Published online May 27, 2023. doi: 10.4254/wjh.v15.i5.649
Peer-review started: December 31, 2022
First decision: January 30, 2023
Revised: February 17, 2023
Accepted: April 6, 2023
Article in press: April 6, 2023
Published online: May 27, 2023
Processing time: 143 Days and 15.1 Hours
Although the frequency of metabolic risk factors for cirrhosis and hepatocellular carcinoma (HCC) is increasing, chronic hepatitis B (CHB) and chronic hepatitis C (CHC) remain the most relevant risk factors for advanced liver disease worldwide. In addition to liver damage, hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are associated with a myriad of extrahepatic manifestations including mixed cryoglobulinaemia, lymphoproliferative disorders, renal disease, insulin resistance, type 2 diabetes, sicca syndrome, rheumatoid arthritis-like polyarthritis, and autoantibody production. Recently, the list has grown to include sarcopenia. Loss of muscle mass or muscle function is a critical feature of malnutrition in cirrhotic patients and has been found in approximately 23.0%-60.0% of patients with advanced liver disease. Nonetheless, among published studies, there is significant heterogeneity in the aetiologies of hepatic diseases and measurement methods used to determine sarcopenia. In particular, the interaction between sarcopenia, CHB and CHC has not been completely clarified in a real-world setting. Sarcopenia can result from a complex and multifaceted virus-host-environment interplay in individuals chronically infected with HBV or HCV. Thus, in the present review, we provide an overview of the concept, prevalence, clinical relevance, and potential mechanisms of sarcopenia in patients with chronic viral hepatitis, with an emphasis on clinical outcomes, which have been associated with skeletal muscle loss in these patients. A comprehensive overview of sarcopenia in individuals chronically infected with HBV or HCV, independent of the stage of the liver disease, will reinforce the necessity of an integrated medical/nutritional/physical education approach in the daily clinical care of patients with CHB and CHC.
Core Tip: Sarcopenia is a key feature of malnutrition in liver cirrhosis and has been found in approximately 23.0%-60.0% of patients with advanced hepatic disease. Skeletal muscle loss is associated with poor quality of life and increased mortality, which are significant cirrhosis-related complications. In individuals chronically infected with hepatitis B virus or hepatitis C virus, the muscle-liver-immune crosstalk during the development of sarcopenia has not been completely clarified. Based on these findings, an overview of the concept, prevalence, clinical relevance, and potential mechanisms of sarcopenia in patients with chronic viral hepatitis is of utmost importance.
- Citation: Coelho MPP, de Castro PASV, de Vries TP, Colosimo EA, Bezerra JMT, Rocha GA, Silva LD. Sarcopenia in chronic viral hepatitis: From concept to clinical relevance. World J Hepatol 2023; 15(5): 649-665
- URL: https://www.wjgnet.com/1948-5182/full/v15/i5/649.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i5.649
Globally, chronic hepatitis B (CHB) and chronic hepatitis C (CHC) were responsible for almost 96.0% of the 1.3 million deaths related to hepatitis viruses in 2015[1,2]. Two-thirds of the global burden of cirrhosis could be attributed to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections[2] and approximately 720000 deaths involving chronically infected individuals have occurred, mostly from cirrhosis and hepatocellular carcinoma (HCC)[1,2].
HBV and HCV affect hepatocytes and can cause both acute and chronic diseases[3]. Individuals with long-term chronic infections have a considerable risk of developing cirrhosis and HCC during their lifetime[3]. Although the frequency of metabolic risk factors for cirrhosis and HCC, such as metabolic syndrome, obesity, type II diabetes, and non-alcoholic fatty liver disease (NAFLD) is increasing, HBV and HCV are currently the most relevant global risk factors for severe hepatic diseases[4,5].
In addition to potential liver diseases, HCV infection is associated with several extrahepatic manifestations, including mixed cryoglobulinaemia, lymphoproliferative disorders, renal disease, insulin resistance, type 2 diabetes, sicca syndrome, rheumatoid arthritis-like polyarthritis, and autoantibody production[6]. Similar to CHC, CHB can be associated with extrahepatic systemic and/or autoimmune manifestations such as systemic vasculitis, glomerulonephritis, and cutaneous manifestations[7].
Numerous studies have demonstrated that both CHB and CHC are associated with nutritional disorders, especially in hepatic cirrhosis, and patients with impaired metabolic function of the liver are at a high risk of malnutrition. This nutritional abnormality has been identified in 13.0%-70.0% of patients with liver disease[8,9] and it is associated with poor quality of life[10-12] and relevant cirrhosis-related complications such as sepsis[13], refractory ascites[14], hepatic encephalopathy[15,16], spontaneous bacterial peritonitis[17], reduced survival[18], and high mortality[19-21]. Taken together, malnourishment and liver cirrhosis contribute to skeletal muscle wasting, an important marker of malnutrition. Loss of muscle mass or muscle function is the key feature of malnutrition in cirrhotic patients and has been found in approximately 23.0%-60.0% of patients with advanced liver disease[9,22-25].
Sarcopenia has been considered a relevant topic in clinical hepatology settings, and a comprehensive overview of skeletal muscle loss in individuals chronically infected with HBV or HCV, independent of the stage of the liver disease, will strengthen an integrated medical/nutritional/physical education approach in the daily clinical care of patients with CHB and CHC.
Thus, we first contextualised our review in relation to the connection between liver and nutrient metabolism. We then briefly reviewed the origin of the concept of sarcopenia along with the progress in understanding viral hepatitis biology and its related clinical manifestations. Finally, we performed a review to identify and summarise available data on the prevalence and clinical implications of sarcopenia in patients with chronic viral hepatitis.
It is well known that liver plays a central role in the metabolism of nutrients, including macronutrients/micronutrients, vitamin storage and processing, and oxidant/antioxidant balance[26-29]. Hepatic dysfunction can impair the entire spectrum of metabolic and nutritional processes in the body. Therefore, liver diseases are strongly associated with nutritional disorders[9,23,25]. In fat metabolism, hepatocytes break down fats to generate energy[30]. In carbohydrate metabolism, hepatic cells are capable of storing or releasing glucose and contribute to maintaining a constant blood glucose level in circulation[31].
Additionally, the liver is crucial for maintaining protein and nitrogen metabolism[32]. Hepatic cells perform important functions in the balance between protein synthesis and degradation. In healthy individuals, the blood ammonia level originating from amino acid metabolism is controlled by functional hepatic glutamine metabolism and urea cycle in the liver[33,34]. In the presence of cirrhosis, hepatocyte dysfunction is associated with a state of overall protein deficiency and hyperammonaemia. In this setting, glutamine synthesis from glutamate in skeletal muscle mass plays a significant compensatory role in ammonia disposal[33-35].
Although glutamine synthetase activity is low in skeletal muscle[36] because of its large mass, skeletal muscle is quantitatively the most important site of glutamine synthesis. Ammonia uptake by appendicular muscle has been measured in patients with acute liver failure[37] and was estimated to be 100 nmoL/100 g/min. In chronic liver disease, skeletal muscle also functions as an important extrahepatic site for the removal of ammonia[34,38].
Skeletal muscle encompasses 30.0%–40.0% of the total body mass; thus, this organ is the primary protein store in the human body[39]. The protein turnover balance is responsible for maintaining normal skeletal muscle mass[40]. Increased plasma ammonia levels have been linked to sarcopenia, as a potential mediator of muscle depletion in cirrhosis. Several investigations, including those using animal models, have demonstrated that hyperammonaemia stimulates myostatin expression[41-43]. Myokine is a well known inhibitor of protein synthesis[12]. Furthermore, hyperammonaemia results in muscle mitochondrial dysfunction, increased formation of reactive oxygen species, and oxidative stress, which impair muscle function and repair[44].
This evidence sheds light on the potential pathophysiological mechanisms involved in the liver-muscle axis in hepatic fibrosis[12,35]. Various investigations have shown that skeletal muscle wasting is associated with the progression and poor prognosis of chronic hepatopathy[9,17-21,45-47].
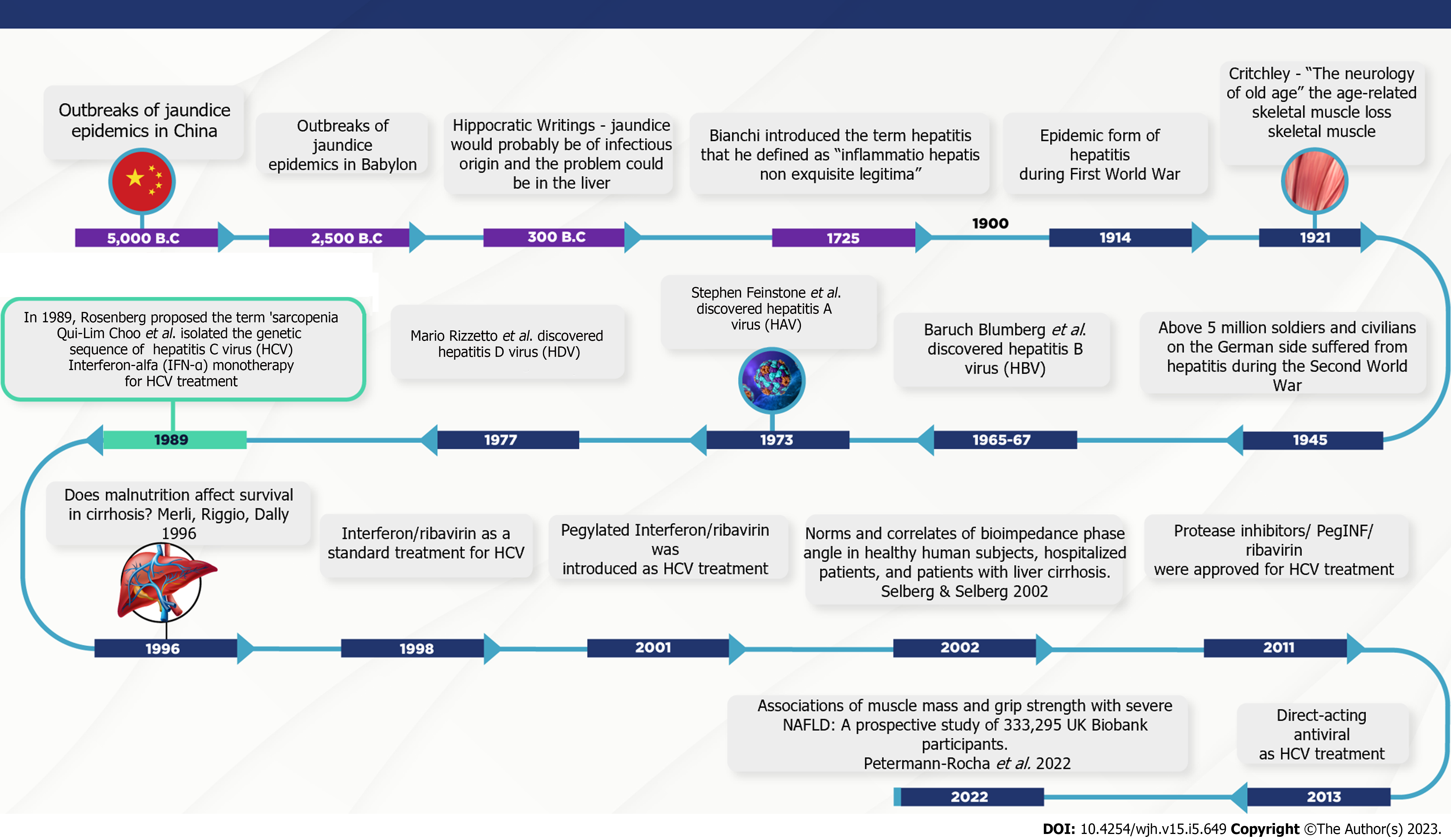
Sarcopenia and chronic viral hepatitis timelines: Understanding the potential interactions between muscle, liver, and chronic viral hepatitis. The neurologist MacDonald Critchley wrote a manuscript 90 years ago titled “The neurology of old age”[48], which is recognised as the first publication demonstrating age-related skeletal muscle loss. Later, in 1970, Nathan Shock conducted the Baltimore Study of Aging, in which functional changes with age were observed in physiological systems such as sensory, cardiovascular, respiratory, and renal systems[49,50]. However, the term sarcopenia from the Greek words “sarx” (flesh or muscle) and “penia” (loss) was first coined by Rosenberg in the late 1980s. According to the author, no decline is more dramatic or potentially more functionally significant than the loss of muscle mass with advancing age[50]. Thus, in the first stage of concept elaboration, sarcopenia was operationally described as a gradual loss of muscle mass based on methods estimating muscle mass[50]. A pioneering study by Baumgartner et al[51] (1998) described sarcopenia as when the appendicular skeletal muscle mass measured by the dual-energy X-ray absorptiometry (DXA) and adjusted for squared height, was less than two standard deviations below the sex-specific means of healthy young adult individuals. However, following studies have shown that the loss of muscle function, defined as muscle strength and power, is two to five times higher than muscle mass wasting and is significantly linked to adverse outcomes[52,53].
The European Working Group on Sarcopenia in Older People (EWGSOP) defined sarcopenia as a syndrome characterised by gradual and generalised loss of skeletal muscle mass and strength[54,55]. Finally, in 2016, sarcopenia was recognised as a disease in the 10th Edition of the International Classification of Diseases with the ICD code 10 - M62.84: Muscle insufficiency[56,57].
Although sarcopenia was originally recognised as an age-related loss of skeletal muscle mass, this clinical condition has been expanded to include loss of muscle function; in addition, it is related to a broad range of chronic diseases[58,59]. Translating this definition into the hepatic disease panorama, several studies have demonstrated that sarcopenia is of utmost significance[9,17-21,58]. Nevertheless, in literature, the term sarcopenia is marked by multiple definitions, diverse methods used to measure skeletal muscle mass, and heterogeneous study designs enrolling patients with cirrhosis of different aetiologies[58,59]. In addition, the major part of the investigations targeting sarcopenia in patients with hepatic diseases evaluated only the skeletal muscle mass[58,59]. Despite these conundrums, we have to bear in mind that most of the cirrhotic patients with skeletal muscle loss included in previous investigations had chronic viral hepatitis.
Although many studies have shown a relationship between liver damage and sarcopenia, the mechanisms underlying skeletal muscle injury have not been completely clarified. Sarcopenia can result from a complex and multifaceted virus-host-environment interplay in individuals chronically infected with HBV or HCV.
Concerning the overlap between viral hepatitis[60-64] and sarcopenia timelines[48-51,65-67] (Figure 1), even before Rosenberg proposed the term sarcopenia[50], Storch (1984) reported a clinical case of 'lupoid' hepatitis with the detection of hepatitis B core antigen (HBcAg) in motor endplates and cross-striations of skeletal muscle in a 12-year-old female patient[67]. Although the diagnostic significance and causes of the described findings were unclear, extrahepatic deposition of this viral marker has been proposed as an indicator of HBV replication in skeletal muscle[67]. Subsequently, inclusion body myositis, a chronic progressive inflammatory myopathy in the elderly, was associated with HCV infection[68,69].
In particular, the prevalence of sarcopenia has been associated with the progression of liver fibrosis[24,70,71]. A study by Hiraoka et al[71] (2016) in Japan, using computed tomography and handgrip strength (based on the EWGSOP criteria), found sarcopenia in 7.1%, 11.8%, and 21.9% of the patients with chronic viral hepatitis B and/or C without cirrhosis, with compensated (Child-Turcotte–Pugh A), and decompensated (Child-Turcotte–Pugh B/C) cirrhosis, respectively. Bering et al[72] (2017), also using the reference values recommended by EWGSOP, identified the presence of sarcopenia in 7.1% and 11.8% of the non-cirrhotic and compensated cirrhotic (Child–Turcotte–Pugh A) Brazilian patients, respectively. In both studies, 7.0% of the CHC subjects had sarcopenia prior to the onset of cirrhosis[71,72]. In line with these findings, a cross-sectional study from the National Health Examination and Nutrition showed that low muscle mass, as evaluated by mid-upper arm circumference measurements, antedates the development of cirrhosis in American patients with CHC[73]. Taken together, these data shed light on putative risk factors for skeletal muscle loss other than advanced hepatopathy-related factors. Among the potential predictors, virus, host, and environmental factors, such as viral load/genotype, nutritional status, and immune response should be highlighted.
Currently, advancements in direct-acting antiviral agents (DAAs) have resulted in outstanding improvements in the management of patients chronically infected with HCV, with sustained virological response rates that surpass 95.0% in real-life scenarios[74]. Treatment with DAAs is safe and effective and has been associated with liver and non-liver benefits, such as the prevention of hepatic disease progression and improvements in quality of life scores[74,75]. However, a study showed that DAA-induced clearance does not completely restore the altered cytokine and chemokine milieu in CHC patients[76]. Hence, in these individuals, cytokine and chemokine signatures vary depending on the stage of the liver disease and the response to antiviral therapy[77-80]. This knowledge can be transferred to the muscle-liver axis in the context of HCV eradication, especially with the introduction of interferon-free (IFN-free) treatments in clinical practice. More recently, results from interventional studies have demonstrated that HCV eradication by DAAs suppresses skeletal muscle loss in patients with CHC[81-84], suggesting a direct role of the virus in muscle mass depletion. However, the role played by the host immune response, especially pro-inflammatory effects, on skeletal muscle cells in CHC should not be overlooked[6,77-79,85]. Future longitudinal and multicentre studies are required to reduce this gap in knowledge.
Concerning extrahepatic manifestations of HCV infection, several studies suggest that an imbalance between pro-inflammatory and anti-inflammatory cytokines might induce immune activation in sites outside the liver, and consequently, generate a wide range of systemic symptoms and signals, including myalgia, weakness, fatigue, nausea, abdominal pain, weight loss, arthralgia, purpura, Raynaud’s phenomenon, xerostomia, dry eyes, depressive feelings, and anxious mood[6,86]. Therefore, CHC has been identified as a systemic disease, and 40%-74% of patients chronically infected with HCV may develop at least one non-liver manifestation throughout the clinical course of the infection[86].
It is generally acknowledged that the mechanisms involved in HCV-related extrahepatic manifestations are attributable to antibody- and cell-mediated immune responses[87-90]. Among these mechanisms, cryoglobulinaemia (type II cryoglobulin) is associated with chronic HCV[6,86]. Cryoglobulins are immunoglobulins that precipitate in vitro at temperatures < 37 °C and solubilise upon warming. HCV can trigger the expansion of B cell clones that secrete monoclonal IgM with rheumatoid factor activity. IgM then binds to polyclonal IgG molecules, which recognise HCV antigens. The resulting immunocomplexes activate complement proteins, which bind cell receptors on endothelial cells, leading to the recruitment of mononuclear and polymorphonuclear cells resulting in vasculitis. Vasculitis may occur in the brain, skin, joints, kidneys, lungs, heart, and digestive tract[6,86]. Another site that may be affected by immune-mediated occurrence is the skeletal muscle. Although secondary sarcopenia is frequently identified in patients with cirrhosis, the mechanisms underlying the interaction between the loss of skeletal mass, inflammatory mediators, and chronic viral hepatitis are still unclear. Given the potential role of circulating pro-inflammatory cytokines in mediating age-related sarcopenia[91,92], the effects of these inflammatory mediators on the pathogenesis of skeletal muscle loss occurring in HBV and HCV should be evaluated.
Other potential predictors of sarcopenia in chronic viral hepatitis should be considered, such as environmental factors, which are strongly linked to nutritional disorders and muscle homeostasis. Several lifestyle aspects in individuals with CHB and CHC may contribute to muscle damage, such as dietary patterns, diet-related non-communicable diseases, sedentarism, cigarette smoking, alcohol, and non-alcohol substance use. Analysing data from the Korean National Health and Nutrition Examination Surveys 2008-2011, Han et al[93] observed that sarcopenia was independently associated with liver fibrosis in patients with CHB. The authors also observed that when the study population was stratified according to metabolic factors, sarcopenia was independently associated with fibrosis among subgroups with obesity, insulin resistance, metabolic syndrome, and liver steatosis.
More recently, Santos et al[94] (2022), used DXA, handgrip strength, and Timed Up and Go test to show that in patients with CHB, the presence of metabolic-associated fatty liver disease and central obesity was associated with low muscle mass and strength. Although secondary sarcopenia is a well-known predictor of liver fibrosis in patients with NAFLD[95], the interaction between sarcopenia and CHB is poorly understood. These findings encourage the evaluation of metabolic and skeletal muscle loss among individuals chronically infected with HBV and reinforce the need for further large-scale case-control studies.
Few studies have examined the effects of antiviral treatment on muscle mass in CHB patients. In an investigation centred on the measurement of psoas major muscle using computed tomography before and after long-term entecavir therapy, no significant change in the muscular area was identified in any of the patients, but a significant increase was detected in the group of patients with serum albumin < 4 g/dL before treatment[96]. In contrast, Kim et al[97] (2020) investigated the dynamic association between changes in fibrosis and muscle mass during antiviral therapy and reported that appendicular skeletal muscle mass (ASM) significantly decreased during treatment of HBV infection.
Approximately 462 million adults worldwide are underweight, whereas 1.9 billion are either overweight or obese[98,99]. According to the World Health Organization definition, the double burden of malnutrition is characterised by the coexistence of undernutrition along with overweight, obesity, or diet-related non-communicable diseases within individuals, households, and populations across the course of life[98,99]. Furthermore, a growing body of evidence has shown that excessive food intake and lack of physical exercise, considered serious characteristics of the modern lifestyle, have also been verified in patients with liver disease[100,101]. Health professionals face a great challenge particularly in the management of CHB and/or CHC patients, because malnutrition and overweight can simultaneously be present in a patient[47,102]. Sarcopenic obesity, which is characterised by a decrease in ASM and excess body fat, is associated with increased mortality and influences the metabolic profile and physical performance compared with clinical manifestations alone[47,100]. Consequently, an improvement in the comprehension of body composition and nutritional status of chronically infected HBV and HCV individuals, regardless of the severity of the liver disease, is highly relevant for clinicians, dieticians, and specialists in hepatic diseases[101,102].
Sarcopenia is a relevant risk factor for adverse outcomes in cirrhotic patients[12,18]. As mentioned earlier, among the objectives of this review, we aimed to identify and summarise the available data on the prevalence and adverse clinical outcomes of sarcopenia in patients with chronic viral hepatitis. The steps involved in the review process are as follows:
We first performed a sequential electronic search using PubMed, Embase, Biblioteca Virtual em Saúde, Cochrane Library, Scopus, Web of Science, and Cumulative Index to Nursing and Allied Health on September 1, 2022 to identify published scientific reports on sarcopenia in patients with chronic viral hepatitis. The search included studies that were published between January 1995 and September 2022. To do the research, a combination of the following descriptors was used: “hepatitis C”, “chronic hepatitis C”, “hepatitis B”, “chronic hepatitis B”, “sarcopenia”, “low muscle mass”, “sarcopenic obesity”, “skeletal muscle mass”, and “skeletal muscle” (Supplementary material).
The eligibility of the articles was evaluated by two independent reviewers (MPPC and TPV). Duplicate articles were excluded from the analysis. The articles were selected by title, abstract, and full text in separate and sequential steps, following the predefined inclusion and exclusion criteria. To evaluate whether the articles met all previously established criteria, each article was analysed individually. A third reviewer resolved the disagreements between the two reviewers.
We used the Patients, Intervention, Comparison, Outcome model to develop literature search strategies[103]. Eligible manuscripts included adults aged ≥ 18 years who were chronically infected with HBV or HCV. We also considered the following conditions: presence of inpatients and outpatients, sample size of at least 30 subjects, and loss of skeletal muscle mass and/or function as the variable of interest. In addition, the clinical outcomes included infectious and noninfectious complications (clinical outcomes), increased length of hospital stay, mortality, survival, and health-related quality of life scores. Moreover, data on the prevalence of low skeletal muscle mass and/or function, including pre-sarcopenia, sarcopenia, and sarcopenic obesity, independent of the grade of liver fibrosis, were also assessed.
The methodological quality of the studies was assessed by two independent reviewers using the Joanna Briggs Institute Critical Appraisal tools applicable to each specific study design[104]. Each criterion was assessed as ‘‘yes’’ (fulfilled), ‘‘no’’ (not fulfilled), or ‘‘unclear’’. Any differences in opinion between the reviewers regarding the methodological quality were resolved by consensus through direct discussion. Disagreements were resolved through discussion with a third research member.
A total of 1427 articles were identified in the aforementioned databases. After discarding duplicates, non-English language papers, and non-relevant articles, 17 full-length published articles were selected for appraisal and were retained in the current mini-review (Supplementary material).
One of the most remarkable consequences of aging is the involuntary loss of muscle mass, strength, and function, termed sarcopenia[54-56]. Various attempts have been made to apply this operational definition to hepatic disease settings, as summarised in Table 1[47,71-73,93,94,105-109,110-114,115]. The designs of the 17 included studies were retrospective cohort (n = 8), cross-sectional (n = 8), and prospective cohort (n = 1). Most of the studies were performed in Asia (7/17, six in Japan and one in Korea[71,93,108,109,111,112,114]) and America (four in the United States[73,106,107,115], two in Canada[47,105], and two in South America[72,94]), while one each was performed in Europe[113], and in Australia/Oceania[110]. The overall sarcopenia prevalence varied from 3.8% to 53.7% in the 17 studies.
| Ref. | Study location | Study design | Diagnosis of sarcopenia | Study population (n) | Age, yr1 | Sex (M/ F) (n) | Aetiology of liver disease, n (%) | Overall prevalence of sarcopenia (%) | Prevalence of sarcopenia according to the severity of the liver disease (%) | Clinical outcome/main results |
| Montano-Loza et al[105], 2012 | Canada | Retrospective cohort | CT at the level of the third lumbar vertebrae (L3 SMI, ≤ 38.5 cm2/m2 for women and ≤ 52.4 cm2/m2 for men) | 112 cirrhotic patients evaluated for LT | 54.0 ± 1.0 | 78/34 | Alcohol 25 (22.0); HCV 32 (29.0); Alcohol + HCV 18 (16.0); HBV 2 (2.0); Autoimmune 21 (19.0); Others 14 (13.0) | 40 | Not mentioned | Sarcopenia, Child-Pugh score, and MELD score were associated with mortality |
| Krell et al[106], 2013 | United States | Retrospective cohort | CT-measured psoas muscle; Sex-stratified TPA terciles; Criteria for cutoff: Lowest TPA tercile | 207 adult patients who underwent LT | 51.7 ± 9.8 | 129/78 | HCV 54 (26.1); HBV 9 (4.4); Alcohol 30 (14.5); Autoimmune 47 (22.7); NASH 8 (3.9); HCC 52 (25.1); Others 28 (13.5); More than one indication for liver transplantation 21 (10.1) | - | Not mentioned | Sarcopenia was associated with a heightened risk for post-transplant infections and mortality |
| Gowda et al[73], 2014 | United States | Cross-sectional | MUAC below the 10th percentile for age- and sex-matched reference values | 18513 NHANES participants | HCV –39.3 ± 8.5; HCV + 47 ± 5.8 | HCV-8923/9287; HCV+ 197/106 | 303 (1.6%) had CHC | Low MUAC HCV+ 42/303 (13.8); HCV-1220/18210 (6.7) | HCV+ without significant liver fibrosis (APRI < 1.5) | CHC was associated with low MUAC, even in the absence of advanced liver disease |
| Yadav et al[107], 2015 | United States | Prospective cohort | CT at the level of the third lumbar vertebrae; (L3 SMI, ≤ 38.5 cm2/m2 for women and ≤ 52.4 cm2/m2 for men) | 213 cirrhotic patients evaluated for LT | 55.3 ± 8.6 | 129/84 | HCV 94 (44.0); Alcohol 34 (16.0); NASH 29 (13.6); PBC/PBS 16 (7.5); Cryptogenic cirrhosis 13 (6.1); Others 26 (12.2) | 22.2 | Not mentioned | Sarcopenia was not associated with mortality, poor quality of life, and functional capacity |
| Hiraoka et al[71], 2016 | Japan | Cross-sectional | CT-measured psoas muscle and HGS-measured muscle strength AWGS and EWGSOP criteria | 807 | 67.1 ± 10.0 | 466/341 | HCV 511 (63.3); HBV 134 (16.6); HBV and HCV 3 (3.7); Alcohol 45 (5.6); Others 114 (14.1); Previous or current HCC 256 (31.7) | 3.9–16.7 (AGWS); 7.1–21.9 (EWGSOP) | [CH, LC Child-Pugh (A, and B/C)]; AGWS; 3.9, 4.8, 16.7; EWGSOP; 7.1, 11.8, 21.9 | Prevalence of sarcopenia increased with the progression of chronic liver disease |
| Montano-Loza et al[47], 2016 | Canada | Retrospective cohort | CT at the level of the third lumbar vertebrae; (L3 SMI, ≤ 41.0 cm2/m2 for women and ≤ 53.0 cm2/m2 for men) | 678 | 56.0 ± 1.0 to 58.0 ± 1.0 | 457/221 | HCV 269 (40.0), alcohol 153 (23.0), NASH and cryptogenic cirrhosis 96 (14.0); Autoimmune liver disease 55 (8.0); HBV 43 (6.0); Others not specified 5 (1.0); Concomitant HCC 291 (43.0) | Sarcopenia 292 (43.0), Sarcopenic obesity 135 (20.0), Myosteatosis 353 (52.0), Sarcopenia and myosteatosis 176 (26.0) | Child-Pugh (A, B, C); Sarcopenia 12.7, 51.0, 36.3; Sarcopenic obesity; 8.9, 47.4, 43.7; Myosteatosis 12.2, 51.0, 36.8 | Sarcopenia and myosteatosis were independently associated with a higher long-term mortality in cirrhosis |
| Nishikawa et al[108], 2017 | Japan | Cross-sectional | BIA-measured upper limb skeletal muscle mass (kg) AWGS cutoff (SMI, ≤ 7.0 kg/m2 for men and ≤ 5.7 kg/m2 for women) | 383 | 65.2 ± 10.3 | 205/178 | HBV 32 (8.3); HCV 235 (61.4); Others 116 (30.3) | 136 (35.5) | No association with Child-Pugh score | Sarcopenia was associated with low overall survival in male patients |
| Bering et al[72], 2018 | Brazil | Cross-sectional | DXA-measured ASMI with EWGSOP cutoff (ASMI, ≤ 7.26 kg/m2 for men and ≤ 5.45 kg/m2 for women)HGS-measured muscle strength - EWGSOP criteria | 104 | 50.5 ± 11.3 | 78/26 | CHC patients without cirrhosis 70 (67.3), with compensated cirrhosis 34 (32.7) | Low muscle strength 29 (27.9), Low ASMI 15 (14.4); Sarcopenia 9 (8.7); Sarcopenic obesity 3 (3.8) | Sarcopenia without cirrhosis 5 (7.1) with compensated cirrhosis 4 (11.8) | Sarcopenia was associated with bone mineral content and malnutrition. BMI was normal in 88.9% of sarcopenic patients and in all patients with sarcopenic obesity. The mid-arm muscle circumference was positively correlated with ASMI |
| Han et al[93], 2018 | Korea | Cross-sectional | DXA-measured ASMI with sarcopenia defined as the lowest quintile for sex-specific sarcopenia index cutoff values (< 0.89 for men and < 0.58 for women) modified from the criteria, were adapted from the FNIH Consensus | 506 | Non-sarcopenic 48.5 ± 12.9; Sarcopenic 48.5 ± 12.9 | 258/248 | CHB significant fibrosis according to FIB4without sarcopenia160/407 (39.3)with sarcopenia57/99 (57.6) | 99 (19.6) | Not mentioned | Sarcopenia was associated with significant fibrosis, specifically in CHB patients with obesity, insulin resistance, metabolic syndrome, and liver steatosis |
| Kamo et al[109], 2019 | Japan | Retrospective cohort | CT at the level of the third lumbar vertebrae; Sarcopenic obesity as the combination of low SMI (< 40.31 cm2/m2 for men; < 30.88 cm2/m2 for women) and either VFA ≥ 100 cm2 or BMI ≥ 25 kg/m2 | 277 | 54.0 [18.0–69.0] | 134/143 | HCC 74 (26.7), HCV and/or HBV 60 (21.7), Cholestatic disease 56 (20.2); Others 87 (31.4) | Groups divided according to SMI and VFA or BMI; Without sarcopenia/non-obesity (NN); n = 167 (60.0)/n = 179 (65.0); Without sarcopenia/obesity (NO); n = 55 (20.0)/n = 43 (15.0); Sarcopenia/ non- obesity (SN); n = 46 (17.0)/n = 49 (18.0); Sarcopenia/obesity (SO); n = 9 (3.0)/n = 6 (2.0) | Groups divided according to SMI and VFA Child-Pugh A, B/C; Sarcopenia/ non-obesity (SN); 13 (28.3)/33 (71.7); Sarcopenia/obesity (SO) 4 (44.4)/5 (55.6); Groups divided according to SMI and BMIChild-Pugh A, B/C; Sarcopenia/ non- obesity (SN); 12 (24.5)/37 (75.5); Sarcopenia/obesity (SO) 5 (8.3)/1 (1.7) | Patients with sarcopenic obesity showed worse survival after LDLT compared to non-sarcopenic/non- obesity patients |
| Sinclair et al[110], 2019 | Australia | Retrospective cohort | DXA-measured ASMI - cutoff (ASMI, ≤ 7.26 kg/m2 for men) | 420 | 55.4 [49.1–59.4] | Male, 420 | HCC 119 (28.3), HCV 102 (24.3), Alcoholic cirrhosis 53 (12.6), Primary sclerosing cholangitis 43 (10.2), NAFLD 26 (6.2); Others autoimmune and metabolic conditions, 77 (18.3) | 130 (30.9) | Not mentioned | Low ASMI is strongly associated with mortality in men awaiting liver transplantation |
| Ohashi et al[111], 2019 | Japan | Cross-sectional | CT at the level of the third lumbar vertebrae; JHS criteria (L3 SMI, ≤ 38.0 cm2/m2 for women and ≤ 42.0 cm2/m2 for men) | 335 | 69.5 ± 10.2 | 169/166 | HCV 139 (41.5), HBV 57 (17.0), NAFLD 44 (13.1), Alcoholic liver disease 40 (11.9) Others 55 (16.4)HCC 86/335 (25.7) | 180 (53.7) | Child-Pugh A, B, C169 (94.0), 10 (5.5), 1 (0.5) | Sarcopenia was associated with low scores of quality of life using the Medical Outcomes Short-Form Health Survey (SF-36) |
| Saeki et al[112], 2019 | Japan | Cross-sectional | BIA-measured SMISarcopenia was diagnosed using the following criteria: JSH criteria: Low HGS (< 26 kg for men and < 18 kg for women) and low SMI (< 7.0 kg/m2 for men and < 5.7 kg/m2 for women); AWGS criteria: Low HGS (< 26 kg for men and < 18 kg for women) and/or low gait speed (≤ 0.8 m/s both for men and women) and low SMI (< 7.0 kg/m2 for men and < 5.7 kg/m2 for women); FWGSOP2 criteria: Low HGS (< 27 kg for men and < 16 kg for women) and low SMI (< 7.0 kg/m2 for men and < 5.5 kg/m2 for women). Low gait speed (≤ 0.8 m/s for both men and women) is an indicator for defining severe sarcopenia | 142 | 70.5 [58.8–76.0] | 90/52 | HCV 45 (31.7), HBV 16 (11.3), Alcoholic liver disease 48 (33.8); Others 33 (23.2) | JSH or AWGS criteria; 48 (33.8); EWGSOP2 criteria; 40 (28.2) | Child-Pugh A/B, C; 32 (66.7)/16 (33.3) | Sarcopenia, osteoporosis, osteosarcopenia, and vertebral fracture were highly prevalent and closely associated with one another in patients with liver cirrhosis. Specifically, patients with osteosarcopenia had the highest risk of vertebral fractures |
| Pinto dos Santos et al[113], 2019 | Germany | Retrospective cohort | CT-measured PMA and bilateral ESA as well as the combined PSMA. Muscle areas were subsequently normalised to the patient’s height squared - PMI, ESI, and PSMI | 368 | 49.2 [36.9–61.5] | 255/113 | HCC 164 (44.6), Alcoholic liver disease 147 (39.9), HCV 91 (24.7), HBV 55 (14.9), Biliary liver disease 38 (10.3) Others (11.1) | Median PSMI was used to divide the study population into high and low muscle index subgroups, which were further compared | Child-Pugh A, B, C; 53 (14.4), 92 (25.0), 197 (53.5) | Sarcopenia was a predictor of early post-OLT survival in male patients |
| Nishikawa et al[114], 2021 | Japan | Retrospective cohort | BIA-measured SMI; Sarcopenia was diagnosed using criteria: JSH criteria: low HGS (< 26 kg for men and < 18 kg for women) and SMI (< 7.0 kg/m2 for men and < 5.7 kg/m2 for women); AWGS criteria: Low calf circumference (CC) (< 34 cm for men and < 33 cm for women); Japanese criteria: High waist circumference (WC) (> 85 cm for men and > 90 cm for women) | 631 CLD | 65.0 [52.0–71.0] | 309/322 | HCV 286 (45.3), HBV 90 (14.3), Others 255 (40.4) | Sarcopenia; Low HGS + Low SMI; 73/631 (11.6); Low HGS; men 49 (15.9); women 89 (27.6); Low SMI; men 76 (24.6); women 107 (33.2); Low CC; men 49 (15.9); women 81 (25.2); High WC; men 106 (66.7); women 103 (32.0) | Not mentioned | Multivariate analysis showed that men, presence of LC, presence of HCC, low-GS, low-CC, serum albumin, estimated glomerular filtration rate, hepatitis B virus, and hepatitis C virus were significant factors contributing to the overall survival. CC can be an alternative marker for muscle mass in CLD patients |
| Van Dongen et al[115], 2022 | United States | Retrospective cohort | BIA-measured SMI; EWGSOP2 criteria: With sarcopenia if their SMI > 1 SD below the gender-specific meanfor young adults (aged 20–39 y) in NHANES III (≥ 36.7% in men and ≥ 26.6% in women) | 12032 NHANES participants (NHANES III, 1988–1994); 4200 (34.9%) CLD; 7832 (65.1%) controls | NAFLD 46.01(0.47); ALD 43.92 (1.33); HCV 39.49 (0.94); HBV 41.12 (1.70); Control; 41.56 (0.40) | 6049/5983 | NAFLD 3238 (77.1%); ALD 685 (16.3%); HCV 218 (5.2%); HBV 59 (1.4%) | Prevalence of sarcopenia was higher among NAFLD than other; CLDs and controls (40.7% in NAFLD, 27.2% in ALD, 22.4% in HCV, 16.8% in HBV, and 18.5% in controls) | Not mentioned | Among 4 patients with CLDs and the controls, all-cause cumulative mortality was: 35.2% HCV, 34.7% ALD, and 29.6% NAFLD. The presence of sarcopenia was associated with a higher risk of all-cause mortality only among subjects with NAFLD. Attainment of ideal LS7 metrics (ideal body mass index, ideal blood pressure, ideal physical activity, and ideal glycaemic control) provides protection against sarcopenia in NAFLD |
| Santos et al[94], 2022 | Brazil | Cross-sectional | DXA-measured ALMBMI and patients in the first sex-specific quintile (< 0.767 for men and < 0.501 for women) were considered to have low ALMBMI adapted from FNIH Consensus criteria, HGS-measured muscle strength, and physical performance - TUG | 105 CHB outpatients | 48.5 ± 12.0 | 61/44 | 105 CHB outpatients without cirrhosis 76.2% with compensated cirrhosis 23.8% | - | Not mentioned | MAFLD and central obesity were associated with low muscle mass and strength in patients with chronic hepatitis B, independent of the stage of the liver disease |
The median age of the participants ranged from 49.2 to 70.5 years[112,113]. One study included only men[110] while all the others were mixed-sex investigations, with the number of women varying between 26[72] and 9287[73]. Different aetiologies of liver diseases were observed in these studies[47,71-73,93,94,105-109,110-114,115]; with respect to chronic viral hepatitis, two studies included only patients chronically infected with HBV[93,94] and HCV[72]. In 53.0% of the investigations, sarcopenia was diagnosed according to one of the four consensus diagnostic criteria for age-related sarcopenia proposed by the Asian Working Group for Sarcopenia, EWGSOP, the Foundation for the National Institutes of Health Sarcopenia Project, and the Japan Society of Hepatology[71,72,93,108,111,112,114,115]. Muscle mass was measured using computed tomography in the majority of studies (8/17 studies[47,71,105-107,109,111,113]), followed by bioelectrical impedance analysis (4/17[108,112,114,115]), DXA (4/17 studies[72,93,94,110]), and anthropometric measurements (1/17 studies[73]). Muscle strength was measured using handgrip strength in 5/17 studies[71,72,94,112,114]. Physical performance was evaluated in two studies[94,112].
The studies reported results for approximately six different types of outcomes: mortality (five studies[47,105,106,110,113]), decreased survival (four studies[108,109,113,114]), severity of liver fibrosis (two studies[71,93]), osteopenia/osteoporosis and vertebral fractures (two studies[72,112]), while one each identified poor quality of life[111], and malnutrition[72]. In HBV scenario, sarcopenia was associated with metabolic derangements, central obesity, and metabolic syndrome[93,94].
Globally, sarcopenia is a research hotspot [116] and its clinical significance in patients with chronic liver disease is of utmost importance. In cirrhosis, sarcopenia intensely affects the health status and health-related quality of life [10,13-17,18-21]. Muscle wasting that affects cirrhotic patients is accelerated, and losses greater than 3.0% annually have been related to adverse outcomes[59].
Despite the awareness and clinical recognition of sarcopenia in cirrhotic patients, large heterogeneity permeates studies focused on sarcopenia in these individuals. It should be highlighted that in literature, the term sarcopenia is marked by multiple definitions, diverse measurement methods, and heterogeneous study designs enrolling patients with cirrhosis of diverse aetiologies and different stages of liver fibrosis[59]. In addition, most investigations targeting sarcopenia in patients with hepatic diseases have evaluated only skeletal muscle mass[59].
In the current review, the overall prevalence of sarcopenia varied from 3.8% to 53.7%. This difference can be attributed to the different criteria used to detect sarcopenia. In patients with chronic liver disease, there is neither a gold-standard definition nor a universal operational criterion for identifying sarcopenic cases. Additionally, the aetiology of the liver disease and severity of hepatic fibrosis varied among the investigations included in this minireview. Using computed tomography and hand grip strength, Hiraoka et al[71] (2016) (based on EWGSOP1 criteria) found that sarcopenia was present in 7.1%, 11.8%, and 21.9% of Japanese patients with chronic B and/or C viral hepatitis with non-cirrhosis, compensated cirrhosis (Child-Turcotte-Pugh A), and decompensated cirrhosis (Child-Turcotte-Pugh B/C), respectively. The authors observed that the prevalence of sarcopenia increased with the progression of hepatic fibrosis. Of particular concern was the finding that patients with CHC had sarcopenia prior to the onset of cirrhosis[71]. These findings reinforce the need for further research focusing on the biological mechanisms underlying the concurrent occurrence of sarcopenia in patients with chronic viral hepatitis.
Concerning the clinical outcomes associated with sarcopenia in patients chronically infected with HBV or HCV, skeletal muscle loss has been considered an independent prognostic marker of mortality in cirrhotic patients and is associated with an increased risk of complications, such as sepsis[13], refractory ascites[14], hepatic encephalopathy[15,16], and spontaneous bacterial peritonitis[17].
Considering other clinical implications of sarcopenia in patients with CHC, an association between skeletal muscle loss and bone loss was verified, independent of the severity of liver fibrosis[72]. In cirrhosis settings, bone disorders have been linked to hypogonadism[117], vitamin D deficiency[118], and low levels of insulin-like growth factor[119]. Nevertheless, little is known about the bone status of patients with CHC, especially before the onset of cirrhosis. Among the potential factors, chronic inflammation, inadequate diet and nutrition, and weight and muscle loss may contribute to low bone mineral density in subjects chronically infected with HCV. Taken together, muscle mass and muscle strength stimulate osteogenesis through connections between the bone and skeletal muscle[120]. In addition, skeletal muscle mass is recognised as an independent predictor of bone mineral density in healthy[121] and diseased individuals[122,123].
In the current review, metabolic derangements, central obesity, and metabolic syndrome were associated with sarcopenia in patients with CHB[93,94]. However, there are few studies exploring skeletal muscle loss in CHB patients. To date, among the various aetiologies implicated in liver diseases, liver-muscle interaction has been the most studied in patients with NAFLD/NASH[66,70]. Of particular concern in fatty liver disease is the fact that various evidence point to the complexity of the mechanisms implicated in skeletal muscle damage. In a previous investigation, Lee et al. observed that up to 12.0% of patients diagnosed with NAFLD had sarcopenia independent of obesity and insulin resistance, and approximately 30.0% of sarcopenic individuals without metabolic syndrome and obesity had NAFLD[124,125].
There is no universal consensus regarding the diagnosis of sarcopenia in patients with chronic viral hepatitis. Although the prevalence of sarcopenia increased in parallel with the progression of hepatic fibrosis, interestingly, sarcopenia was observed in patients chronically infected with HCV before the onset of cirrhosis. Even in studies not focused on evaluating only patients with chronic viral hepatitis, relevant adverse health-related outcomes were associated with sarcopenia in CHB or CHC patients. These findings highlight the importance of addressing skeletal muscle mass and strength loss in patients with chronic viral hepatitis. Effective strategies should be implemented to screen for sarcopenia in these patients, independent of the stage of the liver disease. An integrated medical/nutritional/physical education approach will enable greater understanding of the significance of musculoskeletal changes in patients chronically infected with HBV or HCV.
The authors acknowledge researchers in the fields of sarcopenia and chronic viral hepatitis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Nutrition and dietetics
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S, Russia; Yang SS, Taiwan S-Editor: Ma YJ L-Editor: A P-Editor: Cai YX
| 1. | World Health Organization. Global hepatitis report 2017. Geneva, Switzerland. Available from: https://www.who.int/publications/i/item/9789241565455. |
| 2. | Alberts CJ, Clifford GM, Georges D, Negro F, Lesi OA, Hutin YJ, de Martel C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:724-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
| 3. | Odenwald MA, Paul S. Viral hepatitis: Past, present, and future. World J Gastroenterol. 2022;28:1405-1429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (21)] |
| 4. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 852] [Article Influence: 213.0] [Reference Citation Analysis (1)] |
| 5. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3867] [Article Influence: 966.8] [Reference Citation Analysis (3)] |
| 6. | Cacoub P, Saadoun D. Extrahepatic Manifestations of Chronic HCV Infection. N Engl J Med. 2021;384:1038-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Cacoub P, Asselah T. Hepatitis B Virus Infection and Extra-Hepatic Manifestations: A Systemic Disease. Am J Gastroenterol. 2022;117:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Ferreira LG, Anastácio LR, Lima AS, Correia MI. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant. 2011;25:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. 2020;51:64-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 10. | Ando Y, Ishigami M, Ito T, Ishizu Y, Kuzuya T, Honda T, Ishikawa T, Fujishiro M. Sarcopenia impairs health-related quality of life in cirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31:1550-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Silva LD, Bering T, Rocha GA. The impact of nutrition on quality of life of patients with hepatitis C. Curr Opin Clin Nutr Metab Care. 2017;20:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 431] [Article Influence: 47.9] [Reference Citation Analysis (1)] |
| 13. | Lucidi C, Lattanzi B, Di Gregorio V, Incicco S, D'Ambrosio D, Venditti M, Riggio O, Merli M. A low muscle mass increases mortality in compensated cirrhotic patients with sepsis. Liver Int. 2018;38:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Namba M, Hiramatsu A, Aikata H, Kodama K, Uchikawa S, Ohya K, Morio K, Fujino H, Nakahara T, Murakami E, Yamauchi M, Kawaoka T, Tsuge M, Imamura M, Chayama K. Management of refractory ascites attenuates muscle mass reduction and improves survival in patients with decompensated cirrhosis. J Gastroenterol. 2020;55:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Di Cola S, Nardelli S, Ridola L, Gioia S, Riggio O, Merli M. Ammonia and the Muscle: An Emerging Point of View on Hepatic Encephalopathy. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Tateyama M, Naoe H, Tanaka M, Tanaka K, Narahara S, Tokunaga T, Kawasaki T, Yoshimaru Y, Nagaoka K, Watanabe T, Setoyama H, Sasaki Y, Tanaka Y. Loss of skeletal muscle mass affects the incidence of minimal hepatic encephalopathy: a case control study. BMC Gastroenterol. 2020;20:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Zeng X, Shi ZW, Yu JJ, Wang LF, Luo YY, Jin SM, Zhang LY, Tan W, Shi PM, Yu H, Zhang CQ, Xie WF. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. 2021;12:1948-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 18. | Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S147-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (1)] |
| 19. | Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, Engelmann C, Zhang P, Jeong JY, van Vugt JLA, Xiao H, Deng H, Gao X, Ye Q, Zhang J, Yang L, Cai Y, Liu N, Li Z, Han T, Kaido T, Sohn JH, Strassburg C, Berg T, Trebicka J, Hsu YC, IJzermans JNM, Wang J, Su GL, Ji F, Nguyen MH. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;76:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 243] [Article Influence: 81.0] [Reference Citation Analysis (1)] |
| 20. | Kallwitz ER. Sarcopenia and liver transplant: The relevance of too little muscle mass. World J Gastroenterol. 2015;21:10982-10993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 21. | Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061-8071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Guarino M, Cossiga V, Becchetti C, Invernizzi F, Lapenna L, Lavezzo B, Lenci I, Merli M, Pasulo L, Zanetto A, Burra P, Morisco F; Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF). Sarcopenia in chronic advanced liver diseases: A sex-oriented analysis of the literature. Dig Liver Dis. 2022;54:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 671] [Article Influence: 111.8] [Reference Citation Analysis (2)] |
| 24. | Bhanji RA, Montano-Loza AJ, Watt KD. Sarcopenia in Cirrhosis: Looking Beyond the Skeletal Muscle Loss to See the Systemic Disease. Hepatology. 2019;70:2193-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Plauth M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr. 2020;39:3533-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (2)] |
| 26. | Siddiqui ATS, Parkash O, Hashmi SA. Malnutrition and liver disease in a developing country. World J Gastroenterol. 2021;27:4985-4998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (4)] |
| 27. | Kozeniecki M, Ludke R, Kerner J, Patterson B. Micronutrients in Liver Disease: Roles, Risk Factors for Deficiency, and Recommendations for Supplementation. Nutr Clin Pract. 2020;35:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Wu J, Meng QH. Current understanding of the metabolism of micronutrients in chronic alcoholic liver disease. World J Gastroenterol. 2020;26:4567-4578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 29. | Warner ER 2nd, Aloor FZ, Satapathy SK. A narrative review of nutritional abnormalities, complications, and optimization in the cirrhotic patient. Transl Gastroenterol Hepatol. 2022;7:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1426] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 31. | Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48:e218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 503] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 32. | Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147-R1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 899] [Article Influence: 128.4] [Reference Citation Analysis (0)] |
| 33. | Jindal A, Jagdish RK. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019;25:270-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Ponziani FR, Picca A, Marzetti E, Calvani R, Conta G, Del Chierico F, Capuani G, Faccia M, Fianchi F, Funaro B, Josè Coelho-Junior H, Petito V, Rinninella E, Paroni Sterbini F, Reddel S, Vernocchi P, Cristina Mele M, Miccheli A, Putignani L, Sanguinetti M, Pompili M, Gasbarrini A; GuLiver study group. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int. 2021;41:1320-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 36. | Lund P. A radiochemical assay for glutamine synthetase, and activity of the enzyme in rat tissues. Biochem J. 1970;118:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Clemmesen JO, Kondrup J, Ott P. Splanchnic and leg exchange of amino acids and ammonia in acute liver failure. Gastroenterology. 2000;118:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. 2002;36:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 935] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 40. | Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021;12:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 517] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 41. | Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C, Stark GR, Welle S, Naga Prasad SV, Dasarathy S. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162-18167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 42. | García PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Brief-reports: elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Merli M, Giusto M, Molfino A, Bonetto A, Rossi M, Ginanni Corradini S, Baccino FM, Rossi Fanelli F, Costelli P, Muscaritoli M. MuRF-1 and p-GSK3β expression in muscle atrophy of cirrhosis. Liver Int. 2013;33:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Kosenko E, Venediktova N, Kaminsky Y, Montoliu C, Felipo V. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res. 2003;981:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Ridola L, Gioia S, Faccioli J, Riggio O, Nardelli S. Gut liver muscle brain axis: A comprehensive viewpoint on prognosis in cirrhosis. J Hepatol. 2022;77:262-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Tantai X, Yeo YH, Wang J, Ji F. Reply to: "Gut liver muscle brain axis: A comprehensive viewpoint on prognosis in cirrhosis". J Hepatol. 2022;77:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 47. | Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 401] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 48. | Critchley M. Neurological disabilities in extreme old age. Pa Med J. 1963;66:35-37. [PubMed] [DOI] [Full Text] |
| 49. | Shock NW. Physiologic aspects of aging. J Am Diet Assoc. 1970;56:491-496. [PubMed] |
| 50. | Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 51. | Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2670] [Cited by in RCA: 2748] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 52. | Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207-B218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95:1851-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1174] [Cited by in RCA: 1337] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 54. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8454] [Article Influence: 563.6] [Reference Citation Analysis (0)] |
| 55. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7782] [Article Influence: 1297.0] [Reference Citation Analysis (1)] |
| 56. | Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 2283] [Article Influence: 380.5] [Reference Citation Analysis (0)] |
| 57. | Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 560] [Cited by in RCA: 601] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 58. | Hari A. Muscular abnormalities in liver cirrhosis. World J Gastroenterol. 2021;27:4862-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 59. | Tandon P, Montano-Loza A J. Frailty and Sarcopenia in Cirrhosis. The Basics, the Challenges, and the Future. 1st edn. Springer Nature Switzerland AG, 2020. [DOI] [Full Text] |
| 60. | Reuben A. The thin red line. Hepatology. 2002;36:770-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Bianchi GB. Historia hepatica seu theoria et praxis omnium morborum hepatis et bilis, cum eiusdem visceris anatome. In: Reuben A. Landmarks in hepatology: the thin red line. |
| 62. | Fonseca JC. History of viral hepatitis. Rev Soc Bras Med Trop. 2010;43: 322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (5)] |
| 63. | Burki T. Nobel Prize for hepatitis C virus discoverers. Lancet. 2020;396:1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? Hepatology. 1996;23:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 65. | Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 311] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 66. | Petermann-Rocha F, Gray SR, Forrest E, Welsh P, Sattar N, Celis-Morales C, Ho FK, Pell JP. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J Hepatol. 2022;76:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 67. | Storch W. Hepatitis B core antigen on endplates and cross-striations of skeletal muscle in 'lupoid' hepatitis. Histochem J. 1984;16:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 68. | Alexander JA, Huebner CJ. Hepatitis C and inclusion body myositis. Am J Gastroenterol. 1996;91:1845-1847. [PubMed] |
| 69. | Tsuruta Y, Yamada T, Yoshimura T, Satake M, Ogata K, Yamamoto T, Furuya H, Kira J. Inclusion body myositis associated with hepatitis C virus infection. Fukuoka Igaku Zasshi. 2001;92:370-376. [PubMed] |
| 70. | Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, Marchesini G, Craxì A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 71. | Hiraoka A, Michitaka K, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Yamago H, Suga Y, Tomida H, Miyamoto Y, Azemoto N, Mori K, Miyata H, Tsubouchi E, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2016;28:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | Bering T, Diniz KGD, Coelho MPP, Vieira DA, Soares MMS, Kakehasi AM, Correia MITD, Teixeira R, Queiroz DMM, Rocha GA, Silva LD. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J Cachexia Sarcopenia Muscle. 2018;9:255-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Gowda C, Compher C, Amorosa VK, Lo Re V 3rd. Association between chronic hepatitis C virus infection and low muscle mass in US adults. J Viral Hepat. 2014;21:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Hayes CN, Imamura M, Tanaka J, Chayama K. Road to elimination of HCV: Clinical challenges in HCV management. Liver Int. 2022;42:1935-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 75. | Ogawa E, Chien N, Kam L, Yeo YH, Ji F, Huang DQ, Cheung R, Nguyen MH. Association of Direct-Acting Antiviral Therapy With Liver and Nonliver Complications and Long-term Mortality in Patients With Chronic Hepatitis C. JAMA Intern Med. 2023;183:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 76. | Hengst J, Falk CS, Schlaphoff V, Deterding K, Manns MP, Cornberg M, Wedemeyer H. Direct-Acting Antiviral-Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients With Chronic Hepatitis C. J Infect Dis. 2016;214:1965-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 77. | Carlin AF, Aristizabal P, Song Q, Wang H, Paulson MS, Stamm LM, Schooley RT, Wyles DL. Temporal dynamics of inflammatory cytokines/chemokines during sofosbuvir and ribavirin therapy for genotype 2 and 3 hepatitis C infection. Hepatology. 2015;62:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Ribeiro IG, Coelho-Dos-Reis JGA, Fradico JRB, Costa-Rocha IAD, Silva LD, Fonseca LADS, Stancioli RCS, Teixeira-Carvalho A, Martins-Filho OA, Teixeira R. Remodeling of immunological biomarkers in patients with chronic hepatitis C treated with direct-acting antiviral therapy. Antiviral Res. 2021;190:105073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Holmes JA, Carlton-Smith C, Kim AY, Dumas EO, Brown J, Gustafson JL, Lauer GM, Silva ST, Robidoux M, Kvistad D, Alatrakchi N, Tonnerre P, Cohen DE, Zhang H, Shulman NS, Chung RT. Dynamic changes in innate immune responses during direct-acting antiviral therapy for HCV infection. J Viral Hepat. 2019;26:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, Koike M, Nakano Y, Honda T, Yajima H, Uehara R, Miyazaki O, Kuribayashi Y, Kira K, Taura N, Nakao K. Direct-acting Antivirals Improved the Quality of Life, Ameliorated Disease-related Symptoms, and Augmented Muscle Volume Three Years Later in Patients with Hepatitis C Virus. Intern Med. 2020;59:2653-2660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Tokuchi Y, Suda G, Kimura M, Maehara O, Kitagataya T, Ohara M, Yamada R, Shigesawa T, Suzuki K, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Sakamoto N. Changes in the estimated renal function after hepatitis C virus eradication with direct-acting antiviral agents: Impact of changes in skeletal muscle mass. J Viral Hepat. 2021;28:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Yoh K, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, Aizawa N, Sakai Y, Ikeda N, Takashima T, Takata R, Iijima H, Nishiguchi S. Predictors Associated with Increase in Skeletal Muscle Mass after Sustained Virological Response in Chronic Hepatitis C Treated with Direct Acting Antivirals. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Mihai F, Trifan A, Stanciu C, Huiban L, Muzîca C, Lupașcu-Ursulescu C, Negru D, Savin ML, Gîrleanu I, Cuciureanu T, Sîngeap AM. L3 Skeletal Muscle Index Dynamics in Patients with HCV-Related Compensated Cirrhosis Following Sustained Virological Response after Direct Acting Antiviral Treatment. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 84. | Tokuchi Y, Suda G, Kimura M, Maehara O, Kitagataya T, Kubo A, Yoshida S, Fu Q, Yang Z, Hosoda S, Ohara M, Yamada R, Suzuki K, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Ohnishi S, Sakamoto N. Possible correlation between increased serum free carnitine levels and increased skeletal muscle mass following HCV eradication by direct acting antivirals. Sci Rep. 2021;11:16616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Kamimura H, Sato T, Natsui K, Kobayashi T, Yoshida T, Kamimura K, Tsuchiya A, Murayama T, Yokoyama J, Kawai H, Takamura M, Terai S. Molecular Mechanisms and Treatment of Sarcopenia in Liver Disease: A Review of Current Knowledge. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int. 2016;10:415-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 87. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577-594.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 88. | Li H, Huang MH, Jiang JD, Peng ZG. Hepatitis C: From inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J Gastroenterol. 2018;24:5297-5311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 89. | Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol. 2014;20:3418-3430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 90. | Menezes EG, Coelho-Dos-Reis JG, Cardoso LM, Lopes-Ribeiro Á, Jonathan-Gonçalves J, Porto Gonçalves MT, Cambraia RD, Soares EB, Silva LD, Peruhype-Magalhães V, Rios M, Chancey C, Teixeira-Carvalho A, Martins-Filho OA, Teixeira R. Strategies for serum chemokine/cytokine assessment as biomarkers of therapeutic response in HCV patients as a prototype to monitor immunotherapy of infectious diseases. Antiviral Res. 2017;141:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Wang T. Searching for the link between inflammaging and sarcopenia. Ageing Res Rev. 2022;77:101611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 92. | Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, Manzato E, Sergi G, Veronese N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas. 2017;96:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 511] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 93. | Han E, Lee YH, Kim BK, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH, Kim SU. Sarcopenia is associated with the risk of significant liver fibrosis in metabolically unhealthy subjects with chronic hepatitis B. Aliment Pharmacol Ther. 2018;48:300-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Santos CML, Brito MD, de Castro PASV, de Vries TP, Viana NL, Coelho MPP, Malheiro OB, Bering T, Gonzalez MC, Teixeira R, Cambraia RD, Rocha GA, Silva LD. Metabolic-associated fatty liver disease is associated with low muscle mass and strength in patients with chronic hepatitis B. World J Hepatol. 2022;14:1652-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Chakravarthy MV, Siddiqui MS, Forsgren MF, Sanyal AJ. Harnessing Muscle-Liver Crosstalk to Treat Nonalcoholic Steatohepatitis. Front Endocrinol (Lausanne). 2020;11:592373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 96. | Iwasa M, Sugimoto R, Yoshikawa K, Miyachi H, Mifuji-Moroka R, Tanaka H, Kobayashi Y, Hasegawa H, Takei Y. Change in skeletal muscle mass after administering entecavir in patients with hepatitis B. Nutrition. 2015;31:1173-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Kim KH, Joo DJ, Lee YH, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. Association between liver fibrosis and appendicular skeletal muscle mass during antiviral therapy in chronic hepatitis B. Dig Liver Dis. 2020;52:1338-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | World Health organization. The double burden of malnutrition. Policy brief. Geneva: World Health Organization; 2017. (Accessed March 2022) Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-17.3. |
| 99. | NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3952] [Cited by in RCA: 3516] [Article Influence: 390.7] [Reference Citation Analysis (0)] |
| 100. | Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 101. | Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther. 2020;51:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 102. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2291] [Article Influence: 381.8] [Reference Citation Analysis (0)] |
| 103. | Boudin F, Nie JY, Bartlett JC, Grad R, Pluye P, Dawes M. Combining classifiers for robust PICO element detection. BMC Med Inform Decis Mak. 2010;10:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173, 173.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 608] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 106. | Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, Malani PN. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 107. | Yadav A, Chang YH, Carpenter S, Silva AC, Rakela J, Aqel BA, Byrne TJ, Douglas DD, Vargas HE, Carey EJ. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clin Transplant. 2015;29:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 108. | Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Takata R, Iijima H, Nishiguchi S. Comparison of Prognostic Impact between the Child-Pugh Score and Skeletal Muscle Mass for Patients with Liver Cirrhosis. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Kamo N, Kaido T, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, Yao S, Yagi S, Uemoto S. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin Nutr. 2019;38:2202-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 110. | Sinclair M, Hoermann R, Peterson A, Testro A, Angus PW, Hey P, Chapman B, Gow PJ. Use of Dual X-ray Absorptiometry in men with advanced cirrhosis to predict sarcopenia-associated mortality risk. Liver Int. 2019;39:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 111. | Ohashi K, Ishikawa T, Imai M, Suzuki M, Hoshii A, Abe H, Koyama F, Nakano T, Ueki A, Noguchi H, Hasegawa E, Hirosawa S, Kobayashi M, Hirosawa H, Sato K, Munakata M, Yoshida T. Relationship between pre-sarcopenia and quality of life in patients with chronic liver disease: a cross-sectional study. Eur J Gastroenterol Hepatol. 2019;31:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Saeki C, Takano K, Oikawa T, Aoki Y, Kanai T, Takakura K, Nakano M, Torisu Y, Sasaki N, Abo M, Matsuura T, Tsubota A, Saruta M. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet Disord. 2019;20:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 113. | Pinto Dos Santos D, Kloeckner R, Koch S, Hoppe-Lotichius M, Zöller D, Toenges G, Kremer WM, Zimmermann T, Mittler J, Lang H, Düber C, Galle PR, Weinmann A, Sprinzl MF. Sarcopenia as prognostic factor for survival after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2020;32:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 114. | Nishikawa H, Yoh K, Enomoto H, Nishimura T, Nishiguchi S, Iijima H. Combined grip strength and calf circumference as a useful prognostic system in patients with liver diseases: a large cohort study. Ann Transl Med. 2021;9:624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Van Dongen C, Paik JM, Harring M, Younossi Y, Price JK, Kabbara K, Golabi P, Younossi ZM. Sarcopenia, healthy living, and mortality in patients with chronic liver diseases. Hepatol Commun. 2022;6:3140-3153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 116. | Xiao Y, Deng Z, Tan H, Jiang T, Chen Z. Bibliometric Analysis of the Knowledge Base and Future Trends on Sarcopenia from 1999-2021. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 117. | Chen CC, Wang SS, Jeng FS, Lee SD. Metabolic bone disease of liver cirrhosis: is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol. 1996;11:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 119. | Gallego-Rojo FJ, Gonzalez-Calvin JL, Muñoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 120. | Gries KJ, Zysik VS, Jobe TK, Griffin N, Leeds BP, Lowery JW. Muscle-derived factors influencing bone metabolism. Semin Cell Dev Biol. 2022;123:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 121. | Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study J Bone Miner Res 2001;16:1343-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 122. | Lee N, Radford-Smith GL, Forwood M, Wong J, Taaffe DR. Body composition and muscle strength as predictors of bone mineral density in Crohn's disease. J Bone Miner Metab. 2009;27:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 123. | Santos LA, Lima TB, Augusti L, Franzoni Lde C, Yamashiro Fda S, Bolfi F, Nunes Vdos S, Dorna Mde S, de Oliveira CV, Caramori CA, Silva GF, Romeiro FG. Handgrip strength as a predictor of bone mineral density in outpatients with cirrhosis. J Gastroenterol Hepatol. 2016;31:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology. 2016;63:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 125. | Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, Kang ES, Han KH, Lee HC, Cha BS. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol. 2015;63:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |