Published online Jul 27, 2023. doi: 10.4254/wjh.v15.i7.883
Peer-review started: March 26, 2023
First decision: April 28, 2023
Revised: May 15, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: July 27, 2023
Processing time: 116 Days and 15.7 Hours
Liver diseases after kidney transplantation range from mild biochemical abnormalities to severe hepatitis or cirrhosis. The causes are diverse and mainly associated with hepatotropic viruses, drug toxicity and metabolic disorders. Over the past decade, the aetiology of liver disease in kidney recipients has changed significantly. These relates to the use of direct-acting antiviral agents against hepatitis C virus, the increasing availability of vaccination against hepatitis B and a better understanding of drug-induced hepatotoxicity. In addition, the emer
Core Tip: Liver disease is a common complication after kidney transplantation and can present in a variety of forms, from asymptomatic biochemical abnormalities to fibrosis/cirrhosis/decompensation/malignancy. Early recognition and referral to a hepatologist are crucial for effective treatment, as they can otherwise lead to impaired quality of life and increased morbidity. Screening of kidney transplant recipients for liver disease, including viral disease, metabolic disorders and drug toxicity, should be prioritised by healthcare providers.
- Citation: Kosuta I, Ostojic A, Vujaklija Brajkovic A, Babel J, Simunov B, Sremac M, Mrzljak A. Shifting perspectives in liver diseases after kidney transplantation. World J Hepatol 2023; 15(7): 883-896
- URL: https://www.wjgnet.com/1948-5182/full/v15/i7/883.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i7.883
The efficacy of kidney transplantation (KT) in the treatment of end-stage renal disease (ESRD) has been demonstrated by numerous studies, which have shown that KT is associated with significantly higher survival rates and better quality of life compared to dialysis. As such, KT is now considered the gold standard in the treatment of ESRD[1,2]. Prevalence of chronic kidney disease (CKD) is estimated at 13.4% (11.7%-15.1%) and is on the rise due to increasing rates of diabetes, arterial hypertension, obesity and ageing. This is reflected in the increasing rates of KT; 92532 KT performed in 2021, which is an estimated 40% increase from 2008[2,3]. Advances in organ procurement, surgical techniques, immunosuppression regimens targeting rejection, and prophylactic antibiotic therapies have enabled excellent short-term survival rates after KT, and long-term outcomes are steadily improving over time[4]. This has led to a prolongation of life expectancy of both the graft and the recipient, which is now appreciable well beyond the first year following transplan
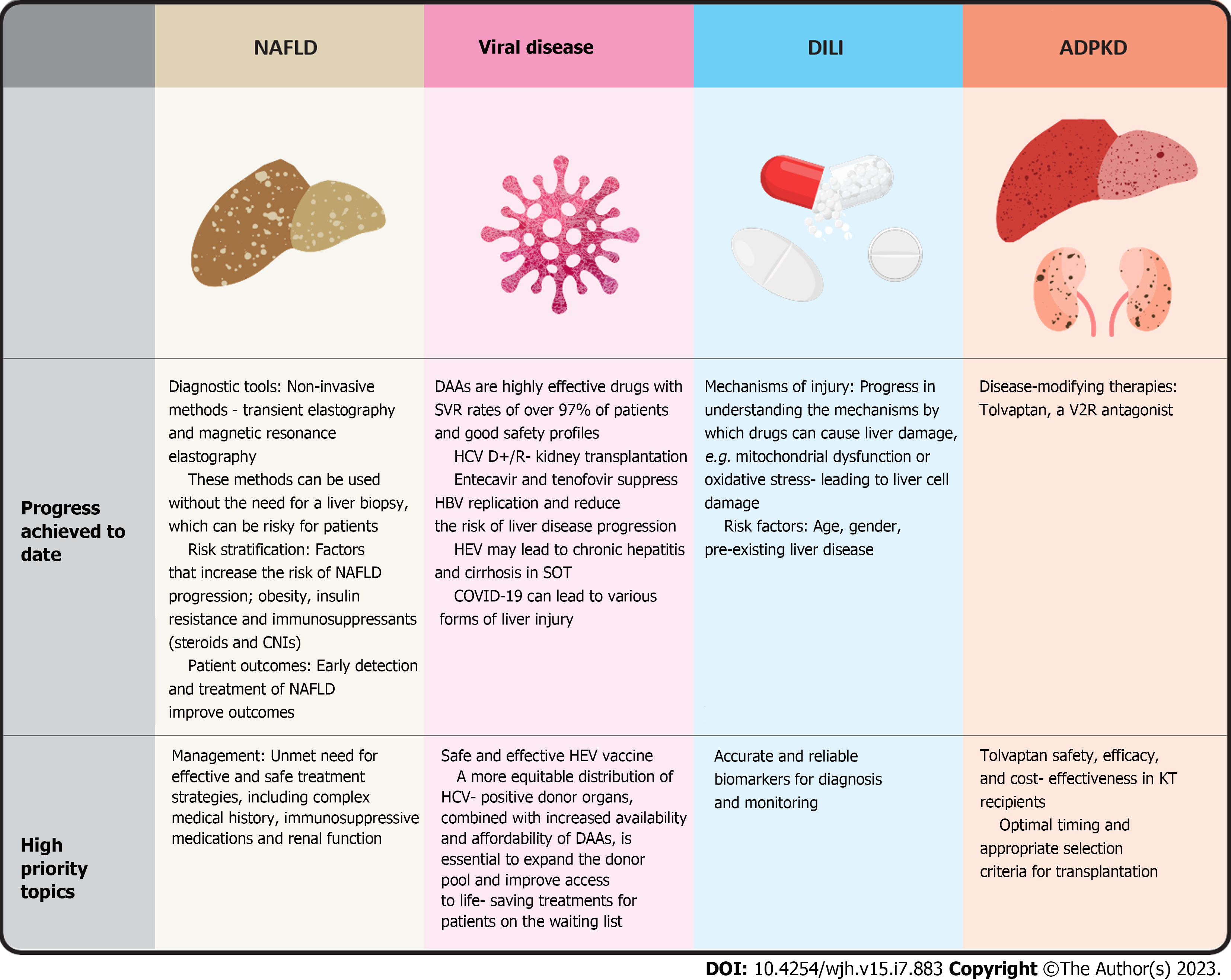
Liver disease after KT occurs in a variety of forms ranging from asymptomatic biochemical abnormalities to severe hepatitis, cirrhosis or malignancy[6]. Causes are heterogeneous and mainly related to hepatotropic viruses, drug toxicity and metabolic disorders[6]. Genetic conditions, such as polycystic kidney disease, which lead to the need for KT are also associated with polycystic liver disease[6]. Significant advances in understanding and treating liver disease in kidney transplant recipients have been made in the last decade. The development of direct-acting antiviral drugs (DAAs) for the treatment of hepatitis C virus (HCV) infection has led to a marked improvement in the treatment of HCV-related liver disease. Hepatitis E has been identified as a causative agent of chronic hepatitis and cirrhosis following solid organ transplantation (SOT). In addition to these advances, the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its impact on SOT has highlighted the need for further research in this field. The impact of coronavirus disease 2019 (COVID-19) on liver function in kidney transplant recipients is not yet fully understood, and the long-term consequences of the virus on liver health in this population are still the subject of ongoing investigation. In addition, the prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing worldwide and it is also taking its toll in transplant population due to changes in eating habits, sedentary lifestyle and unavoidable effect of life-long immunosuppression[7]. This review aims to provide healthcare professionals with a comprehensive understanding of recent advances in the management of liver complications in KT recipients, allowing them to make informed decisions about the risks and impact of liver disease in this population (Figure 1).
NAFLD is a complex and multifactorial metabolic disorder influenced by a variety of genetic, environmental and lifestyle factors. The pathogenesis of NAFLD is characterised by a cascade of events that result in the accumulation of fat in the liver (hepatic steatosis), which can trigger an inflammatory response that further contributes to liver damage and leads to more severe liver diseases such as cirrhosis and hepatocellular carcinoma (HCC). However, the mechanisms underlying this progression remain unclear and are currently the subject of intense research efforts. Recent studies have highlighted the importance of gut microbiota dysbiosis, oxidative stress and immune system dysfunction in the development and progression of NAFLD[8]. Dysbiosis in the gut microbiota, characterised by a decrease in beneficial bacteria and an increase in harmful bacteria, can lead to increased gut permeability, endotoxaemia and inflammation, contributing to the development of NAFLD. Oxidative stress, an imbalance between reactive oxygen species and antioxidant defences, can also promote the progression of NAFLD by damaging hepatocytes and increasing the release of pro-inflammatory cytokines. Immune dysfunction, characterised by an imbalance between pro-inflammatory and anti-inflammatory metabolic pathways, can lead to an accumulation of immune cells in the liver, thus promoting the progression of NAFLD. In addition, genetic factors have been identified as important contributors to the development and progression of NAFLD. Variants in genes involved in lipid metabolism, insulin resistance and inflammation have been linked to an increased risk of NAFLD[8]. However, the interactions between genetic and environmental factors are complex and not fully understood, and more research is needed to fully elucidate the role of genetics in NAFLD.
NAFLD is now considered the most frequent chronic liver disease worldwide, with an estimated 25%-30%of the general population affected, particularly due to the rising prevalence of insulin resistance, obesity and hypertension[7]. Because type 2 diabetes mellitus (T2DM), obesity and dyslipidaemia often coexist with NAFLD, it is considered a hepatic manifestation of the metabolic syndrome[9]. While some experts suggest replacing the term NAFLD with metabolic-associated liver disease (MAFLD) to emphasise the importance of metabolic dysfunction in development and progression of the disease, the term is not yet universally accepted, as it can occur in patients without metabolic disorders[10]. In KT recipients, the high disease burden of NAFLD carries an increased risk of graft dysfunction and patient mortality, making early detection and treatment essential to reducing its impact on transplant outcomes. The increasing prevalence of NAFLD worldwide has led to a growing need for effective diagnostic and therapeutic strategies. Non-invasive diagnostic tools such as imaging and serum biomarkers have been developed to assess the severity of NAFLD and monitor disease progression. Various pharmacological and lifestyle interventions have also been investigated for the treatment of NAFLD, including weight loss, exercise, insulin-sensitising agents and antioxidants[11]. However, the optimal treatment for NAFLD remains uncertain, and more research is needed to find effective treatments that can prevent or reverse disease progression.
Patients with NAFLD and/or CKD share the same risk factors, including visceral obesity, arterial hypertension, prediabetes or T2DM, systemic insulin resistance, dyslipidaemia and low-grade inflammatory states[12]. Recently, several studies have shown that NAFLD is significantly associated with an increased prevalence of CKD. For example, the prevalence of CKD in patients with NAFLD ranged from 20% to 55%, while the prevalence of CKD in patients without NAFLD was 5% to 30%[13]. In addition, several retrospective and prospective cohort studies have found that the association between NAFLD and an increased incidence of CKD persists after adjustment for age, sex, obesity, hypertension and T2DM[14,15].
CVD is the main cause of mortality and graft loss after KT[16]. A meta-analysis of 16 studies found that patients with NAFLD had a higher risk of fatal and non-fatal cardiovascular events than patients without NAFLD (OR 1.64, 1.26-2.13) and that patients with severe NAFLD had a higher risk of fatal and non-fatal cardiovascular events (OR 2.58, 1.78-3.75)[17]. In addition, traditional risk factors (T2DM, obesity, hypertension, dyslipidaemia) for CVD and NAFLD may be exacerbated by immunosuppressive drugs, including calcineurin inhibitor and steroids in renal recipients[18]. In 2017, Kemmer and Buggs[19] reported the presence of NAFLD on ultrasound in 22% of KT candidates. Grupper et al[20] conducted a study of 341 consecutive KT recipients and showed that 36.4% of kidney recipients had sonographic evidence of NAFLD before transplantation. In the same study, recipients with NAFLD had a higher prevalence of new-onset diabetes before transplantation, and NAFLD was independently associated with cardiovascular mortality after KT (HR 4.4), even after adjustment for known CVD risk factors. It is still unclear whether NAFLD is associated with renal graft dysfunction. Grupper et al[20] found no correlation between graft function and NAFLD. However, from the studies already mentioned, NAFLD is significantly associated with a higher incidence of CKD in non-transplanted patients. This means that NAFLD and reduced graft function may also be the case in KT recipients[20]. The results of the above studies emphasise the need to diagnose NAFLD in KT recipients and to undertake aggressive risk factor modification early. The treatment of NAFLD in KT recipients is particularly challenging due to the complex interplay of metabolic disorders, immunosuppression and other comorbidities. Immunosuppressants used to prevent allograft rejection may exacerbate the metabolic disorder and contribute to the development and progression of NAFLD. Therefore, individualised treatment strategies that take into account each patient's unique needs are critical to achieving optimal outcomes according to KT. In addition, physicians caring for patients before and after KT should refer patients to a hepatologist as soon as NAFLD is diagnosed.
The natural course of hepatitis B virus (HBV) infection is a dynamic process that reflects the interaction between HBV replication and the host immune response[21]. In immunocompetent adults, HBV infection is usually acute and self-limiting with more than 90% of patients achieving hepatitis B surface antigen (HBsAg) seroconversion within 6 mo, indicating natural resolution of the infection; however, infection in infancy or childhood often leads to chronicity[21]. Today, an estimated 240 million people are affected by chronic HBV infection[21]. Patients with detectable HBV DNA, especially those over 30 years of age and with a family history of HCC and cirrhosis, are typically considered for antiviral therapyand require lifelong treatment after KT[22,23]. Although modern antivirals such as entecavir or tenofovir result in viral suppression in almost all patients, treatment is long-term, possibly lifelong, and minimises the possibility of seroconversion[24]. Therefore, in chronically infected patients without signs of hepatitis, liver fibrosis or cirrhosis, without extrahepatic manifestations of HBV and with low viral replication rates (as determined by HBsAg and HBV DNA), treatment is not usually recommended by professional society guidelines[22,23]. In immunosuppressive states, e.g. due to chemotherapy or after organ transplantation, patients with cleared or inactive HBV may experience an abrupt increase in viral replication, which is called HBV reactivation.
Symptoms of HBV reactivation can range from asymptomatic to severe acute hepatitis, progression to chronic inflammation and fibrosis or HCC, and even acute liver failure. Where HBV is endemic, reported HBV reactivation rates with immunosuppression are as high as 41.5% (resolved HBV) and 70% (chronic HBV infection)[25,26]. As HBV prevalence in KT recipients ranges from 2.2 to 20.9% it is crucial that healthcare professionals understand their patient’s status to determine appropriate monitoring or antiviral prophylaxis measures to prevent reactivation[27].
The patient with resolved HBV infection (HBsAg-negative, anti-HBc IgG positive recipient): Up to 30% of KT candidates may have resolved HBV infection [defined as HBsAg-negative and hepatitis B core antibody (anti-HBc) -positive], and 1.4%-9.6% may experience HBV reactivation after KT[27]. The American Association for the Study of Liver Diseases guidelines recommend monitoring every three months for the first year (alanine transaminase and HBV DNA) and antiviral prophylaxis only as an alternative[24]. However, since the risk factors for HBV reactivation are not precisely known, it is not clear which patients should be treated and which should be monitored. In a study by Mei et al[28] 52 patients with resolved HBV infection were retrospectively analysed. Five (9.6%) cases of HBV reactivation occurred, and anti-HBcAg titre (P = 0.042) and age (P = 0.037) were identified as risk factors for HBV reactivation. Interestingly, ATG treatment, steroid pulse doses and low-dose rituximab were not associated with HBV reactivation[28]. Shaikh et al[27] found that only 3.4% of KT recipients who did not receive antiviral prophylaxis experienced HBV reactivation, and delayed graft function was identified as a significant risk factor. Still, there were no significant adverse graft-related outcomes among those who experienced reactivation[27].
The patient with chronic HBV infection (HBsAg-positive recipient): Chronic HBV is defined by persistent HBsAg in serum for at least 6 mo. Although current guidelines recommend prophylactic treatment for HBsAg-positive patients if they require immunosuppressive therapy for transplantation, most evidence is based on patients undergoing chemotherapy[22,23]. A systematic review and meta-analysis by Thongprayoon et al[29] included a total of 87623 KT patients and found significant association between HBsAg-positive status and poor outcomes, including mortality (pooled OR = 2.48; 95%CI: 1.61-3.83) and allograft failure (pooled OR = 1.46; 95%CI: 1.08-1.96). There was also a significant negative correlation between study year and risk of allograft failure, suggesting a possible improvement in patient and graft survival in HBsAg-positive recipients over time[29]. A recent study by Mo et al[30] confirmed high rates of viral reactivation in a real-world study of HBsAg-positive KT recipients, with inappropriate antiviral agents (all other than lifelong prophylaxis) (HR = 7.34, 95%CI 1.51-35.69, P = 0.01) and high levels of HBV DNA (≥ 1000 IU/mL) pre-transplant being the main risk factors (HR = 4.39, 95%CI 1.08-17.81, P = 0.04). However, the study found no difference in patients' or graft outcomes between patients who experienced reactivation and those who did not[30].
The kidney graft from donors with resolved HBV infection (HBsAg-negative, anti-HBc IgG positive donor): Donors with resolved HBV infection may alleviate organ shortage in KT. A study by Yamada et al[31] found a low risk of reactivation - 45 cases of KT from donors with resolved HBV infection to HBV-naive recipients were analysed, and one patient (2.2%) became seropositive for anti-HBc, and one patient (2.2%) had detectable HBV-DNA levels after transplantation. In the same study, the presence of covalently closed circular DNA in transplanted organs from donors with resolved HBV infection and the capability of HBV replication in kidney cell lines was demonstrated[31]. Accordingly, further research is needed to fully evaluate the safety and efficacy of this approach.
HCV infection is a global health problem affecting an estimated 71 million people worldwide[32]. The prevalence of HCV tends to be higher in patients with ESRD and in KT recipients than in the general population[33]. Although transplantation of HCV-infected patients offers a clear survival advantage over dialysis, HCV positivity has often been a barrier to KT in the past. Possible reasons for this include concerns about impaired survival of grafts and patients associated with certain HCV-related causes, such as increased rates of liver fibrosis and glomerulonephritis[34]. Highly effective, all-oral DAAs have revolutionised HCV treatment with cure rates of > 95%[35]. DAAs not only improve outcomes in HCV-positive KT patients, but also open new opportunities to increase scarce graft availability by safely transplanting HCV-positive organs into HCV-negative recipients[36]. The focus of interest today is the efficacy and safety of DAAs after KT and the challenges of using HCV-positive grafts in HCV-negative recipients.
DAA therapy for KT recipients: Sofosbuvir, a second-generation DAA approved in 2013, paved the way for efficient interferon/ribavirin-free treatment of HCV. Today, DAAs have proven their efficacy and safety both in clinical trials and in practice[37-39]. Chute et al[37] reported sustained viral response (SVR) rates of 97% in 418 of KT recipients, most of whom were treated with sofosbuvir-based therapies[37]. In the phase-3, open-label, single arm MAGELLAN-2 study, which evaluated a 12-wk course of the pangenotypic regimen of glecaprevir/pibrentasvir SVR was achieved in 98% of patients[38]. In addition to confirming the efficacy of the different DAA regimens used (23 patients, 100% SVR rate), Alkadi et al[40] found that there were no significant changes in renal function or calcineurin inhibitor levels during or after therapy[40]. In another study, DAAs were found to reduce HCV prevalence from 1.97% to 0.43% among recipients of KT when used over a five-year period[41]. Current DAA regimen guidelines for KT recipients are readily available through the American Association for the Study of Liver Diseases/Infectious Diseases Society of America (AASLD/IDSA) and the European Association for the Study of the Liver (EASL) guidelines[35,42].
ESRD and advanced liver disease: The prevalence and clinical-epidemiological profile of HCV infection in ESRD have changed over time. In particular, patients today tend to be older than in the past; more deceased donors are used for transplantation; there are fewer co-infections with HBV, but a higher percentage of cirrhotic patients are treated. Additionally, decompensation was found to be more frequent in recent years as well as patient survival rate being lower than before[32]. Since cirrhosis is an important predictor of poor survival after KT, it is advisable sess the stage of liver fibrosis in all KT candidates. In patients with established cirrhosis and portal hypertension in whom antiviral HCV treatment fails or is not an option, combined liver and KT must be considered[43].
Utilisation of HCV-positive donors: One of the most important advances in the last decade to increase transplantation rates has been the practise of KT from HCV-positive donors to HCV-negative patients (HCV D+/R-), followed by DAA therapy. After the pioneering studies THINKER and EXPANDER, which demonstrated good graft function and found little evidence of adverse outcomes after HCV D+/R- KT, this practice steadily increased in the United States[44,45]. United Network for Organ Sharing (UNOS) data showed and found increasing rates (0.3% to 6.9%) of HCV D+/R- from 1/1/2017 to 12/12/2020[46]. A growing number of real-world studies confirm the safety and efficacy of the HCV D+/R- approach and highlight its advantages, including shorter waiting times, access to younger donors with excellent allograft function, and similar survival compared to HCV D-/R- KT[46,47]. Substantial additional administrative work may be required outside of clinical trials for insurance approval of DAA therapy. A study by Edmonds et al[47] found that the median time from KT to the first dose of DAA was 45 d[47]. However, even delayed initiation of DAA treatment, with a median of 70 d after KT, had no negative impact on SVR rates or liver histology[48].
Understanding gaps and benefits/risks of HCV D+/R- KT may lead to greater acceptance. The majority of patients are willing to accept HCV-positive organs after being educated, citing shorter waiting time, DAA efficacy and faster return to higher functional status[49]. However, Nguyen et al[50] revealed disparities in HCV D+/R- KT access based on race/ethnicity, gender, and education level; minorities were 15%-60%, women over 20%, and those with elementary school degrees or less half as likely to receive a HCV nucleic acid amplification technique (NAT) positive kidney compared to those with bachelor's degrees. These findings underscore unequal distribution of breakthrough treatments, which can take years before recognition or reporting occurs[50]. In conclusion, use of HCV-positive kidney grafts for HCV-negative recipients followed by DAA therapy is a potential solution to organ shortage that can improve quality of life of ESRD patients.
The understanding of hepatitis E virus (HEV) has evolved over the past decade. Genotypes (gt) 1 and 2 are restricted to developing countries and cause large epidemics via the faecal-oral route, while gt 3 and 4 are endemic zoonoses in high-income countries. Although HEV usually causes an acute and mild form of hepatitis in imunocompetent hosts, it can lead to more severe and long-lasting infections in individuals who have undergone a SOT[51]. This has become an increasingly important issue because HEV is now considered one of the primary causes of acute viral hepatitis and cirrhosis in transplant recipients. Data on the seroprevalence of HEV in haemodialysis patients are inconsistent, ranging from 0 to 44%, possibly due to significant differences between the geographical regions studied[52].
In 2008, the first report of chronic hepatitis due to gt 3 and 4 was published in SOT[53]. Subsequent studies showed that organ recipients are at increased risk of HEV infection, with prevalence ranging from 0.44% to 24% depending on the organ transplanted and the geographic region[54]. Interestingly, HEV seropositivity was significantly higher in transplant recipients compared to patients on the waiting list (24% vs 16.4%, P = 0.042)[55]. Still, it is possible that HEV infection is underdiagnosed in SOT recipients, as serological assays with low sensitivity are often used[56]. Clinical course is inconspicuous in most cases with mild but often persistent abnormalities observed in liver function tests[53]. To properly diagnose chronic HEV infection, serum or plasma samples and, if possible, stool samples must be examined using NATs[51]. High clinical suspicion of chronic HEV is important because undetected infection, especially with gt 3, can lead to rapid progression of liver fibrosis and in some cases decompensation and death[57].
KT recipients are particularly susceptible to chronic HEV infections, which can lead to serious complications such as earlier graft rejection. KT recipients with positive ELISA tests or PCR results for HEV are more likely than controls to experience earlier rejection[58]. It is known that HEV infection can cause hepatic and extrahepatic symptoms. A case study of a KT recipient with HEV and membranous nephropathy (MN) illustrates the possible causal relationship between the two conditions. Treatment with ribavirin (RV) as monotherapy proved effective in treating HEV infection and resulted in complete remission of the nephrotic syndrome. This suggests that RV may also have beneficial effects on MN via non-specific immunomodulatory mechanisms[59].
Although chronic HEV was originally defined as viral replication lasting longer than 6 mo, in an observational study of SOT recipients, clearance did not occur 3-6 mo after infection, but only within the first 3 mo. These results suggest that SOT patients who are viraemic for longer than 3 mo should be considered for treatment[60]. A recent study that investigated the efficacy of dose reduction of mycophenolic acid in eight KT patients diagnosed with chronic HEV infection showed that only one patient achieved HEV clearance, while most patients required antiviral treatment with RV. The study provided the clinical evidence that reducing mycophenolic acid therapy alone is not sufficient to control viral replication in transplant patients. In addition, rituximab has been identified as a risk factor for chronic HEV that is complicated to treat[61].
Meta-analysis data show that RV is a safe and effective treatment for chronic HEV infection in SOT recipients. RV induces a sustained virological response in 76% of patients and represents a first-line therapy for this patient group[62]. However, it is important to note that the guidelines recommend monitoring HEV RNA for at least six months after completion of RV therapy, as reinfection or relapse can occur even if the virus has been eliminated by therapy. This is especially true for immunosuppressed transplant patients, including those with positive anti-HEV IgG.
Trials in China have shown the efficacy of recombinant HEV genotype 1 vaccine (Hecolin®, Xiamen Innovax Biotech). However, the vaccine is not available outside China and its efficacy against other genotypes is unknown. Further studies in developed countries are needed to assess its efficacy, durability of immune response and cost-effectiveness before it can be recommended[63]. For now, avoiding contaminated food/water and thorough cooking are the only preventive measures.
Since identified in December 2019, SARS-CoV-2 has infected more than 660 million people to date[64]. SARS-CoV-2 causes COVID -19, which typically presents as an upper respiratory tract infection but can lead to severe pneumonia with acute respiratory distress syndrome and multiorgan failure[65]. The virus infects target cells by binding the spike surface glycoprotein (S) to angiotensin-converting enzyme 2 (ACE2), which is mainly expressed in cells of the alveolar epithelium type II[66]. However, it was also found in the heart, ileum, kidney, bladder and liver, with cholangiocytes being the major ACE2-expressing cell population, with a 20-fold higher expression rate than in hepatocytes[67]. This could potentially make the liver susceptible to SARS-CoV-2 virus, but evidence for specific viral hepatotropism is limited.
Abnormalities in liver function tests have been observed in up to 78% of hospitalized COVID-19 patients, with some individuals developing acute liver injury (ALI) and reports of liver failure due to SARS-CoV-2 infection[68,69]. Given the significantly higher expression of ACE2 receptors on cholangiocytes, one might generally expect a cholestatic laboratory pattern to predominate in patients with COVID-19, but this is not the case. Therefore, it has been hypothesised that liver injury is not primarily due to direct virus-induced damage to hepatocytes, but rather to indirect causes such as hepato
COVID-19 and Kidney transplant recipients: KT recipients are often older, have multiple comorbidities and require chronic immunosuppression, and as such represent a high risk group for more serious COVID-19 disease. Since the onset of the pandemic, a large amount of data on recipients of SOT and KT have been reported, mainly from case series, small cohorts and larger registries. However, large multicentre studies with appropriate control groups are lacking. So, it remains a challenge how to appropriately treat KT recipients with COVID-19 and to understand what factors influence the outcomes. Clinical presentation, including abnormalities in liver function tests, has been shown to be similar in SARS-CoV-2 infected KT patients and in the general population[75].
In a multicentre cohort study the incidence of hepatitis was 20.2% among KT recipients, who received some form of antiviral therapy for SARS-SoV-2[76]. As in immunocompetent patients, the liver disease in KT recipients is usually mild and transient, characterised mainly by elevated levels of AST and ALT[76]. AST abnormality is more pronounced in patients with a severe form of the disease, and higher AST values have been shown to be a marker of poor outcome[77,78]. Early results from national and international registries and multicentre studies found a variable mortality rate of 18% to 32% among KT recipients hospitalised for COVID-19[77,79].
One distinctive feature that must be considered when assessing the possible aetiology of liver damage in patients with COVID-19 is the possibility of drug-induced liver injury (DILI) associated with certain treatments. Data on adverse events are sparse and often cannot be attributed with certainty exclusively to drugs for COVID-19, as a variety of other medications such as antibiotics and antipyretics are widely used in the treatment of such patients. To date various medications have been tried in treatment of SARS-CoV-2 infection – glucocorticoids, antimalarials, immunomodulatory agents (JAK2 inhibitors, IL-6 and IL-1 inhibitors), monoclonal antibodies, antiviral drugs and all of them may theoretically exhibit hepatotoxicity[80,81]. Table 1 Lists the hepatic contraindications and risk of drug induced liver injury (DILI) for drugs approved for the treatment of SARS-CoV-2 infection in the European Union and the United States of America[80-82].
| Drug | Liver Contraindication | Risk of DILI |
| Systemic corticosteroids | Caution in liver failure | + |
| Remdesivir | ALT > 5 × ULN | ++ |
| Tocilizumab | ALT > 5 × ULN | ++ |
| Sarilumab | ALT > 1.5 × ULN | ++ |
| Anakinra | Efficacy and safety in patients with AST/ALT ≥ 1.5 × ULN not been evaluated. | + |
| Not recommended with severe hepatic impairment (Child-Pugh C) | ||
| Baricitinib | Not recommended with severe hepatic impairment (Child-Pugh C) | + |
| Tofacitinib | Not recommended with severe hepatic impairment (Child-Pugh C) | + |
| Nirmatrelvir/ritonavir | Not recommended with severe hepatic impairment (Child-Pugh C) | + |
| Lopinavir/ritonavir | Not recommended with severe hepatic impairment (Child-Pugh C) | ++ |
| Molnupiravir | Limited experience of the use with any degree of hepatic impairment. | + |
| Casirivimab/imdevimab | Not recommended with severe hepatic impairment (Child-Pugh C) | + |
| Sotrovimab | No data in patients with ALT 5 to < 10 × ULN). | + |
| Tixagevimab/cilgavimab | Not been evaluated in patients with hepatic impairment | + |
| Regdanvimab | Not been evaluated in patients with hepatic impairment | + |
DILI, defined as liver injury caused by various drugs, herbs or other xenobiotics, results in abnormalities in liver tests or liver dysfunction, where other causes can be reasonably excluded[83]. The incidence of DILI is increasing worldwide due to the development of new drugs in the West and herbal preparations in Asia[83]. The classes of drugs most commonly associated with DILI include antibiotics, anticonvulsants and psychotropic drugs[84]. Polypharmacy, a common occurrence in KT recipients, increases the risk of drug interactions that may contribute to a higher incidence of DILI[84].
The severity of DILI can be associated with the dose of the consumed substance, e.g., acetaminophen overdose/poisoning. However, most medications cause DILI in a non-dose dependent way, i.e., idiosyncratically. Recent research proposed a 3-step model of DILI in which direct cell stress, direct mitochondrial inhibition and/or specific immune reactions can lead to mitochondrial permeability transition. Various environmental (age-related changes of pharmacokinetics, induction/inhibition of CYP450 enzymes, impaired antioxidant defence, immunological sensitisation, pre-existing liver disease, concomitant infections (e.g. HBV, HCV), mitochondrial dysfunction) and genetic factors may influence each sequence of the proposed model. In KT recipients, there are few potential environmental risk factors, e.g. drug interactions (antibiotics, antifungal and antiviral prophylaxis, immunosuppressive therapy), immunological sensitisation, malnutrition, infections and mitochondrial dysfunction due to diabetes, as well as all possible genetic risk factors that make them more susceptible to developing DILI than the general population. Previous research reported the occurrence of suspected DILI in 23% of KT recipients with possible causality in 57% of cases, and the associated drugs were antimicrobials, immunosuppressants and diuretics[84].
Assessing causality of DILI remains a challenge due to the lack of reliable biomarkers for hepatotoxicity. Several scoring systems are available, but there are discrepancies between scales. The best agreement seems to be between the Roussel-Uclaf Causality Assessment Method (RUCAM) scale and the Digestive Disease Week-Japan (DDW-J) scale, which includes an in vitro drug lymphocyte stimulation test (DLST) and is based on the RUCAM scale. Newer methods such as bile acid metabolomics could be used to assess the severity of DILI[85].
Despite some advances in the diagnosis and prognosis of DILI, the mainstay of treatment is to assess the need for immediate discontinuation of the suspect drug. N-acetylcysteine is currently a worldwide accepted treatment for acetaminophen hepatotoxicity. Bicyclol is a substance that may attenuate ALI by several mechanisms, including induction of autophagy, inhibition of oxidative stress and inactivation of the NLRP3 inflammasome. The results of the research in China show a satisfactory safety profile of the substance with a potentially favourable clinical outcome[86,87].
Autosomal dominant polycystic kidney disease (ADPKD) is a prevalent genetic disorder causing uninhibited cyst growth in the kidneys, liver, and pancreas. It is one of the top causes of ESRD with an estimated occurrence rate ranging from 1:400 to 1000 live births due to mutations in PKD1 and PKD2 genes[88]. In addition, patients may present with other abnormalities, such as cerebral aneurysms, cardiac valve malformations, colonic diverticulosis, hernias of the abdominal wall and inguinal region, and cysts of the seminal vesicles[88]. Given the frequency of the disease, patients with ADPKD make up a large percentage of KT recipients. In rare cases, patients with ADPKD are referred for simultaneous liver and KT due to an enlarged liver leading to early satiety, abdominal pain and cachexia. In the majority of cases, however, synthetic liver function remains intact, so that most patients receive only a KT. After transplantation, liver cysts may continue to grow but rarely cause problems. Nevertheless, cyst complications must be included in the differential diagnosis of both fever of unknown origin in KT recipients with ADPKD (due to cyst inflammation) and abdominal pain (e.g. rupture).
For patients with symptoms of liver enlargement that persist after KT, there are several treatment options: surgical resection, fenestration or sclerotherapy of cysts, or drug treatment[89,90]. Liver transplantation and combined liver/KT have been performed in patients with severe, symptomatic disease. Sirolimus appears to reduce the volume of the polycystic liver, possibly through an antiproliferative effect. Liver volume was significantly lower in seven KT patients receiving sirolimus-mycophenolate-prednisone than in nine recipients receiving tacrolimus-mycophenolate-prednisone[91].
Recently, tolvaptan, an antidiuretic antagonist, was approved as the first therapy for ADPKD after the TEMPO and TEMPO-R trials demonstrated significantly less eGFR loss compared to the placebo group[92]. It is expected that more and more patients with ADPKD will be treated with tolvaptan in the future. Tolvaptan is a V2R antagonist that blocks vasopressin signalling, which plays an important role in cyst growth in ADPKD due to the resulting intracellular increase in cyclic adenosine monophosphate[92]. The main adverse effect of tolvaptan is liver toxicity, requiring frequent monitoring, and polyuria is a logical consequence of V2R blockade. However, adherence to tolvaptan appears to be well-feasible in the majority of patients[93]. Additional ongoing studies will determine whether the benefits are long-term, whether they can be observed in patients with advanced kidney disease and whether they can be translated into quality of life and cost/effectiveness parameters[94]. Tolvaptan may also be considered for patients after KT according to preliminary reports, taking into account the possible adverse effects, including hepatotoxicity, hypernatremia and alteration of serum tolvaptan concentration when used concomitantly with cyclosporine[95].
Other, rare cystic diseases, such as Autosomal Recessive Polycystic Kidney Disease, Autosomal Dominant Tubulointerstitial Kidney Disease and Carolli disease should also be considered in KT recipients[96].
Iron overload is a common problem in ESRD patients and transplant recipients, often resulting from the use of iron supplements to treat anaemia, the use of iron-based phosphate binders, and disruption of iron utilization due to chronic inflammation associated with CKD. Anaemia, defined as a hemoglobin concentration < 130 g/L in men and < 120 g/L in women, is a frequent complication of CKD[97]. The prevalence is 50%-70% in ESRD patients before transplantation and decreases to 51% at 6 mo and 37% at 2 years after KT[98]. Erythropoietin deficiency and impaired iron homeostasis, including absolute and functional iron deficiency, are major contributors to CKD-associated anaemia. Standard therapies for the treatment of anemia include exogenous substitution of erythropoietin, iron supplementation, and blood transfusion. The availability of erythropoiesis-stimulating agents has reduced the need for blood products and the risk of blood-borne infections, iron overload, and allosensitization. Recently, inhibitors of the hypoxia-inducible factor prolyl hydroxylase (HIF-PHI) have emerged as a new therapeutic option for anaemia. HIF-PHIs mediate both the erythropoietin and iron metabolism pathways. HIF-PHIs stabilize hypoxia-inducible factor (HIF), which stimulates endogenous erythropoietin production and affect the transcription of several iron metabolism and transport genes, leading to a decrease in ferritin levels and hepcidin levels[99-102]. HIF-PHIs can reduce the need for iron supplements by mobilizing stored iron[101]. Several randomized control trials in CKD patients regardless of dialysis status of roxadustat, vadadustat, and daprodustat showed no inferiority compared to ESA therapy with a similar safety profile[99,102-105]. These promising results could have significant clinical relevance as iron supplementation could be reduced.
Adequate iron stores are necessary for normal functioning of ESA and maintenance of stable hemoglobin levels. However, iron overload can lead to accumulation of iron in various organs. Iron-related side effects, mostly mediated by the formation of reactive oxygen species, can affect the liver (secondary hemochromatosis, cirrhosis), the heart (heart failure and arrhythmias), and endocrine organs (hypogonadism, diabetes mellitus). Excess iron can aggravate inflammation and shift the immunoregulatory balance, negatively affecting the immune system.
Serum ferritin and serum transferrin saturation (TSAT) are commonly used tests for iron status in CKD patients, including transplant recipients. Hepcidin measurement is not clinically more useful or better than TSAT and ferritin. Serum ferritin levels are affected by inflammation as it acts as an acute phase reactant. Serum ferritin levels ≤ 30 µg/L indicate iron deficiency. However, the ferritin value at which the iron stores in the bone marrow are filled is controversial. Currently, the 2012 KDIGO guidelines recommend iron supplementation therapy based on the combination of TSAT (≤ 30%) and ferritin (≤ 500 µg/L)[106].
Liver disease is a frequent occurrence following KT, estimated to affect 20%-50% of cases, and has a substantial impact on the survival and quality of life of these patients. Symptoms vary from asymptomatic, transient elevations of liver enzymes to the development of fibrosis and chronic liver failure. In severe cases, liver failure can have an acute onset or, in the chronic form, progress to cirrhosis with clinical indications of decompensation, or even the development of malignant disease such as HCC. Vigilant monitoring of KT recipients is crucial for the early detection of liver disease, allowing for timely intervention and improved outcomes. The incidence of viral diseases such as HBV and HCV has declined due to the effectiveness of antiviral medications and HBV vaccination programs. Globally, the prevalence of metabolic diseases such as NAFLD is rising, especially in transplant patients who are vulnerable due to lifelong immunosuppressive therapy. HEV and, more recently, SARS-CoV-2 have also been implicated as potential causes of liver disease. Additionally, healthcare professionals should be aware of the possibility of DILI, as many immunosuppressive and adjunct drugs can cause liver damage. Patients should be monitored closely for signs of drug toxicity, and medications should be adjusted accordingly. Screening KT recipients for liver disease is a priority for healthcare professionals. Early diagnosis and referral to a hepatologist are critical for managing underlying liver problems, which can adversely impact quality of life and general health.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Liver Transplantation Society; European Society of Intensive Care Medicine; European Society for Organ Transplantation; United European Gastroenterology; European Association for the Study of the Liver; Croatian Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ouyang S, China; Sun X, China; Wang XL, China S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Marbun MBH, Susalit E, Susilowati U, Andina T. Long-term outcomes and prognostic factors in kidney transplant recipients in Jakarta, Indonesia: a cohort study. BMJ Open. 2022;12:e059631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Wang JH, Hart A. Global Perspective on Kidney Transplantation: United States. Kidney360. 2021;2:1836-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Lv JC, Zhang LX. Prevalence and Disease Burden of Chronic Kidney Disease. Adv Exp Med Biol. 2019;1165:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 566] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 4. | Poggio ED, Augustine JJ, Arrigain S, Brennan DC, Schold JD. Long-term kidney transplant graft survival-Making progress when most needed. Am J Transplant. 2021;21:2824-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 5. | Vieira GA, Amaral ACC, Carvalho Filho RJ, Souza ALDS, Medina-Pestana JO, Ferraz MLG. Hepatic alterations in kidney transplant recipients from the largest kidney transplant center in brazil. Arq Gastroenterol. 2022;59:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Gunderson A, Said A. Liver disease in kidney transplant recipients. Transplant Rev (Orlando). 2015;29:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7536] [Article Influence: 837.3] [Reference Citation Analysis (0)] |
| 8. | Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 9. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 804] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 10. | Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, Zelber-Sagi S, Hallsworth K, Busetto L, Frühbeck G, Dicker D, Woodward E, Korenjak M, Willemse J, Koek GH, Vinker S, Ungan M, Mendive JM, Lionis C. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep. 2021;3:100322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 11. | Ahmed IA, Mikail MA, Mustafa MR, Ibrahim M, Othman R. Lifestyle interventions for non-alcoholic fatty liver disease. Saudi J Biol Sci. 2019;26:1519-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72:785-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 13. | Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64:638-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 14. | Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J Intern Med. 2019;286:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Wilechansky RM, Pedley A, Massaro JM, Hoffmann U, Benjamin EJ, Long MT. Relations of liver fat with prevalent and incident chronic kidney disease in the Framingham Heart Study: A secondary analysis. Liver Int. 2019;39:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 590] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 17. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1005] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 18. | Kasiske BL. Risk factors for accelerated atherosclerosis in renal transplant recipients. Am J Med. 1988;84:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 274] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Kemmer N, Buggs J. NAFLD in Kidney Transplant Candidates. In: 2017 American Transplant Congress. 2017: May 1; Tampa, FL, United States. Am J Transplant. 2017;17 (suppl 3). |
| 20. | Grupper A, Rabinowich A, Ben Shabat I, Tzadok R, Schwartz D, Schwartz IF, Goykhman Y, Kliuk Ben-Bassat O, Baruch R, Shashar M, Cohen-Hagai K, Katchman H. Nonalcoholic Fatty Liver Disease before Kidney Transplantation Correlates with New Onset Diabetes and Poor Metabolic Outcomes. Am J Nephrol. 2022;53:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 21. | Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics. Cold Spring Harb Perspect Med. 2014;4:a024935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 23. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2844] [Article Influence: 406.3] [Reference Citation Analysis (0)] |
| 24. | Suk-Fong Lok A. Hepatitis B Treatment: What We Know Now and What Remains to Be Researched. Hepatol Commun. 2019;3:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 26. | Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, Gill H, Lam YF, Lie AK, Lai CL, Kwong YL, Yuen MF. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3736-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 27. | Shaikh SA, Kahn J, Aksentijevic A, Kawewat-Ho P, Bixby A, Rendulic T, Park JM. A multicenter evaluation of hepatitis B reactivation with and without antiviral prophylaxis after kidney transplantation. Transpl Infect Dis. 2022;24:e13751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Mei T, Noguchi H, Hisadome Y, Kaku K, Nishiki T, Okabe Y, Nakamura M. Hepatitis B virus reactivation in kidney transplant patients with resolved hepatitis B virus infection: Risk factors and the safety and efficacy of preemptive therapy. Transpl Infect Dis. 2020;22:e13234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Thongprayoon C, Kaewput W, Sharma K, Wijarnpreecha K, Leeaphorn N, Ungprasert P, Sakhuja A, Cabeza Rivera FH, Cheungpasitporn W. Outcomes of kidney transplantation in patients with hepatitis B virus infection: A systematic review and meta-analysis. World J Hepatol. 2018;10:337-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Mo H, Min S, Han A, Jung IM, Ha J. Outcome after kidney transplantation in hepatitis B surface antigen-positive patients. Sci Rep. 2021;11:11744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Yamada R, Morikawa K, Hotta K, Iwami D, Tanabe T, Murai S, Shinohara N, Yoshida S, Hosoda S, Kubo A, Tokuchi Y, Kitagataya T, Kimura M, Yamamoto K, Nakai M, Sho T, Suda G, Natsuizaka M, Ogawa K, Sakamoto N. Incidence of post-transplant hepatitis B virus reactivation with the use of kidneys from donors with resolved hepatitis B virus infection. J Viral Hepat. 2022;29:976-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Popping S, El-Sayed M, Feld J, Hatzakis A, Hellard M, Lesi O, Ninburg M, Ward J, Boucher C. Report from the International Viral Hepatitis Elimination Meeting (IVHEM), 17-18 November 2017, Amsterdam, the Netherlands: gaps and challenges in the WHO 2030 hepatitis C elimination framework. J Virus Erad. 2018;4:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Emori CT, Uehara SNO, Carvalho-Filho RJ, Amaral AC, de Souza E Silva IS, Lanzoni VP, Moreira SR, Silva-Souza AL, Gama RA, Nunes EJS, Leopércio APS, Appel F, Silva AEB, Medina-Pestana JO, Ferraz MLG. Changing pattern of chronic hepatitis C in renal transplant patients over 20 years. Eur J Gastroenterol Hepatol. 2019;31:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Scott DR, Wong JK, Spicer TS, Dent H, Mensah FK, McDonald S, Levy MT. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 36. | Shetty A, Ariyamuthu VK, Gungor AB, Tanriover B. Utilization of hepatitis C virus-positive donors in kidney transplantation. Curr Opin Organ Transplant. 2023;28:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Chute DF, Chung RT, Sise ME. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney Int. 2018;93:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Reau N, Kwo PY, Rhee S, Brown RS Jr, Agarwal K, Angus P, Gane E, Kao JH, Mantry PS, Mutimer D, Reddy KR, Tran TT, Hu YB, Gulati A, Krishnan P, Dumas EO, Porcalla A, Shulman NS, Liu W, Samanta S, Trinh R, Forns X. Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection. Hepatology. 2018;68:1298-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 39. | Fabrizi F, Alonso C, Palazzo A, Anders M, Reggiardo MV, Cheinquer H, Zuain MGV, Figueroa S, Mendizabal M, Silva M, Ridruejo E; Latin American Liver Research, Educational and Awareness Network (LALREAN). 'Real-life' experience with direct-acting antiviral agents for HCV after kidney transplant. Ann Hepatol. 2021;25:100337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Alkadi MM, Abuhelaiqa EA, Elshirbeny MF, Hamdi AF, Fituri OM, Asim M, Alkaabi SR, Derbala MF, Jarman ME, Ashour AM, Nauman A, Al Maslamani YK, Butt AA, Al-Malki HA. Eradication of hepatitis C virus infection in kidney transplant recipients using direct-acting antiviral therapy: Qatar experience. Immun Inflamm Dis. 2021;9:246-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Devresse A, Delire B, Lazarus JV, Kabamba B, De Meyer M, Mourad M, Buemi A, Darius T, Cambier JF, Goffin E, Jadoul M, Kanaan N. Eliminating Hepatitis C Virus From a Prevalent Kidney Transplant Recipient Population: A Single-Center Study in Belgium in the Direct-Acting Antivirals Era. Transplant Proc. 2020;52:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 43. | Van Wagner LB, Baker T, Ahya SN, Norvell JP, Wang E, Levitsky J. Outcomes of patients with hepatitis C undergoing simultaneous liver-kidney transplantation. J Hepatol. 2009;51:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, Bloom RD, Nazarian SM, Sawinski D, Porrett P, Naji A, Hasz R, Suplee L, Trofe-Clark J, Sicilia A, McCauley M, Farooqi M, Gentile C, Smith J, Reese PP. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;376:2394-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 45. | Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N, Wesson R, Reyad A, Naqvi FF, Ostrander D, Sugarman J, Segev DL, Sulkowski M, Desai NM. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med. 2018;168:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 46. | Concepcion BP, Binari LA, Schaefer H, Rega S, Feurer I, Shawar S, Naik R, Hickman L, Walker J, Kapp M, Birdwell KA, Langone A, Helderman JH, Ann Sarrell B, Kochar G, Dubray B, Smith K, O'Dell H, DeMers A, Shelton P, Perri R, Shaffer D, Forbes RC. Kidney Transplantation From Hepatitis C Viremic Deceased Donors to Aviremic Recipients in a Real-world Setting. Transplant Direct. 2021;7:e761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Edmonds C, Carver A, DeClercq J, Choi L, Peter M, Schlendorf K, Perri R, Forbes RC, Concepcion BP. Access to hepatitis C direct-acting antiviral therapy in hepatitis C-positive donor to hepatitis C-negative recipient solid-organ transplantation in a real-world setting. Am J Surg. 2022;223:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Kothadia JP, Bhalla A, Molnar MZ, Mohan R, Balaraman V, Talwar M, Helmick R, Eymard C, Clark I, Jain R, Faust TW, Vanatta JM, Eason JD, Nair SP. Liver Outcome in Renal Transplant Recipients Who Acquired Hepatitis C Infection From an Infected Graft: Study Based on Liver Biopsy Findings. Transplant Direct. 2022;8:e1342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 49. | Cohen S, Cowan V, Rohan V, Pavlakis M, Curry MP, Adler JT, Safa K, Fleishman A, Shenkel J, Rodrigue JR. Willingness of Kidney and Liver Transplant Candidates to Receive HCV-Infected Organs. J Surg Res. 2022;278:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Nguyen T, Sise ME, Delgado C, Williams W, Reese P, Goldberg D. Race, Education, and Gender Disparities in Transplantation of Kidneys From Hepatitis C Viremic Donors. Transplantation. 2021;105:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 52. | Mrzljak A, Dinjar-Kujundzic P, Knotek M, Kudumija B, Ilic M, Gulin M, Zibar L, Hrstic I, Jurekovic Z, Kolaric B, Jemersic L, Prpic J, Tomljenovic M, Vilibic-Cavlek T. Seroepidemiology of hepatitis E in patients on haemodialysis in Croatia. Int Urol Nephrol. 2020;52:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 999] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 54. | Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 489] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 55. | Samala N, Wang RY, Auh S, Balla AK, Dakhoul L, Alter HJ, Farci P, Ghabril M, Lucey MR, Rangnekar AS, Reddy KR, Ghany MG. Hepatitis E prevalence and infection in solid-organ transplant recipients in the United States. J Viral Hepat. 2022;29:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 56. | Hansrivijit P, Trongtorsak A, Puthenpura MM, Boonpheng B, Thongprayoon C, Wijarnpreecha K, Choudhury A, Kaewput W, Mao SA, Mao MA, Jadlowiec CC, Cheungpasitporn W. Hepatitis E in solid organ transplant recipients: A systematic review and meta-analysis. World J Gastroenterol. 2021;27:1240-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 57. | Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssière L, Cointault O, Ribes D, Cardeau I, Nogier MB, Mansuy JM, Muscari F, Peron JM, Izopet J, Rostaing L. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation. 2010;89:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 58. | Wasuwanich P, Sirisreetreerux P, Ingviya T, Kraus ES, Brennan DC, Sue PK, Jackson AM, Oshima K, Philosophe B, Montgomery RA, Karnsakul W. Hepatitis E virus infection and rejection in kidney transplant recipients. Transpl Immunol. 2022;70:101517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 59. | Taton B, Moreau K, Lepreux S, Bachelet T, Trimoulet P, De Ledinghen V, Pommereau A, Ronco P, Kamar N, Merville P, Couzi L. Hepatitis E virus infection as a new probable cause of de novo membranous nephropathy after kidney transplantation. Transpl Infect Dis. 2013;15:E211-E215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 60. | Kamar N, Rostaing L, Legrand-Abravanel F, Izopet J. How should hepatitis E virus infection be defined in organ-transplant recipients? Am J Transplant. 2013;13:1935-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 61. | Affeldt P, Di Cristanziano V, Grundmann F, Wirtz M, Kaiser R, Benzing T, Stippel D, Kann M, Kurschat C. Monitoring of hepatitis E virus RNA during treatment for chronic hepatitis E virus infection after renal transplantation. Immun Inflamm Dis. 2021;9:513-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Gorris M, van der Lecq BM, van Erpecum KJ, de Bruijne J. Treatment for chronic hepatitis E virus infection: A systematic review and meta-analysis. J Viral Hepat. 2021;28:454-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, Guo M, Liu XH, Li JX, Yang CL, Tang Q, Jiang RJ, Pan HR, Li YM, Shih JW, Ng MH, Zhu FC, Xia NS. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 64. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4388] [Article Influence: 877.6] [Reference Citation Analysis (1)] |
| 65. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6518] [Article Influence: 1303.6] [Reference Citation Analysis (0)] |
| 66. | Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281-292.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4743] [Cited by in RCA: 6156] [Article Influence: 1231.2] [Reference Citation Analysis (0)] |
| 67. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: bioRxiv: 931766. [DOI] [Full Text] |
| 68. | Sobotka LA, Esteban J, Volk ML, Elmunzer BJ, Rockey DC; North American Alliance for the Study of Digestive Manifestation of COVID-19. Acute Liver Injury in Patients Hospitalized with COVID-19. Dig Dis Sci. 2022;67:4204-4214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Harapan H, Fajar JK, Supriono S, Soegiarto G, Wulandari L, Seratin F, Prayudi NG, Dewi DP, Monica Elsina MT, Atamou L, Wiranata S, Aprianto DP, Friska E, Sari Firdaus DF, Alaidin M, Wardhani FA, Husnah M, Hidayati NW, Hendriyanti Y, Wardani K, Evatta A, Manugan RA, Pradipto W, Rahmawati A, Tamara F, Mahendra AI, Nainu F, Santoso B, Irawan Primasatya CA, Tjionganata N, Budiman HA. The prevalence, predictors and outcomes of acute liver injury among patients with COVID-19: A systematic review and meta-analysis. Rev Med Virol. 2022;32:e2304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Li X, Wang W, Yan S, Zhao W, Xiong H, Bao C, Chen J, Yue Y, Su Y, Zhang C. Drug-induced liver injury in COVID-19 treatment: Incidence, mechanisms and clinical management. Front Pharmacol. 2022;13:1019487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Durazo FA, Kristbaum K, Miller J, Saeian K, Selim M, Hong JC. De Novo Autoimmune Hepatitis after COVID-19 Infection in an Unvaccinated Patient. Case Rep Hepatol. 2022;2022: e8409269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 72. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol 2021; 116: 1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 73. | Ge J, Pletcher MJ, Lai JC; N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487-1501.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 74. | Mondello C, Roccuzzo S, Malfa O, Sapienza D, Gualniera P, Ventura Spagnolo E, Di Nunno N, Salerno M, Pomara C, Asmundo A. Pathological Findings in COVID-19 as a Tool to Define SARS-CoV-2 Pathogenesis. A Systematic Review. Front Pharmacol. 2021;12:614586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Monfared A, Akhondzadeh L, Mousazadeh M, Jafari A, Khosravi M, Lebadi M, Aghajanzadeh P, Haghdar-Saheli Y, Movassaghi A, Ramezanzadeh E, Shobeirian F, Kazemnezhad E, Esmaeili S. COVID-19 in renal transplant recipients and general population: a comparative study of clinical, laboratory, and radiological features, severity, and outcome. Virol J. 2021;18:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Favà A, Cucchiari D, Montero N, Toapanta N, Centellas FJ, Vila-Santandreu A, Coloma A, Meneghini M, Manonelles A, Sellarés J, Torres I, Gelpi R, Lorenzo I, Ventura-Aguiar P, Cofan F, Torregrosa JV, Perelló M, Facundo C, Seron D, Oppenheimer F, Bestard O, Cruzado JM, Moreso F, Melilli E. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: A multicentric cohort study. Am J Transplant. 2020;20:3030-3041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 77. | Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, Redondo-Pachón MD, Murphy B, Florman S, Cyrino LG, Grafals M, Venkataraman S, Cheng XS, Wang AX, Zaza G, Ranghino A, Furian L, Manrique J, Maggiore U, Gandolfini I, Agrawal N, Patel H, Akalin E, Riella LV. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140-3148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 78. | Duarsa GWK, Sugianto R, Yusari IGAAA, Tirtayasa PMW, Situmorang GR, Rasyid N, Rodjani A, Daryanto B, Seputra KP, Satyagraha P. Predictor factor for worse outcomes in kidney transplant recipients infected with coronavirus disease 2019: A systematic review and meta-analysis. Transpl Immunol. 2023;76:101739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 79. | Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, Thaunat O, Legris T, Moal V, Westeel PF, Kamar N, Gatault P, Snanoudj R, Sicard A, Bertrand D, Colosio C, Couzi L, Chemouny JM, Masset C, Blancho G, Bamoulid J, Duveau A, Bouvier N, Chavarot N, Grimbert P, Moulin B, Le Meur Y, Hazzan M; French SOT COVID Registry. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 80. | Gabrielli M, Franza L, Esperide A, Gasparrini I, Gasbarrini A, Franceschi F, On Behalf Of Gemelli Against Covid. Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 81. | Karlafti E, Paramythiotis D, Pantazi K, Georgakopoulou VE, Kaiafa G, Papalexis P, Protopapas AA, Ztriva E, Fyntanidou V, Savopoulos C. Drug-Induced Liver Injury in Hospitalized Patients during SARS-CoV-2 Infection. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Delgado A, Stewart S, Urroz M, Rodríguez A, Borobia AM, Akatbach-Bousaid I, González-Muñoz M, Ramírez E. Characterisation of Drug-Induced Liver Injury in Patients with COVID-19 Detected by a Proactive Pharmacovigilance Program from Laboratory Signals. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | . Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol 2012; 18: 249–257 . [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 84. | Berradia A. Descriptive Study of Drug Induced Liver Injury in Kidney Transplant Patients. Adv Pharmacoepidemiol Drug Saf. 2020;9:234. [DOI] [Full Text] |
| 85. | Xie Z, Zhang L, Chen E, Lu J, Xiao L, Liu Q, Zhu D, Zhang F, Xu X, Li L. Targeted Metabolomics Analysis of Bile Acids in Patients with Idiosyncratic Drug-Induced Liver Injury. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Naiqiong W, Liansheng W, Zhanying H, Yuanlin G, Chenggang Z, Ying G, Qian D, Dongchen L, Yanjun Z, Jianjun L. A Multicenter and Randomized Controlled Trial of Bicyclol in the Treatment of Statin-Induced Liver Injury. Med Sci Monit. 2017;23:5760-5766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Wang Y, Lai R, Zong P, Xu Q, Shang J, Zhang X, Zhong W, Tang J, Han X, Chen C, Mao Y. Bicyclol for the treatment of drug-induced liver injury: a propensity score matching analysis using a nationwide inpatient database. J Int Med Res. 2021;49:3000605211005945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 375] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 89. | Itou C, Koizumi J, Hashimoto T, Myojin K, Kagawa T, Mine T, Imai Y. Foam sclerotherapy for a symptomatic hepatic cyst: a preliminary report. Cardiovasc Intervent Radiol. 2014;37:800-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Chebib FT, Harmon A, Irazabal Mira MV, Jung YS, Edwards ME, Hogan MC, Kamath PS, Torres VE, Nagorney DM. Outcomes and Durability of Hepatic Reduction after Combined Partial Hepatectomy and Cyst Fenestration for Massive Polycystic Liver Disease. J Am Coll Surg. 2016;223:118-126.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 92. | Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1146] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 93. | Perrone RD, Chapman AB, Oberdhan D, Czerwiec FS, Sergeyeva O, Ouyang J, Shoaf SE. The Nocturne Randomized Trial Comparing 2 Tolvaptan Formulations. Kidney Int Rep. 2020;5:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Sans-Atxer L, Joly D. Tolvaptan in the treatment of autosomal dominant polycystic kidney disease: patient selection and special considerations. Int J Nephrol Renovasc Dis. 2018;11:41-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Akabane M, Ishii Y, Nakamura Y, Yokoyama T, Miki K. Usefulness of Tolvaptan for the Postoperative Control After Kidney Transplantation in a Patient With Cardiac Hypofunction: A Case Report. Transplant Proc. 2021;53:1288-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O; Kidney Disease: Improving Global Outcomes. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int. 2015;88:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 97. | Locatelli F, Nissenson AR, Barrett BJ, Walker RG, Wheeler DC, Eckardt KU, Lameire NH, Eknoyan G. Clinical practice guidelines for anemia in chronic kidney disease: problems and solutions. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2008;74:1237-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 98. | Hanna RM, Streja E, Kalantar-Zadeh K. Burden of Anemia in Chronic Kidney Disease: Beyond Erythropoietin. Adv Ther. 2021;38:52-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 99. | Singh AK, Carroll K, McMurray JJV, Solomon S, Jha V, Johansen KL, Lopes RD, Macdougall IC, Obrador GT, Waikar SS, Wanner C, Wheeler DC, Więcek A, Blackorby A, Cizman B, Cobitz AR, Davies R, DiMino TL, Kler L, Meadowcroft AM, Taft L, Perkovic V; ASCEND-ND Study Group. Daprodustat for the Treatment of Anemia in Patients Not Undergoing Dialysis. N Engl J Med. 2021;385:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 100. | Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Cai GY, Hao L, Leong R, Liu C, Neff T, Szczech L, Yu KP. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 101. | Kaplan JM, Sharma N, Dikdan S. Hypoxia-Inducible Factor and Its Role in the Management of Anemia in Chronic Kidney Disease. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Fishbane S, El-Shahawy MA, Pecoits-Filho R, Van BP, Houser MT, Frison L, Little DJ, Guzman NJ, Pergola PE. Roxadustat for Treating Anemia in Patients with CKD Not on Dialysis: Results from a Randomized Phase 3 Study. J Am Soc Nephrol. 2021;32:737-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 103. | Provenzano R, Shutov E, Eremeeva L, Korneyeva S, Poole L, Saha G, Bradley C, Eyassu M, Besarab A, Leong R, Liu CS, Neff TB, Szczech L, Yu KP. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant. 2021;36:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 104. | Chertow GM, Pergola PE, Farag YMK, Agarwal R, Arnold S, Bako G, Block GA, Burke S, Castillo FP, Jardine AG, Khawaja Z, Koury MJ, Lewis EF, Lin T, Luo W, Maroni BJ, Matsushita K, McCullough PA, Parfrey PS, Roy-Chaudhury P, Sarnak MJ, Sharma A, Spinowitz B, Tseng C, Tumlin J, Vargo DL, Walters KA, Winkelmayer WC, Wittes J, Eckardt KU; PRO2TECT Study Group. Vadadustat in Patients with Anemia and Non-Dialysis-Dependent CKD. N Engl J Med. 2021;384:1589-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 105. | Eckardt KU, Agarwal R, Aswad A, Awad A, Block GA, Bacci MR, Farag YMK, Fishbane S, Hubert H, Jardine A, Khawaja Z, Koury MJ, Maroni BJ, Matsushita K, McCullough PA, Lewis EF, Luo W, Parfrey PS, Pergola P, Sarnak MJ, Spinowitz B, Tumlin J, Vargo DL, Walters KA, Winkelmayer WC, Wittes J, Zwiech R, Chertow GM. Safety and Efficacy of Vadadustat for Anemia in Patients Undergoing Dialysis. N Engl J Med. 2021;384:1601-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 106. | McMurray J, Parfrey P, Adamson JW, Aljama P, Berns JS, Bohlius J, Drüeke TB, Finkelstein FO, Fishbane S, Ganz T, MacDougall IC. Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;279-335. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |