Published online Jan 27, 2024. doi: 10.4254/wjh.v16.i1.17
Peer-review started: September 16, 2023
First decision: November 22, 2023
Revised: December 2, 2023
Accepted: January 3, 2024
Article in press: January 3, 2024
Published online: January 27, 2024
Processing time: 128 Days and 17.7 Hours
Fecal microbiota transplantation (FMT) offers a potential treatment avenue for hepatic encephalopathy (HE) by leveraging beneficial bacterial displacement to restore a balanced gut microbiome. The prevalence of HE varies with liver disease severity and comorbidities. HE pathogenesis involves ammonia toxicity, gut-brain commu
Core Tip: Hepatic encephalopathy (HE) is a reversible neurocognitive dysfunction and a frequent complication in patients with chronic liver disease. HE results from synergistic interaction between various mechanisms like increased ammonia production, systemic inflammation, disruption of the blood-brain barrier, and impairment of neurotransmission, leading to altered gut-brain-liver axis. Lactulose and rifaximin are the current mainstays of management of HE as they are known to decrease ammonia production. Fecal microbiota transplant is being studied as a potential microbiome targeted therapy that can improve the symptoms of HE by decreasing ammonia production, decreasing systemic inflammation, and improving intestinal barrier function.
- Citation: Shah YR, Ali H, Tiwari A, Guevara-Lazo D, Nombera-Aznaran N, Pinnam BSM, Gangwani MK, Gopakumar H, Sohail AH, Kanumilli S, Calderon-Martinez E, Krishnamoorthy G, Thakral N, Dahiya DS. Role of fecal microbiota transplant in management of hepatic encephalopathy: Current trends and future directions. World J Hepatol 2024; 16(1): 17-32
- URL: https://www.wjgnet.com/1948-5182/full/v16/i1/17.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i1.17
Hepatic encephalopathy (HE) is a neurological condition that manifests in advanced liver disease, resulting in significant morbidity and mortality[1]. In the United States, 7–11 million people are affected by HE, with approximately 150000 new diagnoses reported annually. Among recently diagnosed cases, approximately 20% are associated with cirrhosis[2]. The prevalence of HE can vary depending on the severity of liver disease and the specific patient population under investigation. Table 1 provides a general overview of prevalence rates for common hepatic pathologies leading to HE[3,4]. It is important to note that these prevalence figures may differ depending on the study population, the diagnostic criteria employed, and other influencing factors. The occurrence of HE can also be influenced by comorbidities such as alcohol consumption, infections, and other complications associated with liver disease.
| Condition | Total, n = 166192 | Did not develop HE, n = 117433 | Developed HE, n = 48759 |
| Alcoholic cirrhosis | 54194 (33) | 30011 (26) | 24183 (50) |
| Hepatitis C cirrhosis | 49599(30) | 31247(27) | 18352(38) |
| Nonalcoholic cirrhosis | 78111 (47) | 62433 (53) | 15678 (32) |
Clinical intervention holds promise for reversing HE, particularly in acute cases. Contributing factors leading to HE include ammonia toxicity, disrupted gut-brain communication, and inflammation. Increased ammonia levels resulting from liver disease exert neurotoxic effects. Altered gut microbiota and increased gut permeability facilitate the entry of toxins into the bloodstream, affecting brain function through neurotransmitter imbalances. Inflammatory processes in the body and brain further exacerbate the condition. Addressing these underlying factors is critical in the management of HE[5-7]. Effective management improves symptoms and quality of life, thereby significantly improving the well-being of patients, encompassing the treatment of underlying liver disease and the reduction of ammonia levels. Medications such as lactulose or rifaximin are approved by the Food and Drug Administration for the treatment of HE. These medications exert their therapeutic effects by modulating gut microbiota composition and decreasing gut ammonia levels[8]. Recently, fecal microbiota transplantation (FMT) has emerged as an alternative approach for modulating gut microbiota and ameliorating symptoms of HE. Kao et al[9] published a pioneering case report that documented the initial utilization of FMT as a therapeutic approach for the treatment of HE. While FMT is currently primarily used for Clostridium difficile infection, its application in HE is still evolving[10].
This review article aims to provide a comprehensive and scientifically rigorous overview of FMT. It will elucidate the pathogenesis of gut dysbiosis leading to HE, and discuss the efficacy, safety, limitations, and future prospects for the implementation of FMT therapy in managing patients with HE.
The stages of HE can be assessed using the clinical grading system called West Haven Criteria (WHC) as recommended by the American Association for Study of Liver Diseases. HE can be clinically classified into four grades based on the symptoms at presentation, as shown in Table 2[11]. Grade I includes subtle personality changes. Grade II involves gross disorientation, inappropriate behavior, and lethargy. Grade III includes stupor and disorientation, while Grade IV represents a comatose state with or without decorticate or decerebrate posturing[11,12]. Other etiologies that can lead to changes in mentation should be evaluated and ruled out[8]. Based on the etiology, HE can be broadly classified into three types. Type A is HE secondary to acute liver failure, type B occurs in patients with a portosystemic shunt, and type C in patients with cirrhosis[11]. HE can be categorized as episodic if there is one episode over a 6-mo period, recurrent if there are multiple episodes in 6 mo, or persistent if the patient does not return to baseline[11].
| Grade1 | Explanation2 | Suggested operational criteria3 | |
| Covert | Minimal | Tests measuring psychomotor speed, executive function, or neurophysiological abilities may change psychometrically or neuropsychological without showing any signs of a mental shift | A non-phenomenological abnormality on recognized psychometric or neuropsychological tests |
| Grade 1 | Trivial lack of awareness; Euphoria or anxiety; Shortened addition or subtraction | Despite being spatially and temporally oriented, this individual appears to have some cognitive/behavioral issues. decay concerning his clinical assessment that meets her standards, or to the carers | |
| Overt | Grade 2 | Lethargy or apathy; Gross disorientation; Obvious personality change; Inappropriate behavior | Disorientation with regard to time (at least three of the following are incorrect: day of the week, month, season, and year) plus/minus the other symptoms stated) |
| Grade 3 | Marked confusion; Somnolence to semi-stupor; Responsive to stimuli; Bizarre behavior | Disoriented also in terms of space (at least three of the incorrectly reported terms: nation, state or area, cities, location, plus/minus the other indicators) | |
| Grade 4 | Comatose state; Unresponsive to pain; Decorticate or decerebrate posturing | Never react, not even with painful stimuli | |
HE greatly impacts patients' quality of life and prognosis. The severity can range from mild cognitive impairment to severe neurological dysfunction, affecting memory, cognition, and daily functioning[3]. Challenges in activities, social interactions, and employment are common. Frustration, anxiety, and depression are prevalent for patients and caregivers. Patients with HE need comprehensive psychological and social support and management strategies due to its significant negative impact on quality of life[3]. Apart from cognitive dysfunction, HE can also present with physical manifestations, including tremors, muscle stiffness, coordination difficulties, and asterixis. These physical symptoms can restrict a patient's mobility and hinder their performance of tasks that require precise motor skills[11,13]. Recurrence and pro
The microbiome targeted therapies that have been proposed as a therapeutic option in the management of patients with hepatic cirrhosis include prebiotics, probiotics, FMT, antibiotics, and synbiotics[15]. Probiotics are live microbial supplements of human origin which have shown to benefit the host by improving intestinal microbial balance when consumed adequately[15,16]. Prebiotics are nondigestible food ingredients that can selectively stimulate the growth of beneficial bacteria in the human gut and thereby improving the host’s health[16]. Synbiotic is the synergistic combination of prebiotics and probiotics[16]. A meta-analysis of 9 randomized control trials showed that prebiotics and probiotics were associated with significantly reduced relative risk of no improvement in minimal HE without any significant adverse events[16]. There are no studies in the literature that have compared the direct outcomes and adverse events of prebiotics, probiotics, synbiotics, and FMT.
FMT refers to the transfer of stool from healthy donors to patients with a dysbiotic gut environment in order to restore eubiosis[17]. Since the fourth century in China, human fecal material has been used in the form of a yellow soup to manage conditions such as diarrhea, constipation, and abdominal pain[18]. In 2013, the first human randomized controlled trial (RCT) was conducted to test the efficacy of FMT in patients with recurrent Clostridium difficile infection (CDI)[18]. The first successful use of FMT in non-infectious conditions like ulcerative colitis (UC) was reported in 1989[19]. Over the past decade, the range of applications for FMT has significantly expanded[18]. The efficacy of FMT has been tested in various gastrointestinal infectious and noninfectious etiologies including CDI, UC, irritable bowel syndrome (IBS), primary sclerosing cholangitis, metabolic syndromes, HE, and D-lactic acidosis[20].
The human gut microbiome consists of a diverse range of bacteria, fungi, viruses, and protozoa, all of which can have proinflammatory or anti-inflammatory effects, thereby influencing the inflammatory environment[21]. Patients with HE are particularly susceptible to disturbances in gut microbiota due to frequent antibiotic use[22]. Disruption of the gut-liver-brain axis is the primary cause of neurocognitive dysfunction in individuals with HE[6]. In recent years, culture-independent studies have revealed a link between alterations in the gut microbiome, cognitive function, and systemic inflammation. Changes in the microbiota in both minimal HE and overt HE have been associated with impaired cognition, endotoxemia, and inflammation[23].
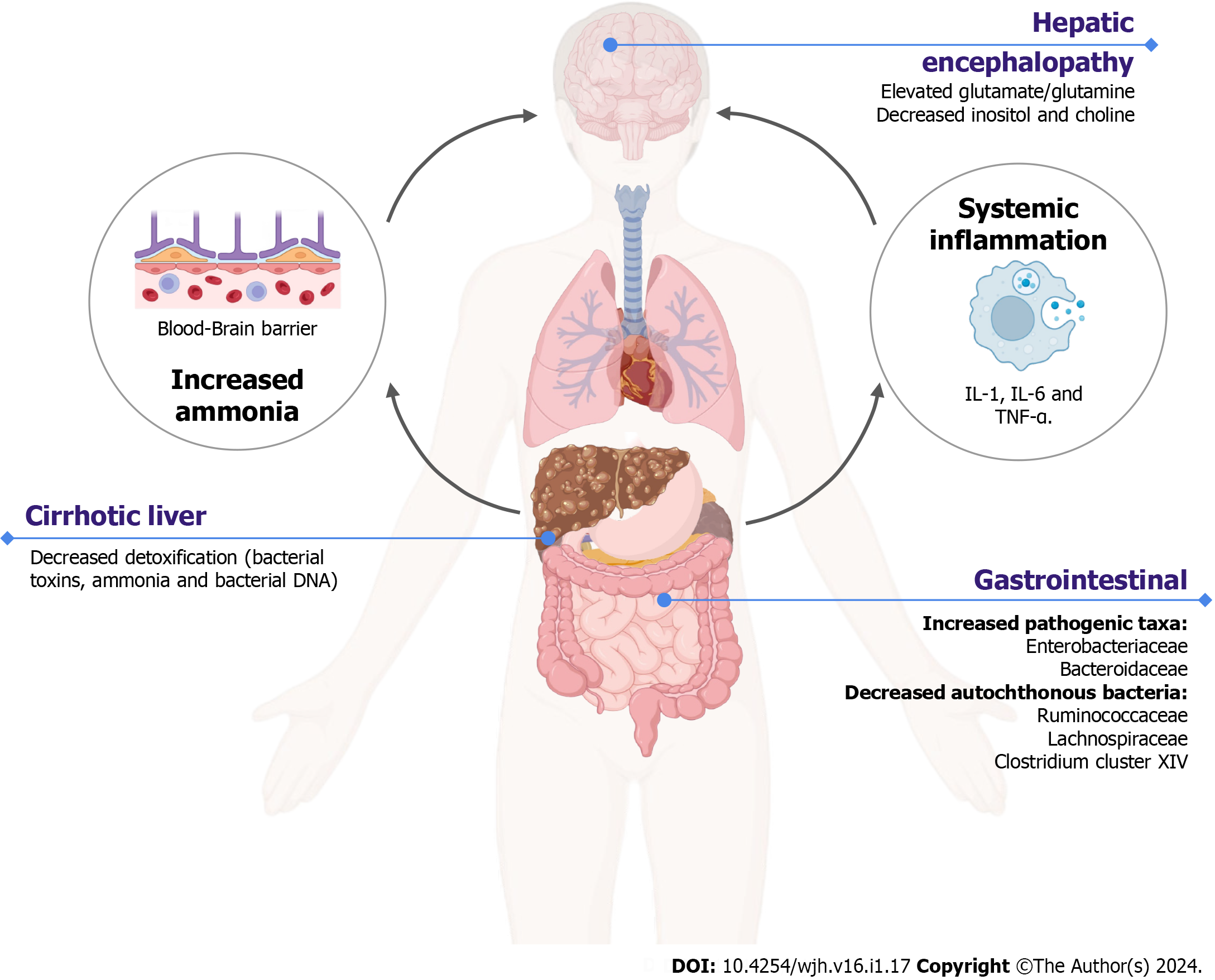
A potential mechanism explaining the association between gut dysbiosis, the severity of cirrhosis, and cognitive function involves reduced production of bile acids in cirrhosis patients which can alter the indigenous gut microbiota[23]. Healthy gut microbiota such as Lachnospiraceae, Ruminococcaceae, and Clostridiales Cluster XIV contribute to the production of short-chain fatty acids and maintenance of the gut barrier integrity[23]. In individuals with liver dysfunction, the liver’s reduced detoxification ability or the bypassing of bacterial products (including endotoxins, ammonia, and bacterial DNA) through portosystemic shunts can lead to systemic inflammation and cognitive decline[24]. Disruptions in the inflammatory environment and the presence of toxins promote neuroinflammation, resulting in elevated levels of intra-astrocytic ammonia. This, in turn, leads to increased concentrations of osmotically active glutamate or glutamine, along with decreased levels of myoinositol and choline[25]. The possible mechanism of HE in cirrhotic patients with gut dysbiosis is displayed in Figure 1 (created with BioRender.com). A study conducted on a rat model demonstrated that FMT can alleviate intestinal edema, mucosal damage, and inflammatory infiltration induced by HE[26]. FMT was also associated with reduced ammonia levels and systemic inflammation, as evidenced by decreased levels of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α[26]. Another study in rats with D-galactosamine-induced liver injury and FMT with B. adolescentis revealed significant alterations in the gut microbial community, including a decrease in pathogenic taxon Proteus and an enrichment of taxa responsible for lipid and amino acid metabolism, such as Coriobacteriaceae, Bacteroidales, and Allobaculum[27].
A study revealed a positive correlation between the severity of cirrhosis, as measured by the Child-Turcotte-Pugh Score (CTP), and the presence of the taxon Enterobacteriaceae, while a negative correlation was observed with Ruminococcaceae[28]. In a larger study involving 219 patients with liver cirrhosis, progressive changes in the gut microbiome were found to be associated with decompensated cirrhosis. The cirrhosis dysbiosis ratio represents the ratio of autochthonous bacteria (such as Ruminococcaceae, Lachnospiraceae, and Clostridales cluster XIV) to non-autochthonous bacteria (including Enterobacteriaceae and Bacteroidaceae), was correlated with the Model for End-Stage Liver Disease (MELD) score and endotoxin levels[29].
An overabundance of Streptococcus salivarius, which has been implicated in increased ammonia production due to its urease activity, was associated with elevated ammonia levels and cognitive impairment in patients with minimal HE. No significant difference was observed in the stool microbiome between minimal HE and overt HE, however, significant differences were observed in the colonic mucosal microbiome of these patients[30]. Autochthonous genera (such as Lachnospiraceae Roseburia, Lachnospiraceae Dorea, and Ruminococcaceae Faecalibacterium) were associated with better cognitive function compared to non-autochthonous genera (including Burkholderiaceae Other, Veillonellaceae Megasphaera, Rikenellaceae Alistipes, Streptococcaceae Streptococcus, Alcaligenaceae Sutterella, and Porphyromonadaceae Parabacteroides), which were linked to poorer cognitive function in patients with both overt and non-overt HE[23]. Currently, Lactulose and rifaximin are commonly used as prophylaxis to reduce ammonia-producing bacteria and prevent recurrent episodes of HE[31,32]. However, long-term use of rifaximin can lead to drug resistance. FMT has shown outstanding clinical efficacy in the management of various conditions like CDI, inflammatory bowel disease, IBS, etc. It has also been explored in the management of HE and has shown promising results[33].
Several techniques have been developed and tested for FMT, including colonoscopy, enema, nasogastric or nasojejunal tubes, and capsules. The outcomes of FMT have been associated with various factors, such as donor selection, sample selection, and delivery techniques[34]. Although there is limited data directly comparing related vs non-related donors, current evidence does not show a significant difference in outcomes[34]. The establishment of stool banks has increased the use of unrelated donors for FMT, providing easier access and availability[35]. Autologous stool FMT is a newer concept with limited evidence, involving the transplant of stool from patients themselves when their disease is in remission[21].
For upper gastrointestinal delivery, standard methods include esophagogastroduodenoscopy, nasogastric or nasojejunal tube, and oral capsules. Colonoscopy and enemas are commonly used for lower gastrointestinal delivery[34]. Oral capsules are the most recent development in stool delivery and are widely used due to their minimal invasiveness, convenience, and acceptance compared to other procedures[36]. The advantages and disadvantages of different delivery methods of FMT are discussed in Table 3[34,37,38].
| Mode of delivery | Advantage | Disadvantage | |
| Upper gastrointestinal tract | Nasogastric | Faster; Comparatively less expensive; Better tolerability | Risk of aspiration; Discomfort; Increased risk of small intestinal bacterial overgrowth |
| Nasojejunal | Faster; Comparatively less expensive; Better tolerability | Risk of aspiration; Risk of bowel perforation; Increased risk of small intestinal bacterial overgrowth | |
| Oral capsule | Least invasive; Cost-effective; Easy to store | Risk of aspiration; Vomiting; Sometimes failure to reach intestinal target | |
| Lower gastrointestinal tract | Colonoscopy | Direct visualization of GI tract; Standard risks of sedation and procedural intervention | Risk of bowel perforation; Higher cost of performing procedure |
| Retention enema | Useful in patients with severe colitis or colon distention to avoid perforation; Less invasive as compared to colonoscopy | Difficulty to retain transplanted stool; Need for repeated small volume infusion; Possible retention in patients with poor sphincter tone | |
Different methods used for fecal preparation include fresh fecal matter, frozen fecal matter, and lyophilized fecal matter (freeze-dried stool)[39]. Fresh FMT can readily be immediately transferred from a donor and has higher microbial load and diversity but it is logistically challenging to find a donor and transfer stool immediately[40]. On the other hand, frozen FMT can be conveniently stored and transported but it can lose efficacy if appropriate preservation and storage techniques are not maintained[15,40]. Lyophilized stool is the easiest to store and administer as it does not warrant invasive procedures for administration[40]. Multiple studies in patients with CDI have shown an overall efficacy of frozen fecal matter ranging from 81% to 100%. However, there are no significant differences in outcomes between fresh and frozen fecal preparations[34]. The efficacy of lyophilized stool also ranges from 78%-100%. The efficacy of lyophilized stool (78%) was significantly lower compared to fresh fecal preparation (100%), but equally effective compared to frozen stool (83%) based on a RCT[41]. No significant data is available comparing the use of different forms and delivery methods of FMT in the management of HE. The method of preparation of FMT can also impact the outcomes. Several studies in patients with UC have shown improved outcomes with anaerobically processed FMT as compared to aerobically processed FMT as many probiotics like Faecalibacterium prausnitzii are lost with aerobic stool processing[42]. Similarly, patients with HE might also benefit from anaerobically processed FMT[42]. The advantages and disadvantages of different delivery methods of FMT are discussed in Table 4[40]. FMT can be a robust technique for treating various conditions. However, there is no consensus on the specific route, dose, and preparation to be used for a particular condition.
| FMT preparation method | Efficacy range (%) | Preservation of microbial diversity | Advantages | Disadvantages |
| Fresh[40] | 85-100 | High | Contains diverse microbial population | Requires immediate availability of the patient |
| Frozen[40] | 83-95 | Moderate | Allows for long term storage | Loss of some microbial diversity during freezing; Comprise on efficacy if not stored properly and use of incorrect thawing techniques |
| Frozen lyophilized[40] | 78-84 | Moderate | Longer shelf life; Can be easily incorporated into a capsule | Loss of some microbial diversity during encapsulation |
Hepatitis B is a viral infection caused by the hepatitis B virus (HBV) and is typically transmitted through sexual, parenteral, or vertical routes. In 2019, it was estimated that there were 316 million infected individuals worldwide[43]. A study using a mouse model demonstrated that FMT can modulate the immune response and affect the host's susceptibility to HBV infection[44]. This finding highlights the crucial role of the gut microbiota in HBV infection. Furthermore, the composition of the gut microbiota varies across the different stages of chronic hepatitis B and these variations may be closely related to liver fibrosis[45]. Moreover, patients with liver cirrhosis experience an imbalance between beneficial and pathogenic bacteria, with a decrease in the abundance of beneficial bacteria such as Dialiste and Alistipes, and an increase in the abundance of pathogenic species within Actinobacteria[45]. This finding suggests that the gut microbiota may be involved in the pathogenesis of chronic hepatitis B progression. Therefore, the modulation of the gut microbiota through FMT could potentially influence the clinical course of HBV infection. Evidence suggests that the gut microbiota plays a critical role in the immune clearance of HBV.
Ren et al[46] conducted a case-control pilot study with an open-label design to assess the effectiveness of FMT in achieving hepatitis B e antigen (HBeAg) clearance. The study included a total of 18 patients, with 5 individuals assigned to the FMT arm and 13 to the control group. The results revealed that among the 5 patients in the FMT arm, 4 successfully achieved HBeAg clearance after undergoing 1-7 rounds of FMT treatment. Similarly, Chauhan et al[47] conducted a non-randomized pilot study where two out of twelve patients achieved HBeAg clearance. These findings indicate a positive association between FMT and the clearance of HBeAg. Guo et al[48] evaluated the efficacy of FMT in 35 patients with different stages of HBV-related chronic liver disease. The results showed that continuous FMT treatment led to improvements in liver function, controlled HBV-DNA replication, enhanced intestinal mucosal barrier function, and delayed the progression of HBV. Additionally, FMT demonstrated the ability to convert HBeAg-positive patients to HBeAg-negative status in 36.4% of cases, and it achieved negative conversion of HBV-DNA in 53.3% of patients[48]. The majority of the studies examined involve a limited number of participants, emphasizing the necessity for larger clinical studies to elucidate the findings pertaining to FMT as a treatment for HBV. Furthermore, there aren’t studies evaluating the effects of FMT on HE resulting from HBV. Hence, further research is required to comprehensively understand the efficacy of FMT in this topic.
Chronic infection with hepatitis C virus (HCV) leads to long-term liver inflammation, potentially resulting in liver fibrosis and cirrhosis, hepatic decompensation and chronic liver failure[49]. Studies have shown that the prevalence of liver cirrhosis 20 years after presumed HCV infection ranges from 7% to 18%[50]. The intestinal microbiota plays a significant role in influencing the onset and progression of HCV infection. Heidrich et al[51], reported a decrease in alpha diversity (observed richness or evenness of a specific taxa in an average sample within the habitat) measured by number of phylotypes and the Shannon Diversity Index associated with HCV infection, which further diminishes in cirrhotic patients. They also identified distinct microbial communities in the intestines of HCV patients, with non-cirrhotic individuals demonstrating a relatively higher abundance of Veillonella spp., Lactobacillus spp., Streptococcus spp., and Alloprevotella spp., while the highest abundance is observed in cirrhotic patients. This positive association of increased abundance in liver fibrosis progression suggests a connection between these genera and liver fibrosis progression in the liver. Conversely, Bilophila spp., Clostridium IV spp., Clostridium XlVb spp., Mitsuokella spp., and Vampirovibrio spp. appear to be negatively associated with fibrosis progression, showing decreased abundance from healthy controls to non-cirrhotic and cirrhotic patients[51]. Furthermore, several studies have investigated the impact of HCV eradication on gut dysbiosis[52-55]. Wellhöner et al[52] analyzed changes in the gut microbiome following direct-acting antivirals treatment and achieved sustained virological response (SVR). The study observed an increase in alpha diversity in non-cirrhotic patients, but not in those with cirrhosis. Bajaj et al[53] also found that systemic inflammation and gut dysbiosis are present in HCV cirrhosis patients irrespective of achieving SVR. Taken together, these studies highlight the complex relationship between the intestinal microbiota, HCV infection, fibrosis progression, and treatment outcomes.
Despite the growing interest in FMT as a potential therapeutic intervention for HE in liver cirrhosis, there is a notable lack of articles specifically assessing its efficacy in patients with liver cirrhosis due to chronic HCV infection. This knowledge gap underscores the need for further research in this area to better understand the effectiveness, safety, and long-term outcomes of FMT in this specific patient population. Such studies would provide valuable insights into the potential benefits and limitations of FMT as a treatment modality for HE in liver cirrhosis associated with chronic HCV infection and help guide future clinical practice and therapeutic decision-making.
Alcohol is a major risk factor for liver cirrhosis with risk increasing exponentially[56]. The prevalence of cirrhosis and heavy alcohol use varies by country, being higher in Europe (16%-78%) and the United States (17%-52%) compared to Asia (0%-41%)[57]. Chronic heavy alcohol consumption, even in the absence of liver disease, affects gut bacterial composition. The composition of the gut microbial community in patients with alcoholic liver cirrhosis (ALC) is characterized by a decrease in the commensal taxa Clostridia, Bacteroidetes, and Ruminococcaceae but an increase in Lactobacillus, Bifidobacterium and oral microbiota[58]. This dysbiosis leads to acetaldehyde-caused increased intestinal permeability, the increased blood concentration of endotoxins, and activation of inflammatory markers (TNF-α, IL-1β, IL-6, IL-8, and IL-10) likely inducing liver damage[59]. In patients with HE and ALC, Escherichia/Shigella, Burkholderiales, and Lactobacillales taxa predominated and led to an enhanced catabolism of arginine through ammonia-producing pathways[60]. This causes further systemic/neuroinflammation, hyperammonemia, endotoxemia, and microglial activation, increasing the risk of development of HE[23,61,62].
A potential role for FMT to improve the altered gut-brain axis in alcoholic liver disease and HE has been described. In a study involving 8 male patients with severe alcohol-associated hepatitis (SAH), FMT demonstrated significant improvements in liver disease severity within 1 wk, which were sustained over a median follow-up of almost a year[63]. HE was resolved in 71.4% of the FMT group[63]. Another similar study aimed to assess the longer-term outcomes (> 1 year) of FMT in SAH and found promising clinical benefits, including significantly lower incidences of HE[64]. The only article evaluating chronic conditions and FMT was a phase 1 randomized control trial with 20 patients with ALC and active drinking, participants were assigned either a placebo or an FMT enema from a donor with an enriched abundance in Lachnospiraceae and Ruminococcaceae[65]. Following a 2-wk period, FMT patients exhibited significant cognitive improvement, as assessed by both the Psychometric HE Score (PHES) and the EncephalApp (mobile application designed to evaluate cognitive function in HE) captured improvements in both off-time (periods of impaired cognitive performance) and on-time (periods of normal cognitive function) among the FMT-treated individuals. In addition, alcohol craving/consumption, quality of life, and diversity of microbiota also improved. These findings highlight the potential of FMT as a promising treatment option for patients with SAH, warranting further investigation through larger controlled studies.
Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD) is a liver disease characterized by the accumulation of fat in the liver. Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent liver diseases worldwide, with a global prevalence of 38.2%[66]. Emerging research has linked the gut microbiome dysbiosis and the pathogenesis of MASLD through the dysregulation of the gut-liver axis[67,68]. In MASLD, there is a disruption in the integrity of the intestinal barrier, resulting in an increase in intestinal permeability. This increased permeability has been associated with the severity of hepatic steatosis[69]. The compromised intestinal barrier function leads to endotoxemia and inflammation, which can further contribute to alterations in bile acid profiles and metabolite levels produced by the gut microbiota[68]. Moreover, dysregulation of bile acids has been closely linked to the progression of MASLD[70]. At the phylum level, patients with MASLD have variations in the gut microbiota composition, characterized by an increase in Proteobacteria and Firmicutes and a decrease in Bacteroidetes abundance[68]. Although obesity is closely associated with NAFLD, the role of dysbiosis caused by obesity in influencing MASLD development remains a subject of debate due to conflicting evidence[68]. Nonetheless, considering the significant role of the gut microbiota in MASLD, it is necessary to explore therapeutic strategies aimed at modulating the gut microbiota such as probiotics, prebiotics, antibiotics, and FMT[71]. A study conducted on a mouse model, in which steatohepatitis was induced through a high-fat diet, demonstrated that FMT effectively attenuated steatohepatitis and restored gut microbiota balance in mice after an 8-wk intervention[72]. This intervention resulted in reduced serum levels of alanine aminotransferase and aspartate aminotransferase, increased expression of zonula occludens-1, associated with improved tight junction integrity, restoration of high-fat diet-induced mucosal damage, and a decrease in serum endotoxin levels[72]. Xue et al[73] conducted a RCT involving 47 patients assigned to the FMT group, wherein the patients received FMT via colonoscopy using donor stool. Remarkably, the results demonstrated that FMT led to a significant increase in the Bacteroidetes/Firmicutes ratio, thereby indicating a positive modulation of the gut microbiota. Furthermore, the FMT group exhibited noteworthy clinical improvements as evidenced by a reduction in hepatic fat attenuation, which was evaluated using FibroScan. Subsequently, Kootte et al[74] examined the effects of FMT on insulin sensitivity in patients with metabolic syndrome. Their findings revealed a significant improvement in insulin sensitivity at 6 wk after allogeneic FMT. However, a clinical trial conducted by Craven et al[75] did not find an improvement in insulin sensitivity but demonstrated that allogeneic FMT improved intestinal permeability after 6 wk. Furthermore, patients with MASLD resulting from dysbiosis often experience disruptions in the gut-brain axis, leading to cognitive deterioration[76]. As MASLD progresses, it can lead to elevated levels of ammonia and exacerbate cognitive impairment in conjunction with a pro-inflammatory environment[76]. FMT has demonstrated efficacy in ameliorating HE among cirrhotic patients[77]. Therefore, FMT holds significant potential as a safe and promising therapeutic approach to enhance cognition in individuals with MASLD.
Table 5 compiles and highlights existing research, showcasing FMT's capacity to rebalance gut equilibrium and mitigate cognitive impairments associated with HE[9,22,42,63,66,77-81]. There are a few studies that have assessed the efficacy of HE based on the severity. Of these studies, the study by Metha et al[81] on 10 patients treated with FMT for recurrent over HE (≥ 2 episodes of WHC criteria II-IV HE in 6 mo as previously described in Table 2) showed that 6 patients had sustained clinical response as well as significant improvement in ammonia levels, CTP and MELD score. On the other hand there were two readmissions for spontaneous bacterial peritonitis and three patients with overt HE[79]. The case report by Kao et al[9] showed improvement in ammonia levels, Inhibitory Control Test (ICT), and Stroop test after FMT enema in a patient with Grade I-II HE which later worsened after stopping the treatment.
| Ref. | Type | Population | Intervention | Comparison | Outcomes | Adverse effects | ||||
| Cognitive impairment | Microbiota | Liver function | Scores | Not specified | ||||||
| Bajaj et al[77], 2017 | RCT | 20 cirrhotic patients experiencing recurrent HE while on lactulose/rifaximin treatment | FMT enema involving donor material enriched in Lachnospiraceae and Ruminococcaceae | SOC (Lactulose and rifaximin) | A significant improvement in both the PHES total score and EncephalApp Stroop was observed within the FMT group but not in the SOC group | Following antibiotic treatment, there was a decline in beneficial taxa and microbial diversity, coinciding with an expansion of Proteobacteria. However, FMT led to an increase in both diversity and beneficial taxa (Lactobacillaceae and Bifidobacteriaceae) | No alterations were observed in AST, ALT, or albumin levels in either study arm | In the SOC arm, MELD scores remained stable. However, in the FMT arm, antibiotics initially worsened the MELD scores, but subsequent FMT intervention successfully restored them to baseline levels | In the SOC arm, the urine metabolic profile remained stable over time. Conversely, the FMT group exhibited altered metabolites due to antibiotics, which were subsequently restored post-FMT | FMT arm: Tolerated treatment with no mental status hospitalizations; two unrelated hospitalizations occurred; SOC arm: Eleven SAEs, with higher incidences of HE and liver-related complications |
| Bajaj et al[65], 2021 | RCT, phase 1 | 10 patients with cirrhosis and alcohol use disorder, with an AUDIT-10 score of ≥ 8 during screening (FMT arm MELD score: 9.3 ± 2.6), and an equivalent of 10 patients in the placebo arm (9.5 ± 2.8) | FMT enema involving donor material enriched in Lachnospiraceae and Ruminococcaceae | Placebo | Cognitively, post-FMT patients exhibited improvements in both PHES and EncephalApp OffTime + OnTime | Post-FMT, an increase in diversity was observed, alongside elevated levels of Odoribacter, Bilophila, Alistipes, and Roseburia; Conversely, no changes were noted in the pre-placebo microbiota | There were no changes in AST, ALT, or albumin levels within the FMT group | The MELD score within the FMT group was similar at the study's conclusion (score at the end of the study: 8.6 ± 2.8) | In the FMT group, a noteworthy decrease in craving was evident among 90% of participants, whereas this reduction was observed in just 30% of the placebo group | A significant decrease in SAEs was observed in the FMT group compared to the placebo group (1 vs 7). The sole SAE in the FMT group was alcohol use disorder related, while 2 placebo-assigned patients required short-term antibiotics |
| Bloom et al[42], 2022 | RCT, phase 2 | A group of 10 cirrhotic patients, each having previously suffered at least one episode of overt HE and currently experiencing ongoing neurocognitive dysfunction | Healthy donors with normal BMI administered 15 oral FMT capsules on days 1, 2, 7, 14, and 21; Antibiotic pretreatment was not employed | None | PHES demonstrated improvement after three doses of FMT (+ 2.1), after five doses of FMT (+ 2.9), and at the 4-wk mark following the fifth dose of FMT (+ 3.1) | Baseline Bifidobacterium abundance was higher in FMT responders compared to nonresponders | Not reported | Not reported | Two taxa, namely Bifidobacterium adolescentis and B. angulatum, displayed a positive correlation with PHES scores. On the contrary, Enterobacter asburiae and B. breve showed a negative correlation with PHES scores | Four minor adverse effects were noted: nausea, bloating, fatigue, and constipation; One SAE involved the transmission of extended-spectrum beta-lactamase-producing Escherichia coli bacteremia through FMT |
| Li et al[78], 2022 | Case series | 2 patients diagnosed with liver cirrhosis resulting from hepatitis B, who faced recurring Grade 2-3 HE following TIPS intervention | Fecal microbiota transplant conducted three times using 50 g of fresh fecal intestinal flora suspension | None | Subsequent hospitalizations due to HE were not reported among the patients | Notable increases in Ruminococcus, Akkermansia, and Oscillospiraceae were observed, alongside decreased abundance of Veillonella and Megasphaera. These changes were accompanied by an overall increase in microbiota diversity | Liver function demonstrated improvement in Case 1, while Case 2 exhibited a nonsignificant enhancement | In Case 1, Child Pugh Score decreased from 10 to 5; In Case 2, it decreased from 11 to 7 | There were no clinical manifestations, and the blood ammonia level decreased significantly | No FMT-related adverse events or infection complications occurred in Case 1. Temporary constipation persisted for 7 d in Case 2 following FMT |
| Bajaj et al[22], 2019 | RCT, phase 1 | 20 cirrhotic patients experiencing recurrent HE and undergoing lactulose and rifaximin treatment. Out of these, ten were assigned to the FMT arm (MELD score of 9.5 ± 2.6) and ten were placed in the placebo arm (MELD score of 10.9 ± 4.2) | Administration of 15 FMT capsules from a single donor enriched in Lachnospiraceae and Ruminococcaceae | Placebo | A noteworthy improvement in OffTime + OnTime was evident within the FMT group compared to baseline. Conversely, significant PHES improvement was not observed in the FMT group, and placebo exhibited no significant changes | After FMT, duodenal mucosal diversity rose, featuring higher Ruminococcaceae and Bifidobacteriaceae, and reduced Streptococcaceae and Veillonellaceae. Similar reductions in Veillonellaceae were seen post-FMT in sigmoid and stool samples | Not reported | The MELD score within the FMT group was similar at the study's conclusion (score at the end of the study: 8.7 ± 2.9) | Following FMT, Duodenal E-cadherin and Defensin A5 increased, while IL-6 and serum LBP reduced | In the placebo group, 6 patients experienced SAEs: Five HE episodes, two infections, and one renal insufficiency case. In addition, 1 patient was transferred to hospice and deceased. In contrast, the FMT group had only one HE episode, with no reported deaths |
| Mehta et al[79], 2018 | Case series | 10 patients, previously treated with FMT for recurrent HE (defined as ≥ 2 episodes of West Haven grade II–IV HE in the last 6 mo) | FMT was introduced via colonoscopy into the right colon 7–10 d after the episode of HE | None | Not reported | Not reported | Not reported | A reduction in both CTP and MELD scores was observed from baseline to post-treatment week 20 | The arterial ammonia concentration showed a considerable decrease at post-treatment week 20 | 1 patient died due to bronchopneumonia complicated by sepsis 2 mo after FMT. Additionally, 2 patients were readmitted due to spontaneous bacterial peritonitis |
| Kao et al[9], 2016 | Case report | A 57-yr-old male with grade 1-2 HE, with liver cirrhosis (MELD score of 10), attributed to alcohol and hepatitis C | Weekly FMT was administered, with the first application performed via colonoscopy and the subsequent sessions through retention enema | None | Mental status was assessed through the ICT and Stroop test. At 4 wk after the third FMT, the ICT score changed from 17 (baseline) to 5, and the Stroop test score changed from 250.9 to 183.5. However, by the 14-wk mark, these values reverted to baseline levels | Following FMT, there was a reduction in the relative abundance of Lachnospiraceae | Not reported | Not reported | Not applicable | No adverse events or infectious complications linked to FMT occurred |
| Bajaj et al[80], 2019 | RCT, long term outcomes (> 12 mo) of a 2017 study | 20 patients with cirrhosis experiencing recurring episodes of HE | FMT enema involving donor material enriched in Lachnospiraceae and Ruminococcaceae | SOC (Lactulose and rifaximin) | The FMT group experienced fewer HE episodes during long-term follow-up compared to SOC. Additionally, cognitive function, evaluated using the PHES total score and EncephalApp Stroop, significantly favored the FMT group | During long-term follow-up, FMT displayed increased Burkholderiaceae and decreased Acidaminococcaceae. However, Lachnospiraceae and Ruminococcaceae remained relatively stable. Microbiota composition remained similar post-FMT, regardless of short or long-term follow-up, when compared to the pre-FMT state | Not reported | Changes in MELD scores exhibited similarity between the two groups | The FMT group experienced significantly fewer hospitalizations compared to the SOC group during the long-term follow-up | The intervention was well-tolerated in the FMT group, demonstrating a favorable long-term safety profile |
| Philips et al[63], 2017 | Pilot study | 8 patients diagnosed with steroid-ineligible severe alcohol-asSOCiated hepatitis (MELD score: 31 ± 5.6) and 18 control subjects (MELD score: 27 ± 5.2) | Thirty grams of donor stool samples infused daily for 7 d through a nasoduodenal tube | SOC (specifics not provided) | HE resolved in 6 out of 8 patients after FMT (71.4%). | 1 yr post-FMT, there was an increase in Firmicutes and a reduction in Proteobacteria and Actinobacteria. Noteworthy species changes included decreased Klebsiella pneumoniae and increased Enterococcus villorum, Bifidobacterium longum, and Megasphaera elsdenii | The mean bilirubin levels significantly decreased from 20.5 ± 7.6 mg/dL to 2.86 ± 0.69 mg/dL after treatment | Child-Turcotte-Pugh, MELD, and MELD Sodium scores showed significant reductions post-treatment in comparison to baseline | Survival was notably better in the FMT group when compared to healthy controls. Additionally, post-FMT improvements were observed in bile, carotenoid, and pantothenate pathways | Excessive flatulence was reported as a complaint by 50% of FMT patients |
| Philips et al[81], 2022 | Retrospective analysis | 47 patients diagnosed with severe alcohol-asSOCiated hepatitis (MELD score: 28.1 ± 4.7) and 25 control subjects (MELD score: 28.2 ± 6.3) | The FMT group received 100 mL of freshly processed stool samples daily via a nasoduodenal tube for 7 d | Pentoxifylline (400 mg thrice daily for 28 d) | During follow-up, the FMT group exhibited significantly lower HE incidences compared to the SOC group | In the FMT group, there was a decrease in Proteobacteria and an increase in Actinobacteria and Bacteroides. Genus-level analysis revealed higher Bifidobacterium and lower Acinetobacter. Within the SOC group, higher levels of Erwinia and Porphyromonas were noted, along with lower beneficial Bifidobacterium at 1-2 yr. Beyond the 2-yr mark, FMT led to higher beneficial Bifidobacterium levels | Not reported | Not reported | During follow-up, the FMT group exhibited lower instances of ascites, infections, hospitalizations, and alcohol relapse in comparison to the SOC group. A longer time to relapse was noted, along with a trend towards improved survival at 3 yr | Acute variceal bleeding was the most common cause of death in the FMT group, whereas infection predominated in the SOC group |
To sum up, significant progress has been achieved in the potential application of FMT as a therapeutic option for HE among cirrhotic patients and careful selection of the donor can lead to improved outcomes in patients with HE. Prospective studies are required to compare the efficacy of FMT in patients with different stages of HE.
FMT is grounded in the concept of bacterial displacement, where beneficial bacteria from a healthy donor are introduced to replace harmful pathogens in the recipient’s gut. This process leverages competitive exclusion[82]. By restoring a balanced microbiome, FMT aims to alleviate disease and its progression. Analysis of cross-sectional stool genomics data has revealed significant dysregulation in the expression levels of specific genomics species between decompensated and compensated hepatic conditions[83]. This provides valuable scientific insights into the molecular alterations associated with disease progression and highlights potential targets for further investigation and therapeutic interventions[9]. Cognitive assessment using the ICT and the Stroop test demonstrated progressive improvement in cognition with consecutive FMT sessions, reaching the plateau after the 4th wk following three FMTs. Notably, cognition regressed to baseline 14 wk after discontinuing FMT which intriguingly was associated with a marked reduction in the levels of lachnospiraceae, a bacterial family associated with improved cognitive function. The promising outcomes of this study paved the pathway for the initiation of the first RCT.
In a notable study by Bajaj et al[77] in 2017, an open-label randomized trial was conducted involving a cohort of 20 cirrhotic patients experiencing recurring episodes of HE. The intervention arm involved the administration of lachnospiraceae and ruminococcaceae enema in addition to standard of care (SOC). The results of the study demonstrated a significant reduction in the occurrence of serious adverse events (SAEs) in the FMT arm (P = 0.02) compared to the SOC arm. In the FMT arm, 2 patients were hospitalized within 5 mo, but their conditions (acute kidney injury and chest pain) were deemed not related to FMT. In contrast, the SOC arm experienced 11 SAEs, including liver-related complications such as mental status changes, pneumonia, chest pain, portal vein thrombosis, anemia, gastroenteritis, and variceal bleeding. The FMT arm also showed significant cognitive improvement, indicated by improved PHES and encephal app Stroop scores, while the SOC did not exhibit similar improvements. The 12 mo follow-up of the patients revealed that FMT appeared to be safe and held potential for long-term efficacy[80].
In 2019, a subsequent study was conducted by Bajaj et al[22] comparing FMT using capsules enriched with lachnospiraceae and ruminococcaceae (administered in a dose of 15 capsules at a time) with a placebo group. The study focused on patients with cirrhosis experiencing recurrent episodes of HE. The placebo group had a higher number of SAEs compared to the FMT group. The FMT group (n = 10) had one reported SAE, whereas the placebo group (n = 10) reported 11 SAE (P < 0.05). Most SAEs in the placebo group were related to liver disease progression and resulted in hospitalizations and emergency room (commonly referred to as ER) visits. Six placebo patients experienced SAEs, with one patient having multiple events including episodes of HE and renal insufficiency without HE. The remaining five patients had one SAE each, including infections (pneumonia and cellulitis), HE and electrolyte abnormalities. Four placebo patients did not have any SAEs. In the FMT group, only one patient had an episode of HE as an SAE, while the remaining nine patients did not experience any SAEs during the follow up period. One placebo patient required ER visits/hospitalization for altered electrolytes, which resolved within 24 h. Unfortunately, one placebo patient with multiple admissions was transferred to hospice and passed away 4 mo after enrollment. None of the FMT assigned patients died during the follow up period. The results also revealed improvements in the microbiota composition, inflammatory markers, and cognitive scores, indicating the potential of FMT. In the FMT group, duodenal microbiota diversity was enhanced and was also marked by a decrease in the levels of serum lipopolysaccharide binding protein and interleukin-6.
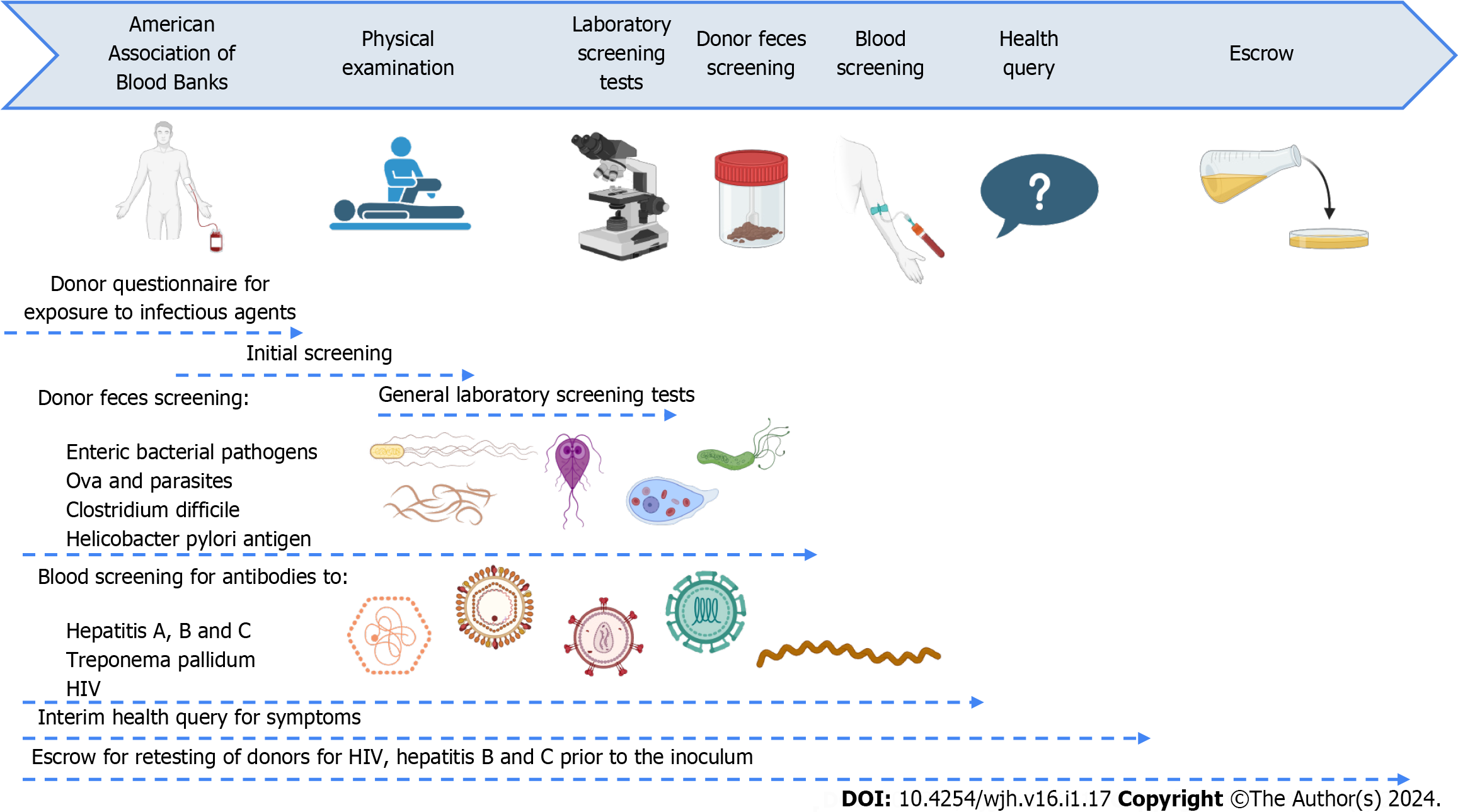
Another study conducted by Bloom et al[42] demonstrated improved cognitive function in patients with a history of cirrhosis and overt HE after 5 doses of oral FMT capsules were given over 3 wk. FMT donors were healthy adults with normal body mass index, carefully chosen through a rigorous screening process (Figure 2: Created with biorender.com) outlined in a previously published study[36]. Following the completion of the FMT treatment, there was a notable average improvement of 3.1 points in the PHES after 4 wk. FMT resulted in mild and transient gastrointestinal side effects in a few patients. However, it is important to note that one patient experienced SAE in the form of esbl-producing E. coli bacteremia following FMT. In spite of the reported cases of FMT-transmitted infections, a recent systematic review encompassing 4241 patients concluded that FMT is generally safe, demonstrating a notably low incidence of SAE related to microbiota[84]. Additionally, there are more promising studies establishing the process of FMT for the treatment of HE in cirrhotic patients[85]. Even though alterations in the efficacy of FMT have been noticed, immunocompromised patients, including those with cirrhosis, may require multiple FMT treatments to achieve a cure. A study by Shogbesan et al[86] found that the success rate of FMT increased from 88% to 93% when multiple FMTs were administered. However, in decompensated cirrhotic patients, the efficacy of FMT may be diminished due to worsened immune deficiencies resulting in a lower success rate compared to other immunocompromised patients or the general population[87].
FMT has several limitations and challenges that need to be addressed. Firstly, the standard microbiota composition for FMT in the donor and the receptor sets a basis for which patients will respond, and the optimal treatment duration remains largely unknown. Large-scale, randomized, and controlled clinical trials are necessary to validate and standardize the clinical application of FMT in HE cases[88].
Several studies have been conducted, including randomized and controlled trials, to evaluate the efficacy and safety of FMT in HE. However, the number of patients enrolled in these studies is relatively small, limiting the generalizability of the findings. Additionally, factors such as the optimal dose, duration and administration route of FMT, long-term effects of FMT, mortality, the need for prior antibiotic use to facilitate engraftment, and donor selection based on their microbiota profile still need further investigation[22,42,77,89].
Furthermore, while some studies have reported decreased hospitalizations and severe adverse events in the FMT group compared to the placebo, these outcomes were not the primary endpoints in those trials[85,90]. Some other evidence of FMT in patients with decompensated cirrhosis has shown a marginally higher rate of death and SAE compared to the average immunocompetent population[87]. FMT as a one-time infusion, has been found to be less effective than expected[87]. Therefore, a true meta-analysis to get a better conclusion by combining the available literature is currently not feasible, because of the scarcity of large research trials and limited published evidence. This also limits the widespread use of FMT, making it primarily available in academic centers. Detailed information regarding the health status of donors and sourcing of donor material is often lacking in the included studies, which could potentially introduce confounding factors[87,88].
In conclusion, although FMT has shown therapeutic efficacy in treating HE in cirrhotic patients, there are limitations and challenges that need to be addressed. Further research with larger cohorts and robust study protocols is necessary to fully understand the role of FMT in cirrhotic patients and establish standardized guidelines for its clinical application[85].
FMT is currently being studied as a treatment option for HE. The evidence is limited due to the quality and the number of studies performed. This review offers a summary of current studies in various clinical conditions, delivery and preparation methods, safety, limitations and future aspects in the field of FMT for management of HE. The review also highlights the important aspects of hepatic conditions associated with HE, and their pivotal role in the pathogenesis and understanding how the microbiome is affected in each pathology, and how the FMT could help in these clinical scenarios. Significant efforts need to be directed towards addressing the doses, delivery methods, and safety of FMT, as well as larger studies performed in humans to better understand and assess the quality and benefit of the intervention. Patients with hepatic conditions that cause HE will greatly benefit from more advances in the medical research of FMT that remains as a promising therapy for HE in different contexts.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhu YY, China S-Editor: Lin C L-Editor: Filipodia P-Editor: Zhao S
| 1. | Elsaid MI, Rustgi VK. Epidemiology of Hepatic Encephalopathy. Clin Liver Dis. 2020;24:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 2. | Acharya C, Bajaj JS. Current Management of Hepatic Encephalopathy. Am J Gastroenterol. 2018;113:1600-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Louissaint J, Deutsch-Link S, Tapper EB. Changing Epidemiology of Cirrhosis and Hepatic Encephalopathy. Clin Gastroenterol Hepatol. 2022;20:S1-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and Risk Factors for Hepatic Encephalopathy in a Population-Based Cohort of Americans With Cirrhosis. Hepatol Commun. 2019;3:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Cordoba J. Hepatic Encephalopathy: From the Pathogenesis to the New Treatments. ISRN Hepatol. 2014;2014:236268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Chen Z, Ruan J, Li D, Wang M, Han Z, Qiu W, Wu G. The Role of Intestinal Bacteria and Gut-Brain Axis in Hepatic Encephalopathy. Front Cell Infect Microbiol. 2020;10:595759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Salgado M, Cortes Y. Hepatic encephalopathy: etiology, pathogenesis, and clinical signs. Compend Contin Educ Vet. 2013;35:E1-8; quiz E9. [PubMed] |
| 8. | Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Kao D, Roach B, Park H, Hotte N, Madsen K, Bain V, Tandon P. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology. 2016;63:339-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Hassouneh R, Bajaj JS. Gut Microbiota Modulation and Fecal Transplantation: An Overview on Innovative Strategies for Hepatic Encephalopathy Treatment. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1406] [Article Influence: 127.8] [Reference Citation Analysis (1)] |
| 12. | Bailey C, Gene Hern H. Hepatic Failure: An Evidence-Based Approach In The Emergency. EB Medicine. 2010;12. |
| 13. | Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 14. | Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl 1:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Jiang H, Peng Y, Zhang W, Chen Y, Jiang Q, Zhou Y. Gut microbiome-targeted therapies in liver cirrhosis: a protocol for systematic review and meta-analysis. Syst Rev. 2022;11:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Shukla S, Shukla A, Mehboob S, Guha S. Meta-analysis: the effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Aliment Pharmacol Ther. 2011;33:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Rocco A, Sgamato C, Compare D, Coccoli P, Nardone OM, Nardone G. Gut Microbes and Hepatic Encephalopathy: From the Old Concepts to New Perspectives. Front Cell Dev Biol. 2021;9:748253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, Hu HM, Hsu PI, Wang JY, Wu DC. Fecal microbiota transplantation: Review and update. J Formos Med Assoc. 2019;118 Suppl 1:S23-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 332] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 19. | Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Antushevich H. Fecal microbiota transplantation in disease therapy. Clin Chim Acta. 2020;503:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 21. | Basson AR, Zhou Y, Seo B, Rodriguez-Palacios A, Cominelli F. Autologous fecal microbiota transplantation for the treatment of inflammatory bowel disease. Transl Res. 2020;226:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, Hayward M, Holtz ML, Matherly S, Lee H, Osman M, Siddiqui MS, Fuchs M, Puri P, Sikaroodi M, Gillevet PM. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019;70:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 23. | Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Zapater P, Francés R, González-Navajas JM, de la Hoz MA, Moreu R, Pascual S, Monfort D, Montoliu S, Vila C, Escudero A, Torras X, Cirera I, Llanos L, Guarner-Argente C, Palazón JM, Carnicer F, Bellot P, Guarner C, Planas R, Solá R, Serra MA, Muñoz C, Pérez-Mateo M, Such J. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology. 2008;48:1924-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | McPhail MJ, Taylor-Robinson SD. The role of magnetic resonance imaging and spectroscopy in hepatic encephalopathy. Metab Brain Dis. 2010;25:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Wang WW, Zhang Y, Huang XB, You N, Zheng L, Li J. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J Gastroenterol. 2017;23:6983-6994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Li Y, Lv L, Ye J, Fang D, Shi D, Wu W, Wang Q, Wu J, Yang L, Bian X, Jiang X, Jiang H, Yan R, Peng C, Li L. Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in D-galactosamine-treated rats. Appl Microbiol Biotechnol. 2019;103:375-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 29. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 836] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 30. | Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, Shi P. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol. 2013;108:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther. 2016;43 Suppl 1:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Gluud LL, Vilstrup H, Morgan MY. Nonabsorbable disaccharides for hepatic encephalopathy: A systematic review and meta-analysis. Hepatology. 2016;64:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | Xie WR, Yang XY, Xia HHX, He XX. Fecal Microbiota Transplantation for Treating Hepatic Encephalopathy: Experimental and Clinical Evidence and Possible Underlying Mechanisms. J Explor Res Pharmacol. 2018;3:119-124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Ramai D, Zakhia K, Ofosu A, Ofori E, Reddy M. Fecal microbiota transplantation: donor relation, fresh or frozen, delivery methods, cost-effectiveness. Ann Gastroenterol. 2019;32:30-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 36. | Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, Kaplan JL, Hohmann EL. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 37. | Verdier C, Denis S, Gasc C, Boucinha L, Uriot O, Delmas D, Dore J, Le Camus C, Schwintner C, Blanquet-Diot S. An Oral FMT Capsule as Efficient as an Enema for Microbiota Reconstruction Following Disruption by Antibiotics, as Assessed in an In Vitro Human Gut Model. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Heath RD, Cockerell C, Mankoo R, Ibdah JA, Tahan V. Fecal microbiota transplantation and its potential therapeutic uses in gastrointestinal disorders. North Clin Istanb. 2018;5:79-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Tian H, Ding C, Gong J, Wei Y, McFarland LV, Li N. Freeze-dried, Capsulized Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. J Clin Gastroenterol. 2015;49:537-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Gangwani MK, Aziz M, Aziz A, Priyanka F, Weissman S, Phan K, Dahiya DS, Ahmed Z, Sohail AH, Lee-Smith W, Kamal F, Javaid T, Nawras A, Hart B. Fresh Versus Frozen Versus Lyophilized Fecal Microbiota Transplant for Recurrent Clostridium Difficile Infection: A Systematic Review and Network Meta-analysis. J Clin Gastroenterol. 2023;57:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Jiang ZD, Ajami NJ, Petrosino JF, Jun G, Hanis CL, Shah M, Hochman L, Ankoma-Sey V, DuPont AW, Wong MC, Alexander A, Ke S, DuPont HL. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection - fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther. 2017;45:899-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 42. | Bloom PP, Donlan J, Torres Soto M, Daidone M, Hohmann E, Chung RT. Fecal microbiota transplant improves cognition in hepatic encephalopathy and its effect varies by donor and recipient. Hepatol Commun. 2022;6:2079-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 43. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 404] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 44. | Wang J, Zhou X, Li X, Guo W, Zhu Q, Zhu B, Lu Y, Zheng X, Yang D, Wang B. Fecal Microbiota Transplantation Alters the Outcome of Hepatitis B Virus Infection in Mice. Front Cell Infect Microbiol. 2022;12:844132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Chen Z, Xie Y, Zhou F, Zhang B, Wu J, Yang L, Xu S, Stedtfeld R, Chen Q, Liu J, Zhang X, Xu H, Ren J. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020;11:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Ren YD, Ye ZS, Yang LZ, Jin LX, Wei WJ, Deng YY, Chen XX, Xiao CX, Yu XF, Xu HZ, Xu LZ, Tang YN, Zhou F, Wang XL, Chen MY, Chen LG, Hong MZ, Ren JL, Pan JS. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Chauhan A, Kumar R, Sharma S, Mahanta M, Vayuuru SK, Nayak B, Kumar S, Shalimar. Fecal Microbiota Transplantation in Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients: A Pilot Study. Dig Dis Sci. 2021;66:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Guo Q, Huang SS, Li J, Tian Y, Zhou Y, Li XA. Fecal Microbiota Transplantation Slows the Progression of HBV-Related Liver Diseases and Induces Virologic Response in Patients with HBV Infection. Austin J Gastroenterol. 2021;8:1117. [DOI] [Full Text] |
| 49. | Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 360] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 50. | Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 51. | Heidrich B, Vital M, Plumeier I, Döscher N, Kahl S, Kirschner J, Ziegert S, Solbach P, Lenzen H, Potthoff A, Manns MP, Wedemeyer H, Pieper DH. Intestinal microbiota in patients with chronic hepatitis C with and without cirrhosis compared with healthy controls. Liver Int. 2018;38:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 52. | Wellhöner F, Döscher N, Woelfl F, Vital M, Plumeier I, Kahl S, Potthoff A, Manns MP, Pieper DH, Cornberg M, Wedemeyer H, Heidrich B. Eradication of Chronic HCV Infection: Improvement of Dysbiosis Only in Patients Without Liver Cirrhosis. Hepatology. 2021;74:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Bajaj JS, Sterling RK, Betrapally NS, Nixon DE, Fuchs M, Daita K, Heuman DM, Sikaroodi M, Hylemon PB, White MB, Ganapathy D, Gillevet PM. HCV eradication does not impact gut dysbiosis or systemic inflammation in cirrhotic patients. Aliment Pharmacol Ther. 2016;44:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Ponziani FR, Putignani L, Paroni Sterbini F, Petito V, Picca A, Del Chierico F, Reddel S, Calvani R, Marzetti E, Sanguinetti M, Gasbarrini A, Pompili M. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther. 2018;48:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Hsu YC, Chen CC, Lee WH, Chang CY, Lee FJ, Tseng CH, Chen TH, Ho HJ, Lin JT, Wu CY. Compositions of gut microbiota before and shortly after hepatitis C viral eradication by direct antiviral agents. Sci Rep. 2022;12:5481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, Neuman MG, Rehm J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019;114:1574-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 57. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 361] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 58. | Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, Govorun VM. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 59. | Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485-E4493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 701] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 60. | Baltazar-Díaz TA, Riggen-Bueno V, Cortina-Romero DB, Del Toro-Arreola S, Haramati J, Bastidas-Ramírez BE, Bueno-Topete MR. Low-diversity microbiota and an increased metabolism of arginine and aromatic amino acids: a hallmark of hepatic encephalopathy in western Mexican patients with alcohol-associated cirrhosis. J Appl Microbiol. 2023;134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 61. | Kang DJ, Betrapally NS, Ghosh SA, Sartor RB, Hylemon PB, Gillevet PM, Sanyal AJ, Heuman DM, Carl D, Zhou H, Liu R, Wang X, Yang J, Jiao C, Herzog J, Lippman HR, Sikaroodi M, Brown RR, Bajaj JS. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice. Hepatology. 2016;64:1232-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 63. | Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, Kumar G, Sharma MK, Maiwall R, Jindal A, Choudhary A, Hussain MS, Sharma S, Sarin SK. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 64. | Philips CA, Ahamed R, Rajesh S, Abduljaleel JKP, Augustine P. Long-term Outcomes of Stool Transplant in Alcohol-associated Hepatitis-Analysis of Clinical Outcomes, Relapse, Gut Microbiota and Comparisons with Standard Care. J Clin Exp Hepatol. 2022;12:1124-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 65. | Bajaj JS, Gavis EA, Fagan A, Wade JB, Thacker LR, Fuchs M, Patel S, Davis B, Meador J, Puri P, Sikaroodi M, Gillevet PM. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology. 2021;73:1688-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 66. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1410] [Article Influence: 705.0] [Reference Citation Analysis (2)] |
| 67. | Song Q, Zhang X. The Role of Gut-Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 68. | Pezzino S, Sofia M, Faletra G, Mazzone C, Litrico G, La Greca G, Latteri S. Gut-Liver Axis and Non-Alcoholic Fatty Liver Disease: A Vicious Circle of Dysfunctions Orchestrated by the Gut Microbiome. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | De Munck TJI, Xu P, Verwijs HJA, Masclee AAM, Jonkers D, Verbeek J, Koek GH. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020;40:2906-2916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 70. | Jackson KG, Way GW, Zhou H. Bile acids and sphingolipids in non-alcoholic fatty liver disease. Chin Med J (Engl). 2022;135:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Gupta M, Krishan P, Kaur A, Arora S, Trehanpati N, Singh TG, Bedi O. Mechanistic and physiological approaches of fecal microbiota transplantation in the management of NAFLD. Inflamm Res. 2021;70:765-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 293] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 73. | Xue L, Deng Z, Luo W, He X, Chen Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front Cell Infect Microbiol. 2022;12:759306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 74. | Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 679] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 75. | Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, Hramiak I, Hegele R, Joy T, Meddings J, Urquhart B, Harvie R, McKenzie C, Summers K, Reid G, Burton JP, Silverman M. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am J Gastroenterol. 2020;115:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 76. | George ES, Sood S, Daly RM, Tan SY. Is there an association between non-alcoholic fatty liver disease and cognitive function? A systematic review. BMC Geriatr. 2022;22:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 77. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 452] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 78. | Li J, Wang D, Sun J. Application of fecal microbial transplantation in hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Medicine (Baltimore). 2022;101:e28584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Mehta R, Kabrawala M, Nandwani S, Kalra P, Patel C, Desai P, Parekh K. Preliminary experience with single fecal microbiota transplant for treatment of recurrent overt hepatic encephalopathy-A case series. Indian J Gastroenterol. 2018;37:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 80. | Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term Outcomes of Fecal Microbiota Transplantation in Patients With Cirrhosis. Gastroenterology. 2019;156:1921-1923.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 81. | Philips CA, Ahamed R, Rajesh S, Singh S, Tharakan A, Abduljaleel JK, Augustine P. Clinical outcomes and gut microbiota analysis of severe alcohol-associated hepatitis patients undergoing healthy donor fecal transplant or pentoxifylline therapy: single-center experience from Kerala. Gastroenterol Rep (Oxf). 2022;10:goac074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 388] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 83. | Bajaj JS, Betrapally NS, Gillevet PM. Decompensated cirrhosis and microbiome interpretation. Nature. 2015;525:E1-E2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 84. | Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 85. | Tun KM, Hong AS, Batra K, Naga Y, Ohning G. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplantation in the Treatment of Hepatic Encephalopathy and Clostridioides difficile Infection in Patients With Cirrhosis. Cureus. 2022;14:e25537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Shogbesan O, Poudel DR, Victor S, Jehangir A, Fadahunsi O, Shogbesan G, Donato A. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplant for Clostridium difficile Infection in Immunocompromised Patients. Can J Gastroenterol Hepatol. 2018;2018:1394379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 87. | Hong AS, Tun KM, Hong JM, Batra K, Ohning G. Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy. Antibiotics (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 88. | Madsen M, Kimer N, Bendtsen F, Petersen AM. Fecal microbiota transplantation in hepatic encephalopathy: a systematic review. Scand J Gastroenterol. 2021;56:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 89. | Afecto E, Ponte A, Fernandes S, Silva J, Gomes C, Correia J, Carvalho J. Fecal microbiota transplantation in hepatic encephalopathy : a review of the current evidence and future perspectives. Acta Gastroenterol Belg. 2021;84:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Chathur A, Nita S, Genta, Masoumeh S, Michael H, Andrew F, Edith AG, Melanie W, Hajime T, Hiroshi N, William MP, Phillip BH, Patrick MG, Jasmohan SB. P: 11 Cognitive Improvement After Capsular Fecal Microbial Transplant in Hepatic Encephalopathy Is Associated With Changes in Microbial Function and Inflammation. Am J Gastroenterol. 2019;114:S4-S5. [DOI] [Full Text] |