease, prioritizing antiviral therapy is crucial to prevent liver-related complications such as decompensation and hepatocellular carcinoma, while simultaneously reducing the systemic inflammatory burden that contributes to atherosclerotic progression[53].
Recent studies highlight the role of HMG CoA reductase inhibitors, commonly known as statins, in modulating hepatic steatosis and fibrosis. Statins have shown promising antiviral and antifibrotic effects in CHC patients, adding another layer of therapeutic potential for managing both liver disease and cardiovascular risk[53].
Lifestyle management is fundamental to prevent atherosclerotic CVD, and its importance is highlighted by all major guidelines. Key aspects of lifestyle modification include smoking cessation, weight loss, dietary improvements, increased physical activity, and stress management[54].
HCV appears to contribute to the development of multiple cardiomyopathy phenotypes, including dilated, hypertrophic, and restrictive forms. Studies have demonstrated a higher prevalence of HCV antibodies in patients with hypertrophic (10.6%) and dilated (6.3%) cardiomyopathies compared to the general population. Over a 10-year period, approximately 9.9% of patients with dilated cardiomyopathy (DCM) were found to have evidence of HCV infection, significantly higher than in patients with ischemic heart disease[55].
A population-based cohort study from 1998 to 2020 further highlighted the prevalence of cardiovascular complications among HCV patients. HCV was identified as the third most prevalent liver disease (6.0 per 100000 person-years), following NAFLD and alcoholic liver disease. The study also revealed that the age group most affected by HCV infection was 40-49 years, indicating that middle-aged individuals are more susceptible to developing HCV-related complications, including cardiomyopathy. Additionally, the study examined 17 types of CVD, with atrial fibrillation being the most common and hypertrophic cardiomyopathy the least prevalent. Table 2 shows a summary of study designs, findings and limitations[56].
| Ref. | Year | Study design | Findings | Limitations |
| Wang et al[74] | 2024 | Cross-sectional study | HCV group without metabolic syndrome had higher odds ratio of 2.75 | While CT is a widely used method, it primarily detects advanced atherosclerosis (calcified plaques) and may miss earlier stages of the disease |
| Chang et al[56] | 2022 | Population-based cohort | HCV patients have a high incidence of CVD, though it is lower than that of ALD and NAFLD | The findings may not be directly generalizable |
| Petta et al[9] | 2011 | Case-control | Severe hepatic fibrosis is associated with a high risk of early carotid atherosclerosis in G1 CHC patients | Selection bias in the recruitment of the control population and limited generalizability |
| Lee et al[72] | 2019 | Systematic review and meta-analysis | Patients with HCV had 28% higher risk compared to those without HCV infection | Limited generalizability on global scale, and significant heterogeneity |
| Wen et al[75] | 2019 | Systematic review and meta-analysis | The overall RR of 1.25 in the cohort studies, higher OR of 1.94 in these studies suggests a stronger association between HCV and CAD compared to the cohort studies | Small sample size between studies and different criteria for diagnosing CHC |
| Badawi et al[76] | 2018 | Survey analysis | HCV infection was significantly associated with a 25%-3.5% absolute risk increases of 10-year CVD after adjusting for sociodemographic and cardiometabolic risk factors | Confounding variables such as alcohol use, and drug use were not controlled |
| Roed et al[65] | 2014 | Cross-sectional study | Higher carotid intima media thickness in CHC patients with small difference of means | Confounding variables such as physical activity, diet, medication use, family history of CAD, and genetic predisposition were not controlled |
HCV has been implicated in the pathogenesis of myocarditis and cardiomyopathy, contributing to both inflammatory and structural damage in the myocardium. Myocarditis, an acute inflammation of the heart muscle, is often associated with viral infections and has been linked to HCV in various studies. The virus can directly invade cardiac tissue, triggering an immune response that results in myocardial injury. In patients with CHC, diastolic dysfunction has been observed, characterized by reduced left ventricular end-diastolic volume and increased myocardial fibrosis. Significant morbidity and mortality are still reported, primarily due to cardiogenic shock and arrhythmia[57].
The cardiotropic nature of HCV is further evidenced by its association with cardiomyopathy, particularly dilated and hypertrophic forms. Studies have demonstrated the presence of HCV antibodies in a significant proportion of patients with idiopathic cardiomyopathy, supporting the virus's role in the disease’s development. Additionally, HCV-related insulin resistance has been shown to contribute to left ventricular hypertrophy, indicating that metabolic dysfunction may exacerbate the virus's impact on cardiac structure[58].
Several studies have implicated HCV infection in the development of cardiomyopathy. Ngu et al[59] found that patients with CHC and histological analyses have confirmed the presence of HCV genomes in the myocardium of patients with myocarditis, suggesting a direct viral effect on cardiac cells. The results demonstrated diastolic dysfunction, characterized by lower left ventricular end-diastolic volume, stroke volume, and increased diffuse myocardial fibrosis. Importantly, these changes occurred in the absence of overt heart failure.
The direct impact of the HCV on cardiomyocytes plays a significant role in the development of cardiomyopathy. HCV has been shown to replicate within cardiac tissues, which leads to significant cellular damage and contributes to cardiac dysfunction. Studies have detected HCV RNA in myocardial tissues, confirming that the virus can infect heart cells directly. This viral replication can cause necrosis and apoptosis of the heart muscle cells (myocytes), leading to the development of conditions like DCM and other related cardiac disorders[60,61].
Moreover, the HCV core protein exerts a cytotoxic effect on myocytes by disrupting essential cellular structures and interferes with calcium handling, a critical process for muscle contraction. This disruption can result in ventricular dilation and reduced contractility, which are key features of DCM[6,62].
Infected cardiomyocytes also trigger local inflammatory responses. The presence of the virus leads to the recruitment of immune cells to the heart muscle, causing chronic inflammation and further damage to the cardiac tissue. Additionally, HCV infection induces the release of pro-inflammatory cytokines such as TNF-α and IL-6, which can exacerbate myocardial injury and contribute to the development of heart failure[6,62].
The immune response to HCV infection is another critical factor in the progression of cardiomyopathy. Both the innate and adaptive immune systems are involved in this process. Upon infection, the innate immune system is activated, leading to the production of inflammatory cytokines by immune cells like macrophages and natural killer (NK) cells. This response causes tissue damage not only in the liver but also in the heart, contributing to the deterioration of cardiac function[63].
Chronic HCV infection can also result in the formation of autoantibodies against myocardial proteins such as troponin I. These autoantibodies further promote inflammation in the heart and exacerbate myocardial dysfunction, thereby worsening cardiac outcomes. Additionally, the immune response often results in an imbalance between pro-inflammatory and anti-inflammatory cytokines. Elevated levels of TNF-α, for example, are linked to impaired cardiac function, as this cytokine disrupts calcium currents in heart cells, compromising their ability to contract properly[60,61].
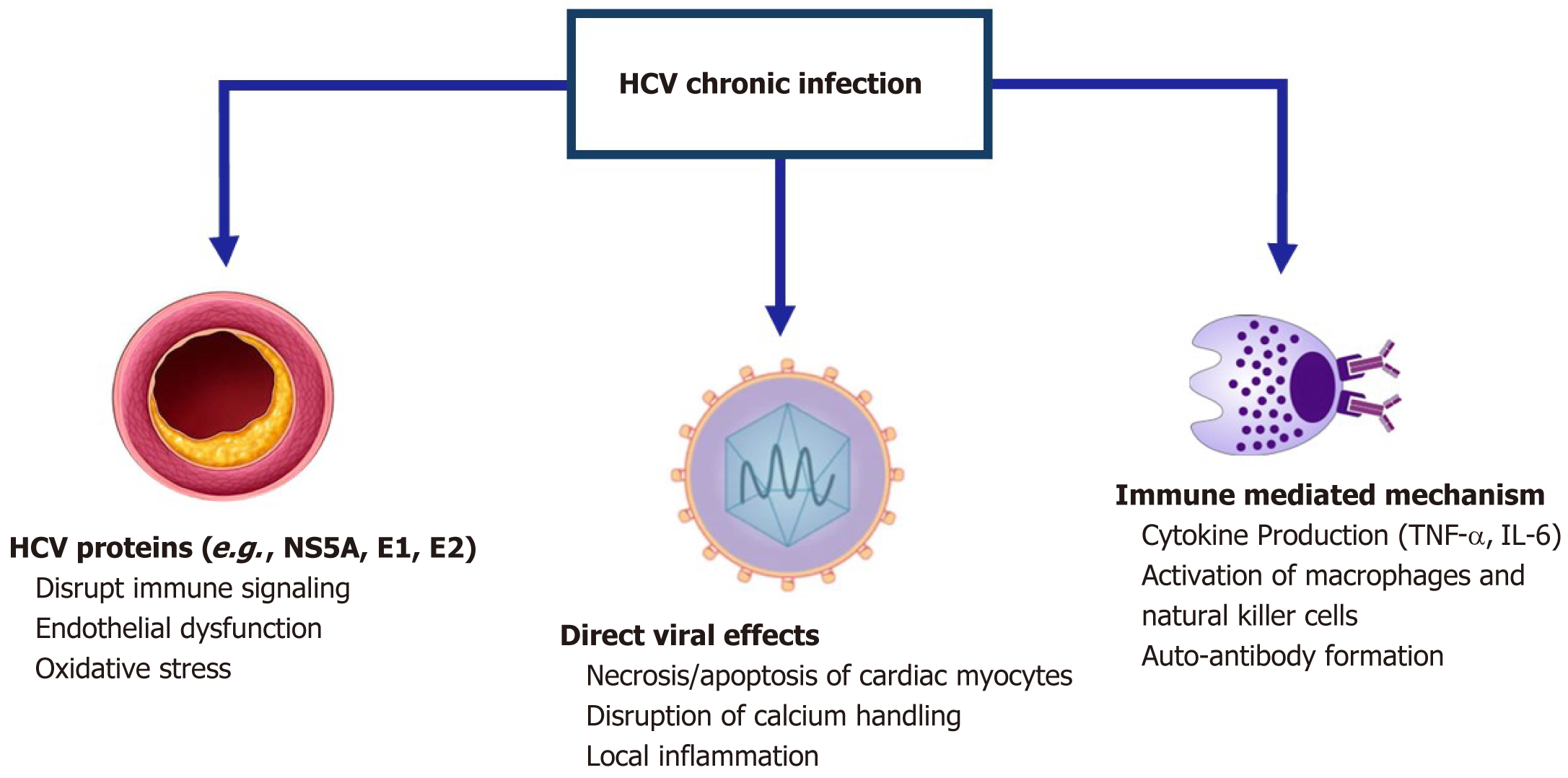
Molecular mimicry is another mechanism by which HCV infection contributes to cardiomyopathy. In this process, the structural similarity between viral proteins and host myocardial proteins triggers an autoimmune response, leading to further damage to the heart muscle. Persistent inflammation caused by ongoing viral replication also contributes to myocardial fibrosis and remodeling, both of which are key factors in the progression toward heart failure in HCV-infected individuals. Figure 1 shows the 3 possible mechanisms of HCV infection on the cardiovascular system[60,61].
HCV proteins play a crucial role in modulating immune responses and can significantly impact cardiovascular health. One notable protein, NS5A, disrupts innate immune signaling pathways, particularly those involving Toll-like receptors. This disruption can lead to chronic inflammation, a condition recognized as a risk factor for various cardiovascular complications. The chronic inflammatory state induced by HCV can create an environment conducive to the development of CVD, including atherosclerosis and coronary artery disease[64].
Additionally, NS5A impairs the function of NK cells, further exacerbating inflammatory responses. NK cells are essential for the innate immune response and play a vital role in controlling viral infections. The dysfunction of these cells due to HCV can promote heightened inflammation and contribute to the progression of cardiovascular complications[64].
The envelope proteins of HCV, specifically E1 and E2, also contribute to cardiovascular risk. These proteins can induce endothelial dysfunction and promote inflammation, which are critical processes in the development of atherosclerosis. The interplay between HCV proteins and the immune response highlights the intricate mechanisms through which HCV infection can adversely affect cardiovascular health[65,66].
One of the most common signs is shortness of breath, which may occur during physical exertion or even at rest as the heart struggles to pump efficiently. Fatigue is another common symptom, where individuals experience unexplained tiredness despite adequate rest. Swelling, known as edema, may appear in the ankles, legs, abdomen, or neck veins due to fluid retention caused by impaired cardiac function[67].
Other symptoms include dizziness and light-headedness, sometimes leading to fainting, particularly during physical activity. Arrhythmias, or irregular heartbeats, may feel like rapid or fluttering sensations in the chest. Some patients experience chest pain, especially after physical exertion or large meals, while bloating in the abdominal area and coughing when lying down can also indicate worsening heart function. These symptoms tend to progress as the condition advances, greatly impacting a patient’s daily life and quality of life[68].
An electrocardiogram is often one of the first diagnostic tools used, as it can detect abnormalities in the heart’s electrical activity due to chamber hypertrophy or dilatation, which are common in cardiomyopathy.
An echocardiogram is a key imaging tool used to visualize the heart’s structure and function. It provides valuable information about heart chamber size, wall motion abnormalities, and whether the heart is functioning normally in terms of systolic and diastolic function. A chest X-ray may also be performed to check for signs of heart enlargement (cardiomegaly) or fluid accumulation in the lungs, though it is not specific for diagnosing cardiomyopathy.
More invasive diagnostic techniques, such as cardiac catheterization, may be used to measure the pressures within the heart chambers and evaluate blood flow. This test often includes coronary angiography to rule out any blockages in the coronary arteries that might contribute to heart dysfunction. In certain cases, a myocardial biopsy may be performed to remove and examine a small sample of heart tissue, which can help identify specific types of cardiomyopathies[67-69].
MicroRNA-21 has gained attention as a promising biomarker in the study of HCV-related conditions, particularly due to its involvement in various pathological processes such as inflammation and cardiac remodeling. Increased levels of microRNA-21 have been associated with cardiac hypertrophy and fibrosis. Notably, microRNA-21 Levels show a strong correlation with serum markers of myocardial fibrosis, indicating its value in tracking cardiac involvement and disease progression in HCV-infected individuals[70].
HCV RNA has been detected in myocardial tissues, supporting the possibility that the virus can replicate within the heart muscle itself. Research has shown that around 19.4% of patients with DCM had detectable levels of HCV RNA in their heart tissue. This presence of the virus correlates with the severity of cardiac symptoms such as heart failure and arrhythmias. As a result, HCV RNA detection in heart tissue could serve as a direct marker for assessing myocardial involvement in patients with cardiomyopathy[6].
Elevated levels of atrial and brain natriuretic peptides have been observed in HCV-infected patients who develop cardiomyopathy. These peptides are typically markers of heart failure, as they rise in response to increased cardiac stress and dysfunction[6].
Another interesting marker is cardiac troponin T, which is a widely recognized biomarker for detecting myocardial injury, and it has been used in patients with HCV-related cardiomyopathy to assess heart muscle damage. Elevated levels of troponin T can indicate ongoing myocardial injury and are often monitored alongside other cardiac markers to provide a more comprehensive understanding of a patient’s cardiac health. In the context of HCV infection, elevated troponin levels serve as a critical marker for cardiac involvement and damage[6].
Treatment of cardiomyopathy, particularly in the context of HCV-related disease, focuses on both managing symptoms and addressing the underlying cause.
Antiviral therapies such as DAAs have revolutionized the management of HCV infection, which can indirectly contribute to cardiomyopathy. These antivirals help reduce systemic inflammation and may limit the progression of cardiomyopathy by addressing the viral cause.
Historically, interferon-based regimens were used in HCV treatment, but their lower efficacy in patients with cirrhosis, combined with significant side effects, have led to their decline in favor of DAAs[52].
For managing cardiomyopathy symptoms directly, beta-blockers and calcium channel blockers are often used to manage heart rate and improve overall cardiac function, though specific evidence for their efficacy in HCV-related cardiomyopathy remains limited. Symptomatic treatment in these patients is aimed at improving quality of life and reducing the risk of cardiac complications[71].
A systematic review and meta-analysis (SR/MA) examined the relationship between HCV infection and CVD on a global scale. The SR/MA estimated 1.5 million DALYs lost annually due to HCV-associated CVD. The highest burden of HCV-associated CVD was observed in low-income and middle-income countries, with regions such as South Asia, eastern Europe, north Africa, and the Middle East accounting for two-thirds of all DALYs. This highlights the disproportionate impact of HCV infection on these regions and underscores the need for targeted interventions to address the burden of CVD in these populations[72].
Despite the large sample size in this study, most of the studies included in the meta-analysis originate from high-income countries, particularly in North America and Western Europe. This limits the generalizability of their results on a global scale. Furthermore, the study observed significant heterogeneity in the risk ratio estimates, likely due to the diverse patient populations, varying viremic statuses, differences in healthcare systems, access to treatment, and geographical locations of the studies. Finally, the study's burden estimation may have been limited by the inability to assess the impact of angina and peripheral artery disease, conditions that are often diagnosed in outpatient settings and less likely to be captured by electronic health records[72].
A study, by Hallsworth et al[73] found that those who engaged in regular physical activity reported significantly higher health-related quality of life (HRQoL) scores across various domains of the Short-Form 36v2 compared to their inactive counterparts. Despite these differences in HRQoL, no significant differences were observed in metabolic and car
The study also examined the Exercise Benefits/Barriers Scale (EBBS) and found that while overall scores were similar between active and inactive participants, active patients scored significantly higher on the psychological outlook and social interaction subscales. Furthermore, significant associations were observed between EBBS scores and HRQoL, suggesting that psychological and social factors might play a role in enhancing quality of life through regular physical activity, even if direct metabolic or cardiovascular improvements were not detected[73-76].
CHC infection is now recognized as a significant contributor to CVD, extending its clinical impact beyond hepatic pathology. This minireview summarizes current evidence linking HCV to both atherosclerotic and cardiomyopathic heart disease, highlighting the multifaceted mechanisms by which the virus influences cardiovascular risk. The interplay of direct viral effects, immune activation, oxidative stress, and metabolic disturbances emphasizes the increased risk of atherosclerosis and cardiomyopathy in affected individuals. Early diagnosis, comprehensive cardiovascular risk assessment, prompt initiation of antiviral therapy, and aggressive management of modifiable risk factors are essential strategies to reduce the burden of CVD in patients with CHC. Further research is needed to elucidate the different pathways, refine risk stratification, and optimize therapeutic approaches for this high-risk population.
| 1. | 2022 Viral Hepatitis Surveillance Report. Hepatitis C Surveillance. Available from: https://www.cdc.gov/hepatitis-surveillance-2022/hepatitis-c/index.html. |
| 2. | Yang J, Qi JL, Wang XX, Li XH, Jin R, Liu BY, Liu HX, Rao HY. The burden of hepatitis C virus in the world, China, India, and the United States from 1990 to 2019. Front Public Health. 2023;11:1041201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 3. | Matsumori A, Shimada T, Chapman NM, Tracy SM, Mason JW. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006;12:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Boyella V, Onyebueke I, Farraj N, Graham-Hill S, El Younis C, Bergasa NV. Prevalence of hepatitis C virus infection in patients with cardiomyopathy. Ann Hepatol. 2009;8:113-115. [PubMed] |
| 5. | Shoeib O, Ashmawy M, Badr S, El Amroosy M. Association between coronary artery disease and hepatitis C virus seropositivity. East Mediterr Health J. 2018;24:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Iorga RA, Bacalbasa N, Bratu OG, Ionita Radu F, Diaconu CC. The impact of infection with hepatitis C virus on cardiovascular risk. Am J Cardiovasc Dis. 2020;10:201-206. [PubMed] |
| 8. | Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, Licata A, Marchesini G, Mazzola A, Parrinello G, Novo S, Licata G, Craxì A. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5:927-946. [PubMed] |
| 11. | Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, Benito-Vicente A, Martín C. Pathophysiology of Atherosclerosis. Int J Mol Sci. 2022;23:3346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 435] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 12. | Yue M, Deng X, Zhai X, Xu K, Kong J, Zhang J, Zhou Z, Yu X, Xu X, Liu Y, Zhu D, Zhang Y. Th1 and Th2 cytokine profiles induced by hepatitis C virus F protein in peripheral blood mononuclear cells from chronic hepatitis C patients. Immunol Lett. 2013;152:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Oliveira CP, Kappel CR, Siqueira ER, Lima VM, Stefano JT, Michalczuk MT, Marini SS, Barbeiro HV, Soriano FG, Carrilho FJ, Pereira LM, Alvares-da-Silva MR. Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol. 2013;164:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Sevastianos VA, Voulgaris TA, Dourakis SP. Hepatitis C, systemic inflammation and oxidative stress: correlations with metabolic diseases. Expert Rev Gastroenterol Hepatol. 2020;14:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Tawadrous GA, Aziz AA, Amin DG, Eldemery A, Mostafa MA. RANTES, TNF-α, oxidative stress, and hematological abnormalities in hepatitis C virus infection. J Investig Med. 2012;60:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Ray M, Autieri MV. Regulation of pro- and anti-atherogenic cytokines. Cytokine. 2019;122:154175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Cavusoglu E, Marmur JD, Yanamadala S, Chopra V, Hegde S, Nazli A, Singh KP, Zhang M, Eng C. Elevated baseline plasma IL-8 levels are an independent predictor of long-term all-cause mortality in patients with acute coronary syndrome. Atherosclerosis. 2015;242:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Ait-Oufella H, Taleb S, Tedgui A. Interleukin 5 Contributes to Human Atherosclerosis Development But not to Thrombotic Complications. JACC Basic Transl Sci. 2019;4:903-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Zekri A, Badr A, Rabah S, El Razky M, El Deeb S. Serum cytokine profile during disease progression stages in male and female hepatitis C patients. J Biosci Appl Res. 2019;5:308-324. [DOI] [Full Text] |
| 21. | Zhang Z, Rong L, Li YP. Flaviviridae Viruses and Oxidative Stress: Implications for Viral Pathogenesis. Oxid Med Cell Longev. 2019;2019:1409582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Almaeen AH, Alduraywish AA, Mobasher MA, Almadhi OIM, Nafeh HM, El-Metwally TH. Oxidative stress, immunological and cellular hypoxia biomarkers in hepatitis C treatment-naïve and cirrhotic patients. Arch Med Sci. 2021;17:368-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, Isaguliants MG. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget. 2017;8:3895-3932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Freekh DA, Helmy MW, Said M, El-Khodary NM. The effect of direct acting antiviral agents on vascular endothelial function in Egyptian patients with chronic hepatitis C virus infection. Saudi Pharm J. 2021;29:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Fekadu J, Modlich U, Bader P, Bakhtiar S. Understanding the Role of LFA-1 in Leukocyte Adhesion Deficiency Type I (LAD I): Moving towards Inflammation? Int J Mol Sci. 2022;23:3578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Macías C, Villaescusa R, del Valle L, Boffil V, Cordero G, Hernández A, Hernández P, Ballester JM. [Endothelial adhesion molecules ICAM-1, VCAM-1 and E-selectin in patients with acute coronary syndrome]. Rev Esp Cardiol. 2003;56:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Matsumori A, Shimada M, Obata T. Leukocytes are the major target of hepatitis C virus infection: Possible mechanism of multiorgan involvement including the heart. Cvd Prev Control. 2010;5:51-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Caballero-Marcos A, Romero-Cristóbal M, Puerto M, Fernández-Yunquera A, Dieguez L, Navarrete C, Clemente A, Diaz-Fontenla F, Catalán P, Rincón D, López-Baena JÁ, Bañares Cañizares R, Salcedo M. HCV eradication in recurrent hepatitis C after liver transplantation normalizes enhanced endothelial activation. Transpl Int. 2021;34:2214-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Li T, Li X, Feng Y, Dong G, Wang Y, Yang J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediators Inflamm. 2020;2020:3872367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Sarhan MA, Chen AY, Michalak TI. Differential expression of candidate virus receptors in human T lymphocytes prone or resistant to infection with patient-derived hepatitis C virus. PLoS One. 2013;8:e62159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Kondo Y, Shimosegawa T. Direct effects of hepatitis C virus on the lymphoid cells. World J Gastroenterol. 2013;19:7889-7895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Tomer S, Arora SK. A juggernaut of innate & adaptive immune cells in chronic hepatitis C. Indian J Med Res. 2020;151:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Cacoub P, Saadoun D. Extrahepatic Manifestations of Chronic HCV Infection. N Engl J Med. 2021;384:1038-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 34. | Sigon G, D'Ambrosio R, Clerici M, Pisano G, Chantarangkul V, Sollazzi R, Lombardi R, Peyvandi F, Lampertico P, Fargion S, Tripodi A, Fracanzani AL. Procoagulant imbalance influences cardiovascular and liver damage in chronic hepatitis C independently of steatosis. Liver Int. 2019;39:2309-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 36. | Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410-3417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 37. | Goossens N, Negro F. Cardiovascular Manifestations of Hepatitis C Virus. Clin Liver Dis. 2017;21:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | McKibben RA, Haberlen SA, Post WS, Brown TT, Budoff M, Witt MD, Kingsley LA, Palella FJ Jr, Thio CL, Seaberg EC. A Cross-sectional Study of the Association Between Chronic Hepatitis C Virus Infection and Subclinical Coronary Atherosclerosis Among Participants in the Multicenter AIDS Cohort Study. J Infect Dis. 2016;213:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Society for Maternal-Fetal Medicine (SMFM). Dotters-Katz SK, Kuller JA, Hughes BL. Society for Maternal-Fetal Medicine Consult Series #56: Hepatitis C in pregnancy-updated guidelines: Replaces Consult Number 43, November 2017. Am J Obstet Gynecol. 2021;225:B8-B18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Domont F, Cacoub P. Chronic hepatitis C virus infection, a new cardiovascular risk factor? Liver Int. 2016;36:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Babiker A, Hassan M, Muhammed S, Taylor G, Poonia B, Shah A, Bagchi S. Inflammatory and cardiovascular diseases biomarkers in chronic hepatitis C virus infection: A review. Clin Cardiol. 2020;43:222-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Adinolfi LE, Rinaldi L, Nevola R. Chronic hepatitis C, atherosclerosis and cardiovascular disease: What impact of direct-acting antiviral treatments? World J Gastroenterol. 2018;24:4617-4621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Onut R, Balanescu AP, Constantinescu D, Calmac L, Marinescu M, Dorobantu PM. Imaging Atherosclerosis by Carotid Intima-media Thickness in vivo: How to, Where and in Whom? Maedica (Bucur). 2012;7:153-162. [PubMed] |
| 44. | Owen DR, Lindsay AC, Choudhury RP, Fayad ZA. Imaging of atherosclerosis. Annu Rev Med. 2011;62:25-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, Ohayon J, Pettigrew R, Sabatine MS, Tearney GJ, Waxman S, Domanski MJ, Srinivas PR, Narula J. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5:941-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 46. | Barakat AAE, Nasr FM, Metwaly AA, Morsy S, Eldamarawy M. Atherosclerosis in chronic hepatitis C virus patients with and without liver cirrhosis. Egypt Heart J. 2017;69:139-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3371] [Cited by in RCA: 3354] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 48. | de Korte CL, Hansen HH, van der Steen AF. Vascular ultrasound for atherosclerosis imaging. Interface Focus. 2011;1:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Rossi L, Pratesi C, Gensini GF, Paperetti L, Zignego AL. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Moccetti F, Weinkauf CC, Davidson BP, Belcik JT, Marinelli ER, Unger E, Lindner JR. Ultrasound Molecular Imaging of Atherosclerosis Using Small-Peptide Targeting Ligands Against Endothelial Markers of Inflammation and Oxidative Stress. Ultrasound Med Biol. 2018;44:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Syed MB, Fletcher AJ, Forsythe RO, Kaczynski J, Newby DE, Dweck MR, van Beek EJ. Emerging techniques in atherosclerosis imaging. Br J Radiol. 2019;92:20180309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Nakamoto S, Kanda T, Shirasawa H, Yokosuka O. Antiviral therapies for chronic hepatitis C virus infection with cirrhosis. World J Hepatol. 2015;7:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Simon TG, Butt AA. Lipid dysregulation in hepatitis C virus, and impact of statin therapy upon clinical outcomes. World J Gastroenterol. 2015;21:8293-8303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | van Trier TJ, Mohammadnia N, Snaterse M, Peters RJG, Jørstad HT, Bax WA. Lifestyle management to prevent atherosclerotic cardiovascular disease: evidence and challenges. Neth Heart J. 2022;30:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Matsumori A, Matoba Y, Sasayama S. Dilated cardiomyopathy associated with hepatitis C virus infection. Circulation. 1995;92:2519-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 108] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Chang WH, Mueller SH, Chung SC, Foster GR, Lai AG. Increased burden of cardiovascular disease in people with liver disease: unequal geographical variations, risk factors and excess years of life lost. J Transl Med. 2022;20:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Haykal M, Matsumori A, Saleh A, Fayez M, Negm H, Shalaby M, Bassuony S. Diagnosis and treatment of HCV heart diseases. Expert Rev Cardiovasc Ther. 2021;19:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Wexler RK, Elton T, Pleister A, Feldman D. Cardiomyopathy: an overview. Am Fam Physician. 2009;79:778-784. [PubMed] |
| 59. | Ngu PJ, Butler M, Pham A, Roberts SK, Taylor AJ. Cardiac remodelling identified by cardiovascular magnetic resonance in patients with hepatitis C infection and liver disease. Int J Cardiovasc Imaging. 2016;32:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Roguljic H, Nincevic V, Bojanic K, Kuna L, Smolic R, Vcev A, Primorac D, Vceva A, Wu GY, Smolic M. Impact of DAA Treatment on Cardiovascular Disease Risk in Chronic HCV Infection: An Update. Front Pharmacol. 2021;12:678546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Poller W, Haghikia A, Kasner M, Kaya Z, Bavendiek U, Wedemeier H, Epple HJ, Skurk C, Landmesser U. Cardiovascular Involvement in Chronic Hepatitis C Virus Infections - Insight from Novel Antiviral Therapies. J Clin Transl Hepatol. 2018;6:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Rezende AGDS, Lopes EP, Markman-filho B. Cardiac Disorder in Chronic Hepatitis C. Int J Cardiovasc Sci. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Fierro NA, Gonzalez-Aldaco K, Torres-Valadez R, Martinez-Lopez E, Roman S, Panduro A. Immunologic, metabolic and genetic factors in hepatitis C virus infection. World J Gastroenterol. 2014;20:3443-3456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 64. | Imran M, Waheed Y, Manzoor S, Bilal M, Ashraf W, Ali M, Ashraf M. Interaction of Hepatitis C virus proteins with pattern recognition receptors. Virol J. 2012;9:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging. 2012;32:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Wong RJ, Kanwal F, Younossi ZM, Ahmed A. Hepatitis C virus infection and coronary artery disease risk: a systematic review of the literature. Dig Dis Sci. 2014;59:1586-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | American Heart Association. Symptoms and Diagnosis of Cardiomyopathy. Available from: https://www.heart.org/en/health-topics/cardiomyopathy/symptoms-and-diagnosis-of-cardiomyopathy. |
| 68. | Ciarambino T, Menna G, Sansone G, Giordano M. Cardiomyopathies: An Overview. Int J Mol Sci. 2021;22:7722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 69. | Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:e159-e240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 451] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 70. | Hussein MA, Radwan AFM, Fawzi MM, Rashed LA, Saad EHAI. MicroRNA 21as a novel biomarker in hepatitis C virus-related hepatocellular carcinoma. Egypt J Intern Med. 2022;34:56. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Spoladore R, Maron MS, D'Amato R, Camici PG, Olivotto I. Pharmacological treatment options for hypertrophic cardiomyopathy: high time for evidence. Eur Heart J. 2012;33:1724-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 72. | Lee KK, Stelzle D, Bing R, Anwar M, Strachan F, Bashir S, Newby DE, Shah JS, Chung MH, Bloomfield GS, Longenecker CT, Bagchi S, Kottilil S, Blach S, Razavi H, Mills PR, Mills NL, McAllister DA, Shah ASV. Global burden of atherosclerotic cardiovascular disease in people with hepatitis C virus infection: a systematic review, meta-analysis, and modelling study. Lancet Gastroenterol Hepatol. 2019;4:794-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 73. | Hallsworth K, Gosrani S, Hogg S, Patel P, Wetten A, Welton R, McPherson S, Campbell MD. Association of exercise participation levels with cardiometabolic health and quality of life in individuals with hepatitis C. BMJ Open Gastroenterol. 2021;8:e000591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Wang Y, Liu Y, Chen L, Zhang X, Li J, Zhao H. The association between hepatitis C virus infection status and blood pressure in US adults: a cross-sectional study. Front Cell Infect Microbiol. 2024;14:1401323. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 75. | Wen D, Du X, Dong JZ, Ma CS. Hepatitis C virus infection and risk of coronary artery disease: A meta-analysis. Eur J Intern Med. 2019;63:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Badawi A, Di Giuseppe G, Arora P. Cardiovascular disease risk in patients with hepatitis C infection: Results from two general population health surveys in Canada and the United States (2007-2017). PLoS One. 2018;13:e0208839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |