Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.103217
Revised: March 10, 2025
Accepted: May 21, 2025
Published online: June 27, 2025
Processing time: 225 Days and 17.2 Hours
Since non-alcoholic fatty liver disease (NAFLD) is associated with abnormal liver function tests, treatment recommendations aim to reduce the level of known markers of liver inflammation, such as alanine transaminase (ALT), aspartate transaminase (AST) and gamma-glutamyl transferase (GGT). Essential phospho
To describe liver enzyme profiles across NAFLD severity to inform a diagnostic staging algorithm and identify who may benefit from EPLs.
This post-hoc analysis of the observational MANPOWER study included 2843 adult patients with newly diagnosed NAFLD. The primary endpoint was assessment of baseline liver enzyme profiles. Secondary endpoints were effectiveness of Essentiale® (an EPL) on liver enzyme levels and ultrasonography findings across three definitions of NAFLD: (1) Statistical distribution of liver enzyme levels; (2) MANPOWER cut-offs; and (3) Presence of physician-diagnosed non-alcoholic steatohepatitis. The best performing algorithm was used to describe the risk factors and profiles associated with increased liver enzyme levels.
Of the 2843 patients included in this post-hoc analysis, most were female (62.2%), with a mean age of 48.4 years (SD 8.59 years). Overall, mean levels of ALT, AST and GGT increased with NAFLD severity for all three subgroups, with the rate of chronic comorbidities correlated with NAFLD severity. Across each subgroup of interest, Essentiale significantly reduced average liver enzyme levels and improved ultrasonography features, including diffuse liver hyperechogenicity and heterogeneous liver structure (P < 0.05), with greater benefit associated with increased severity. Compared with all algorithms tested, the algorithm based on the statistical distribution of liver enzymes displayed the highest accuracy, sensitivity and specificity for the grading and staging of NAFLD and could form the basis of a diagnostic algorithm.
Liver enzyme profiles may identify NAFLD severity and allow monitoring of therapeutic response. Essentiale may improve liver enzyme levels and ultrasonography features. An algorithm could aid in the diagnosis/staging of NAFLD.
Core Tip: Essentiale Forte N®, administered as adjunctive therapy in patients with non-alcoholic fatty liver disease (NAFLD), reduced liver enzyme levels [alanine transaminase (ALT), aspartate transaminase (AST) and gamma-glutamyl transferase (GGT)] and improved ultrasonographic features of NAFLD, particularly with regard to liver hyperechogenicity and structure, with a greater reduction noted with increasing NAFLD severity. These data indicate that Essentiale, alone or in combination with other treatment, is effective for the management of NAFLD. Additionally, a non-invasive diagnostic algorithm based on the statistical distribution of three liver enzymes (ALT, AST, and GGT) could be used to increase the accuracy of diagnosis, staging, and monitoring of patients with NAFLD.
- Citation: Dajani AI, Popovic B, Amand C, Tong S, Starostin KM, Goncharuk V. MANPOWER study: Real-world post-hoc analysis assessing essential phospholipids for non-alcoholic fatty liver disease from the Russian registry. World J Hepatol 2025; 17(6): 103217
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/103217.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.103217
Non-alcoholic fatty liver disease (NAFLD) is a major public health problem that impacts approximately 25% of adults worldwide[1,2]; with prevalence increasing to 75%-93% in patients with obesity[2]. NAFLD, defined by the presence of fat (≥ 5%) in the liver, encompasses a broad spectrum of clinical pathologies, ranging from simple steatosis without substantial liver inflammation or injury, to the most severe form, non-alcoholic steatohepatitis (NASH), which is characterised by liver inflammation and injury[2]. Progression of NASH can lead to advanced fibrosis and cirrhosis, which may trigger the development of hepatocellular carcinoma[3,4].
Based on the heterogeneous nature of NAFLD, a consensus panel of international experts recommended that NAFLD be redefined as metabolic (dysfunction)-associated fatty liver disease (MAFLD); the updated diagnostic criteria were based on the presence of metabolic dysfunction[5,6]. However, the use of NAFLD has been retained throughout this manuscript as the NAFLD criteria were used for data collection.
Current treatment recommendations for NAFLD aim to reduce the levels of known markers of liver inflammation, such as alanine transaminase (ALT), aspartate transaminase (AST) and gamma-glutamyl transferase (GGT), by focusing on lifestyle modifications (e.g., diet, exercise and weight loss); however, successful management of NAFLD is highly dependent on patient adherence and, as such, is largely ineffective in real-world settings[2,3]. Consequently, pharmacotherapies targeting the main pathways involved in the development and progression of NAFLD, such as lipotoxicity, insulin resistance, along with inflammatory and immune pathways, are under investigation[2,7]. Among these emerging pharmacological options, essential phospholipids (EPLs), which contain a highly purified extract of polyenyl phosphatidylcholine (PPC) molecules from soybean, have been shown to target fat accumulation in the liver and improve liver enzyme levels[8]. Previous studies have shown that the use of EPLs as monotherapy, or in conjunction with other treatment, not only improves the serum levels of liver function markers (ALT, AST and GGT) and lipids, but also improves/resolves fatty liver, as assessed by ultrasonography[9,10]. As a result, EPLs have been incorporated into several national guidelines for the diagnosis and management of NAFLD[11,12]. Essentiale Forte N® is a leading commercially available PPC that supports the management of damaged liver due to acute or chronic liver disease such as NAFLD, MAFLD, alcoholic liver disease, drug-induced liver disease, excess iron and copper, autoimmune hepatitis and cirrhosis[13].
Multiple modalities can be used to diagnose and monitor NAFLD, including clinical, biochemical and imaging techniques; however, liver biopsy remains the gold standard for the diagnosis and staging of NAFLD. Although liver biopsy demonstrates a high degree of accuracy for distinguishing the different stages of NAFLD, it is an expensive and invasive technique and is associated with morbidity and mortality[2,14]. As a result, ultrasonography is extensively used in the diagnostic workup of patients with NAFLD as it is readily available, cost-effective and well-tolerated[14,15]. However, as the diagnostic performance of ultrasonography is limited by multiple factors (e.g., patient characteristics, accessibility, operator skill and an inability to grade NAFLD fibrosis)[16], research has focused on the development of non-invasive diagnostic tools for the diagnosis and staging of NAFLD.
Since NAFLD is associated with abnormal liver function tests, the measurement of serum liver enzymes, such as ALT, AST and GGT, has been adopted into clinical practice to improve diagnostic accuracy[17]. Several non-invasive digital tools based on liver enzymes have been developed to stage liver inflammation, but these were not specifically designed for patients with NAFLD[18]. Additionally, although elevated levels of ALT, AST and GGT are associated with NAFLD, they do not correlate with NAFLD severity, and have displayed poor predictive ability for NASH[19]. This highlights the need to describe the fluctuating profile of ALT, AST and GGT across the different levels of NAFLD severity to form a diagnostic algorithm for the grading and staging of NAFLD and prediction of NASH, and to identify those who may benefit most from treatment with a commercially available PPC.
This was a post-hoc analysis of pre-existing data from the MANPOWER study, which was a 6-month, observational, multicenter, prospective study carried out in 174 medical sites across six major federal districts of the Russian Federation. A detailed description of the study design has been previously published[3]. Briefly, patients aged 18-60 years with newly diagnosed NAFLD (within 30 days of study entry) and receiving PPC (Essentiale Forte N, containing 300 mg of EPLs (hereafter referred to as Essentiale) as adjunctive treatment to standard of care were enrolled between September 2015 and September 2016 and prospectively observed. NAFLD was diagnosed by a physician and based on clinical exa
Data on patient characteristics were collected using paper-based case report forms at baseline, week 12 and week 24. At baseline, information on demographics, comorbidities, severity/histologic features of NAFLD and previous/current treatments and PPC therapy were collected.
All laboratory parameters, including fasting blood glucose, lipid profile, liver function tests (ALT, AST and GGT), routine urine analysis and ultrasonography parameters, including diffuse liver hyperechogenicity and heterogeneous structure of the liver, were obtained at baseline, week 12 and week 24.
The primary endpoint was to describe the baseline enzyme levels (ALT, AST and GGT) of patients diagnosed with NAFLD across the spectrum of disease severity, from early NAFLD through to NASH.
The key secondary endpoint was to assess the effectiveness of Essentiale, according to various NAFLD severity spectrum subgroups of interest, as categorised by ALT, AST and GGT levels, in reducing liver enzyme levels and improving ultrasonography parameters through the 24-week follow-up period. The co-secondary endpoints were to develop and test the diagnostic accuracy of various algorithms (Supplementary Table 1), to define the severity spectrum of NAFLD using the three liver enzymes (ALT, AST and GGT) and to assess the risk factors and profiles associated with increased liver enzyme levels using the definitions derived from the best-performing algorithms.
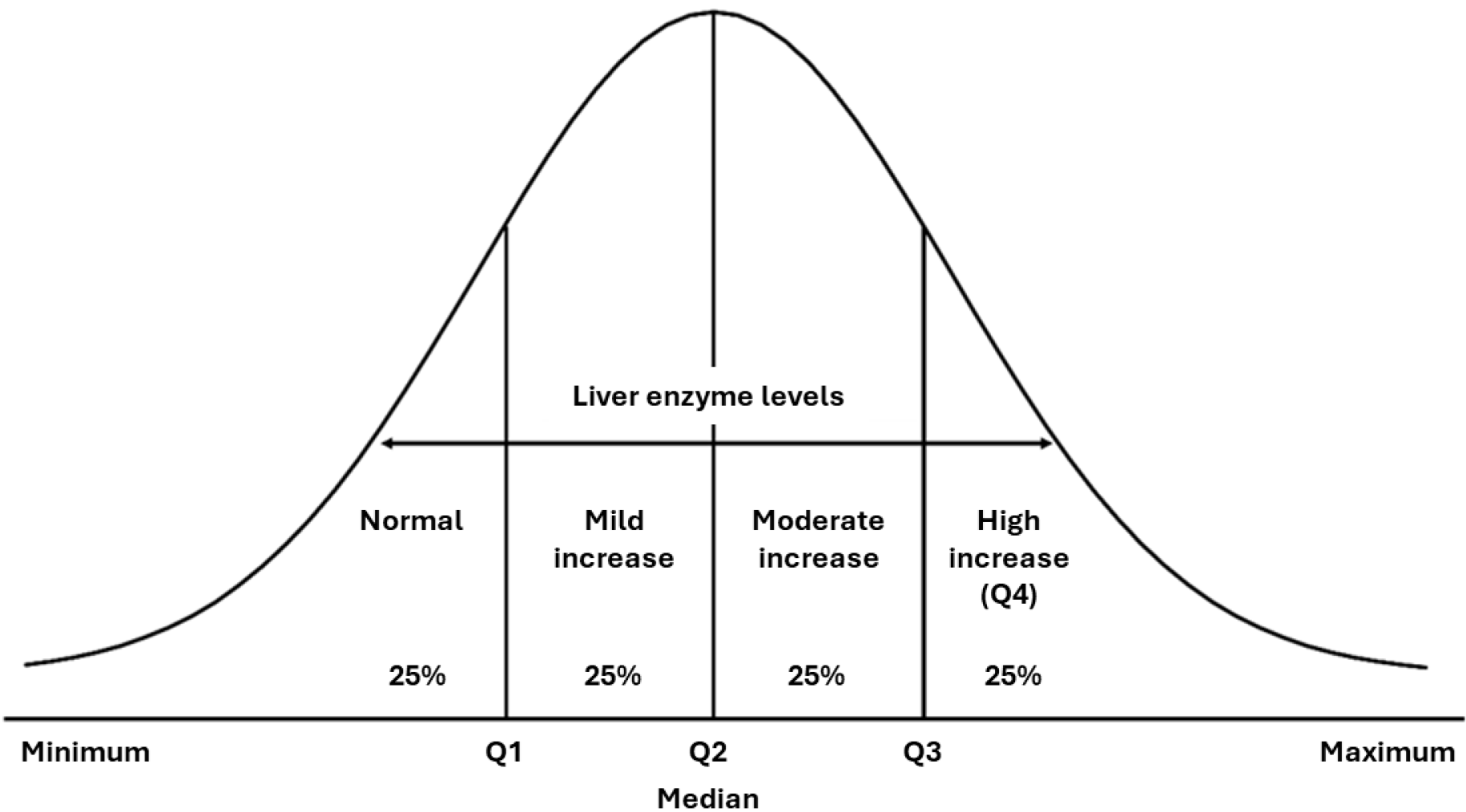
Subgroups of interest: Three concepts (herein described as the subgroups of interest) were used to define the severity spectrum of NAFLD. Subgroup of interest 1 (disease severity spectrum) defined the spectrum of NAFLD severity based on the statistical distribution of liver enzyme levels, as shown in Figure 1, using the following medians and quartiles (Q): ”Normal” (minimum-Q1); ”Mild increase” (Q1-median); “Moderate increase” (median-Q3); and “High increase” [Q3–maximum (denoted as Q4)]. Subgroup of interest 2 (based on MANPOWER cut-offs) defined increased/normal levels of liver enzymes according to the MANPOWER cut-off values in units per liter (ALT: ≥ 33 female, ≥ 41 male; AST: ≥ 35 female, ≥ 50 male; GGT: ≥ 32 female, ≥ 49 male). Subgroup of interest 3 (based on physician diagnosis) determined the presence of physician-diagnosed NASH (yes/no) using the following three diagnostic methods: Clinical examination (percussion and palpation of the liver) and ultrasonography examination, both of which were performed in all patients; transient elastography, which was performed in 125 patients; and liver biopsy, which was performed in two patients.
Outcomes: Baseline demographic characteristics and laboratory data were described using mean and SD for the overall population and for the three subgroups of interest. Categorical variables were reported as numbers and percentages. At week 12 and week 24, mean (SD) changes from baseline for each enzyme (ALT, AST and GGT) were calculated. Mean changes from baseline were estimated using generalised linear regression models and adjusted for baseline variables, such as age, BMI, number of comorbidities, presence of diabetes, obesity, hypertension and NASH diagnosis.
Statistical comparisons (McNemar test) were performed for the change in ultrasonography status by NAFLD severity level from baseline to week 12 and week 24. A χ2 test was performed to assess the significance of the difference between the subgroups of interest and the different NAFLD severity levels. The diagnostic accuracy of the algorithms, based on the MANPOWER cut-offs and the statistical distribution of liver enzymes, were assessed using the sensitivity, specificity, positive predictive value (PPV), negative predicative value, and accuracy. The reference standard used to assess the diagnostic accuracy of each algorithm was physician-diagnosed NASH; all algorithms were tested in patients without missing data. In patients with diabetes, algorithms based on the MANPOWER cut-off were tested against physician-diagnosed NASH (the diagnostic accuracy measures for the main algorithms assessed are shown in Supplementary Table 2). Multivariate logistic regression models were used to assess the risk factors associated with increased, abnormal serum levels of the three liver enzymes, ALT, AST and GGT.
Overall, a total of 2843 patients with newly diagnosed NAFLD were included in this post-hoc analysis. Demographic characteristics were similar across the different subgroups of interest analyzed (Table 1). The majority of patients were female (62.2%), with a mean age of 48.4 years (SD 8.59 years). Obesity was the most common comorbidity (80.1%), followed by elevated cholesterol (75.6%) and hypertension (57.8%); type 2 diabetes was present in only 16.8% of patients. However, chronic comorbidities were higher in patients with severe NAFLD, based on the statistical distribution of liver enzymes, increased liver enzymes based on the MANPOWER cut-off, and those with physician-diagnosed NASH (Table 1). Patients with physician-diagnosed NASH (n = 712) were more likely to be obese (85.7% vs 79.2%) and diabetic (18.8% vs 16.1%) than those with physician-diagnosed no NASH (n = 2131), respectively (Table 1). Similar trends were noted across the different subgroups of interest (the statistical distribution of enzymes and the MANPOWER cut-offs) (Table 1). Data on concomitant medications have been previously published[3]. Laboratory parameters increased similarly across the different levels of NAFLD severity in all subgroups of interest (data not shown).
| Variables | All patients (n = 2843) | Disease severity spectrum | MANPOWER cut-offs | Physician diagnosis | |||||
| Normal | Mild increase | Moderate increase | High increase (Q4) | Increased | Normal | NASH | No NASH | ||
| ALT, n = 682 | ALT, n = 740 | ALT, n = 702 | ALT, n = 719 | ALT, n = 1815 | ALT, n = 1028 | n = 712 | n = 2131 | ||
| AST, n = 732 | AST, n = 719 | AST, n = 738 | AST, n = 654 | AST, n = 1356 | AST, n = 1487 | – | - | ||
| GGT, n = 475 | GGT, n = 549 | GGT, n = 530 | GGT, n = 522 | GGT, n = 1249 | GGT, n = 827 | – | - | ||
| Female | 1767 (62.15) | – | – | – | – | – | – | 371 (52.11) | 1396 (65.51) |
| ALT | – | 478 (70.09) | 500 (67.57) | 422 (60.11) | 367 (51.04) | 1139 (62.75) | 628 (61.09) | – | – |
| AST | – | 502 (68.58) | 467 (64.95) | 460 (62.33) | 338 (51.68) | 971 (71.61) | 796 (53.53) | – | – |
| GGT | – | 345 (72.63) | 363 (66.12) | 316 (59.62) | 260 (49.81) | 855 (68.45) | 429 (51.87) | – | – |
| Age in years, mean (SD) | 48.4 (8.59) | – | – | – | – | – | – | 47.58 (8.54) | 48.67 (8.60) |
| ALT | – | 48.37 (9.23) | 48.87 (8.44) | 48.63 (7.94) | 47.72 (8.72) | 48.35 (8.39) | 48.48 (8.95) | – | – |
| AST | – | 48.42 (9.30) | 48.71 (8.45) | 48.47 (8.21) | 47.95 (8.35) | 48.74 (8.18) | 48.09 (8.94) | – | – |
| GGT | – | 48.57 (8.69) | 48.26 (8.36) | 48.92 (8.29) | 47.8 (7.94) | 48.76 (7.93) | 47.81 (8.85) | – | – |
| Obesity | 2298 (80.83) | – | – | – | – | – | – | 610 (85.67) | 1688 (79.21) |
| ALT | – | 512 (75.07) | 576 (77.84) | 583 (83.05) | 627 (87.20) | 1521 (83.80) | 777 (75.58) | – | – |
| AST | – | 556 (75.96) | 569 (79.14) | 593 (80.35) | 580 (88.69) | 1140 (84.07) | 1158 (77.87) | – | – |
| GGT | – | 379 (79.79) | 443 (80.69) | 454 (85.66) | 445 (85.25) | 1056 (84.55) | 665 (80.41) | – | – |
| Hypertension | 1642 (57.76) | – | – | – | – | – | – | 364 (51.12) | 1278 (59.97) |
| ALT | – | 403 (59.09) | 423 (57.16) | 398 (56.70) | 418 (58.14) | 1045 (57.58) | 597 (58.07) | – | – |
| AST | – | 409 (55.87) | 401 (55.77) | 438 (59.35) | 394 (60.24) | 807 (59.51) | 835 (56.15) | – | – |
| GGT | – | 232 (48.84) | 304 (55.37) | 309 (58.30) | 302 (57.85) | 724 (57.97) | 423 (51.15) | – | – |
| Elevated cholesterol | 2063 (72.56) | – | – | – | – | – | – | 504 (70.79) | 1559 (73.16) |
| ALT | – | 467 (68.48) | 528 (71.35) | 516 (73.50) | 552 (76.77) | 1354 (74.60) | 709 (68.97) | – | – |
| AST | – | 508 (69.40) | 492 (68.43) | 576 (78.05) | 487 (74.46) | 1031 (76.03) | 1032 (69.40) | – | – |
| GGT | – | 334 (70.32) | 419 (76.32) | 397 (74.91) | 394 (75.48) | 942 (75.42) | 602 (72.79) | – | – |
| Type 2 diabetes mellitus | 477 (16.78) | – | – | – | – | – | – | 134 (18.82) | 343 (16.10) |
| ALT | – | 92 (13.49) | 114 (15.41) | 127 (18.09) | 144 (20.03) | 330 (18.18) | 147 (14.30) | – | – |
| AST | – | 105 (14.34) | 116 (16.13) | 123 (16.67) | 133 (20. 34) | 235 (17.33) | 242 (16.27) | – | – |
| GGT | – | 56 (11.79) | 72 (13.11) | 92 (17.36) | 107 (20.50) | 233 (18.65) | 94 (11.37) | – | – |
| Physician-diagnosed NASH | 712 (25.04) | – | – | – | – | – | – | N/A | N/A |
| ALT | – | 39 (5.72) | 73 (9.86) | 224 (31.91) | 376 (52.29) | 645 (35.54) | 67 (6.52) | – | – |
| AST | – | 43 (5.87) | 92 (12.80) | 247 (33.47) | 330 (50.46) | 515 (37.98) | 197 (13.25) | – | – |
| GGT | – | 61 (12.84) | 86 (15.66) | 161 (30.38) | 277 (53.07) | 457 (36.59) | 128 (15.48) | – | – |
| ALT, U/L, mean (SD) | 50.22 (33.19) | – | – | – | – | – | – | 72.58 (38.58) | 42.76 (27.39) |
| ALT | – | 19.81 (7.12) | 35.86 (4.12) | 51.56 (5.74) | 92.56 (37.08) | 64.91 (32.95) | 24.30 (8.83) | – | – |
| AST | – | 23.44 (10.79) | 39.41 (20.47) | 54.70 (17.91) | 87.04 (39.09) | 68.93 (36.02) | 33.17 (17.71) | – | – |
| GGT | – | 38 (25.63) | 40.62 (25.93) | 53.98 (27.21) | 73.32 (39.74) | 58.92 (34.63) | 40.68 (27.74) | – | – |
| AST, U/L, mean (SD) | 43.64 (25.69) | – | – | – | – | – | – | 58.75 (27.79) | 38.59 (22.83) |
| ALT | – | 21.49 (9.77) | 34.42 (13.02) | 46.87 (11.22) | 70.99 (30.19) | 54.04 (25.17) | 25.28 (13.37) | – | – |
| AST | – | 19.44 (6.32) | 33.52 (3.58) | 46.93 (4.71) | 78.14 (28.56) | 61.13 (26.14) | 27.70 (10.29) | – | – |
| GGT | – | 33.67 (20.47) | 37.00 (16.10) | 46.30 (17.54) | 59.48 (29.37) | 49.94 (24.51) | 35.69 (19.29) | – | – |
| AST/ALT ratio, mean (SD) | 0.95 (0.44) | – | – | – | – | – | – | 0.90 (0.39) | 0.97 (0.46) |
| ALT | – | 1.15 (0.70) | 0.96 (0.39) | 0.91 (0.20) | 0.79 (0.22) | 0.87 (0.22) | 1.10 (0.65) | – | – |
| AST | – | 0.92 (0.38) | 0.94 (0.30) | 0.96 (0.55) | 0.99 (0.50) | 0.98 (0.53) | 0.92 (0.34) | – | – |
| GGT | – | 0.95 (0.35) | 0.99 (0.48) | 0.95 (0.46) | 0.87 (0.29) | 0.94 (0.45) | 0.95 (0.32) | – | – |
| GGT, U/L, mean (SD) | 51.74 (37.64) | – | – | – | – | – | – | 71.4 (49.39) | 44.03 (28.39) |
| ALT | – | 35.38 (26.30) | 41.01 (27.01) | 55.21 (33.40) | 71.92 (47.01) | 59.50 (40.65) | 36.40 (24.46) | – | – |
| AST | – | 37.19 (29.83) | 41.91 (26.32) | 56.20 (34.55) | 70.74 (46.87) | 61.88 (42.22) | 41.42 (28.89) | – | – |
| GGT | – | 21.03 (6.11) | 35.33 (3.66) | 50.19 (5.61) | 98.52 (47.17) | 67.74 (40.63) | 27.58 (9.68) | – | – |
Baseline enzyme levels were analyzed in the overall population (Table 1), and results were stratified by gender (Supplementary Table 3). ALT and AST values were available for all patients; however, GGT values were only available for 2076 (73%) patients. In the overall population, the mean (SD) ALT, AST and GGT levels were 50.2 (33.2) U/L, 43.6 (25.7) U/L and 51.7 (37.6) U/L (Table 1), respectively, with males displaying consistently higher levels of all three enzymes compared with females (Supplementary Table 3).
Patients with physician-diagnosed NASH displayed higher mean levels of ALT [72.6 (38.6) vs 42.8 (27.4) U/L], AST [58.8 (27.8) vs 38.6 (22.8) U/L] and GGT [71.4 (49.4) vs 44.0 (28.4) U/L] than those with physician-diagnosed no NASH, respectively (Table 1). Similarly, mean levels of ALT, AST and GGT increased with NAFLD severity for patients categorized based on the statistical distribution of liver enzyme levels, with lower levels displayed by patients with normal enzymes levels at baseline, and higher levels displayed by those with highly increased enzyme levels at baseline (Table 1). The same pattern was noted for patients categorized according to the MANPOWER cut-off values, with those categorized as having ”increased” value displaying higher mean levels of ALT [64.9 (33.0) vs 24.3 (8.8) U/L], AST [54.0 (25.2) vs 25.3 (13.4) U/L] and GGT [59.5 (40.7) vs 36.4 (24.5) U/L], than those categorized as having ”normal” values, respectively (Table 1).
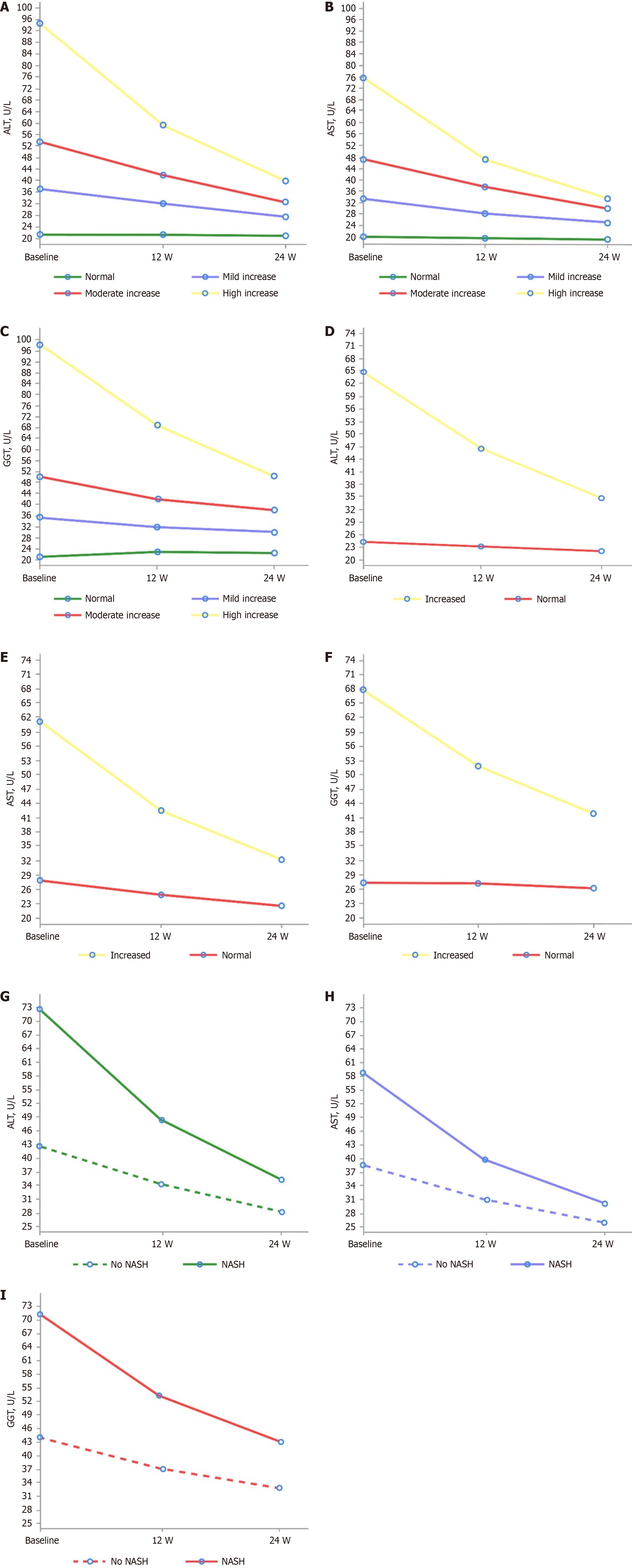
Effect on liver enzyme levels: Overall, after treatment with Essentiale, the average level of each enzyme decreased across all levels of NAFLD severity in all subgroups of interest (NAFLD severity, MANPOWER cutoffs and physician-diagnosed NASH) over the 24-week follow-up (Figure 2). Across the different subgroups of interest, as defined by ALT, AST and GGT levels, Essentiale led to statistically significant reductions (P < 0.05) in each liver enzyme from baseline to week 12 and week 24 (Supplementary Tables 4 and 5). At week 12, there was a 12.3, 10.6 and 10.6 U/L reduction from baseline in the adjusted mean levels of ALT, AST and GGT, respectively (Supplementary Table 4). By week 24, there was a 20.4, 16.9 and 17.1 U/L reduction from baseline in the adjusted mean levels of ALT, AST and GGT, respectively (Supplementary Table 5).
For each subgroup of interest, a higher significant reduction in ALT, AST, and GGT correlated with increasing NAFLD severity levels and increased liver function test values (Supplementary Tables 4 and 5). The highest reduction from baseline was noted with ALT in patients with physician-diagnosed NASH at both week 12 [–24.2 (28.1)] and week 24 [–37.2 (35.3)]. Trends of higher reduction in liver enzyme levels with increasing severity were similar across all three subgroups of interest and the adjusted mean changes confirmed the results (Supplementary Tables 4 and 5).
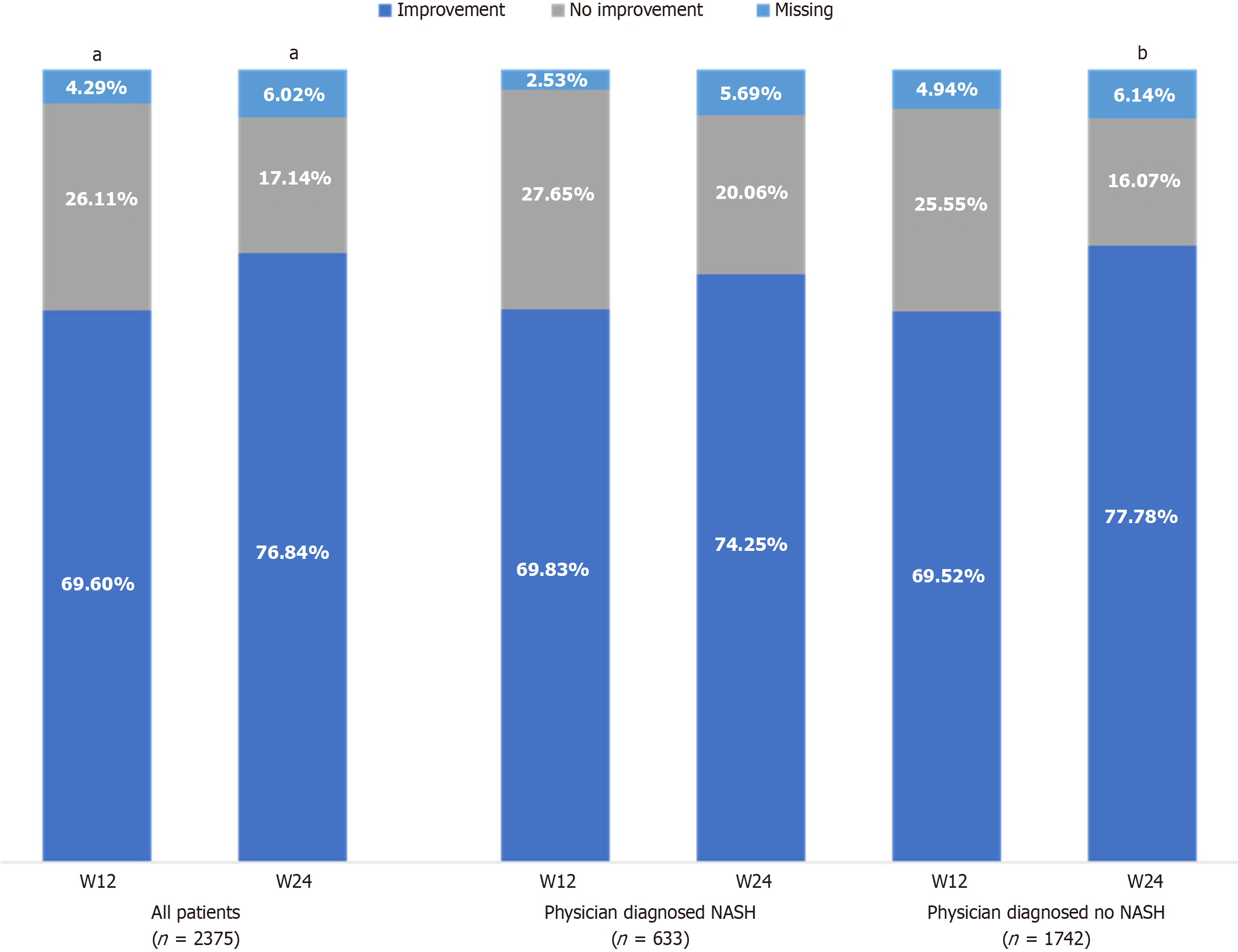
Diffuse liver hyperechogenicity: At baseline, diffuse liver hyperechogenicity was detected in 83.5% of all patients. Essentiale treatment was associated with a significant improvement (P < 0.001) in diffuse liver hyperechogenicity, with improvement reported in 69.6% and 76.8% of all patients at week 12 and week 24, respectively (Figure 3; Supplementary Table 6). The benefit of Essentiale was also assessed according to the three previously defined subgroups of interest. Among those with physician-diagnosed NASH, significant improvement (P < 0.001) was observed in 69.8% and 74.3% of patients at week 12 and week 24, respectively (Figure 3; Supplementary Table 6). Similar results were noted in those with physician-diagnosed no NASH, with 69.5% displaying improvement at week 12 (P = 0.48 vs NASH diagnosis); however, a higher proportion of patients with physician-diagnosed no NASH (77.8%) displayed significant improvement at week 24 compared with patients with physician-diagnosed NASH (P = 0.025) (Figure 3; Supplementary Table 6). Similarly, among patients with increased ALT enzyme levels, based on the MANPOWER cut-offs or the statistical distribution of enzymes, the percentage of patients reporting improvement ranged from 65.9% to 75.3% at week 12, and from 73.2% to 79.6% at week 24 (Supplementary Table 7). These changes were significant compared with baseline (P < 0.001); however, these ranges were not significantly different (P > 0.5) between patients with normal ALT levels and patients with increased ALT levels at baseline, regardless of disease classification. Similar trends were observed when disease status was classified based on AST and GGT enzyme levels (Supplementary Table 7).
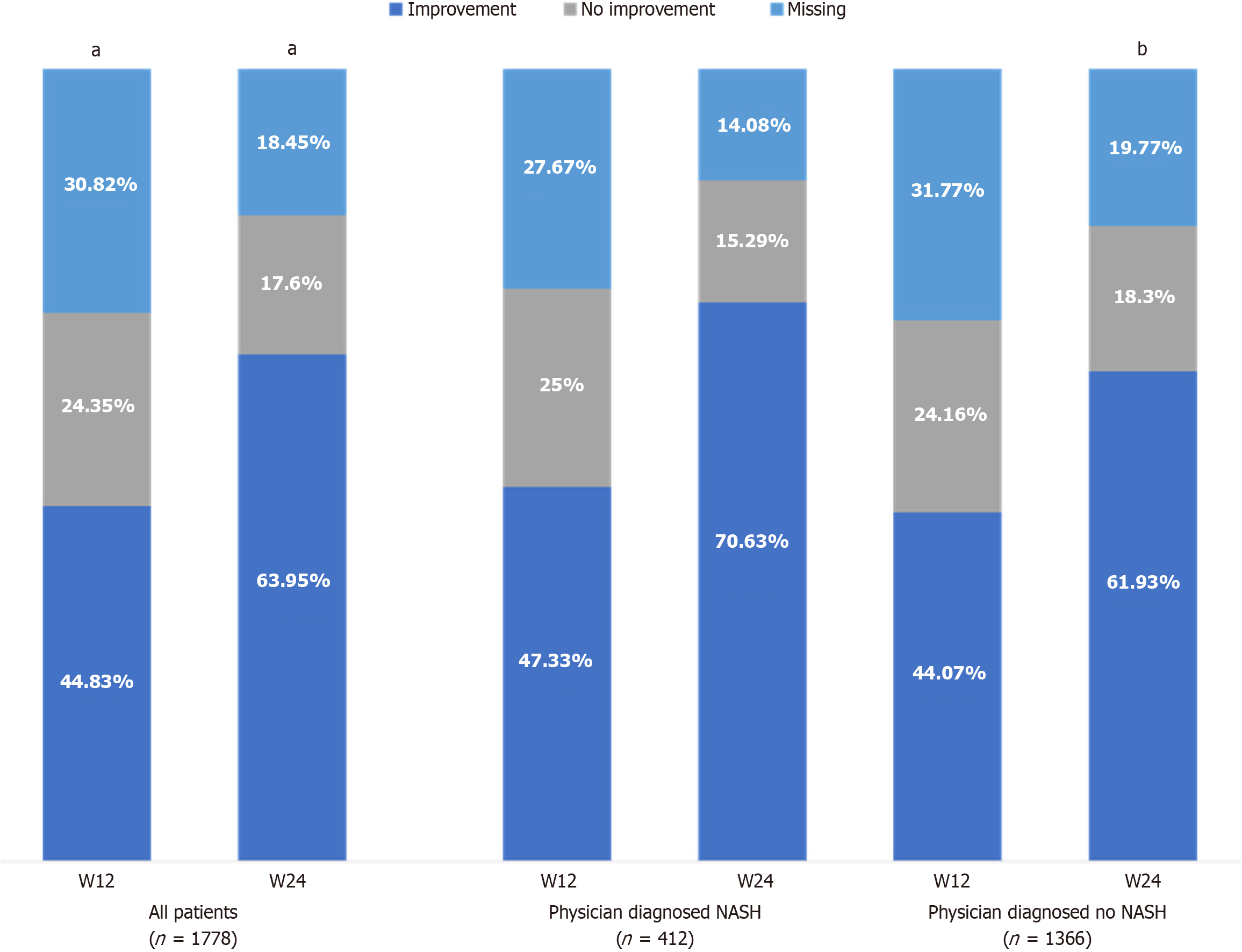
Changes in heterogeneous liver structure were detected in 62.5% of all patients at baseline. Among those with physician-diagnosed NASH, Essentiale led to improvement in heterogenous liver structure in 47.3% and 70.6% of patients at week 12 and week 24, respectively (Figure 4). Among those with physician-diagnosed no NASH, improvement was observed in 44.1% and 61.9% of patients at week 12 and week 24, respectively (Figure 4).
A total of 2076 patients had laboratory values for all three liver enzymes, and were included in the analysis. Overall, PPVs were low in all tested algorithms. Algorithms based on the MANPOWER cut-offs displayed high sensitivity, but low specificity for determining NASH diagnosis. Using physician-diagnosed NASH as the standard, algorithm 2a (Q4 ALT OR Q4 AST OR Q4 GGT vs remaining categories) displayed the highest accuracy (72.3%) for determining physician-diagnosed NASH, with the best trade-off between sensitivity (75.6%) and specificity (71.0%); however, based on reliability and increased sensitivity, algorithm 2 (increased ALT OR increased AST OR increased GGT vs remaining categories) was used for the assessment of risk factors.
Using algorithm 2, two groups were defined: (1) Those with at least one abnormal ALT OR abnormal AST OR abnormal GGT result; and (2) Those without at least one abnormal ALT OR AST OR GGT level. Overall, in the adjusted multivariate analysis (Supplementary Table 8), younger patients (≤ 50 years) displayed significantly higher odds of having abnormal enzyme levels, according to definitions derived from algorithms 2 and 2a (OR: 1.33; 95%CI: 1.06, 1.67). A borderline statistically significant association was also observed in patients with diabetes (OR: 1.61; 95%CI: 0.99, 2.59).
This post-hoc analysis suggests that Essentiale may be an effective adjunctive therapy for patients with NAFLD, and highlights the ability of liver enzyme profiling to determine the severity spectrum of NAFLD. Essentiale led to a reduction in average liver enzyme levels (ALT, AST, and GGT) over the 24-week follow-up period across all patients, regardless of NAFLD severity or NASH diagnosis. When the impact of Essentiale was stratified based on the different subgroups of interest reflecting NAFLD disease severity (MANPOWER cut-offs or statistical distribution of disease severity), a greater reduction in enzyme levels was noted in those with increased baseline enzyme levels compared with those with normal baseline enzyme levels.
Additionally, ultrasonography features (diffuse liver hyperechogenicity and heterogeneous liver structure) improved over the 24-week follow-up period; however, it is important to note that improvement may have occurred earlier in the study. After treatment with Essentiale, a large proportion of those with physician-diagnosed NASH and no NASH showed improvement in diffuse liver hyperechogenicity at week 12 and week 24, which has been shown to correlate with clinically detected steatosis. Furthermore, the improvement in another ultrasonography feature, heterogenous liver structure, further confirmed the treatment benefit of Essentiale.
Overall, this study adds to the growing body of evidence on the role of EPLs in the management of patients with NAFLD, and our results are in line with those of previous studies. For example, Essentiale has proven beneficial in the management of patients with NAFLD and chronic metabolic comorbidities. Obesity, hypertension, hypercholesterolaemia and type 2 diabetes are recognized as the most prevalent comorbidities in patients with NAFLD, with prevalence increasing with NAFLD severity. Treatment with Essentiale led to a significant decrease in liver enzyme levels and a significant improvement in ultrasonography findings in patients with the most prevalent comorbidities[21]. Additionally, a recent meta-analysis found that EPL treatment in patients with NAFLD and diabetes and/or obesity resulted in a significant reduction in ALT levels and improved ultrasonography features[10]. Other analysis of real-world data on the management of patients with NAFLD found that treatment with EPLs not only led to improvements in both laboratory and ultrasonography findings, but also high patient adherence and satisfaction[3,22]. Furthermore, a review of 25 clinical studies that assessed the benefit of EPLs in patients with NAFLD noted an overall positive therapeutic response, with improvements in laboratory and imaging findings correlating with improvements in patient symptoms[8]. The mechanism by which EPLs exert therapeutic effects in patients with NAFLD is likely multifactorial. Recently, three potential mechanisms of action have been proposed: (1) Oxidation of fatty acids; (2) Liponeogenesis inhibition; and (3) Efflux of fatty acids from hepatocytes[23,24]. Additionally, EPLs have been shown to increase the proportion of PPCs in cell membranes, which are essential for membrane functionality, fluidity and repair[9,10]. PPCs have also been shown to improve the hepatic phosphatidylcholine-to-phosphatidylethanolamine ratio in cellular membranes, a decrease of which is a key predictor of NAFLD[8], and to also downregulate enzymes that produce reactive oxygen species, therefore reducing inflammation and fibrogenesis in the liver[10].
Although other hepatoprotective agents have been investigated for the treatment of NAFLD, such as resveratrol, coenzyme Q10 and vitamin E, evidence of efficacy is low, and an increased risk of side effects has been observed[9]. Conversely, EPLs not only display anti-inflammatory, antioxidant, anti-fibrogenic, anti-apoptotic and membrane-protective properties[24]; they also demonstrated a favorable safety profile[8]. As such, EPLs are considered the most promising adjuvant treatment option for patients with NAFLD and have therefore been incorporated into the Russian guidelines for the management of NAFLD[9,12].
Lastly, an algorithm developed based on the statistical distribution of three liver enzymes (ALT, AST, and GGT) displayed the best performance in terms of accuracy, sensitivity and specificity; however, a low PPV was noted. Several non-invasive diagnostic panels have been developed to stage liver inflammation and potentially alleviate the need for an invasive liver biopsy. Currently, the AST-to-platelet ratio index and the Fibrosis-4 score are recommended by the World Health Organization as alternatives to a liver biopsy; however, these panels were not specifically designed to evaluate fibrosis in patients with NAFLD[25]. As such, an algorithm based on the statistical distribution of liver enzymes could form the basis of a diagnostic algorithm for grading and staging NAFLD, and could enable early identification of patients with NAFLD who are at risk for the development of NASH; however, further research is needed to confirm this.
A key strength of this study was the large sample size (n = 2843), which not only allowed the results to be generalized to patients with newly diagnosed NAFLD, but also allowed trends in key patient groups to be examined (e.g., comorbidities, gender and age). Additionally, although a low PPV was noted with the best-performing algorithm, as this study prioritized sensitivity and accuracy, the benefits of identifying true positives should outweigh the negative consequences of identifying more false positives. Ultimately, this should help physicians identify those requiring a liver biopsy.
This study had several limitations that warrant discussion. Firstly, although liver biopsy is considered the ”gold standard” for the diagnosis and staging of NAFLD, it is invasive and difficult to obtain; therefore, it is rarely performed owing to high costs and the risk of complications[2,26]. As such, the diagnosis of NASH in this study was based on the level of serum liver enzymes, ALT, AST and GGT, according to Russian NAFLD/NASH guidelines. Secondly, residual confounding factors could not be excluded despite adjusting for possible influence by using multivariate analyses. For example, the treatment of pre-existing comorbidities may have been a confounding factor that potentially impacted the true treatment effect; however, this related to the observational nature of the study. Thirdly, there were missing values for several ultrasonography findings, but these could potentially be explained by the internal subjective and technical limitations associated with this diagnostic method[27,28]. Lastly, the study population consisted of only patients with newly diagnosed NAFLD, with late-stage disease underrepresented. As such, the findings may or may not apply to those with the highest degree of NAFLD severity.
This study adds to the growing evidence on the profile of liver enzymes in patients with NAFLD and the role of EPLs in the management of these patients. While the findings were based on the Russian population, they can be extrapolated to other ethnicities. Our findings suggest that liver enzymes profiles (ALT, AST and GGT) may be used to identify different NAFLD severity levels and monitor therapeutic response in patients taking commercially available PPCs. These data also suggest that Essentiale may help to improve liver enzyme levels and ultrasonography features in patients with NAFLD across various levels of disease severity (according to ALT and AST levels). Lastly, we showed that a simple algorithm used to define the spectrum of NAFLD could form the basis of a diagnostic algorithm for staging NAFLD, which would avoid the need for an invasive liver biopsy; however, further exploration is required to confirm this.
Nichola Cruickshanks, PhD, and Ella Palmer, PhD, of inScience Communications, Springer Healthcare Ltd., UK, provided medical writing support, which was funded by Sanofi in accordance with the Good Publication Practice 2022 guidelines.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7536] [Article Influence: 837.3] [Reference Citation Analysis (0)] |
| 2. | Ivashkin VT, Maevskaya MV, Zharkova MS, Kotovskaya YV, Tkacheva ON, Troshina EA, Shestakova MV, Maev IV, Breder VV, Gheivandova NI, Doshchitsin VL, Dudinskaya EN, Ershova EV, Kodzoeva KB, Komshilova KA, Korochanskaya NV, Mayorov AY, Mishina EE, Nadinskaya MY, Nikitin IG, Pogosova NV, Tarzimanova AI, Shamkhalova MS. Clinical Practice Guidelines of the Russian Scientific Liver Society, Russian Gastroenterological Association, Russian Association of Endocrinologists, Russian Association of Gerontologists and Geriatricians and National Society for Preventive Cardiology on Diagnosis and Treatment of Non-Alcoholic Liver Disease. Rossijskij žurnal gastroènterologii, gepatologii, koloproktologii. 2022;32:104-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Maev IV, Samsonov AA, Palgova LK, Pavlov CS, Shirokova E, Starostin KM. Real-world comorbidities and treatment patterns among patients with non-alcoholic fatty liver disease receiving phosphatidylcholine as adjunctive therapy in Russia. BMJ Open Gastroenterol. 2019;6:e000307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Tesfay M, Goldkamp WJ, Neuschwander-Tetri BA. NASH: The Emerging Most Common Form of Chronic Liver Disease. Mo Med. 2018;115:225-229. [PubMed] |
| 5. | Nan Y, An J, Bao J, Chen H, Chen Y, Ding H, Dou X, Duan Z, Fan J, Gao Y, Han T, Han Y, Hu P, Huang Y, Huang Y, Jia J, Jiang J, Jiang Y, Li J, Li J, Li R, Li S, Li W, Li Y, Lin S, Liu J, Liu S, Lu L, Lu Q, Luo X, Ma X, Rao H, Ren H, Ren W, Shang J, Shi L, Su M, Wang B, Wang R, Wei L, Wen Z, Wu B, Wu J, Xin S, Xing H, Xu J, Yan M, Yang J, Yang J, Yang L, Yang Y, Yu Y, Zhang L, Zhang L, Zhang X, Zhang Y, Zhang Y, Zhao J, Zhao S, Zheng H, Zhou Y, Zhou Y, Zhuang H, Zuo W, Xu X, Qiao L. The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol. 2021;75:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 552] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 7. | Dufour JF, Anstee QM, Bugianesi E, Harrison S, Loomba R, Paradis V, Tilg H, Wong VW, Zelber-Sagi S. Current therapies and new developments in NASH. Gut. 2022;71:2123-2134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 8. | Gundermann KJ, Gundermann S, Drozdzik M, Mohan Prasad VG. Essential phospholipids in fatty liver: a scientific update. Clin Exp Gastroenterol. 2016;9:105-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Dajani AI, Abuhammour A. Agents for the treatment of fatty liver disease: focus on essential phospholipids. Drugs Ther Perspect. 2021;37:249-264. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Dajani AI, Popovic B. Essential phospholipids for nonalcoholic fatty liver disease associated with metabolic syndrome: A systematic review and network meta-analysis. World J Clin Cases. 2020;8:5235-5249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Russian Scientific Liver Society. Diagnosis and treatment of nonalcoholic fatty liver disease 2015. Available from https://www.rsls.ru/files/Guidelines-RSLS-NASH-2016-01-03.pdf. |
| 12. | Lazebnik LB, Golovanova EV, Turkina SV, Raikhelson KL, Okovityy SV, Drapkina OM, Maev IV, Martynov AI, Roitberg GE, Khlynova OV, Abdulganieva DI, Alekseenko SA, Ardatskaya MD, Bakulin IG, Bakulina NV, Bueverov AO, Vinitskaya EV, Volynets GV, Eremina EY, Grinevich VB, Dolgushina AI, Kazyulin AN, Kashkina EI, Kozlova IV, Konev YV, Korochanskaya NV, Kravchuk YA, Li ED, Loranskaya ID, Makhov VM, Mekhtiev SN, Novikova VP, Ostroumova OD, Pavlov CS, Radchenko VG, Samsonov AA, Sarsenbaeva AS, Sayfutdinov RG, Seliverstov PV, Sitkin SI, Stefanyuk OV, Tarasova LV, Tkachenko EI, Uspensky YP, Fominykh YA, Khavkin AI, Tsyganova YV, Sharhun OO. Non-alcoholic fatty liver disease in adults: clinic, diagnostics, treatment. Guidelines for therapists, third version. jour. 2021;1:4-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Monthly Index of Medical Specialities (MIMS). Essentiale Forte N information Available from: https://www.mims.com/malaysia/drug/info/essentiale%20forte%20n?type=full. |
| 14. | Allan R, Thoirs K, Phillips M. Accuracy of ultrasound to identify chronic liver disease. World J Gastroenterol. 2010;16:3510-3520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Cuenza LR, Razon TLJ, Dayrit JC. Correlation between severity of ultrasonographic nonalcoholic fatty liver disease and cardiometabolic risk among Filipino wellness patients. J Cardiovasc Thorac Res. 2017;9:85-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Zhang YN, Fowler KJ, Ozturk A, Potu CK, Louie AL, Montes V, Henderson WC, Wang K, Andre MP, Samir AE, Sirlin CB. Liver fibrosis imaging: A clinical review of ultrasound and magnetic resonance elastography. J Magn Reson Imaging. 2020;51:25-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 17. | CKS is only available in the UK. NICE. Available from: https://cks.nice.org.uk/topics/non-alcoholic-fatty-liver-disease-nafld/diagnosis/diagnosis/. |
| 18. | Piazzolla VA, Mangia A. Noninvasive Diagnosis of NAFLD and NASH. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 19. | Paul J. Recent advances in non-invasive diagnosis and medical management of non-alcoholic fatty liver disease in adult. Egypt Liver J. 2020;10:37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Ivashkin VT, Mayevskaya MV, Pavlov CS, Tikhonov IN, Shirokova YN, Buyeverov AO, Drapkina OM, Shulpekova YO, Tsukanov VV, Mammayev SN, Mayev IV, Palgova LK. Diagnostics and treatment of non-alcoholic fatty liver disease: clinical guidelines of the Russian Scientific Liver Society and the Russian gastroenterological association. Rossijskij žurnal gastroènterologii, gepatologii, koloproktologii. 2016;26:24-42. [DOI] [Full Text] |
| 21. | Maev IV, Samsonov AA, Palgova LK, Pavlov CS, Vovk EI, Shirokova EN, Starostin KM. Effectiveness of phosphatidylcholine in alleviating steatosis in patients with non-alcoholic fatty liver disease and cardiometabolic comorbidities (MANPOWER study). BMJ Open Gastroenterol. 2020;7:e000341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Gundermann KJ, Kuenker A, Kuntz E, Droździk M. Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacol Rep. 2011;63:643-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Wupperfeld D, Fricker G, Bois De Fer B, Frank L, Wehrle A, Popovic B. Essential phospholipids decrease apoptosis and increase membrane transport in human hepatocyte cell lines. Lipids Health Dis. 2022;21: 91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Küllenberg D, Taylor LA, Schneider M, Massing U. Health effects of dietary phospholipids. Lipids Health Dis. 2012;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 339] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 25. | Yang Z, Ma X, Zhou X, Huang D, Wang Y, Li X, Lu W, Zhang Z, Ding R. Predictive performance of eLIFT for liver inflammation and fibrosis in chronic liver diseases. Int J Med Sci. 2021;18:3599-3608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Liao YY, Yang KC, Lee MJ, Huang KC, Chen JD, Yeh CK. Multifeature analysis of an ultrasound quantitative diagnostic index for classifying nonalcoholic fatty liver disease. Sci Rep. 2016;6:35083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Hussain K. Imaging methods for screening for hepatic steatosis. In: Williams R, Taylor-Robinson SD, editors. Clinical Dilemmas in Non-Alcoholic Fatty Liver Disease. Wiley, 2016. [DOI] [Full Text] |
| 28. | Kahl S, Straßburger K, Nowotny B, Livingstone R, Klüppelholz B, Keßel K, Hwang JH, Giani G, Hoffmann B, Pacini G, Gastaldelli A, Roden M. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One. 2014;9:e94059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |