Published online Jun 27, 2023. doi: 10.4254/wjh.v15.i6.775
Peer-review started: February 15, 2023
First decision: March 9, 2023
Revised: March 22, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: June 27, 2023
Processing time: 130 Days and 2.2 Hours
Hepatocellular (HCC) and intrahepatic cholangiocarcinoma (ICC), the most common primary tumors of the liver, are among the most important causes of cancer deaths worldwide. Because patients with primary liver tumors are frequently diagnosed at an advanced stage and have high mortality, many efforts have been made to identify new markers to determine their behavior and treatment, similar to those in other solid organ tumors. Recently, morphological assessment of tumor budding (TB) has been revealed as a promising prognostic finding to predict tumor behavior and survival across several different tumor types. Currently, the TB score in colorectal cancer has been revealed as an important parameter in pathology report protocols to determine the course of the disease. Regarding the liver, despite enormous data showing that many mechanisms involved in TB are associated with tumor behavior in both HCC and ICC, studies focusing on the role of TB in predicting the behavior and prognosis of these tumors have started to be investigated very recently. The purpose of this review is to present data about TB in primary tumors of the liver, pointing out the potential role of this parameter in determining the course of the disease, and emphasize the need to increase the number of further studies focusing on the evaluation of this parameter with an overview of the mechanisms involved in TB.
Core Tip: This review aims to present recent data on the potential of tumor budding (TB) in determining tumor behavior in hepatocellular carcinoma and cholangiocarcinoma. Although the evidence from the published literature indicates that TB may be a promising prognostic factor for primary liver tumors, more multidisciplinary studies are needed to draw a conclusion. Besides, different assessment techniques in previous investigations indicate that a standard method should be established to provide a solid basis for further studies that may clarify whether this parameter will be included in pathology report protocols as in colorectal carcinoma in the near future.
- Citation: Unal B, Celik MY, Gedik EO, Bassorgun CI, Elpek GO. Tumor budding as a potential prognostic marker in determining the behavior of primary liver cancers. World J Hepatol 2023; 15(6): 775-785
- URL: https://www.wjgnet.com/1948-5182/full/v15/i6/775.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i6.775
Primary liver cancer is the seventh most common cancer worldwide and the fourth leading cause of cancer death[1]. Two types of liver cancer constitute a significant majority of cases: Hepatocellular carcinoma (HCC), originating from hepatocytes and usually accompanied by another underlying disease (75%-85%), and intrahepatic cholangiocarcinoma (ICC), arising from the bile duct epithelium (12%-15%)[2]. Their incidence rates are increasing in many countries and are expected to continue to rise in the next decade[3,4]. Considering that many patients are diagnosed at an advanced stage, there is a lack of current systemic therapy, especially for HCC, and the mortality rates are high, similar to that of other solid organ tumors. Thus, many efforts have been made to identify new markers to determine the course of the disease and the choice of treatment.
Recently, TB has emerged as a promising prognostic parameter to predict tumor behavior and survival across several tumor types[5,6]. After the international TB consensus conference, the first guideline for reporting TB was published in 2017[7]. Subsequently, the TB score in colorectal carcinoma (CRC) has been included as an important parameter in pathology report protocols[8]. These guidelines have also been confirmed to be helpful in cancers of the lung, stomach cancers, and ductal adenocarcinoma of the pancreas[9]. However, regarding primary liver tumors, studies focusing on the relationship between TB and clinicopathological parameters and prognosis are relatively new. Nevertheless, numerous studies have shown that many mechanisms involved in TB are associated with tumor behavior in HCC and ICC[10,11].
Therefore, this review aims to provide an overview of the events involved in TB, which is also observed in primary liver tumors. Additionally, this review presents the latest data in these tumors to draw attention to the potential role of this parameter in determining behavior and prognosis and underlines the need to increase the number of further studies focusing on the evaluation of this parameter.
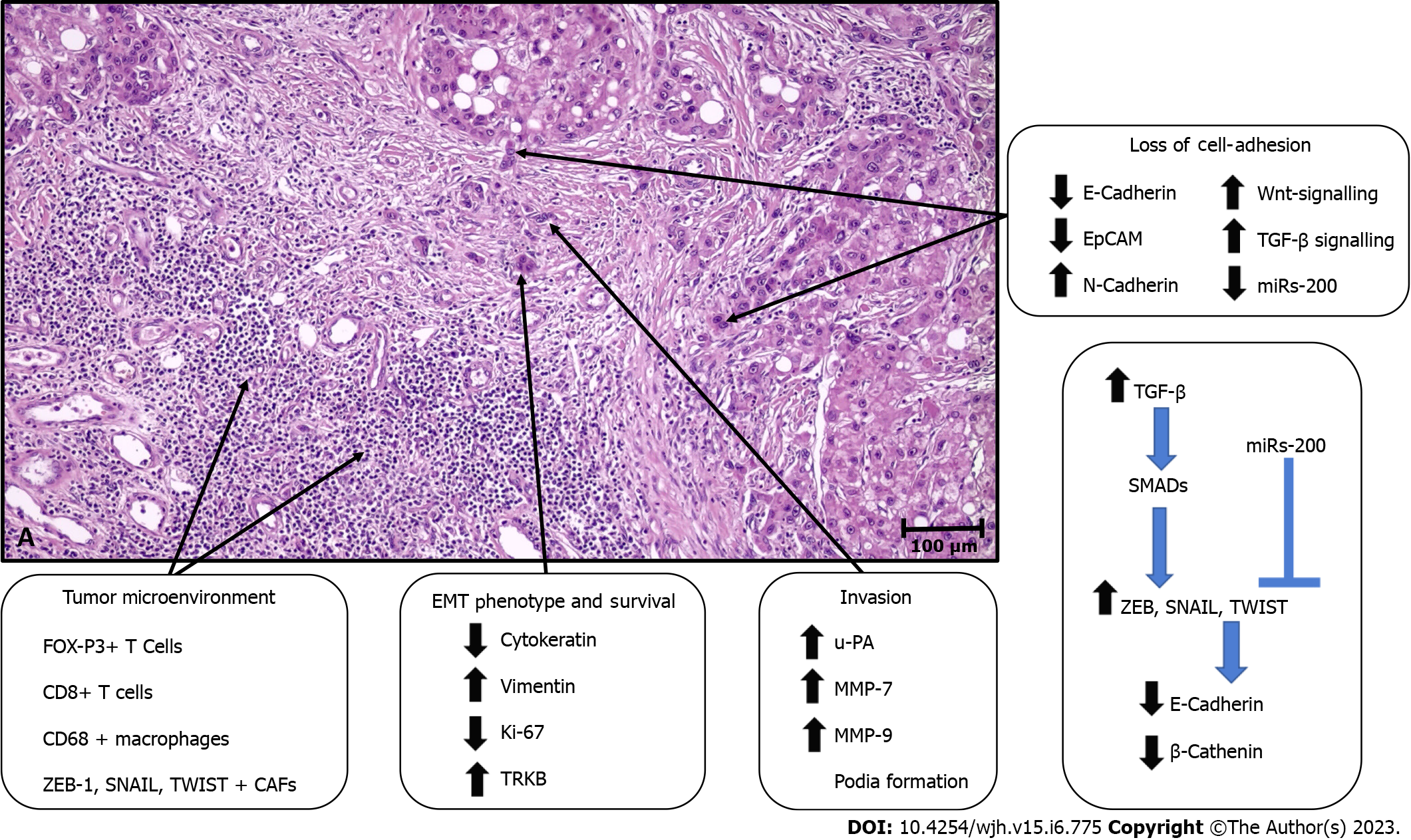
During the invasion-metastasis process in cancers, tumor cells must undergo various changes to invade the surrounding tissue, transition to the vascular system, and finally engage in a parenchymal invasion of metastatic organs[12]. The mechanisms involved in TB are presented in Figure 1.
The epithelial-mesenchymal transition (EMT) program, which contributes to developmental events throughout embryogenesis, has been hypothesized to play a fundamental role in TB formation, particularly in the steps of cell dissociation and cell migration[10-14]. Indeed, accumulating evidence indicates that budding tumor cells might display the properties of cells undergoing EMT to acquire more invasive and migratory capacity.
E-cadherin, an essential cell-cell adhesion protein, plays a pivotal role in cellular dissociation. Therefore, the reports indicating a decrease or loss of expression of E-cadherin in the invasive margin and bud areas in many solid organ tumors, including esophageal, colon, pancreas, endometrial, and oral cancers, are not surprising[15-18]. In addition, the increase in the expression of EMT-related transc
Moreover, data have also shown that TGFβ signaling activation in buds can induce transcriptional repression of E-cadherin by inducing E-cadherin repressors, such as ZEB, TWIST, and SNAIL, via deregulation of SMADs[18,23]. However, the observation that different subtypes of EMT transcription factors are increased in some tumors highlights that not all of them should be expected to be increased together in tumor buds[24]. It has been shown that both E-cadherin and molecules such as CD44 and EpCam are lost in TB areas[25-27]. Signature changes in some miRNAs have also been shown to contribute to TB. In particular, changes in the miR-200 family have been noted[28-31]. The levels of miR-200, which has a suppressive effect on the ZEB family that induces E-cadherin expression, were significantly decreased in tumor buds of colorectal and pancreatic ductal adenocarcinoma[32,33].
In TB, the effect of EMT is not limited to cell dislocation; moreover, it significantly affects cell migration through cytoskeletal reorganization, increased cell-associated proteolytic activity, and reprogramming of gene expression[34]. Recently, many studies have shown that these changes are found in budding tumor cells, and marked differences in the expression of genes involved in integrin-mediated cell adhesion, cell migration, cytoskeletal changes, and extracellular matrix degradation have been noted[35].
A monomeric form of laminin 5 gamma 2, which plays a role in the anchorage of epithelial cells to the underlying basement membrane, has been found to increase during tumor invasion and in tumor buds[35,36]. This finding was associated with aggressive tumor behavior, especially in pulmonary[37,38] and colorectal cancers[39-42]. Moreover, in the latter, the dendritic extensions of budding tumor cells are positive for laminin 5 gamma 2, which is associated with vascular invasion[43,44]. In addition, in line with the findings that β-catenin induces gene expression of this protein by binding to TCF and LEF family transcription factors, decreased membranous β-catenin levels, increased nuclear β-catenin levels, positivity for laminin 5 gamma 2, and decreased E-cadherin expression were associated with TB[40,45]. These data indicate that altered expression of β-catenin may participate in multiple events in TB. In addition, other cell migration markers, including motility class III β-tubulin and high-mobility Group A family proteins, are more abundant in invasive and TB sites[46,47]. Furthermore, the expression of proteins such as matrix metalloproteinase 7, matrix metalloproteinase 9 urokinase plasminogen activator and cathepsin B, which degrade the matrix of cells, was found to be significantly increased in tumor buds[41,48-50]. In this region, various metastasis suppressors (such as rapidly accelerated fibrosarcoma kinase inhibitor protein and maspin) are frequently disrupted and/or downregulated in tumor buds compared to the primary tumor mass[51-54].
The survival of malignant cells in the tumor bud largely depends on their adaptation to a hypoxic environment. Studies have shown that these cells overexpress TRKB, a marker of resistance to cell death, and hypoxia-inducible factor 1α[55,56]. In addition, cells in tumor buds have either shallow levels or the absence of proliferation markers (such as Ki-67)[57,58]. These findings support the view that cell proliferation and migration are mutually exclusive processes and that the transition from cell proliferation to invasion may be triggered by hypoxia. Moreover, the fact that budding tumor cells frequently overexpress stem cell markers, such as LGR5, ALDH1, and CD44, suggests the self-renewal capacity of these cells, including those at metastatic sites[26,59-62].
There are also data showing that T cells in the peritumoral stroma (CD8+ T cells and FOXP3+ T cells)[63-65], EMT marker-positive cancer-associated fibroblasts[66-68], the engulfment of budding tumor cells by CD68+ macrophages, and the loss of MHC class I expression may play roles in TB[69-71].
From a morphological point of view, TB is defined as small clusters of 4 or fewer tumor cells at the interface of invasive carcinoma. Although different methods are performed, TB is usually evaluated by determining the most invasive area of the tumor (hot spot) at 20x magnification on hematoxylin and eosin-stained slides. Regardless of tumor type, buds in these areas are counted, and according to the recommendation of TBCC, TB is classified into three grades: Low, intermediate, and high[7].
In the context of HCC and ICC, there is evidence from numerous studies focusing on the mechanisms involved in TB outlined above. Among these, it is noteworthy that the number of studies focusing on the EMT in primary liver tumors is over 200 per year[10,11].
This is not surprising, given the considerable roles of the EMT in tumor behavior and progression[72-74]. Accordingly, the number of studies aiming to detect tumor aggression using comprehensive immunohistochemical and molecular methods far exceeds the number of studies focusing on TB, which can be easily detected as a simple, cost-effective morphological finding from resection materials.
Unfortunately, according to the literature, there are very few studies on the relationship between TB and tumor behavior and prognosis in HCC (Table 1). Kairaluoma et al[75] studied the prognostic value of TB, including 259 patients with HCC, in a retrospective cohort study from a single institution. TB is evaluated according to the hot spot method, which is recommended when investigating TB in CRC. The overall 5-year survival in bud-negative patients was higher (72.1%) than that in bud-positive patients (29.2%) (P = 0.009). In addition, the difference between the disease-specific 5-year survival rates of these two groups was also significant, 86.5% (in bud-positive patients) vs 35.1% (in bud-negative patients) (P = 0.002). Multivariate analysis demonstrated that TB is an independent prognostic factor in surgically treated cases.
| Ref. | Tumor | No. | Correlations | Prognosis |
| Kairaluoma et al[75] | HCC | 47-R; 212-NR | Not observed; Not observed | OS: TB negative vs TB positive; DSS: TB negative vs TB positive |
| Wei et al[76] | HCC | 423 | Tumor subtypes, EMT related marker expression, FOXP3, PD-L1 and CD68 expressions; Frequent mast cell infiltration, p53 mutation (IS-TB type I); CTNNB1 mutation (IS-TB type IV) | DFS: Type II vs Type I + Type IV; Type III vs Type I + Type IV; OS: Type II vs Type I + Type IV; Type III vs Type I + Type IV |
| Okubo et al[77] | CCC | 299 | Dif G1/G2vs Dif G3 | OS: TB negative vs TB positive |
| Ogino et al[78] | EHCC-PH; EHCC-DC | 195; 115 | Grade, T, LI, VI, PN, LNM, RSM; Grade, Higher T, LI, VI, PN, LNM | OS: TB low vs TB ıntermediate vs TB high; OS: TB low vs TB high |
| Tanaka et al[80] | ICC | 107 | Stage, Hilar invasion, Grade, VI, LNM, SM | RFS: TB negative vs TB positive; OS: TB negative vs TB positive |
| Type 1 | 49 | NP | RFS: Not prognostic; OS: Not prognostic | |
| Type 2 | 58 | NP | RFS: Not prognostic; OS: TB negative vs TB positive | |
| EHCC-PH | 54 | LI | RFS TB negative vs TB positive; OS TB negative vs TB positive | |
| EHCC-DC | 40 | VI | RFS: Not prognostic; OS: Not prognostic | |
| Ito et al[81] | EHCC-PH | 78 | Grade, T, LNM, M | |
| 36 NT | Combined HA/PV Resection, Grade, T, LNM, M | DSS: TB low vs TB high; RFS: TB low vs TB high | ||
| 42 WT | Not observed | DSS: TB low vs TB high; RFS: Not prognostic | ||
| Agostini-Vulaj et al[83] | EHCC; ICC | 58; 54 | Gender, Location, Grade, LNI, PNI, RSM; Gender, Location, Grade, LNI, PNI | DSS: TB ıntermediate vs TB high; RFS: TB ıntermediate vs TB high |
| Budau et al[84] | ICC | 89 | NP | OS: TB Low vs TB Intermediate vs TB High; RFS TB Low vs TB Intermediate vs TB High ITTB, PTTB, TB |
| Kosaka et al[85] | ICC | 235 | Size, Tumor type, Grade, VI, MBI, LNM | DSS: TB Low/Intermediate vs TB High; RFS: TB Low/Intermediate vs TB High |
| Nakayama et al[82] | EHCC-DC | 65 | T, LNM, LI, VI, ZEB-1 expression, stage | OS: TB Low vs TB High |
However, this parameter was not correlated with clinicopathological factors. This is the only study investigating TB in HCC in a Western population, although it had some limitations, as noted by the authors. There were relatively few patients, yielding wide confidence intervals in the surgical cohort. Additionally, instead of looking for the optimal threshold value, the analysis was performed by making a negative/positive distinction in TB. Again, the absence of significant results in biopsy samples warrants further studies.
Another study was performed in China by Wei et al[76] to classify HCC based on TB and immune scores in 423 patients. The authors developed a prognosis-relevant immune score based on five types of immune cells. A classification based on TB grade and immune type was established (IS-TB type). To explore the association between IS-TB type and molecular alterations of HCC, tumor samples and adjacent nontumor tissues from 100 patients were investigated by whole-exome sequencing. TB was classified into three grades. In addition, cases were also divided into high-grade TB (with ≥ 10 buds) and low-grade TB (with 0 to 9 buds) groups. TB was an independent prognostic indicator for overall survival (OS) and disease-free survival (DFS) in the training and validation cohorts. They also observed that high-grade TB was significantly associated with EMT markers and had higher incidences in patients with nonsteatotic, nonfibrolamellar HCC, stromal active (high α-SMA expression), and immature tumors. A link between TB and EMT markers (E-cadherin and vimentin) confirmed the hypothesis that TB might represent the EMT process.
Because the role of the immune milieu of HCC as a prognostic feature is only starting to emerge, they also divided cases by an immune score established based on Z scores that included five parameters (CD8 stromal, PD-L1 stromal, mast-cell stromal, CD68 stromal, and FOXP3 stromal) for each patient. According to the cutoff value (0.04), patients were divided into immune type A and B groups. DFS and OS were better in the type A group than in the type B group in both the training and validation cohorts. The combination of TB grade and immune type cases was also divided into four groups: ISA-TBhigh (type I), ISB-TB high (type II), ISA-TBlow (type III), and ISB- TBlow (type IV). While cases within IS-TB type II showed the worst long-term survival, cases within IS-TB type III had the best OS and DFS. These findings are in line with previous observations that indicated that a high lymphocyte-to-TB ratio was a good prognostic factor and that the integration of both TILs and TB was advantageous in the prediction of long-term prognosis in colorectal cancers. These findings provide a rationale for the pathological evaluation of the TME in addition to the current pathological classifications of HCC.
Another interesting finding of this study was the association between IS-TB type and molecular alterations. TP53 (mainly within IS-TB type I) and CTNNB1 (mainly within IS-TB type IV) mutations in two distinct HCC phenotypes exhibit different immune and pathological characteristics. While TP53 mutations were related to poor differentiation and a thick trabecular pattern, CTNNB1 mutations were associated with impaired antitumor immunity (immune type B), well-differentiated morphology, a pseudoglandular pattern, mature stroma, and low α-SMA (fibroblast activation protein) expression.
As noted above, despite the scarcity of studies examining TB in HCC, there is a wealth of data on the processes involved in this phenomenon.
Several studies focusing on TB in cholangiocarcinomas have recently been performed. The number of studies, including extrahepatic perihilar (EHCC-PH) and distal cholangiocarcinoma (EHCC-D) cases, exceeded the number of studies that included ICC cases in the study group. The characteristics and results of these studies are summarized in Table 1.
In an earlier investigation of cholangiocarcinomas from all anatomical locations (CCC), TB was associated with the grade but not with the course of the disease[77]. However, in a more recent study, in addition to high grade, high TB was more frequently observed in males and patients with extrahepatic localization, perineural and lymphatic invasion, and presentation in settings with positive resection margins[78]. Moreover, TB is an independent prognostic factor for CCC. However, since TB scoring differed in these two studies, it is not possible to compare the results of one with the other (Tables 1 and 2), as noted by Regmi et al[79], who performed a meta-analysis of CCC samples from different locations, including tumors of the ampulla and gallbladder.
| Ref. | Tumor | Tumor budding criteria |
| Kairaluoma et al[75] | HCC | Evaluation was performed according to median values; Negative: No buds were found; Positive: At least one bud was present |
| Wei et al[76] | HCC | Association between TB and clinicopathological parameters; Grade 1 (0-4), Grade 2 (5-9), Grade 3 (≥ 10); For survival analysis; Low grade (0-9), High grade (≥ 10) |
| Okubo et al[77] | CCC | Negative: < 5 budding focus; Positive: ≥ 5 budding focus |
| Ogino et al[78] | EHCC-PH, EHCC-DC | Cut-off values of TB obtained by recursive partioning technique; For EHCC-PH; Low grade (0-4), Intermediate grade (5-11), High grade (≥ 12); For EHCC-DC; Low grade (0-4), High grade (≥ 5) |
| Tanaka et al[80] | ICC, EHCC-PH, EHCC-DC | Low grade (0-4), Intermediate grade (5-9), High grade (≥10) |
| Ito et al[81] | EHCC-PH | Low TB: < 5 budding focus; High TB: ≥ 5 budding focus |
| Agostini-Vulaj et al[83] | ICC, EHCC | Grade 1 (0-4), Grade 2 (5-9), Grade 3 (≥ 10) |
| Budau et al[84] | ICC | Grade 1 (0-4), Grade 2 (5-9), Grade 3 (≥ 10) |
| Kosaka et al[85] | ICC | Low grade (0-4), Intermediate grade (5-9), High grade (≥ 10) |
| Nakayama et al[82] | EHCC-DC | Low TB (0-4), High [TB Grade 2 (5-9) and 3 (≥ 10)] |
In EHCC-PH, TB is associated with tumor invasion, lymph node metastasis, perineural invasion, lymphovascular invasion, and positive resection margin status. It has also been shown to be an independent prognostic factor in determining the course of the disease in all of the studies[78,80,81]. In EHCC-D, higher TB was more frequent in tumors with deeper invasion, lymph node metastasis, and lymphovascular and perineural invasion[78,80,82]. The correlation between TB and stage and ZEB-1 expression was also noted[83]. Similar to EHCC-P, all but one study[81] showed that TB effectively determines the course of the disease, as shown by both univariate and multivariate analyses[77,78,81-84].
Regarding ICC, TB was shown to be correlated with stage, hilar invasion, grade, venous invasion, lymph node metastasis, and positive surgical margins, which are important parameters for determining the behavior of these tumors. Moreover, when ICCs were analyzed according to growth patterns, it was noted that 80% of mass-forming tumors had high TB. In contrast, this ratio was 16% and 2.3% in periductal infiltrating and intraductal growing subtypes, respectively[85]. In addition, the prognostic role of TB has been described[77,80,81,85]. Budau et al[84] analyzed TB using a three-tier grading system: high, intermediate, and low. While patients with low TB had the most favorable recurrence survival, high TB was associated with the most unfavorable outcomes.
Similarly, TB correlated significantly with the overall survival of patients in univariate and multivariate analyses (P < 0.001). In addition, their data demonstrated that in ICC, TB is significantly independent of the area of investigation (intratumoral or peritumoral). These findings indicate the possibility that TB assessment in preoperative tissue biopsies and in cases that would not be suitable for resection could be used to predict tumor behavior. Nevertheless, the evidence for intratumoral TB is still weak.
In another study, TB was observed to be a powerful prognostic factor for RFS and OS in ICC[80]. In patients stratified into negative and positive TB status, the median time to recurrence in cases with positive TB was 10.26 mo. This was significantly shorter than that of subjects with negative TB (35.57 mo), and the difference among median survival times was significant (P < 0.001). Furthermore, the results of the same study indicated that TB was a decisive and powerful prognostic factor for OS (HR: 4.547). Although these findings need to be supported by further large-scale studies, they suggest that TB may be an important prognostic parameter in these tumors.
Tanaka et al[80] presented an interesting finding about TB in ICC in an elegant study. When they evaluated TB by dividing ICC into two subgroups, Type 1 (hilar) and Type 2 (peripheral), according to the combined scores of mucin productivity and immunoreactivity of S100P, N-cadherin, and neural cell adhesion molecule, this parameter was determined to be a decisive prognostic factor in Type 2 but not in Type 1. They suggested that some differences exist in the biological behavior of these subtypes and pointed out that despite the prognostic importance of TB in ICC, its pathogenetic role in biliary tract carcinomas might differ by anatomic location. However, this finding needs to be supported in further studies. Nevertheless, the results of TB studies in ICC are similar and support the suggestion that TB is a relevant prognostic factor in the histopathological evaluation of these tumors.
Generally, different scoring methods have been used to investigate TB in cholangiocarcinomas. In a few studies, unlike the recommendation of TTBC, five cells were taken as the cutoff for the definition of TB[77,78,81]. The analyses were performed by categorizing the cases as negative vs. positive or low vs. high TB. In most other studies, including ICC cases, patients were assessed following the three-tiered system recommended by the TTBC for colorectal cancer[80,82-84]. However, different stratifications were used for further evaluations (Table 2). More recently, in an elegant study, Zlobec et al[86] observed that CRC without TB (TB0) is relatively frequent and provided additional information on tumor behavior, suggesting a new “zero budding” category for TB. There is currently no evidence about the prognostic value of TB0 in cholangiocarcinomas, and it would be interesting to conduct further studies in which this category is addressed separately.
Accumulated data indicate that the preferred staining method for scoring TB is HE. Recently, some studies on TB have reported that IHC is superior to HE regarding reproducibility and interobserver agreement in assessing this parameter in CRC. Regarding CCC, Ogino et al[78] obtained TB scores in HE-stained whole-tissue sections and PanCK immunostained tissue microarray (TMA) sections from 266 patients. They observed that the number of tumor buds in HE-stained slides was almost equal to that in PanCK-stained slides from TMA, with a strong correlation between them (R = 0.763, P < 0.001). This finding also supports that evaluating TB in HE-stained sections is a simple and reproducible method. Nevertheless, more studies are needed to standardize the assessment of TB in ICC because grading systems for this parameter vary between different types of cancer.
In CRC, TB, combined with other established biomarkers, may allow us to discriminate between patients who would benefit from oncological resection and patients who will receive adjuvant therapy and to classify different therapeutic options, especially in advanced-stage patients[87]. Thus, TB can predict prognosis and regulate treatment options in primary liver cancers. However, the role of TB in the treatment of these tumors remains to be investigated.
This review highlights that TB may be a promising prognostic factor for primary liver tumors. However, its clinical value in managing patients should be established in multidisciplinary studies. Evidence also suggests that TB in HCC can identify and reclassify tumors of molecular subtypes with different behavioral characteristics. The differences in the classification of TB in primary liver tumors indicate that a standard and validated method should be established to provide a solid basis for large-scale clinicopathological studies for further evaluation. In addition, the precise determination of the value of budding tumor assessment with multiple further studies may allow us to clarify whether this parameter will be included in pathology report protocols as in CRC in the near future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Pathology, No. 11959.
Specialty type: Pathology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maffeis V, Italy; Qin Y, China S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY
| 1. | Global Cancer Observatory, Lyon, France: International Agency for Research on Cancer, 2018. Accessed: November 22, 2018. Available from: http://gco.iarc.fr/today/. |
| 2. | Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer. 2016;139:1534-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 3. | Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 4. | Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Zlobec I, Lugli A. Tumour budding in colorectal cancer: molecular rationale for clinical translation. Nat Rev Cancer. 2018;18:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. Tumor Budding: The Name is EMT. Partial EMT. J Clin Med. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 7. | Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 725] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 8. | Loughrey MB, Webster F, Arends MJ, Brown I, Burgart LJ, Cunningham C, Flejou JF, Kakar S, Kirsch R, Kojima M, Lugli A, Rosty C, Sheahan K, West NP, Wilson RH, Nagtegaal ID. Dataset for Pathology Reporting of Colorectal Cancer: Recommendations From the International Collaboration on Cancer Reporting (ICCR). Ann Surg. 2022;275:e549-e561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 10. | Gurzu S, Kobori L, Fodor D, Jung I. Epithelial Mesenchymal and Endothelial Mesenchymal Transitions in Hepatocellular Carcinoma: A Review. Biomed Res Int. 2019;2019:2962580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Vaquero J, Guedj N, Clapéron A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J Hepatol. 2017;66:424-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47048] [Article Influence: 3360.6] [Reference Citation Analysis (5)] |
| 13. | Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2456] [Cited by in RCA: 2571] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 14. | Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1356] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 15. | Nakagawa Y, Ohira M, Kubo N, Yamashita Y, Sakurai K, Toyokawa T, Tanaka H, Muguruma K, Shibutani M, Yamazoe S, Kimura K, Nagahara H, Amano R, Ohtani H, Yashiro M, Maeda K, Hirakawa K. Tumor budding and E-cadherin expression are useful predictors of nodal involvement in T1 esophageal squamous cell carcinoma. Anticancer Res. 2013;33:5023-5029. [PubMed] |
| 16. | Lee SJ, Choi SY, Kim WJ, Ji M, Lee TG, Son BR, Yoon SM, Sung R, Lee EJ, Youn SJ, Park SM. Combined aberrant expression of E-cadherin and S100A4, but not β-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol. 2013;8:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Kohler I, Bronsert P, Timme S, Werner M, Brabletz T, Hopt UT, Schilling O, Bausch D, Keck T, Wellner UF. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol. 2015;30 Suppl 1:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jønson L, Vikesaa J, Nielsen FC, von Buchwald C. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Galván JA, Zlobec I, Wartenberg M, Lugli A, Gloor B, Perren A, Karamitopoulou E. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br J Cancer. 2015;112:1944-1950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Park JY, Hong DG, Chong GO, Park JY. Tumor Budding is a Valuable Diagnostic Parameter in Prediction of Disease Progression of Endometrial Endometrioid Carcinoma. Pathol Oncol Res. 2019;25:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Baek TH, Kang DW, Kim JH, Son HJ. Gland Attenuation, a Novel Morphological Feature of Colorectal Cancer: Evidence for an Epithelial-Mesenchymal Transition. Ann Coloproctol. 2018;34:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Banias L, Jung I, Bara T, Fulop Z, Simu P, Simu I, Satala C, Gurzu S. Immunohistochemical-based molecular subtyping of colorectal carcinoma using maspin and markers of epithelial-mesenchymal transition. Oncol Lett. 2020;19:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Dardare J, Witz A, Merlin JL, Gilson P, Harlé A. SMAD4 and the TGFβ Pathway in Patients with Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Maffeis V, Cappellesso R, Galuppini F, Guzzardo V, Zanon A, Cazzador D, Emanuelli E, Ventura L, Martini A, Fassina A. Tumor budding is an adverse prognostic marker in intestinal-type sinonasal adenocarcinoma and seems to be unrelated to epithelial-mesenchymal transition. Virchows Arch. 2020;477:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Zheng S, Luo J, Xie S, Lu S, Liu Q, Xiao H, Luo W, Huang Y, Liu K. Tumor budding of cervical squamous cell carcinoma: epithelial-mesenchymal transition-like cancer stem cells? PeerJ. 2022;10:e13745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Xie N, Wang C, Zhuang Z, Hou J, Liu X, Wu Y, Liu H, Huang H. Decreased miR-320a promotes invasion and metastasis of tumor budding cells in tongue squamous cell carcinoma. Oncotarget. 2016;7:65744-65757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Karamitopoulou E, Haemmig S, Baumgartner U, Schlup C, Wartenberg M, Vassella E. MicroRNA dysregulation in the tumor microenvironment influences the phenotype of pancreatic cancer. Mod Pathol. 2017;30:1116-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Møller T, James JP, Holmstrøm K, Sørensen FB, Lindebjerg J, Nielsen BS. Co-Detection of miR-21 and TNF-α mRNA in Budding Cancer Cells in Colorectal Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Knudsen KN, Lindebjerg J, Kalmár A, Molnár B, Sørensen FB, Hansen TF, Nielsen BS. miR-21 expression analysis in budding colon cancer cells by confocal slide scanning microscopy. Clin Exp Metastasis. 2018;35:819-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol Ther. 2010;10:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 33. | Pavlič A, Boštjančič E, Kavalar R, Ilijevec B, Bonin S, Zanconati F, Zidar N. Tumour budding and poorly differentiated clusters in colon cancer - different manifestations of partial epithelial-mesenchymal transition. J Pathol. 2022;258:278-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Derynck R, Weinberg RA. EMT and Cancer: More Than Meets the Eye. Dev Cell. 2019;49:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 35. | Zhou B, Zong S, Zhong W, Tian Y, Wang L, Zhang Q, Zhang R, Li L, Wang W, Zhao J, Chen X, Feng Y, Zhai B, Sun T, Liu Y. Interaction between laminin-5γ2 and integrin β1 promotes the tumor budding of colorectal cancer via the activation of Yes-associated proteins. Oncogene. 2020;39:1527-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Berndt A, Gaßler N, Franz M. Invasion-Associated Reorganization of Laminin 332 in Oral Squamous Cell Carcinomas: The Role of the Laminin γ2 Chain in Tumor Biology, Diagnosis, and Therapy. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Masuda R, Kijima H, Imamura N, Aruga N, Nakazato K, Oiwa K, Nakano T, Watanabe H, Ikoma Y, Tanaka M, Inokuchi S, Iwazaki M. Laminin-5γ2 chain expression is associated with tumor cell invasiveness and prognosis of lung squamous cell carcinoma. Biomed Res. 2012;33:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Taira T, Ishii G, Nagai K, Yoh K, Takahashi Y, Matsumura Y, Kojima M, Ohmatsu H, Goto K, Niho S, Takashima H, Inoue H, Ohe Y, Ochiai A. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer. 2012;76:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 39. | Shinto E, Baker K, Tsuda H, Mochizuki H, Ueno H, Matsubara O, Foulkes WD, Jass JR. Tumor buds show reduced expression of laminin-5 gamma 2 chain in DNA mismatch repair deficient colorectal cancer. Dis Colon Rectum. 2006;49:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Kevans D, Wang LM, Sheahan K, Hyland J, O'Donoghue D, Mulcahy H, O'Sullivan J. Epithelial-mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int J Surg Pathol. 2011;19:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Masaki T, Matsuoka H, Sugiyama M, Abe N, Izumisato Y, Goto A, Sakamoto A, Atomi Y. Laminin-5 gamma 2 chain and matrix metalloproteinase-2 may trigger colorectal carcinoma invasiveness through formation of budding tumor cells. Anticancer Res. 2003;23:4113-4119. [PubMed] |
| 42. | Sordat I, Rousselle P, Chaubert P, Petermann O, Aberdam D, Bosman FT, Sordat B. Tumor cell budding and laminin-5 expression in colorectal carcinoma can be modulated by the tissue micro-environment. Int J Cancer. 2000;88:708-717. [PubMed] [DOI] [Full Text] |
| 43. | Shinto E, Jass JR, Tsuda H, Sato T, Ueno H, Hase K, Mochizuki H, Matsubara O. Differential prognostic significance of morphologic invasive markers in colorectal cancer: tumor budding and cytoplasmic podia. Dis Colon Rectum. 2006;49:1422-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Prall F, Ostwald C. High-degree tumor budding and podia-formation in sporadic colorectal carcinomas with K-ras gene mutations. Hum Pathol. 2007;38:1696-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T. Beta-catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer. 2004;108:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Arora S, Singh P, Rahmani AH, Almatroodi SA, Dohare R, Syed MA. Unraveling the Role of miR-20b-5p, CCNB1, HMGA2 and E2F7 in Development and Progression of Non-Small Cell Lung Cancer (NSCLC). Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Rizzi C, Cataldi P, Iop A, Isola M, Sgarra R, Manfioletti G, Giancotti V. The expression of the high-mobility group A2 protein in colorectal cancer and surrounding fibroblasts is linked to tumor invasiveness. Hum Pathol. 2013;44:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Sugai T, Yamada N, Eizuka M, Sugimoto R, Uesugi N, Osakabe M, Ishida K, Otsuka K, Sasaki A, Matsumoto T. Vascular Invasion and Stromal S100A4 Expression at the Invasive Front of Colorectal Cancer are Novel Determinants and Tumor Prognostic Markers. J Cancer. 2017;8:1552-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Märkl B, Renk I, Oruzio DV, Jähnig H, Schenkirsch G, Schöler C, Ehret W, Arnholdt HM, Anthuber M, Spatz H. Tumour budding, uPA and PAI-1 are associated with aggressive behaviour in colon cancer. J Surg Oncol. 2010;102:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Guzińska-Ustymowicz K. MMP-9 and cathepsin B expression in tumor budding as an indicator of a more aggressive phenotype of colorectal cancer (CRC). Anticancer Res. 2006;26:1589-1594. [PubMed] |
| 51. | Banias L, Gurzu S, Kovacs Z, Bara T, Bara T Jr, Jung I. Nuclear maspin expression: A biomarker for budding assessment in colorectal cancer specimens. Pathol Res Pract. 2017;213:1227-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Kovacs Z, Jung I, Szalman K, Banias L, Bara TJ, Gurzu S. Interaction of arylsulfatases A and B with maspin: A possible explanation for dysregulation of tumor cell metabolism and invasive potential of colorectal cancer. World J Clin Cases. 2019;7:3990-4003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Gurzu S, Jung I. Subcellular Expression of Maspin in Colorectal Cancer: Friend or Foe. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Karamitopoulou E, Zlobec I, Gloor B, Kondi-Pafiti A, Lugli A, Perren A. Loss of Raf-1 kinase inhibitor protein (RKIP) is strongly associated with high-grade tumor budding and correlates with an aggressive phenotype in pancreatic ductal adenocarcinoma (PDAC). J Transl Med. 2013;11:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Tanaka K, Shimura T, Kitajima T, Kondo S, Ide S, Okugawa Y, Saigusa S, Toiyama Y, Inoue Y, Araki T, Uchida K, Mohri Y, Kusunoki M. Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br J Cancer. 2014;110:2923-2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Righi A, Sarotto I, Casorzo L, Cavalchini S, Frangipane E, Risio M. Tumour budding is associated with hypoxia at the advancing front of colorectal cancer. Histopathology. 2015;66:982-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Hacking S, Sajjan S, Angert M, Ebare K, Jin C, Chavarria H, Kataria N, Zhang L, Cho M, Thomas R, Lee L, Nasim M. Tumor Budding in Colorectal Carcinoma Showing a Paradoxical Mitotic Index (Via PHH3) With Possible Association to the Tumor Stromal Microenvironment. Appl Immunohistochem Mol Morphol. 2020;28:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Rubio CA. Arrest of cell proliferation in budding tumor cells ahead of the invading edge of colonic carcinomas. A preliminary report. Anticancer Res. 2008;28:2417-2420. [PubMed] |
| 59. | Xiang Z, He Q, Huang L, Xiong B, Xiang Q. Breast Cancer Classification Based on Tumor Budding and Stem Cell-Related Signatures Facilitate Prognosis Evaluation. Front Oncol. 2021;11:818869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 60. | Sadek SA, A Rehim DM, Fatima S. The role of tumor budding in colorectal adenocarcinoma: Possible involvement of the intestinal cancer stem cell marker Lgr5. Indian J Pathol Microbiol. 2020;63:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Zheng Z, Yu H, Huang Q, Wu H, Fu Y, Shi J, Wang T, Fan X. Heterogeneous expression of Lgr5 as a risk factor for focal invasion and distant metastasis of colorectal carcinoma. Oncotarget. 2018;9:30025-30033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Zhou Y, Xia L, Wang H, Oyang L, Su M, Liu Q, Lin J, Tan S, Tian Y, Liao Q, Cao D. Cancer stem cells in the progression of colorectal cancer. Oncotarget. 2018;9:33403-33415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 63. | Dawson H, Christe L, Eichmann M, Reinhard S, Zlobec I, Blank A, Lugli A. Tumour budding/T cell infiltrates in colorectal cancer: proposal of a novel combined score. Histopathology. 2020;76:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Nearchou IP, Lillard K, Gavriel CG, Ueno H, Harrison DJ, Caie PD. Automated Analysis of Lymphocytic Infiltration, Tumor Budding, and Their Spatial Relationship Improves Prognostic Accuracy in Colorectal Cancer. Cancer Immunol Res. 2019;7:609-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 65. | Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L, Eichmann MD, Worni M, Gloor B, Perren A, Karamitopoulou E. Integrated Genomic and Immunophenotypic Classification of Pancreatic Cancer Reveals Three Distinct Subtypes with Prognostic/Predictive Significance. Clin Cancer Res. 2018;24:4444-4454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 66. | Shin N, Son GM, Shin DH, Kwon MS, Park BS, Kim HS, Ryu D, Kang CD. Cancer-Associated Fibroblasts and Desmoplastic Reactions Related to Cancer Invasiveness in Patients With Colorectal Cancer. Ann Coloproctol. 2019;35:36-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Ueno H, Shinto E, Shimazaki H, Kajiwara Y, Sueyama T, Yamamoto J, Hase K. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol. 2015;22:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Galván JA, Helbling M, Koelzer VH, Tschan MP, Berger MD, Hädrich M, Schnüriger B, Karamitopoulou E, Dawson H, Inderbitzin D, Lugli A, Zlobec I. TWIST1 and TWIST2 promoter methylation and protein expression in tumor stroma influence the epithelial-mesenchymal transition-like tumor budding phenotype in colorectal cancer. Oncotarget. 2015;6:874-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Wang M, Su Z, Amoah Barnie P. Crosstalk among colon cancer-derived exosomes, fibroblast-derived exosomes, and macrophage phenotypes in colon cancer metastasis. Int Immunopharmacol. 2020;81:106298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, Zlobec I. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 2016;5:e1106677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 71. | Zlobec I, Minoo P, Terracciano L, Baker K, Lugli A. Characterization of the immunological microenvironment of tumour buds and its impact on prognosis in mismatch repair-proficient and -deficient colorectal cancers. Histopathology. 2011;59:482-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Kim H, Lee S, Shin E, Seong KM, Jin YW, Youn H, Youn B. The Emerging Roles of Exosomes as EMT Regulators in Cancer. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 73. | Jiang J, Li J, Zhou X, Zhao X, Huang B, Qin Y. Exosomes Regulate the Epithelial-Mesenchymal Transition in Cancer. Front Oncol. 2022;12:864980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 74. | Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1887] [Article Influence: 269.6] [Reference Citation Analysis (0)] |
| 75. | Kairaluoma V, Kemi N, Pohjanen VM, Saarnio J, Helminen O. Tumour budding and tumour-stroma ratio in hepatocellular carcinoma. Br J Cancer. 2020;123:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Wei L, Delin Z, Kefei Y, Hong W, Jiwei H, Yang Z. A classification based on tumor budding and immune score for patients with hepatocellular carcinoma. Oncoimmunology. 2020;9:1672495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Okubo S, Mitsunaga S, Kato Y, Kojima M, Sugimoto M, Gotohda N, Takahashi S, Hayashi R, Konishi M. The prognostic impact of differentiation at the invasive front of biliary tract cancer. J Surg Oncol. 2018;117:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Ogino M, Nakanishi Y, Mitsuhashi T, Hatanaka Y, Amano T, Marukawa K, Nitta T, Ueno T, Ono M, Kuwabara S, Yamada T, Hirano S. Impact of tumour budding grade in 310 patients who underwent surgical resection for extrahepatic cholangiocarcinoma. Histopathology. 2019;74:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Regmi P, Paudyal A, Paudyal P, Hu HJ, Liu F, Ma WJ, Jin YW, Li FY. Prognostic significance of tumor budding in biliary tract cancer. Eur J Surg Oncol. 2022;48:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Tanaka M, Yamauchi N, Ushiku T, Shibahara J, Hayashi A, Misumi K, Yasunaga Y, Morikawa T, Kokudo T, Arita J, Sakamoto Y, Hasegawa K, Fukayama M. Tumor Budding in Intrahepatic Cholangiocarcinoma: A Predictor of Postsurgery Outcomes. Am J Surg Pathol. 2019;43:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Ito T, Kuriyama N, Kozuka Y, Komatsubara H, Ichikawa K, Noguchi D, Hayasaki A, Fujii T, Iizawa Y, Kato H, Tanemura A, Murata Y, Kishiwada M, Mizuno S, Usui M, Sakurai H, Isaji S. High tumor budding is a strong predictor of poor prognosis in the resected perihilar cholangiocarcinoma patients regardless of neoadjuvant therapy, showing survival similar to those without resection. BMC Cancer. 2020;20:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Nakayama G, Hisaka T, Sakai H, Akashi M, Yuichi G, Sato T, Naito Y, Akiba J, Yano H, Akagi Y. Tumour Budding as an Independent Prognostic Factor for Survival in Patients With Distal Bile Duct Cancer. Anticancer Res. 2022;42:4079-4087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Agostini-Vulaj D, Cates JMM, Bratton LE, Gonzalez RS. Increasing tumor budding in cholangiocarcinoma is associated with decreased disease-specific survival. Hum Pathol. 2021;111:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 84. | Budau KL, Sigel CS, Bergmann L, Lüchtenborg AM, Wellner U, Schilling O, Werner M, Tang L, Bronsert P. Prognostic Impact of Tumor Budding in Intrahepatic Cholangiocellular Carcinoma. J Cancer. 2022;13:2457-2471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 85. | Kosaka H, Ishida M, Ueno M, Komeda K, Hokutou D, Iida H, Hirokawa F, Matsui K, Sekimoto M, Kaibori M. Tumor budding may be a promising prognostic indicator in intrahepatic cholangiocarcinoma: A multicenter retrospective study. Ann Gastroenterol Surg. 2023;7:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 86. | Zlobec I, Bächli M, Galuppini F, Berger MD, Dawson HE, Nagtegaal ID, Lugli A. Refining the ITBCC tumor budding scoring system with a "zero-budding" category in colorectal cancer. Virchows Arch. 2021;479:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Zlobec I, Berger MD, Lugli A. Tumour budding and its clinical implications in gastrointestinal cancers. Br J Cancer. 2020;123:700-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |