Published online Jun 27, 2023. doi: 10.4254/wjh.v15.i6.725
Peer-review started: February 27, 2023
First decision: March 28, 2023
Revised: April 3, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: June 27, 2023
Processing time: 118 Days and 8.8 Hours
Non-alcoholic fatty liver disease (NAFLD) or metabolic (dysfunction)-associated fatty liver disease is the leading cause of chronic liver diseases defined as a disease spectrum comprising hepatic steatosis, non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatic carcinoma. NASH, characterized by hepatocyte injury, steatosis, inflammation, and fibrosis, is associated with NAFLD prognosis. Ductular reaction (DR) is a common compensatory reaction associated with liver injury, which involves the hepatic progenitor cells (HPCs), hepatic stellate cells, myofibroblasts, inflammatory cells (such as macrophages), and their secreted substances. Recently, several studies have shown that the extent of DR parallels the stage of NASH and fibrosis. This review summarizes previous research on the correlation between DR and NASH, the potential interplay mechanism driving HPC differentiation, and NASH progression.
Core Tip: This is the first review focusing on recent advances in the relationship of hepatic cells with ductular reaction (DR), in fatty liver-related steatohepatitis and fibrosis. Recent advances in DR, a common compensatory reaction in liver injury, shed light on the effects of hepatic progenitor cells, hepatic stellate cells, myofibroblasts, inflammatory cells, and their secreted substance. In particular, hepatic progenitor cell differentiation was thoroughly discussed in developing steatohepatitis and fibrosis. This review summarizes the correlation between DR and steatohepatitis and fibrosis, the advanced stages of non-alcoholic fatty liver disease, or metabolic (dysfunction) related fatty liver disease.
- Citation: He YH, Pan JX, Xu LM, Gu T, Chen YW. Ductular reaction in non-alcoholic fatty liver disease: When Macbeth is perverted. World J Hepatol 2023; 15(6): 725-740
- URL: https://www.wjgnet.com/1948-5182/full/v15/i6/725.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i6.725
Non-alcoholic fatty liver disease (NAFLD), which affects approximately 25% of adults worldwide, is the leading cause of chronic liver diseases[1]. NAFLD refers to a disease spectrum including hepatic steatosis, non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatic carcinoma[2]. In early 2020, an international expert group led a consensus-driven process to develop a more appropriate term for NAFLD, and the term “metabolic (dysfunction) related fatty liver disease (MAFLD)” was recommended[3]. NASH/MASH is characterized by ≥ 5% hepatic steatosis, hepatocyte injury or necrosis, and inflammation[2,4]. NASH is a critical stage in NAFLD development and is associated with NAFLD prognosis; thus, it has become the focus of NAFLD research. NASH is the second most common indication for liver transplantation in the United States[1]. The occurrence and progress of NASH are related to several factors such as glucose and lipid metabolism, immune response, and gut microbiota[5-7]. The diagnosis and severity classification of NASH depends on histopathological examination. The main pathological features of NASH are hepatocyte balloon degeneration, inflammatory infiltration, Mallory-Den K corpuscle, and zone 3 fibrosis[2,8]. Some studies have shown that neutrophil infiltration and portal inflammatory infiltration are also characteristics of NASH[9,10].
Ductular reaction (DR) is a compensatory reaction commonly detected in various liver injuries[11], involving the participation of hepatic progenitor cells (HPCs), hepatic stellate cells (HSCs), myofibroblasts, inflammatory cells (such as macrophages), and their secreted substances. Among them, the proliferation and differentiation of HPCs are the core of DR[12]. DR is commonly found in the livers of NASH patients. Moreover, there is a parallel relationship between DR and the severity of inflammation and fibrosis in NASH patients[13-15], suggesting that DR has an important role in the progression of NASH.
Based on clinical investigations, the present review summarizes the correlation between DR and NASH. It discusses the shaped HPC differentiation fate in the context of NASH and its influence on NASH progression.
DR is a compensatory reaction in the portal area caused by biliary diseases, viral hepatitis, NAFLD, acute fulminant liver failure, etc[16]. DR is heterogeneous in both pathology and pathophysiology. Desmet divided DR into four types based on pathology: Type 1, Type 2A, Type 2B, and Type 3[17].
Type 1 is predominant in acute complete bile duct (BD) obstruction, alpha-naphtyl isothiocyanate intoxication, and cytokine (e.g., interleukin 6)-induced ductular increase. It results from the proliferation of preexisting cholangiocytes. Type 1 causes the biliary tubes to elongate, branch out, and widen their lumens, allowing them to adjust to the swelling and inflammation of the portal mesenchyme. Type 2A has been interpreted as “ductular metaplasia of hepatocytes.” It is often detected in periportal areas, most characteristically, in chronic cholestatic conditions. In lasting cholestasis, bile acids increase the number of cholangiocytes, which promote the development of pericellular fibrosis, and in this way, it enhances bile ductular metaplasia of hepatocytes. Of note, Type 1 and Type 2A can be reversed when the causative trigger is eliminated; the ductular structures are cleared by apoptosis; and the associated fibrosis is ameliorated to a considerable extent. Prolonged hypoxia induces Type 2B, which manifests in areas of parenchymal hypoxia, specifically in the centrolobular region of liver lobules and the centronodular region of cirrhotic nodules. Although often slower in development, its microscopic pattern is comparable to that of Type 2A in terms of ductular metaplasia or dedifferentiation of mature hepatocytes, which is associated with myofibroblast-induced fibrosis. Type 3 occurs in cases of massive loss of parenchymal cells and is characterized by the activation and proliferation of HPCs located in the ductules and canals of Hering. As bipotential cells, HPCs can differentiate into hepatocytes and BD cells[17].
There is consensus that the fate of HPC differentiation is the core of DR, determining the pathological type of DR and affecting disease development[18]. Epithelial cell adhesion molecule and the neural cell adhesion molecule/sex-determining region Y-Box 9 (SOX9) have been previously considered markers of HPCs, cytokeratin-7 (CK7) and CK19 have been used to identify cholangiocytes, and albumin and hepatic nuclear factor 4-alpha have been considered markers of hepatocytes[19-21]. HPCs located in the Hering canal typically differentiate into biliary cells in a normal liver[18] but do not lead to DR. HPCs are activated and differentiate into hepatocytes or biliary cells during liver injury. For example, HPCs differentiate into hepatocytes in acute fulminant hepatic failure and contribute to liver regeneration[22,23]. CK7 immunohistochemistry is also positive in HPCs, which can predict liver injury severity; for instance, HPCs differentiate into CK7+ cells in the portal area in chronic hepatitis C and exacerbate liver injury[13,14,24-26]. Furthermore, a similar phenomenon has been found in hepatitis B virus-injected murine models[27]. In addition, DR is significantly associated with hepatocellular carcinoma peritumoral hepatic inflammation, liver fibrosis, tumor node metastasis classification stage, and poor prognosis[28]. Hepatocyte-derived ductular HPCs can give rise to hepatocellular carcinoma via concomitant activation of yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif transcription factors. Autophagy suppresses the formation of hepatocyte-derived cancer-initiating HPCs in the liver[29].
HPCs are activated in the majority of liver diseases[30]. During liver injury, a ubiquitous DR affects the differentiation vs dedifferentiation type of HPCs, depending on the severity of the liver injury[31]. Proliferating BDs in DR are misshapen, lack an apparent lumen, and are associated with increased portal inflammation and fibrosis[19,32]. It has been previously demonstrated that HPC activation is sufficient to regenerate a large proportion of the liver parenchyma using targeted deletion of mouse double minute 2 (MDM2) in mouse hepatocytes. This kind of HPC activation may be induced by the tumor necrosis factor-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor-inducible 14 pathway[33]. Interestingly, in the hepatocyte-specific β-catenin knockout model, hepatocytes lose their regenerative capacity, and cholangiocytes still express β-catenin. β-catenin-positive cholangiocytes (differentiated HPCs) differentiate into β-catenin-positive small hepatocytes, which then proliferate and repopulate the liver[34,35]. A previous study reported that YAP levels are increased in NAFLD patients and NAFLD mouse models[36]. A recent study showed that the DR reaction is more intense and hepatocytes trans-differentiate into cholangiocytes protected from cholestatic damage by activating Hippo-YAP in the Tjp2 cKO mouse model (more susceptible to cholic acid-induced liver injury) fed 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)[37]. A murine BD ligation model of liver fibrosis showed that heme oxygenase-1-mediated pro-resolution M2 polarization of macrophages protects the liver from excessive DR and fibrosis with the ligand of numb protein X1 as the key downstream factor[38]. Interestingly, recent studies have shown that HPCs can promote angiogenesis by secreting vascular endothelial growth factor (VEGF) via the secretin/secretin receptor/microRNA 125b (miR-125b) axis[39]. However, recent studies have shown that DR cells can promote angiogenesis through slit guidance ligand 2-roundabout 1 signaling channels in various chronic liver diseases (CLDs), contrary to VEGF[40]. Another study showed that the signaling of apelin/APJ (G protein-coupled apelin receptor) can promote intrahepatic angiogenesis[41].
The impact of DR on liver diseases is a double-edged sword. HPCs can be activated and differentiated into hepatocytes to participate in liver regeneration in the case of massive loss of parenchymal cells. Conversely, the activation of HPCs may play a role in the activation of HSCs and the infiltration of inflammatory cells in DR in most CLDs, which can lead to further liver injury, including cirrhosis and tumorigenesis[14,25,42,43].
A state of NAFLD begins with healthy liver parenchyma (steatosis in < 5% of hepatocytes) and then progresses to steatosis in > 5% of hepatocytes with the initiation of DR. The condition progresses to a severe stage with scar tissue accumulation, elevated steatosis, and hepatic ballooning[43]. In recent years, DR has attracted considerable attention in NASH research. It is worth noting that although DR can assist in repairing liver injury by aiding in HPC activation and differentiation, its impact on the progression of chronic liver disease associated with NASH may not always be favorable, especially when liver regeneration capacity is impaired. In fact, in some cases, DR-induced differentiation may even contribute to the occurrence and progression of inflammation and liver fibrosis in NASH. In 2007, Richardson et al[14] analyzed data from 118 liver specimens (107 from NAFLD patients and 11 from normal liver) and found that DR commonly existed in NASH, especially in patients with fibrosis. Multivariate analysis demonstrated that the extent of DR was independently associated with hepatocyte replicative arrest [odds ratio (OR) = 6.5] and fibrosis stage (OR = 17.9). Moreover, they further found that the expansion of HPCs was significantly correlated with NASH activity score[14]. In 2013, based on biopsy specimens from 56 adults with NAFLD (10 with steatosis and 46 with NASH) from Austria and the United States, Skoien et al[44] found that both centrilobular fibrosis and portal fibrosis stages were positively associated with the extent of DR. In 2018, multicenter observational studies of 90 NAFLD patients showed that DR was identified in 90% of biopsy samples, and its extent was correlated with fibrosis stage[15]. Similarly, Gadd et al[13] also found that DR appeared in almost all NASH patients, and its grade was significantly associated with pathological liver progression. Similar to the results in adult NAFLD, DR can also be found in pediatric NAFLD, and its extent and/or HPC expansion were significantly correlated with fibrosis degree[44-46].
DR also exists in animal NAFLD models. In an 8-wk methionine/choline-deficient (MCD) diet mouse model and a 16-wk western diet mouse model, the number of YAP+, CK19+ reactive-appearing ductular cells, and HPCs were significantly increased with the severity of hepatocyte injury and inflammation[47]. A recent study based on mouse models indicated that during NASH development, YAP activation occurred earlier than DR but they were spatiotemporally correlated. Murine YAP activation may promote hepatocyte dedifferentiation during NASH development[48]. Morell et al[49] also established an 8-wk MCD diet mouse model and found that DR extent and HPC number increased steadily over time in the portal and lobular areas. Furthermore, the extent of DR rose significantly in a 12-wk western diet and carbon tetrachloride-treated mouse model, which led to severe NASH-related fibrosis. DR can also occur in other NAFLD animal models, such as rats and monkeys[50,51]. Although some animal models are particularly useful, especially for studying liver regeneration, many features of DR in humans are significantly different from those of animals[18]. The contrasting anatomical features of the two species likely account for this distinction. In humans, cholangiocytes are classified based on the diameter of the biliary tract, which can vary from small to medium to large, resulting in different sizes of the cells. Unlike humans, rodents have small BDs and large BDs, lined by small BDs and large BD cells, respectively, with distinct functional properties[52].
Interestingly, the location of DR varies in different NAFLD patient populations. In pediatric NAFLD patients, DR often appears in the portal/periportal area. In a retrospective study involving 30 children and adolescents with biopsy-proven NAFLD, CK7-positive HPCs localized at the portal-parenchymal interface, i.e. the periportal site[45]. Similarly, a cohort study of 32 children and adolescents with biopsy-proven NAFLD showed that DR commonly occurred in the portal area[46]. In another pediatric NAFLD study, the authors gathered 38 biopsy specimens from NASH children in three United Kingdom medical centers. They found DR at the interface between the parenchyma and portal areas in 36 NASH patients[44]. Similarly, portal DR can also occur in adult NAFLD patients[13-15]. However, in adult NAFLD patients, CK7+ cells and/or CK7+ structures can be found in the centrilobular area. Interestingly, CK7+ cells and/or CK7+ structures in centrilobular zones universally occurred in several other CLDs (including chronic viral hepatitis, autoimmune hepatitis, drug-induced liver injury, etc), which was termed centrilobular DR[53-55]. Both centrilobular DR and periportal DR were also found in adult NAFLD studies and showed a significant correlation with NASH progression[15,55,56]. Importantly, centrilobular DR was also located, and the correlation of fibrosis stage with centrilobular DR was much stronger than with periportal DR (regression coefficient: 1.856 vs 0.646)[15].
The difference in DR localization between pediatric NAFLD and adult NAFLD is plausible. In children, pediatric NASH is characterized by portal inflammation and/or fibrosis[57-59]. Since it is acknowledged that periportal DR is closely related to NASH progression in pediatric NAFLD, the localization of DR in the portal area is reasonable. The concept of centrilobular DR seemingly contradicts the localization characteristic (portal area) in the classic DR definition in adults. However, this phenomenon might be explained from the following two perspectives. From the pathology standpoint, centrilobular fibrosis, i.e. zone 3 fibrosis, is one of the typical pathological features of adult NASH[8]. Therefore, DR – a process related to fibrosis – would emerge in the centrilobular area by fibrosis location. Regarding the underlying pathophysiological mechanism, it has been postulated that CK7+ cells/structures in centrilobular DR might stem from hepatocytes through metaplastic response and/or dedifferentiation[55,60]. Hence, the concept of DR in NAFLD should be expanded to cover centrilobular DR[17]. In a cross-sectional analysis, it was found that centrilobular DR was highly correlated with the stage of fibrosis in adult non-alcoholic steatohepatitis[15]. In addition, centrilobular was the dominant injury pattern, presumably due to pressure induced by mechanical injury[53]. Besides, in NASH, the different underlying impact between centrilobular DR and periportal DR on disease development remains to be clarified.
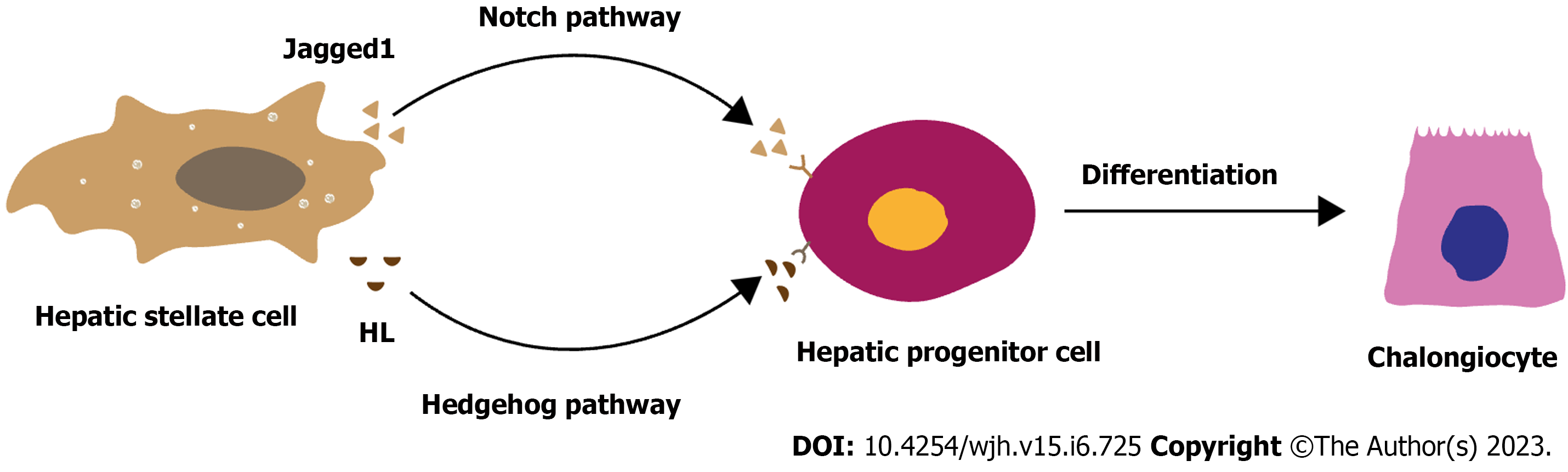
The DR microenvironment, composed of parenchymal cells, mesenchymal cells, inflammatory cells, and their secreted substances, participates in the activation, proliferation, and differentiation of HPCs[12,61,62]. Different components drive HPC differentiation fate in different directions (Figure 1). Previous studies have indicated that HPCs reside in a specialized microenvironment (niche), which is crucial in determining their cell fate. Laminins, as part of the extracellular matrix (ECM), control the expansion of HPCs in an undifferentiated state, and hence DR, during liver injury. Other studies have demonstrated that HSCs and myofibroblasts might play an essential role in the differentiation of HPCs towards the cholangiocyte cell phenotype, while macrophages may participate in HPC differentiation into hepatocyte phenotypes[12,63]. A previous study showed that estimated glomerular filtration rate (EGFR) ligands were present in the liver microenvironment. In animal models lacking EGFR catalytic activity, the expansion of HPCs can be observed after DDC-induced liver damage, indicating that the lack of EGFR may promote HPC differentiation into hepatocytes, and thus liver regeneration[64]. However, it is noteworthy that the differentiation of HPCs is not modulated by a single factor but by a complicated cellular and molecular network in liver diseases. HPCs tend to differentiate into biliary cell phenotypes in NASH, which may involve the participation of HSCs, myofibroblasts, macrophages, and natural killer T (NKT) cells[13-15,18,44]. At the molecular level, Notch and Hedgehog pathways may be the critical pathways in HPC differentiation into the biliary cell phenotype in NASH patients and mice[16,19,65] (Figure 1).
HSCs, located in the space of Disse, are the critical cells for liver fibrosis development and progression[66,67]. HSCs maintain a quiescent phenotype in normal liver but they can be activated by multiple factors in NAFLD, such as inflammatory cells, damaged hepatocytes, oxidative stress, etc[66]. Activated HSCs can acquire a myofibroblast phenotype and increase ECM production, contributing to NASH progression[67].
HSC fibrogenic activation promotes HPC differentiation into hepatocytes to restore mass and function[68]. A subfamily of the inhibitor of apoptosis protein family, survivin (also called baculoviral inhibitor of apoptosis repeat containing-5), has minimal expression in differentiated cells and is associated with cell division. Activated HSCs and HPCs can express survivin. Survivin protein is upregulated with increasing fibrogenic activation of HSCs from their quiescent state. Survivin protein can suppress the fibrotic response of HSCs. At this point, the regenerative capacity of hepatocytes is diminished, followed by replenishment with survivin-expressing HPCs, which differentiate into hepatocytes to promote liver regeneration[68].
HSCs also play an essential role in NAFLD-related DR, possibly by inducing HPCs to differentiate into CK7+ and/or CK19+ cells[12,17,69,70]. In NAFLD, the emergence of DR is accompanied by a significant increase in HSCs and ECM in the DR microenvironment, and the number of HSCs is associated with the DR stage and CK7+ HPC expansion[13]. A similar association between HSC and DR can also be found in other liver diseases, such as hepatitis C infection and primary biliary cirrhosis[13,16]. Further studies have partially explained the underlying mechanism of HSC-mediated HPC differentiation[25,69].
Primary studies have shown that HSC-mediated HPC differentiation may involve the Notch and Hedgehog pathways. In the DR microenvironment, activated HSCs can upregulate the Notch pathway in HPCs by expressing Jagged1 (a Notch pathway ligand)[60,63], leading to the expression of Notch pathway target genes such as hes-related family bHLH transcription factor with YRPW motif 1 and hairy and enhancer of split homolog-1[63,71,72]. Increased Notch target gene expression can further increase the expression of hepatic nuclear factor 1β (HNF1β) and HNF6, consequently contributing to HPC differentiation into biliary cells and BD formation[73-75]. Similarly, activated HSCs can upregulate the Hedgehog pathway in HPCs by expressing HL (a ligand of the Hedgehog pathway), leading to an increase in the Gli transcription factor family (Gli1, Gli2, and Gli3)[76]. Furthermore, Gli2 can translocate to the nucleus and promote target gene transcription[77,78], whose activation can promote the proliferation and differentiation of HPCs into CK7+ cells[79-83]. Elevated activity of Notch and Hedgehog pathways was analogous to disease severity in studies of both mouse models of NASH and patients with NASH[48,79,84], indicating the potential role of Notch and Hedgehog pathways in HSC-mediated HPC differentiation (Figure 2).
Emerging evidence suggests that macrophages are a heterogeneous population of cells. There are two types of macrophages: Resident macrophages, i.e. Kupffer cells, originating from yolk sac-derived erythroid, myeloid progenitors in the fetal liver; and infiltrating macrophages originating from bone marrow-derived circulating monocytes[7]. In NAFLD, macrophages can be activated and differentiated into two types of macrophages: M1 and M2 macrophages[7]. M1 macrophages secrete pro-inflammatory cytokines and have high phagocytic activity, whereas M2 macrophages secrete immune-suppressive but pro-fibrogenic cytokines[85,86].
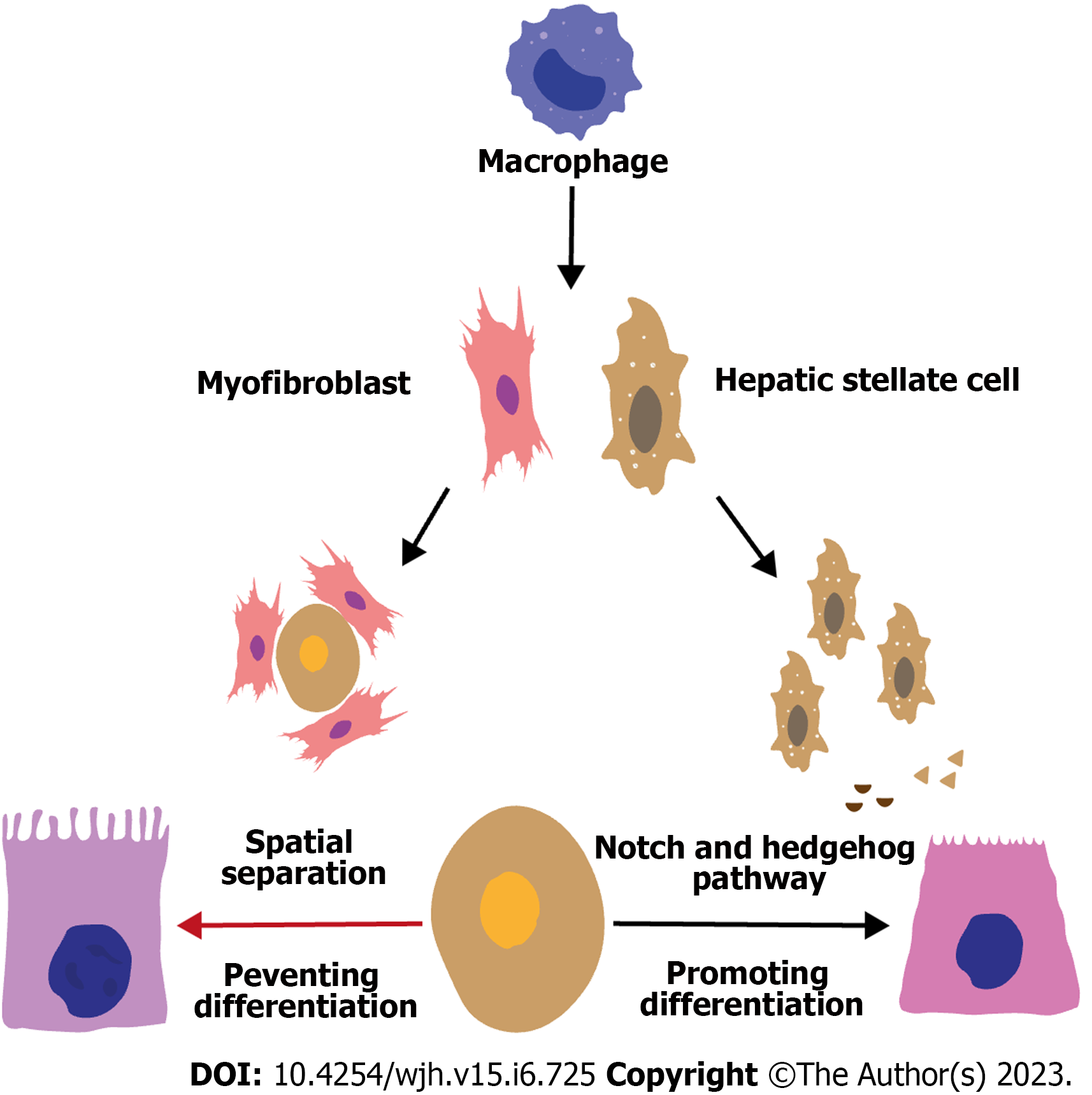
Although it is universally acknowledged that macrophages play a critical role in NAFLD progression, the relationship between macrophages and HPC differentiation in NAFLD-related DR remains elusive. Macrophages were found to promote HPC differentiation into hepatocytes in the DDC diet mouse model, and the Wnt/β-catenin pathway was the key mechanism in this process[69,83,87]. After phagocytosis of the hepatocyte debris, macrophages increase the expression and secretion of Wnt3a (a ligand of the Wnt/β-catenin pathway), activating the Wnt/β-catenin pathway in HPCs[12,63]. Therefore, β-catenin can translocate to the nucleus and bind its co-activators (e.g., CREB-binding protein), promoting the expression of target genes such as SOX9, MYC, and Twist-related protein 1, all of which are associated with HPC differentiation into hepatocytes[63,88]. Studies have shown that HPCs activate during chronic liver injury when hepatocyte proliferation is insufficient to reach homeostasis. During transforming growth factor (TGF)-induced apoptosis in a fibrogenic environment, HPC expands due to a balance between proliferation and apoptosis, which is favorable in a fibrogenic climate. Mitogens that trigger HPC expansion overlap significantly with pro-inflammatory cytokines released by hepatic macrophages including tumor necrosis factor, interferon gamma (IFN-γ), interleukin 6 (IL-6), and TWEAK. Human amnion epithelial cell-treated NASH mice showed a reduction in both HPC and macrophage numbers and expression levels of HPC mitogens and macrophage-released cytokines[89]. In NAFLD patients, macrophages increased significantly in the DR area, and macrophage infiltration was mainly related to the expansion of CK7+ HPCs and fibrosis stage, indicating the potential role of the macrophage in the HPC differentiation fate[13,46]. However, in the context of liver diseases, the role of macrophages in determining HPC differentiation fate is still unclear. Deduced from the aforementioned basic studies, the increased macrophage infiltration in the DR area of NAFLD patients may promote the differentiation of HPCs into hepatocytes. Nonetheless, according to pathological findings, the actual characteristic of NAFLD-related DR is HPC differentiation into cholangiocytes. Therefore, this seemingly contradictory phenomenon might be explained from the following two perspectives.
The regulation of macrophage-mediated HPC differentiation fate may vary across different disease contexts, which is one potential explanation. Disease pathogenesis in the DDC diet mouse model is highly distinct from NAFLD pathogenesis. Therefore, the functional state of macrophages in NAFLD might be correspondingly specific to that in the DDC diet mouse model. Second, the crosstalk between macrophages and HSCs in NAFLD may predominantly contribute to the differentiation of HPCs into cholangiocytes. It has been well established in NAFLD that macrophages can express multiple pro-fibrotic factors (such as platelet-derived growth factors subunit B and TGF-β), contributing to the proliferation and activation of HSCs and myofibroblasts[7,66,90-92]. Notably, macrophages were near HSCs in the DR area in NAFLD patients, indicating a potential promotive effect of macrophages in driving HPC differentiation into cholangiocytes by activating HSCs[13,46].
Conversely, HSCs might hinder macrophage-mediated HPC differentiation into hepatocytes by interrupting the interaction between macrophages and HPCs in spatial separation. In a biliary regeneration model, HPCs were surrounded by a thick sheath-like layer of myofibroblasts and collagen I, which excluded macrophages from forming a close association with HPCs[63]. Similar sheath-like structures might also exist in NAFLD; however, further studies in NAFLD patients are needed to validate the potential existence of this structure in the DR area. In summary, macrophages may participate in NAFLD-related DR onset and development through crosstalk with cells such as HPCs and HSCs. However, its specific role and related mechanisms warrant further investigation (Figure 3).
According to recent studies, NAFLD/NASH development is primarily influenced by the interaction between DR and mast cells (MCs)[93,94]. MCs may promote NAFLD/NASH progression by activating Kupffer cells and HSCs with histamine[94]. Recruitment of MCs is a characteristic of BD injury. It has been proven that knocking down or inhibiting the expression of MCs can effectively reduce DR[95,96]. MC-derived TGF-β1 is a critical regulator of hepatobiliary damage, and blockage of TGF-β1 can ameliorate DR and other features of cholestatic liver injury[97]. MCs were found to promote microve
Moreover, miR-144-3p showed increased expression in insulin resistance in NASH. Meanwhile, DR expansion in mouse models of Western diet with NASH is more sensitive. The phenotypic changes are associated with the secretion of insulin-like growth factor 1 by cholangiocytes, driving peribiliary infiltration and MC activation. Consistent with this finding, MCs from NASH patients accumulate in the portal area, directly correlating with fibrosis stage[93]. A more relevant study discovered that inhibiting MCs reduced DR, inflammation, fibrosis, and recovery from liver injury after MC injection[94].
Previous studies have demonstrated that elevated farnesoid X receptor (FXR) expressed by MCs can be detected in primary sclerosing cholangitis, primary biliary cholangitis, and NAFLD[100-102]. MC-FXR plays a critical role in liver injury and DR in a cholestasis model, where MCs express FXR and infiltrate the liver promoting liver fibrosis during cholestasis and triggering biliary injury. After migration and activation, MCs induce DR and senescence through paracrine interactions with cholangiocytes. Moreover, the MC-FXR signaling pathway modulates the biliary senescence/senescence-associated secretory phenotype and histamine H1- and H2-receptor signaling pathways to regulate total bile acid and then affects DR and liver injury[103]. According to these studies, MCs are corrected with DR in various liver diseases and may affect the differentiation of HPCs through macrophages, HSCs, and fibroblasts. However, the mechanism by which MCs influence HPC differentiation remains obscure.
ECM – a supporting structure for organs, tissues, and cells-represents a complex protein network including fibrillar and non-fibrillar collagen, laminin, fibronectin, etc[104]. ECM proteins can play a vital role in HPC differentiation fate. For example, loss of the basement membrane, a cell-supporting structure, is correlated with the increased level of HNF4 in HPCs, indicating the differentiation of HPCs into hepatocytes[105]. In addition, laminin can upregulate the expression of the biliary marker gene and downregulate hepatocyte transcription factor C/EBPa in HPCs, driving HPC differentiation into cholangiocytes[106]. A recent study based on mouse models of chronic parenchymal damage showed that iloprost reduces laminin deposition and enhances the differentiation of HPCs into hepatocytes[107]. The disruption of integrin β6, an adhesion receptor that interacts with fibronectin and TGF-β1, inhibits the response of HPCs to tissue damage. Significant ECM deposition, such as collagen deposition, is commonly found in NAFLD-related fibrosis[67,108]. Therefore, the accumulation of ECM during the development of NAFLD may contribute to HPC differentiation and the formation of DR.
Cellular senescence, a cell cycle arrest response, is mediated by the induction of cyclin-dependent kinase inhibitors p21 and p16[109,110]. In NAFLD, hepatocyte senescence involves multiple factors, such as oxidative stress and inflammation, and is characterized by increased p21 levels[111,112]. Interestingly, hepatocyte senescence, i.e. replicative arrest, may activate HPC proliferation and differentiation. Oxidative stress induces hepatocyte senescence with consequent cell cycle arrest and impaired regeneration[113]. A recent study demonstrated that oxidative stress can affect HPC differentiation, and the redox is regulated by various transcription factors, of which nuclear factor (erythroid-derived 2)-like 2 (NRF2) plays a crucial role in HPC differentiation, and its activation can inhibit oxidative stress. As stemness is maintained in HPCs through constitutive NRF2 activation, it is inhibited when HPCs are activated during liver injury, e.g., NASH.
Interestingly, NRF2 inhibition increases the transplantation efficiency of human HPCs[114]. In an MDM2-deleted mouse model, server hepatocyte senescence was characterized by a high p21 level and resulted in significant HPC proliferation and differentiation into hepatocytes[33]. However, in NAFLD patients and the choline-deficient and ethionine-supplemented (CDE) diet mouse model, mild hepatocyte senescence was also identified by a lower p21 level and was positively correlated with DR stage and CK7+ HPC expansion, conversely indicating a potential role of hepatocyte senescence in HPC differentiation into cholangiocytes[14,33]. To reconcile these apparently conflicting findings, some experts have suggested that the absence of hepatocyte senescence may enable hepatocytes to undergo self-regeneration without relying on HPC-mediated regeneration[33]. In addition, hepatocytes are the primary source of liver regeneration in a healthy liver, while HPCs do not participate in normal liver regeneration. Therefore, it might be further speculated that aging and healthy hepatocytes may regulate HPC differentiation. Nevertheless, the mechanism by which aging hepatocytes and/or healthy hepatocytes regulate HPC differentiation fate is yet to be elucidated.
NKT cells – a type of innate immune cell in the liver – can participate in the development of liver inflammation and fibrosis[115]. In NAFLD, NKT cells significantly increase in the DR area, and their infiltration extent correlates with both NASH severity and DR stage[80,116]. Conversely, liver biopsies of HBV patients often reveal a pronounced DR and diminished expression of IFN-γ, which is caused by NKT cells. Nevertheless, treatment with IFN-γ has been shown to ameliorate DR in these patients[117]. However, the role of NKT cells in HPC differentiation fate is unclear in NAFLD-related DR. There is evidence suggesting a promotive role of NKT cells in HPC differentiation into cholangiocytes in liver injury models. In these studies, NKT cells increased the expression of IL-13 and the production of Hedgehog ligands, which may drive HPC differentiation into cholangiocytes[80,118-121]. Nevertheless, it is unclear whether NKT cells are required for HPC differentiation into biliary cells in NASH.
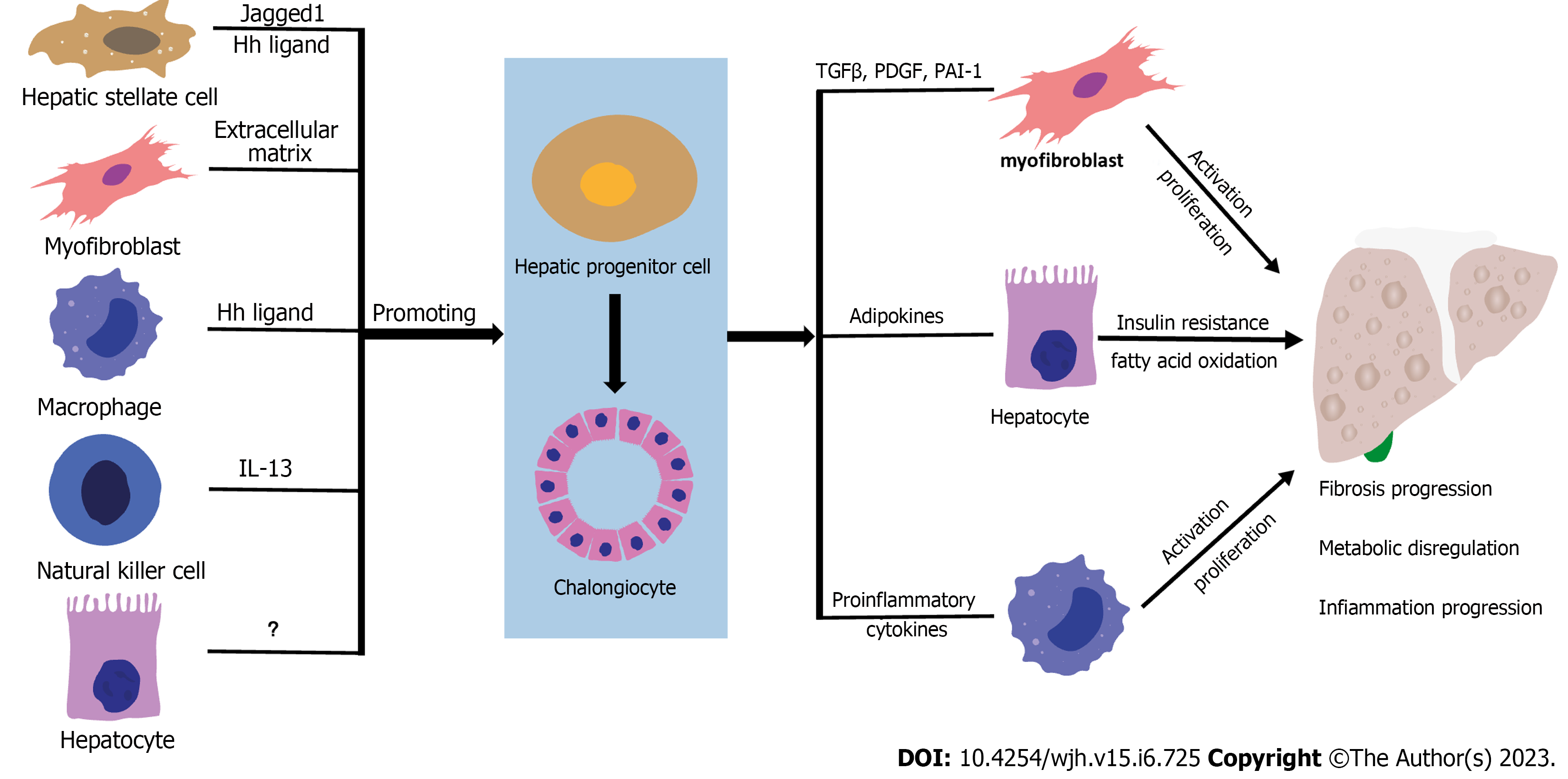
In addition to the impact of the NASH-related DR microenvironment on HPC differentiation fate, differentiated HPCs can aggravate inflammation and fibrosis progression in NASH. As aforementioned, there is a close correlation between HPC expansion and NASH progression, indicating the potential role of differentiated HPCs in aggravating NASH. Moreover, the promotive role of differentiated HPCs in NASH inflammation and fibrosis progression has been proven in NASH-related animal models. Although the underlying mechanism has yet to be fully understood, it may involve the participation of HSCs, macrophages, adipokines, and the epithelial-mesenchymal transition (EMT) (Figure 1).
Differentiated HPCs may participate in HSC-mediated NASH-related fibrosis by promoting HSC activation and proliferation. Increased hepatic levels of several factors, such as PDGF, connective tissue growth factor (CTGF), and Hedgehog ligands, have been found in NAFLD animal models[60,122,123]. In basic studies, HPCs are one of the sources of PDGF, CTGF, and Hedgehog ligands[81,122]. The promotive role of these molecules in enhancing HSC proliferation, accumulation, and ECM production has been well established[81,124-126]. Therefore, these pathways may be involved in HPC-mediated HSCs activation in NASH aggravation.
In addition to directly promoting HSC and myofibroblast activation, HPCs may undergo the EMT towards myofibroblasts, consequently leading to hepatic fibrosis progression. EMT is a cell reprogramming process from the epithelial to mesenchymal phenotype[76,77,127]. EMT in hepatocytes, cholangiocytes, and HSCs can be found in various liver diseases and is related to hepatic fibrosis[76,128,129]. A proportion of HPCs can go through the EMT, which is characterized by the upregulation of mesenchymal cell markers [such as alpha-smooth muscle actin (α-SMA) and S100 calcium-binding protein A4) and downregulation of epithelial cell markers (such as CK7 and CK19)[130-133]. Differentiated HPCs (CK7+] that highly express α-SMA can be found in NAFLD, indicating the presence of HPC-originated EMT and its potential contribution to fibrosis pathogenesis[79]. The onset of EMT in HPCs may involve the Hedgehog pathway activity and TGF-β[79]. Notably, whether high expression of a-SMA or collagen in HPCs can be regarded as the EMT remains controversial. This is because a recent lineage tracing study, using an α-fetoprotein Cre mouse model, provided strong evidence against the existence of HPC-myofibroblast transition[134]. Therefore, further basic studies regarding the origination of α-SMA and CK7 double-positive cells are warranted.
Differentiated HPCs can promote macrophage-mediated inflammation in NASH. Studies have shown that macrophages play an essential role in NASH aggravation[7]. As previously mentioned, significant macrophage infiltration was detected in the NAFLD-related DR area. The number of macrophages is significantly associated with the extent of DR and HPC expansion, indicating that HPCs have a potential role in macrophage recruitment[13]. Primary studies have proven that multiple factors, such as chemokines and pro-inflammatory cytokines, are involved in HPC-mediated macrophage recruitment[7,135-137]. For example, HPCs can contribute to macrophage recruitment by increasing C-C motif chemokine ligand 2 and C-X3-C motif chemokine ligand 1 expression and promote macrophage polarization into M1-type by secreting IL-1, IL-6, and IFN-γ, consequently exacerbating hepatic inflammation[7,135-137]. Therefore, these cytokines may participate in HPC-mediated macrophage infiltration and activation in NASH.
Metabolic dysregulation is a major hallmark in the pathophysiological process of NAFLD, and differentiated HPCs exacerbate by causing dysregulation of the secretion of adipokines, leading to an increase in NASH progression. Adipokines, including adiponectin, leptin, and resistin, contribute to NAFLD development by modulating glycolipid metabolism, inflammatory response, and HSC activation[138]. Although adipokines are mainly produced by adipose tissues, they have also been found to secrete adiponectin and resistin[45,139]. Notably, in NASH, differentiated HPCs increase resistin expression and downregulate adiponectin expression. Moreover, resistin expression in HPCs is positively correlated with the severity of NAFLD.
By contrast, adiponectin expression in HPCs was found to be negatively correlated with the severity of NAFLD, indicating that adipokines play a role in HPC-mediated NASH progression[45]. Adiponectin can suppress hepatic lipogenesis and the production of proinflammatory cytokines but can stimulate insulin secretion and fatty acid oxidation in the liver[140,141]. By contrast, resistin reduces peripheral insulin sensitivity and promotes the expression of proinflammatory cytokines[138,142]. In NASH, adipokine dysregulation aggravates insulin resistance, worsening liver inflammation and injury, which also increases HSC activation, thereby aggravating NASH[45,143-145]. Therefore, the NAFLD-related microenvironment can cause the dysregulation of adipokine expression in HPCs, leading to NAFLD-related metabolic dysregulation.
Studies conducted in the past 100 years have shown that DR may be a compensatory reaction to liver injury, but the correlation between DR and NAFLD needs to be sufficiently studied. The expected prevalence of DR in NAFLD patients, and more importantly, the close relationship between DR and the progression of inflammation and fibrosis in NASH, remain to be clarified. Although DR promotes liver regeneration[54,146], it remodels the NASH microenvironment, which aggravates rather than alleviates NASH severity, similar to the initially upright “Macbeth” getting perverted under a corruptive lure. In NAFLD, HPC proliferation and differentiation, the core processes in DR pathogenesis, might be triggered by NAFLD-related liver injury. The cells (such as HSCs and macrophages) and their secreted substances may drive the differentiation of HPCs into cholangiocytes. Conversely, differentiated HPCs may, in turn, aggravate NASH through multiple pathways, which may involve the participation of HSCs, macrophages, adipokines, and the EMT. The involvement of these cells in the interaction between DR and NASH pathogenesis may form a ‘vicious circle,’ presumably leading to further progression of hepatic inflammation and fibrosis.
However, the bilateral interaction between DR and NAFLD remains to be further verified. For the DR caused by NAFLD, the majority of previous findings about NAFLD-related DR were primarily obtained through observational studies. Several signaling pathways are involved in DR (e.g., Notch, Hedgehog, TWEAK), and it was recently discovered that long non-coding RNA/p300 could influence DR progression[147]. However, how these pathways promote the pathogenesis of DR in the context of NAFLD remains unclear. We are still determining whether the pathways mentioned above are involved in DR-related NAFLD. The key factors driving HPC differentiation in NAFLD need to be further investigated. In addition, in terms of the impact of DR on the pathogenesis of NAFLD, considering our limited understanding of the core molecular mechanism driving DR, it is difficult to provide a direct and exact intervention towards the DR onset, which hinders establishment of a causal effect of DR on NAFLD progression. Therefore, we need further investigations to deepen our understanding of the core and characteristic pathways of DR, to achieve the development of DR-targeted intervention in NAFLD-related studies. More importantly, the underlying mechanisms of both NAFLD-caused DR and HPC-mediated NAFLD progression may be important targets for treating NAFLD.
We thank Dr. Shuangzhe Lin for his helpful discussions and comments in preparing this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kordzaia D, Georgia; Silva LD, Brazil S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Cai YX
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3775] [Article Influence: 539.3] [Reference Citation Analysis (2)] |
| 2. | Hashimoto E, Tokushige K, Ludwig J. Diagnosis and classification of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Current concepts and remaining challenges. Hepatol Res. 2015;45:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2807] [Article Influence: 561.4] [Reference Citation Analysis (1)] |
| 4. | Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 585] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 5. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4932] [Article Influence: 704.6] [Reference Citation Analysis (9)] |
| 6. | Xu C, Wan X, Xu L, Weng H, Yan M, Miao M, Sun Y, Xu G, Dooley S, Li Y, Yu C. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: One stone hits two birds. J Hepatol. 2015;62:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 8. | Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 950] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 9. | Working Group*; Association of Pathologists** for Guidebook of NASH and NAFLD, 2015: The Japan Society of Hepatology. Pathological Findings of NASH and NAFLD: for Guidebook of NASH and NAFLD, 2015: The Japan Society of Hepatology. Hepatol Res. 2017;47:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, Neuschwander-Tetri BA; NASH Clinical Research NetworkA list of members of the Nonalcoholic Steatohepatitis Clinical Research Network can be found in the Appendix. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Sjöblom N, Boyd S, Kautiainen H, Arola J, Färkkilä M. Novel histological scoring for predicting disease outcome in primary sclerosing cholangitis. Histopathology. 2022;81:192-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Boulter L, Lu WY, Forbes SJ. Differentiation of progenitors in the liver: a matter of local choice. J Clin Invest. 2013;123:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 14. | Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 362] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 15. | Zhao L, Westerhoff M, Pai RK, Choi WT, Gao ZH, Hart J. Centrilobular ductular reaction correlates with fibrosis stage and fibrosis progression in non-alcoholic steatohepatitis. Mod Pathol. 2018;31:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 521] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011;458:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Sato K, Marzioni M, Meng F, Francis H, Glaser S, Alpini G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology. 2019;69:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 20. | Schmelzer E, Pietrosi G, Gridelli B, Gerlach J. Characterization of CD326-positive human hepatic stem cells. Clin Exp Hepatol. 2021;7:101-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Abou Monsef Y, Kutsal O. Immunohistochemical evaluation of hepatic progenitor cells in different types of feline liver diseases. J Vet Med Sci. 2021;83:613-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Turányi E, Dezsö K, Csomor J, Schaff Z, Paku S, Nagy P. Immunohistochemical classification of ductular reactions in human liver. Histopathology. 2010;57:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int. 2006;26:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Misra S, Majumdar K, Sakhuja P, Jain P, Singh L, Kumar P, Dubey AP. Differentiating Biliary Atresia From Idiopathic Neonatal Hepatitis: A Novel Keratin 7 Based Mathematical Approach on Liver Biopsies. Pediatr Dev Pathol. 2021;24:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Svegliati-Baroni G, Faraci G, Fabris L, Saccomanno S, Cadamuro M, Pierantonelli I, Trozzi L, Bugianesi E, Guido M, Strazzabosco M, Benedetti A, Marchesini G. Insulin resistance and necroinflammation drives ductular reaction and epithelial-mesenchymal transition in chronic hepatitis C. Gut. 2011;60:108-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Sato K, Pham L, Glaser S, Francis H, Alpini G. Pathophysiological Roles of Ductular Reaction in Liver Inflammation and Hepatic Fibrogenesis. Cell Mol Gastroenterol Hepatol. 2023;15:803-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 28. | Xu M, Xie F, Qian G, Jing Y, Zhang S, Gao L, Zheng T, Wu M, Yang J, Wei L. Peritumoral ductular reaction: a poor postoperative prognostic factor for hepatocellular carcinoma. BMC Cancer. 2014;14:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Barthet VJA, Brucoli M, Ladds MJGW, Nössing C, Kiourtis C, Baudot AD, O'Prey J, Zunino B, Müller M, May S, Nixon C, Long JS, Bird TG, Ryan KM. Autophagy suppresses the formation of hepatocyte-derived cancer-initiating ductular progenitor cells in the liver. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Cadamuro M, Lasagni A, Sarcognato S, Guido M, Fabris R, Strazzabosco M, Strain AJ, Simioni P, Villa E, Fabris L. The Neglected Role of Bile Duct Epithelial Cells in NASH. Semin Liver Dis. 2022;42:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 31. | Calcagno DM, Chu A, Gaul S, Taghdiri N, Toomu A, Leszczynska A, Kaufmann B, Papouchado B, Wree A, Geisler L, Hoffman HM, Feldstein AE, King KR. NOD-like receptor protein 3 activation causes spontaneous inflammation and fibrosis that mimics human NASH. Hepatology. 2022;76:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Kennedy L, Carpino G, Owen T, Ceci L, Kundu D, Meadows V, Kyritsi K, Franchitto A, Onori P, Isidan A, Zhang W, Ekser B, Alvaro D, Gaudio E, Gershwin ME, Francis H, Glaser S, Alpini G. Secretin alleviates biliary and liver injury during late-stage primary biliary cholangitis via restoration of secretory processes. J Hepatol. 2023;78:99-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, Ridgway RA, Kendall T, Williams MJ, Jamieson T, Raven A, Hay DC, Iredale JP, Clarke AR, Sansom OJ, Forbes SJ. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 372] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 34. | Russell JO, Lu WY, Okabe H, Abrams M, Oertel M, Poddar M, Singh S, Forbes SJ, Monga SP. Hepatocyte-Specific β-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes. Hepatology. 2019;69:742-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 35. | Hu S, Russell JO, Liu S, Cao C, McGaughey J, Rai R, Kosar K, Tao J, Hurley E, Poddar M, Singh S, Bell A, Shin D, Raeman R, Singhi AD, Nejak-Bowen K, Ko S, Monga SP. β-Catenin-NF-κB-CFTR interactions in cholangiocytes regulate inflammation and fibrosis during ductular reaction. Elife. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. [1097-4172 (Electronic)]. |
| 37. | Xu J, Kausalya PJ, Ong AGM, Goh CMF, Mohamed Ali S, Hunziker W. ZO-2/Tjp2 suppresses Yap and Wwtr1/Taz-mediated hepatocyte to cholangiocyte transdifferentiation in the mouse liver. NPJ Regen Med. 2022;7:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 38. | Canesin G, Feldbrügge L, Wei G, Janovicova L, Janikova M, Csizmadia E, Ariffin J, Hedblom A, Herbert ZT, Robson SC, Celec P, Swanson KD, Nasser I, Popov YV, Wegiel B. Heme oxygenase-1 mitigates liver injury and fibrosis via modulation of LNX1/Notch1 pathway in myeloid cells. iScience. 2022;25:104983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 39. | Chen L, Wu N, Kennedy L, Francis H, Ceci L, Zhou T, Samala N, Kyritsi K, Wu C, Sybenga A, Ekser B, Dar W, Atkins C, Meadows V, Glaser S, Alpini G. Inhibition of Secretin/Secretin Receptor Axis Ameliorates NAFLD Phenotypes. Hepatology. 2021;74:1845-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Coll M, Ariño S, Martínez-Sánchez C, Garcia-Pras E, Gallego J, Moles A, Aguilar-Bravo B, Blaya D, Vallverdú J, Rubio-Tomás T, Lozano JJ, Pose E, Graupera I, Fernández-Vidal A, Pol A, Bataller R, Geng JG, Ginès P, Fernandez M, Sancho-Bru P. Ductular reaction promotes intrahepatic angiogenesis through Slit2-Roundabout 1 signaling. Hepatology. 2022;75:353-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Chen L, Zhou T, White T, O'Brien A, Chakraborty S, Liangpunsakul S, Yang Z, Kennedy L, Saxena R, Wu C, Meng F, Huang Q, Francis H, Alpini G, Glaser S. The Apelin-Apelin Receptor Axis Triggers Cholangiocyte Proliferation and Liver Fibrosis During Mouse Models of Cholestasis. Hepatology. 2021;73:2411-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Park YN, Kojiro M, Di Tommaso L, Dhillon AP, Kondo F, Nakano M, Sakamoto M, Theise ND, Roncalli M. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer. 2007;109:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Zhou T, Kundu D, Robles-Linares J, Meadows V, Sato K, Baiocchi L, Ekser B, Glaser S, Alpini G, Francis H, Kennedy L. Feedback Signaling between Cholangiopathies, Ductular Reaction, and Non-Alcoholic Fatty Liver Disease. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Skoien R, Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, O'Brien PE, Tilg H, Moschen AR, Baumann U, Brown RM, Couper RT, Manton ND, Ee LC, Weltman M, Clouston AD. Heterogeneity of fibrosis patterns in non-alcoholic fatty liver disease supports the presence of multiple fibrogenic pathways. Liver Int. 2013;33:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, Onori P, Alvaro D, Gaudio E. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012;56:2142-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Carpino G, Nobili V, Renzi A, De Stefanis C, Stronati L, Franchitto A, Alisi A, Onori P, De Vito R, Alpini G, Gaudio E. Macrophage Activation in Pediatric Nonalcoholic Fatty Liver Disease (NAFLD) Correlates with Hepatic Progenitor Cell Response via Wnt3a Pathway. PLoS One. 2016;11:e0157246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Machado MV, Michelotti GA, Pereira TA, Xie G, Premont R, Cortez-Pinto H, Diehl AM. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J Hepatol. 2015;63:962-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 48. | Jin L, Huang H, Ni J, Shen J, Liu Z, Li L, Fu S, Yan J, Hu B. Shh-Yap signaling controls hepatic ductular reactions in CCl(4) -induced liver injury. Environ Toxicol. 2021;36:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Morell CM, Fiorotto R, Meroni M, Raizner A, Torsello B, Cadamuro M, Spagnuolo G, Kaffe E, Sutti S, Albano E, Strazzabosco M. Notch signaling and progenitor/ductular reaction in steatohepatitis. PLoS One. 2017;12:e0187384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | de Lima VM, Oliveira CP, Alves VA, Chammas MC, Oliveira EP, Stefano JT, de Mello ES, Cerri GG, Carrilho FJ, Caldwell SH. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol. 2008;49:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Nagarajan P, Venkatesan R, Kumar M, Usmani A, Majumdar SS. Macaca radiata (bonnet monkey): a spontaneous model of nonalcoholic fatty liver disease. Liver Int. 2008;28:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Pai RK, Hart JA. Aberrant expression of cytokeratin 7 in perivenular hepatocytes correlates with a cholestatic chemistry profile in patients with heart failure. Mod Pathol. 2010;23:1650-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Eleazar JA, Memeo L, Jhang JS, Mansukhani MM, Chin S, Park SM, Lefkowitch JH, Bhagat G. Progenitor cell expansion: an important source of hepatocyte regeneration in chronic hepatitis. J Hepatol. 2004;41:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Matsukuma S, Takeo H, Kono T, Nagata Y, Sato K. Aberrant cytokeratin 7 expression of centrilobular hepatocytes: a clinicopathological study. Histopathology. 2012;61:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Gill RM, Belt P, Wilson L, Bass NM, Ferrell LD. Centrizonal arteries and microvessels in nonalcoholic steatohepatitis. Am J Surg Pathol. 2011;35:1400-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol. 2019;16:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 58. | Takahashi Y, Inui A, Fujisawa T, Takikawa H, Fukusato T. Histopathological characteristics of non-alcoholic fatty liver disease in children: Comparison with adult cases. Hepatol Res. 2011;41:1066-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Molleston JP. The histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011;458:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Lanzoni G, Cardinale V, Carpino G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology. 2016;64:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 62. | Overi D, Carpino G, Franchitto A, Onori P, Gaudio E. Hepatocyte Injury and Hepatic Stem Cell Niche in the Progression of Non-Alcoholic Steatohepatitis. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 577] [Cited by in RCA: 606] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 64. | Lazcanoiturburu N, García-Sáez J, González-Corralejo C, Roncero C, Sanz J, Martín-Rodríguez C, Valdecantos MP, Martínez-Palacián A, Almalé L, Bragado P, Calero-Pérez S, Fernández A, García-Bravo M, Guerra C, Montoliu L, Segovia JC, Valverde ÁM, Fabregat I, Herrera B, Sánchez A. Lack of EGFR catalytic activity in hepatocytes improves liver regeneration following DDC-induced cholestatic injury by promoting a pro-restorative inflammatory response. J Pathol. 2022;258:312-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Fiorotto R, Raizner A, Morell CM, Torsello B, Scirpo R, Fabris L, Spirli C, Strazzabosco M. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol. 2013;59:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1974] [Article Influence: 246.8] [Reference Citation Analysis (0)] |
| 67. | Schwabe RF, Tabas I, Pajvani UB. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology. 2020;158:1913-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 68. | Sharma S, Ghufran SM, Das B, Roy B, Ghose S, Biswas S. Survivin expression is essential for early activation of hepatic stellate cells and fibrosis progression in chronic liver injury. Life Sci. 2021;287:120119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 69. | Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 70. | Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 71. | Kurooka H, Kuroda K, Honjo T. Roles of the ankyrin repeats and C-terminal region of the mouse notch1 intracellular region. Nucleic Acids Res. 1998;26:5448-5455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 163] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 681] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 73. | Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, Roskams T, Rousseau GG, Lemaigre FP. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 74. | Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 76. | Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331-3342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 77. | Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 78. | Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, Teaberry V, Choi SS, Conde-Vancells J, Karaca GF, Diehl AM. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478-1488.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 80. | Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, Xie G, Philips G, Chan IS, Karaca GF, Pereira Tde A, Chen Y, Mi Z, Kuo PC, Choi SS, Guy CD, Abdelmalek MF, Diehl AM. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 81. | Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 82. | Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, Diehl AM; NASH CRN. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 83. | Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 84. | Zhu C, Kim K, Wang X, Bartolome A, Salomao M, Dongiovanni P, Meroni M, Graham MJ, Yates KP, Diehl AM, Schwabe RF, Tabas I, Valenti L, Lavine JE, Pajvani UB. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 85. | Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol. 2018;68:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 368] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 86. | Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 812] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 87. | Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, Kong XN, Chen C, Liu SQ, Wu MC, Wang HY. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 88. | Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 1273] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 89. | Goonetilleke M, Kuk N, Correia J, Hodge A, Moore G, Gantier MP, Yeoh G, Sievert W, Lim R. Addressing the liver progenitor cell response and hepatic oxidative stress in experimental non-alcoholic fatty liver disease/non-alcoholic steatohepatitis using amniotic epithelial cells. Stem Cell Res Ther. 2021;12:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Morinaga H, Mayoral R, Heinrichsdorff J, Osborn O, Franck N, Hah N, Walenta E, Bandyopadhyay G, Pessentheiner AR, Chi TJ, Chung H, Bogner-Strauss JG, Evans RM, Olefsky JM, Oh DY. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 91. | Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, Jang MK, Guenther ND, Mederacke I, Friedman R, Dragomir AC, Aloman C, Schwabe RF. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 465] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 92. | Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, Portman JR, Matchett KP, Brice M, Marwick JA, Taylor RS, Efremova M, Vento-Tormo R, Carragher NO, Kendall TJ, Fallowfield JA, Harrison EM, Mole DJ, Wigmore SJ, Newsome PN, Weston CJ, Iredale JP, Tacke F, Pollard JW, Ponting CP, Marioni JC, Teichmann SA, Henderson NC. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 868] [Cited by in RCA: 1093] [Article Influence: 182.2] [Reference Citation Analysis (0)] |
| 93. | Lombardo J, Broadwater D, Collins R, Cebe K, Brady R, Harrison S. Hepatic mast cell concentration directly correlates to stage of fibrosis in NASH. Hum Pathol. 2019;86:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Kennedy L, Meadows V, Sybenga A, Demieville J, Chen L, Hargrove L, Ekser B, Dar W, Ceci L, Kundu D, Kyritsi K, Pham L, Zhou T, Glaser S, Meng F, Alpini G, Francis H. Mast Cells Promote Nonalcoholic Fatty Liver Disease Phenotypes and Microvesicular Steatosis in Mice Fed a Western Diet. Hepatology. 2021;74:164-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 95. | Kennedy L, Hargrove L, Demieville J, Bailey JM, Dar W, Polireddy K, Chen Q, Nevah Rubin MI, Sybenga A, DeMorrow S, Meng F, Stockton L, Alpini G, Francis H. Knockout of l-Histidine Decarboxylase Prevents Cholangiocyte Damage and Hepatic Fibrosis in Mice Subjected to High-Fat Diet Feeding via Disrupted Histamine/Leptin Signaling. Am J Pathol. 2018;188:600-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Hargrove L, Kennedy L, Demieville J, Jones H, Meng F, DeMorrow S, Karstens W, Madeka T, Greene J Jr, Francis H. Bile duct ligation-induced biliary hyperplasia, hepatic injury, and fibrosis are reduced in mast cell-deficient Kit(W-sh) mice. Hepatology. 2017;65:1991-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 97. | Kyritsi K, Kennedy L, Meadows V, Hargrove L, Demieville J, Pham L, Sybenga A, Kundu D, Cerritos K, Meng F, Alpini G, Francis H. Mast Cells Induce Ductular Reaction Mimicking Liver Injury in Mice Through Mast Cell-Derived Transforming Growth Factor Beta 1 Signaling. Hepatology. 2021;73:2397-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 98. | Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Ünalp-Arida A, Wilson LA, Chalasani N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol. 2011;55:654-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (3)] |
| 99. | Fromenty B, Berson A, Pessayre D. Microvesicular steatosis and steatohepatitis: role of mitochondrial dysfunction and lipid peroxidation. J Hepatol. 1997;26 Suppl 1:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 100. | Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 101. | Wunsch E, Milkiewicz M, Wasik U, Trottier J, Kempińska-Podhorodecka A, Elias E, Barbier O, Milkiewicz P. Expression of hepatic Fibroblast Growth Factor 19 is enhanced in Primary Biliary Cirrhosis and correlates with severity of the disease. Sci Rep. 2015;5:13462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Armstrong LE, Guo GL. Role of FXR in Liver Inflammation during Nonalcoholic Steatohepatitis. Curr Pharmacol Rep. 2017;3:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 103. | Meadows V, Kennedy L, Ekser B, Kyritsi K, Kundu D, Zhou T, Chen L, Pham L, Wu N, Demieville J, Hargrove L, Glaser S, Alpini G, Francis H. Mast Cells Regulate Ductular Reaction and Intestinal Inflammation in Cholestasis Through Farnesoid X Receptor Signaling. Hepatology. 2021;74:2684-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 104. | Bedossa P, Paradis V. Liver extracellular matrix in health and disease. J Pathol. 2003;200:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 105. | Paku S, Nagy P, Kopper L, Thorgeirsson SS. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Lorenzini S, Bird TG, Boulter L, Bellamy C, Samuel K, Aucott R, Clayton E, Andreone P, Bernardi M, Golding M, Alison MR, Iredale JP, Forbes SJ. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59:645-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 107. | Govaere O, Cockell S, Van Haele M, Wouters J, Van Delm W, Van den Eynde K, Bianchi A, van Eijsden R, Van Steenbergen W, Monbaliu D, Nevens F, Roskams T. High-throughput sequencing identifies aetiology-dependent differences in ductular reaction in human chronic liver disease. J Pathol. 2019;248:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 750] [Cited by in RCA: 1049] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 109. | Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 790] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 110. | Birch J, Gil J. Blunting senescence boosts liver regeneration. Genes Dev. 2020;34:463-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Aravinthan A, Mells G, Allison M, Leathart J, Kotronen A, Yki-Jarvinen H, Daly AK, Day CP, Anstee QM, Alexander G. Gene polymorphisms of cellular senescence marker p21 and disease progression in non-alcohol-related fatty liver disease. Cell Cycle. 2014;13:1489-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, von Zglinicki T, Jurk D. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 736] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 113. | Sun S, Xie F, Xu X, Cai Q, Zhang Q, Cui Z, Zheng Y, Zhou J. Advanced oxidation protein products induce S-phase arrest of hepatocytes via the ROS-dependent, β-catenin-CDK2-mediated pathway. Redox Biol. 2018;14:338-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Bellanti F, di Bello G, Iannelli G, Pannone G, Pedicillo MC, Boulter L, Lu WY, Tamborra R, Villani R, Vendemiale G, Forbes SJ, Serviddio G. Inhibition of nuclear factor (erythroid-derived 2)-like 2 promotes hepatic progenitor cell activation and differentiation. NPJ Regen Med. 2021;6:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Strick-Marchand H, Masse GX, Weiss MC, Di Santo JP. Lymphocytes support oval cell-dependent liver regeneration. J Immunol. 2008;181:2764-2771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 116. | Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, Teaberry V, Pereira FE, Adams DH, Diehl AM. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998-2007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 117. | Weng HL, Feng DC, Radaeva S, Kong XN, Wang L, Liu Y, Li Q, Shen H, Gao YP, Müllenbach R, Munker S, Huang T, Chen JL, Zimmer V, Lammert F, Mertens PR, Cai WM, Dooley S, Gao B. IFN-γ inhibits liver progenitor cell proliferation in HBV-infected patients and in 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet-fed mice. J Hepatol. 2013;59:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 118. | Syn WK, Witek RP, Curbishley SM, Jung Y, Choi SS, Enrich B, Omenetti A, Agboola KM, Fearing CM, Tilg H, Adams DH, Diehl AM. Role for hedgehog pathway in regulating growth and function of invariant NKT cells. Eur J Immunol. 2009;39:1879-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D, Segawa T, Maeda M, Hamuro J, Nakayama T, Taniguchi M, Yagita H, Van Kaer L, Onóe K, Denhardt D, Rittling S, Uede T. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 120. | Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, Diehl AM. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 121. | Gieseck RL 3rd, Ramalingam TR, Hart KM, Vannella KM, Cantu DA, Lu WY, Ferreira-González S, Forbes SJ, Vallier L, Wynn TA. Interleukin-13 Activates Distinct Cellular Pathways Leading to Ductular Reaction, Steatosis, and Fibrosis. Immunity. 2016;45:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 122. | Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, Pereira TA, Zdanowicz M, Malladi P, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenetti A, Abdelmalek MF, Guy CD, Adams DH, Kuo PC, Michelotti GA, Whitington PF, Diehl AM. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 123. | Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 124. | Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33:4857-4866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 125. | Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 126. | Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 127. | Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 128. | Meindl-Beinker NM, Dooley S. Transforming growth factor-beta and hepatocyte transdifferentiation in liver fibrogenesis. J Gastroenterol Hepatol. 2008;23 Suppl 1:S122-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 129. | Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50:2007-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 130. | Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168:1500-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 131. | Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008;88:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 132. | Ji S, Wang X, Shu J, Sun A, Si W, Guo X, Zhao B, Ji W, Jin L. In vitro generation of myofibroblasts-like cells from liver epithelial progenitor cells of rhesus monkey (Macaca mulatta). In Vitro Cell Dev Biol Anim. 2011;47:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 133. | Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47:636-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 134. | Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 135. | Theise ND, Kuwahara R. The tissue biology of ductular reactions in human chronic liver disease. Gastroenterology. 2007;133:350-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 137. | Viebahn CS, Benseler V, Holz LE, Elsegood CL, Vo M, Bertolino P, Ganss R, Yeoh GC. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2065] [Cited by in RCA: 2248] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 139. | Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 307] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 140. | Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 398] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 141. | Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 142. | Singhal NS, Patel RT, Qi Y, Lee YS, Ahima RS. Loss of resistin ameliorates hyperlipidemia and hepatic steatosis in leptin-deficient mice. Am J Physiol Endocrinol Metab. 2008;295:E331-E338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 143. | Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Ginés P, Colmenero J, Parola M, Gelmini S, Tarquini R, Laffi G, Pinzani M, Marra F. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol. 2006;169:2042-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 144. | Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 145. | Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1594] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 146. | Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology. 2014;59:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 147. | Navarro-Corcuera A, Sehrawat TS, Jalan-Sakrikar N, Gibbons HR, Pirius NE, Khanal S, Hamdan FH, Aseem SO, Cao S, Banales JM, Kang N, Faubion WA, LaRusso NF, Shah VH, Huebert RC. Long non-coding RNA ACTA2-AS1 promotes ductular reaction by interacting with the p300/ELK1 complex. J Hepatol. 2022;76:921-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |