Published online May 27, 2023. doi: 10.4254/wjh.v15.i5.585
Peer-review started: November 19, 2022
First decision: February 1, 2023
Revised: March 13, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 27, 2023
Processing time: 186 Days and 7.7 Hours
Over 296 million people are estimated to have chronic hepatitis B viral infection (CHB), and it poses unique challenges for elimination. CHB is the result of hepatitis B virus (HBV)-specific immune tolerance and the presence of covalently closed circular DNA as mini chromosome inside the nucleus and the integrated HBV. Serum hepatitis B core-related antigen is the best surrogate marker for intrahepatic covalently closed circular DNA. Functional HBV “cure” is the durable loss of hepatitis B surface antigen (HBsAg), with or without HBsAg seroconversion and undetectable serum HBV DNA after completing a course of treatment. The currently approved therapies are nucleos(t)ide analogues, interferon-alpha, and pegylated-interferon. With these therapies, functional cure can be achieved in < 10% of CHB patients. Any variation to HBV or the host immune system that disrupts the interaction between them can lead to reactivation of HBV. Novel therapies may allow efficient control of CHB. They include direct acting antivirals and immunomodulators. Reduction of the viral antigen load is a crucial factor for success of immune-based therapies. Immu
Core Tip: Chronic hepatitis B virus (HBV) infection is a result of immune tolerance and the presence of covalently closed circular DNA and the integrated HBV. The currently approved therapies are nucleos(t)ide analogues, interferon-alpha, and pegylated-interferon-alpha, with functional cure achieved in < 10% of patients. Disruption of the interaction between HBV or the host immune system can lead to HBV reactivation. Novel therapies include direct acting antivirals and immunomodulators. Immunomodulators may enhance/restore innate and adaptive immunity against HBV (as toll-like-receptors and retinoic acid inducible gene-1 agonist), checkpoint inhibitors, therapeutic HBV vaccines, and genetically engineered T cells to restore T cell function.
- Citation: Salama II, Sami SM, Salama SI, Abdel-Latif GA, Shaaban FA, Fouad WA, Abdelmohsen AM, Raslan HM. Current and novel modalities for management of chronic hepatitis B infection. World J Hepatol 2023; 15(5): 585-608
- URL: https://www.wjgnet.com/1948-5182/full/v15/i5/585.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i5.585
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). It is a major global health problem. It can cause chronic infection and puts people at high risk of death from cirrhosis and liver cancer[1]. In 2019, the World Health Organization (WHO) reported that there were three million people living with new HBV infection and 296 million people living with chronic HBV (CHB) infection. The target of the Sustainable Development Goals and Global Health Sector Strategy in 2020 to decrease the incidence of hepatitis B has been met, as the global prevalence of CHB infection among children aged < 5 years decreased to less than 1% (0.94%) in 2019 compared to 5% in the pre-vaccine era[2]. The prevalence of CHB ranges from < 2% in the United States and Western Europe to ≥ 8% in western Africa[3]. The endemicity of HBV in Asia is heterogeneous, and most of the region has a moderate to high prevalence of HBV infection, except for a few low endemic areas.
The WHO[4] reported that in 2019 approximately 820000 deaths were related to HBV infection, mainly from cirrhosis and hepatocellular carcinoma (HCC). A National Egyptian study reported that among vaccinated children aged 1-16 years, 1535 (42.8%) were identified to have non-seroprotective levels of anti HBs (< 10 IU/L), and CHB prevalence was 0.39%[5,6]. Nearly, 95% of infections occurring during infancy and early childhood progress to CHB[7]. Over 90% of infections occurring during adulthood resolve as a result of developing robust immune responses[8]. In naïve CHB patients, the cumulative incidence of hepatic cirrhosis within 5 years of infection is about 10%-20% among patients with active CHB, and about 2%-5% develop liver cancer annually[9]. Infections during infancy usually carry a greater risk of developing cirrhosis and liver cancer[10].
HBV can be transmitted by sexual exposure, perinatal (mother-to-child transmission), percutaneous, and direct contact with the blood and other body fluids of HBV-infected persons[11]. Acute HBV infection manifestations range from subclinical to icteric to fulminant hepatitis in few cases. Patients who resolved the acute infection develop an efficient B cell immune response displayed by high levels of antibodies (anti-HBs) to surface antigen (HBsAg). They also develop a vigorous T cell response including CD4 and CD8 T cells to produce antiviral cytokines that kill infected hepatocytes. The difference between patients who resolved the acute HBV infection and those with CHB infection is primarily dependent on the magnitude and effectiveness of their immune response. CD8+ T cells are considered responsible for viral clearance during acute recovery but are impaired by continuous exposure to high viral HBsAg and immunosuppression in CHB[12]. The patient immune response directed towards the virus-infected hepatocytes usually leads to hepatocytes damage with an increasing risk of liver cirrhosis and HCC due to long-term liver inflammation associated with lack of viral clearance[13]. The present review summarized the current and novel diagnostic and therapeutic modalities for HBV infection.
HBV is a DNA hepatotropic virus[14]. HBV is a double-stranded DNA virus that belongs to the Hepadnaviridae family. It consists of an outer envelope containing HBsAg and a capsid core. The capsid core bears the viral genome and DNA polymerase[15]. Inside the host nucleus, the relaxed circular DNA genome is transformed to a covalently closed circular DNA (cccDNA), which is the stable form of the virus acting as a mini-chromosome. The cccDNA produces a template for generating subgenomic mRNAs and pre-genomic RNA (pgRNA) and uses the host transcription factors for viral synthesis[16]. HBV polymerase catalyzes the pgRNA to synthesize the viral genomic DNA, while the mRNA is translated to various viral proteins as a part of the HBV life cycle[17]. The HBV viral genome encodes RNA transcripts and seven proteins. The DNA polymerase gene is the main part of the virus genome sequence. The S gene includes three parts: pre-s1, pre-s2, and s encoding envelope HBsAg. The C gene encodes both hepatitis B core (HBcAg) and “e” antigens (HBeAg). The HBx gene is encoded in both the polymerase gene and the X region[18,19].
There are three different sized proteins derived from the envelope gene: Small from s (SHB), middle from pre-s2 + s (MHB) , and large from pre-s1 + pre-s2 + s (LHB)[20]. The virus secretes many defective particles as enveloped nucleocapsids that are empty or contain defective immature genomes and subviral lipid particles (SVPs) containing the viral surface antigens. These SVPs are noninfectious smaller particles containing the most plentiful form of 17-25 nm spherical particles of SHB. They also contain filamentous (or tubular) particles of variable length and comprised of SHB, MHB, and LHB proteins[21]. HBsAg proteins can gather to noninfectious SVPs approximately 22 nm in diameter containing the HBV envelope proteins (LHB, MHB, and SHB) and are secreted at a level 1000-fold to 100000-fold higher than the infectious particles[22].
During the chronic phase, HBsAg may lead to dysregulation of innate and adaptive host immunity through interacting with either the immune or non-immune cells causing impairment of the immune system and liver damage. SVPs inhibit antibody responses to the virus[23]. HBsAg could activate the unfolded protein response leading to cellular premalignant changes, i.e. it is associated with both hepatic inflammation and HCC[24]. LHB plays a crucial role in the virus envelope and initiates infectivity[25], and the form of the HBV particles appears to be determined by the ratio of the various HBsAg proteins[26]. SHB may have a role in HCC metastasis and progression. Among CHB patients, SHB can be a target for prevention or intervention of HCC progression. A continuous low level of HBsAg in CHB patients is due to failure of the host to eliminate it due to several effects related to the virus, host, and other factors[27]. Moreover, HBeAg and HBx are also major carcinogenesis-related proteins as they are essential to initiate and maintain virus replication. Various cellular events are related to HBx, such as ubiquitination, autophagy, epigenetic modifications, and non-coding RNA regulation. Subsequently, they might contribute to hepatic inflammation, fibrosis, and HCC[28].
After resolution of an acute infection, there is disappearance of serum HBV DNA, appearance of anti-HBc, HBeAg seroconversion to anti-HBe, and finally HBsAg seroconversion to anti-HBs during recovery[11]. Occult HBV infection is defined by the presence of isolated anti-HBc with the absence of HBsAg and anti-HBs[29,30]. HBV DNA is a direct measurement of the viral load. It indicates viral replication activity and disappears at the recovery from HBV infection or gradually diminishes in CHB. High titers of HBV DNA may lead to more quickly to liver cirrhosis and HCC[31]. HBV DNA is immunostimulatory and is recognized by cyclic GMP-AMP synthase and stimulator of interferon genes. Lauterbach-Rivière et al[32] found that in infected hepatocytes HBV passively escapes recognition by cellular sensors of nucleic acids by producing non-immunostimulatory RNAs and avoiding sensing of its DNAs by cyclic GMP-AMP synthase/stimulator of interferon genes without active inhibition of the pathway.
Novel biomarkers such as hepatitis B core-related antigen (HBcrAg) and serum HBV RNA are recognized as important markers to monitor the antiviral effects of the emergent therapies. HBcrAg is made up of three related viral proteins sharing an identical 149 amino acid sequence. These include HBcAg, HBeAg, and a truncated 22 kDa pre-core protein[33]. Several Asian and European studies reported a significant positive correlation between serum HBcrAg levels and the amount of intrahepatic cccDNA[34]. The presence of HBcrAg among patients with undetectable HBV DNA indicates continued transcription of cccDNA and can predict clinical relapse and be an aid for clinicians in identifying patients with a higher risk of HCC development[35,36]. HBcrAg can monitor the response to novel therapy targeting cccDNA[37]. The combination of both HBsAg and HBcrAg was found to be an outstanding biomarker for assessing the risk of HCC[38]. Treated patients with persistently high HBcrAg levels had a higher risk of HCC[39]. In addition, HBcrAg is a good virologic marker differentiating active HBV from inactive HBV in the presence of negative HBeAg[40].
The level of serum HBV RNA, presented as a pgRNA-containing virion, is correlated with intrahepatic pgRNA and cccDNA content, and it signifies high viral replication activities[39,41]. pgRNA is present in serum at lower levels than HBV DNA in treatment-naïve patients. It is enriched during nucleos(t)ide analogue (NA) therapy, which inhibits reverse transcriptase activities by blocking the transcription of pgRNA into HBV DNA. This could explain the presence of HBV RNA in serum after NA therapy, despite the undetectable serum HBV DNA[39,42]. The HBV RNA level was found to be associated with a higher risk of HCC and recurrence in patients treated with NAs[43,44]. However, the way for serum HBV RNA detection and assessment should be standardized before its clinical application.
Yang et al[45] found that HBV can encode HBV-related microRNA and named it HBV microRNA 3 (HBV-miR-3). It is used in the monitoring of HBV infection, and it is positively correlated to HBV DNA, HBsAg, and pgRNA. HBV-miR-3 was secreted by HBV-infected hepatocytes and existed in the peripheral blood exosomes of CHB patients. It is positioned at site 373–393 in the HBV genome and can be encoded by three mRNAs, (except for HBx mRNA). HBV-miR-3 and pgRNA are synthesized using cccDNA. Little effect on HBsAg, pgRNA, and HBV-miR-3 was found after antiviral drug treatment[46].
The integrated HBV DNA in the host genome can produce viral RNAs and proteins, although it does not produce progeny virus[47]. In the early HBV life cycle, HBV DNA integration occurs at the double stranded breaks throughout the whole host genome. Mutants of HBV genes may be the products of HBV DNA integration, which can stably express mutant HBV proteins. This may lead to HCC and might be a useful biomarker to monitor disease progression from CHB to HCC[48]. HBV reactivation may occur among HBV patients with resolved infection or inactive carrier state and spontaneously or as a complication of immunosuppressive therapy[7]. The basic initial step is the loss of HBV immune control. The Asian Pacific Association for the Study of the Liver (APASL) recommends the need to screen all patients for hepatitis B preceding the initiation of immunosuppressive therapy and to administer protective NAs to those patients with a considerable risk of hepatitis and acute on chronic liver failure due to hepatitis B reactivation[49].
The current available treatments can control viral replication and decrease HCC progression, but these regimens are not curative and may require lifelong therapy[50].
Interferon-alpha (IFN-α) and pegylated-IFN-α (PEG-IFN-α) act as immune modulators of finite duration. They work through inducing immune-mediated control of HBV infection with long-lasting viral replication suppression after therapy[10,50]. They inhibit viral cell entry, increase host immune response, induce cccDNA degradation, inhibit pgRNA encapsidation, and exert epigenetic modification of cccDNA transcription. Epigenetically they suppress cccDNA transcription and viral protein secretion[51], with a higher success rate shown in PEG-IFN compared to IFN[52]. IFN-α and PEG-IFN-α are the only licensed anti-HBV therapies capable of eliminating cccDNA[53]. PEG-IFN-α-2a and PEG-IFN-α-2b improve the expression of innate antiviral genes and proteins, stimulate natural killer (NK) T and CD8 immune cells, and enhance a non-cytolytic viral clearance through cytokines or cytolysis of the infected cells[54].
They have high HBsAg clearance rates, especially with genotypes A and B. However, the rate of success of IFN therapy is still low with major side effects. The long-term 5-year HBsAg loss post treatment is less than 20%[55]. A study by Wu et al[27] demonstrated that after a short-course of PEG-IFN-α-2b re-treatment in patients with HBsAg recurrence a high rate of functional cure could be achieved after therapy withdrawal, which was relatively safe.
NAs are nucleos(t)ide reverse transcriptase inhibitors. As an oral therapy targeting viral reverse transcriptase activity, it inhibits viral replication and prevents new HBV DNA formation from pgRNA[56]. However, it has minimal effects on the existing or newly formed cccDNA because cccDNA formation does not depend on the viral reverse transcriptase activity. This allows viral relapse after NA withdrawal[57]. Trials with finite treatment duration have been conducted[58]. There are currently seven approved NAs, mainly tenofovir disoproxil fumarate (TDF), tenofovir alafenamide, and entecavir. They have high effectiveness in decreasing the risk of cirrhosis and HCC with good tolerability and minimal side effects[10,11]. However, HBV disease progression can still occur even with sustained viral suppression[39]. The probability of HBsAg loss after NA cessation varies according to patient ethnicity, HBV genotype, and viral antigen levels at the end of treatment. Non-Asian patients are more likely to achieve favorable outcomes[59]. Irrespective of ethnicity, lower serum levels of HBcrAg and HBsAg and HBV genotype C are associated with higher rates of viral response, HBsAg loss, and lower rates of alanine aminotransferase (ALT) flares[60].
NAs inhibit viral replication but not viral transcription or translation[61]. The newly developed generation of NAs should be safer and more efficient through novel prodrug methods. ATI-2173 is a noncompetitive non-chain terminating clevudine derivative that can inhibit HBV polymerase[62]. After dosing for 28 d, the mean HBV DNA reduced to 2.8 Log10 IU/mL without any serious adverse events in phase I studies[63]. But during the short dosing interval, no changes were seen in HBsAg levels. Pradefovir, HS-10234, and NCO-48 fumarate are other new NA prodrugs derived from adefovir and tenofovir and increase antiviral potency and reduce metabolite toxicity[64,65]. Similar effectiveness is derived from TDF. Agents targeting the RNase H function of the HBV polymerase are in preclinical development[66].
When HBV DNA was undetectable, NAs had little effect on the HBV-miR-3 levels. PEG-IFN following NA therapy had a positive impact on the decrease of HBV-miR-3, pgRNA, and HBsAg[46].
The three major liver societies [the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver, and APASL] strongly agree that patients with active disease (defined as those with elevated HBV DNA and ALT levels), those with decompensated liver disease, and those with cirrhosis should receive treatment[67]. Table 1 summarized the current therapy regimens for CHB as recommended by AASLD 2023[68].
| For adult patients | Treatment |
| Immune-active CHB (HBeAg negative or positive) | Antiviral therapy as PEG-IFN, tenofovir, or entecavir to decrease the risk of liver complications |
| Immune-tolerant CHB | Against antiviral therapy. Continuous monitoring of ALT levels at least every 6 mo for potential transition to immune-active or inactive CHB |
| Immune-active CHB HBeAg-negative | Indefinite antiviral therapy, unless there is a strong indication for treatment withdrawal |
| Compensated cirrhosis with low levels of viremia (< 2000 IU/mL) | Antiviral therapy to reduce the risk of decompensation, regardless of ALT level |
| Decompensated cirrhosis with positive HBsAg | Indefinite antiviral therapy, irrespective of the level of HBV DNA, ALT, or HBeAg status to decrease risk of worsening the condition |
| HBeAg-positive CHB without cirrhosis seroconvert to anti-HBe on NA therapy | Discontinue NAs after a period of treatment consolidation |
| HBeAg-positive CHB with cirrhosis seroconvert to anti-HBe on therapy | Indefinite antiviral therapy unless there is a strong indication for treatment withdrawal |
| For children (2 to < than 18 years) | Treatment |
| HBeAg positive with elevated ALT and HBV DNA levels | Antiviral therapy (IFN-α, PEG-IFN, and NAs) aiming for achieving sustained HBeAg seroconversion. PEG-IFN-α is recommended for use compared to NAs due to absence of viral resistance and finite duration of treatment |
| HBeAg-positive with persistently normal ALT, regardless of HBV DNA level | Against antiviral therapy |
After seroconversion and consolidation therapy, drug withdrawal may be considered as recommended by guidelines in HBeAg-positive patients[69]. The European Association for the Study of the Liver guidelines in 2017[10] recommended a minimum of 3 years of viral inhibition, while the AASLD guidelines in 2018[70] recommended at least 2 years of combined therapy and viral inhibition. In 2021, APASL guidelines recommended NA withdrawal in HBeAg-positive patients who experienced HBeAg seroconversion and a 3-year consecutive treatment in HBeAg-negative patients who have had undetectable HBV DNA with normalization of ALT[71]. The disagreement of when antiviral therapy should be discontinued in CHB patients is due to the lack of effective methods for assessing cccDNA in the hepatocyte nucleus[72,73]. Moreover, stopping NAs carries a high incidence of virological relapse and surge of ALT levels, with high risk of fibrosis progression, decompensation, and HCC. Over 40% of CHB patients who stopped NAs eventually received re-treatment[71].
Complete cure is defined as an undetectable HBsAg and HBV DNA in the serum and complete eradication of both the intrahepatic cccDNA and integrated HBV DNA. Functional cure is defined as sustained undetectable levels of both HBsAg and HBV DNA in the serum with or without anti-HBs seroconversion and the persistence of low amounts of intrahepatic cccDNA and HBV DNA integration[74]. Since it is currently not possible to eradicate the cccDNA mini-chromosome or the integrated HBV DNA from the infected cell, functional cure is more attainable with the approved therapies. In addition, to reach high rates of HBsAg loss, there is a crucial need for short duration regimens because current treatments do not restore the immune dysfunction occurring in CHB.
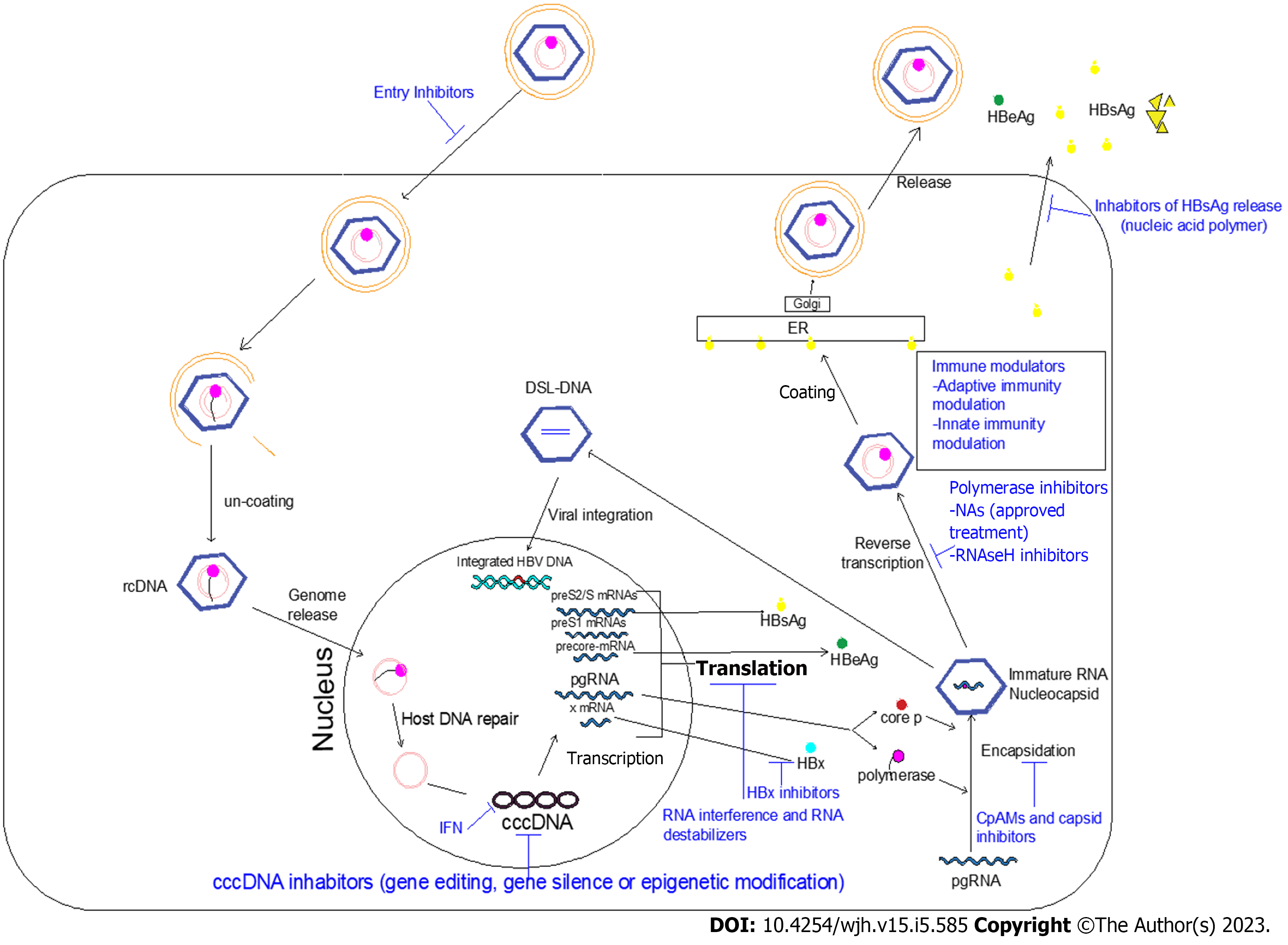
Ongoing efforts are directed towards developing novel anti-HBV drugs that achieve a complete cure. Two main classes of novel therapies are recognized: (1) Agents that directly interfere with the HBV life cycle; and (2) Agents that reinforce the host immune response against HBV infection (immune modulators). The combination of these two novel agents is expected to be more effective. Antiviral immune responses should be restored to allow elimination of both cccDNA and integrated HBV. Figure 1 shows the HBV life cycle and the effects of current and novel therapies. Table 2 shows the new HBV treatment modalities and its clinical trial phases.
| Class | Drug name | Mechanism |
| HBV entry inhibitors | Hepcludex (Bulevirtid, formerly known as Myrcludex B) | Block HBV binding to NTCP receptor; clinical trial phase II |
| Betulin derivatives and cyclosporin derivatives | ||
| IFN-α, γ | Decreases cccDNA transcription via epigenetic modifications | |
| ZFNs | Site-specific cleavage of DNA creates DSBs to target the viral cccDNA; Pre-clinical phase | |
| Agents targeting cccDNA | TALENs | |
| CRISPR and CRISPR-Cas9 as EBT106 HBV CRISPR Cas9 lipid nanoparticle | Target and reduce the viral cccDNA reservoir; | |
| Arginine methyltransferase 5 (PRMT5) | Restriction of HBV transcription and replication through cccDNA transcription suppression and pgRNA encapsidation inhibition | |
| Targeting HBx | SMC5/6 complex | Block all HBV mRNA transcription except HBx mRNA transcription |
| Nitazoxanide | Restore SMC5/6 protein levels and block HBV transcription by inhibiting the HBx-DDB1 binding; clinical trial phase II | |
| CRV-431 | Pan-cyclophilin inhibitor that inhibits liver HBV DNA and HBsAg; clinical trial phase I | |
| Agents targeting viral transcripts | ASO: IONIS-HBVRx (GSK3228836) IONIS-HBVLRx (GSK33389404) RG6004 RO7062931 | HBV RNA degradation, inhibit the expression of the corresponding gene, and bind viral mRNA to prevent viral protein production; clinical trial phase II |
| siRNAs: AB-729, ARB-1467, ARB-1740, Lunar-HB Vir-2218 (also known as ALN HBV02), JNJ-3989 (ARO-HBV), RG6346 (DCR-HBVS), GSK3228836 (IONIS-HBVRx), and Hepbarna (BB-HB-331) | HBV RNA degradation and reduce viremia, antigenemia, core, and HBx protein inside the hepatocyte; clinical trial phase I and II | |
| asRNA agent | Block HBV translation; clinical trial phase II | |
| Capsid assembly modulators (core protein assembly modulators) | HAPS as Morphothiadin (GLS4) | Core protein binding that inhibits encapsidation of pgRNA or nucleocapsid assembly, to an extent that the HBV pgRNA could not enter inside the capsid resulting in morphologically normal capsids with no nucleic acid content and therefore the virus will be noninfectious; clinical trial phase I and II |
| Phenylpropenamides derivatives as 3711, AT-61, and AT-130 | ||
| Sulfamoylbenzamide derivatives as AB-423, AB 506, JNJ-6379, JNJ-0440, NVR 3-778 | ||
| Morphothiadin, JNJ 56136379, and ABI-H0731 | Core protein binding led to a significant decrease in HBV DNA, but with smaller reductions of HBV | |
| RO7049389, ABI-H2158, GLS4JHS, ABI-H0731, ABI-H3733, RG7907, QL-007, EDP-514, CB-HBV-001 | Core protein binding; clinical trial phase I and II | |
| Targeting HBsAg | DNA based NAPs (REP-2055 and REP- 2031) or RNA-based NAPs (REP-2139 and REP-2165) | Block HBsAg release and improve the HBV-specific immune response; clinical trial phase II |
| NAPs: REP 301, REP 301-LFT and REP 401 | Functional cure in the form of undetectable HBV DNA and HBV RNA, decrease HBsAg and HBcrAg, normalize ALT levels, and detect anti-HBsAg; clinical trial phase II | |
| STOPS | Disrupt HBsAg secretion, ALG-010133; further development of this compound has been discontinued | |
| mAb against HBsAg, mAb E6F6A, and VIR-3434 | Overcome persistent HBV replication; clinical trial phase I and II | |
| E6F6 immunotherapy | Restore anti-HBV T cell response; clinical trial phase II | |
| GC1102 mAb and HBIg | Anti-HBs (against HBsAg) and boost humoral immunity; clinical trial phase II | |
| Apoptosis inducer as APG-1387 and Cyclophilin Inhibitor as CRV 431 (CPI 431-32) | Target host pathways; clinical trial phase II | |
| Immune modulators | ||
| -Therapeutic vaccination | GS-4774 | Enhance antiviral cytokine production by HBV-specific T cells as CD8+ cells; clinical trial phase III |
| DNA vaccines as INO-1800, HB-110, and JNJ-64300535 | Encode HBsAg, HBcAg, and polymerase proteins; clinical trial phase I | |
| NASVAC, Sci-B-Vac derivative BRII-179, and HepTcell | T cell vaccines; clinical trial phase I and II | |
| TG1050/T101 and TomegaVax HBV | Non-replicative adenovirus serotype 5 encode three HBV proteins; clinical trial phase II | |
| ChAdOx1 HBV | Adjuvanted ChAd and MVA vector; clinical trial phase I | |
| AIC 649 | iPPVO nonspecific vaccine; clinical trial phase I | |
| ABX-203 | Contain both HBsAg and HBcAg; clinical trial phase I | |
| -Checkpoint inhibitors | Tim-3, CTLA-4 (anti-CTLA-4), Nivolumab, ASC22 (KN035), and Cemiplimab (REGN2810) | Anti-PD-1/anti-PD-L1 antibodies that restore virus-specific CD8+ T cell responses that boost adaptive immunity; clinical trial phase I and II |
| -Genetically engineered T cells | cTCR with anti-HBs antibody or HBV specific T cell receptor gene | Recognize HBV-infected cells carrying HBV proteins on their surfaces resulting in disappearance of HBV-infected cells and decreasing cccDNA; Pre-clinical trial |
| LTCR-H2-1 and CAR-T cells | LTCR-H2-1 targets TCR gene transfer, which boosts adaptive immunity; Preclinical trial | |
| -Pathogen recognition receptors | Vesatolimod (GS-9620), RO7020531 (RG7854), RO6864018 (RG7795), ANA773, and JNJ-4964 (AL-034/TQ-A3334) | TLR-7 agonists activate intrahepatic dendritic cells, NK cells, and mucosal-associated invariant T cells and trigger the production of type I and II IFNs causing inhibition of HBV replication and boost innate immunity; clinical trial phase I and II |
| Selgantolimod (GS-9688) | TLR-8 agonists activate intrahepatic dendritic cells, NK cells, and mucosal-associated invariant T cells and enhance the production of antiviral cytokines (IL-12/IL-18) and boost innate immunity; clinical trial phase II | |
| STING agonist and RIG-I and NOD2 agonist as Inarigivir (SB9200) | Induce production of IFN-stimulated genes and proinflammatory cytokines that can cytopathically or noncytopathically clear virus, inhibit HBV replication, and boost innate immunity; clinical trial phase II | |
| -Other immune approaches | IMC-I109V | Immune mobilizing monoclonal T cell receptors against the virus; clinical trial phase I |
The direct-acting antiviral (DAA) therapy should be non-toxic; however, off-target effects may inevitably occur. Clinical trials have been carried out on novel NAs targeted to overcome the drawbacks of the currently approved NAs. Besifovir, a prodrug of tenofovir comprised of tenofovir exalidex and tenofovir disoproxilorotate, is currently under investigation[75-78]. The following are the novel DAAs that are currently in experimental or clinical trials.
HBV enters the hepatocyte through binding irreversibly to the Na+-taurocholate co-transporting polypeptide (NTCP), which is a receptor exclusively expressed on hepatocytes with high-affinity to HBV and hepatitis D virus (HDV). The myristoylated preS1 domain (myr- preS12-48 lipopeptide) of the LHB is important for this binding complex, triggering viral endocytosis[79]. It is believed that de novo infection through the NTCP receptor is required to maintain the cccDNA pool. Hepcludex (bulevirtid, formerly known as myrcludex B) is a synthetic analogue of the myr-preS12-48 lipopeptide and specifically blocks NTCP HBV/HDV binding. It is in a clinical phase II trial[80].
They found that bulevirtide monotherapy or with PEG-IFN-α resulted in a significant, undetectable level of HDV RNA within 24 wk of treatment. For these results, it was approved for HDV treatment by the European Medicine Agency[81,82]. On the other hand, it was not expected that bulevirtide would provide a sustained decrease of hepatocyte HBV DNA levels, especially when used as monotherapy[83]. The reported adverse effect for this drug was interfering with the physiological function of NTCP (reuptake of circulating bile acids from the portal blood into the liver), leading to the elevation of plasma bile acids[84]. Further studies are required before bulevirtide can be applied for the treatment of HBV infection[85].
Betulin derivatives and cyclosporin derivatives were found to have a promising potent and selective inhibition of NTCP to HBV without affecting its role as a bile acid transporter[86]. Neutralizing antibodies are another promising entry inhibitor directed toward the antigenic S, preS1-domain, cellular heparan sulphate proteoglycans (e.g., poly-Lysin), or attachment inhibitors that bind the virus (e.g., heparin)[80].
Rather than targeting the receptor, several monoclonal/polyclonal antibody preparations are being developed that bind to the N-terminal region of pre-S1, the site of viral interaction with NTCP[87]. In addition to blocking viral entry, monoclonal antibodies can lower viremia and the level of subviral particles and may cross-present viral antigens with stimulation of T cells leading to HBsAg loss[67].
In the hepatocyte nucleus, the relaxed circular DNA genome is converted into cccDNA, the mini-chromosome for HBV transcription and replication, resulting in persistence of HBV infection. IFN-α decreases cccDNA transcription via epigenetic modifications[88]. Gene therapy including editing, silencing, or epigenetics are currently assessed to modify HBV, most of them aiming to permanently disable cccDNA and lead to HBV infection cure.
Gene editing introduces genome targeted modifications using engineered nucleases for cutting a specific genomic target sequence[89]. They induce double strand breaks (DSBs) at a particular site in the HBV genome resulting in activation of the repair mechanisms of cellular DNA, aiding in producing targeted genome modifications at specific areas[90]. Three classes of nucleases have been developed: zinc finger nucleases (ZFN); transcription activator-like effector nucleases (TALENs); and the clustered regularly interspaced short palindromic repeats (CRISPR) system[91]. They have the ability to make DSBs and target cccDNA. The DSBs are repaired by nonhomologous end-joining pathways, producing insertions and deletions and disrupting the genes open reading frames. Moreover, they lead to degradation of cccDNA[92-94].
ZFNs are designed with zinc finger domains and Fok1 nuclease domain. The zinc finger domains bind to specific sites on DNA, which facilitate the introduction of a DSB into a specific targeted locus. It stimulates gene targeting 100-fold to 10000-fold via the activation of DNA repair mechanisms[95,96]. However, ZFN production needs extensive protein engineering, and for every new genome target a new ZFN must be created. Moreover, its production is time-consuming and costly. Therefore, it is not an attractive therapeutic agent[97].
TALENs are superior to ZFNs, regarding simplicity. Instead of binding to three nucleotides, they only bind to one nucleotide, executing an increased specificity with a decline in the off-target effects. TALENs use a catalytic or functional domain of a nonspecific FokI endonuclease, which can effectively induce the formation of DSBs to the target[98,99]. The DNA binding domains of TALENs are the repeat region of transcriptional activator-like effector protein[100].
CRISPR and CRISPR-associated protein nine nuclease (Cas9) is produced from Streptococcus pyogenes[101]. It directly targets and reduces the viral cccDNA reservoir. CRISPR/Cas9 is simply formed of Cas, and it precisely allows gene sequence editing with specific cleavage of cccDNA[75]. Unfortunately, it bears the risk of host genomic rearrangement and damage when targeting the integrated HBV DNA causing DSBs of the host genome[102,103]. The CRISPR/Cas9 approach revealed a potent effect in destroying the HBV genome and reducing HBsAg levels both in vitro and in animal models[104]. It is more efficient and more cost-effective than ZFNs and TALENs[62]. However, this strategy has several unwanted effects such as accurate targeting of the DNA site, off-target results on the host genome, mutation introduction, and resistance development towards the Cas9/single guide RNA system[105,106].
Targeting HBV DNA using HBV-infected HepG2-NTCP cells with CRISPR-Cas9 resulted in cleavage and the subsequent appearance of episomal HBV DNA variants. The effects of Cas9 were sustainable, signifying permanent changes in the HBV genome (not just transient effects owing to transcriptional interference)[107].
A novel technology of the CRISPR system has been developed to allow permanent damage to the HBV genome and finally reach a complete cure. This was accomplished by producing premature stop codons, without causing a DSB of the host genome[108]. Persistent high levels of HBsAg are considered a critical risk factor for the development of HCC. In vitro experiments of the CRISPR/Cas9 system showed significant functional inactivation of cccDNA by mutagenesis, inducing suppression of viral replication and potent synergy with NAs[109,110]. Results suggest that additive modulation of host DNA repair pathways enhances the antiviral activity of CRISPR/Cas9 and causes profound reduction of intracellular pgRNA[111]. However, fragments of viral DNA appear to be repaired in transcriptionally active “small cccDNA” formations[110]. The HBV DNA variants were generated after dual pgRNA targeting, highlighting the importance of understanding the fate of cccDNA after gene editing.
It is expected that cccDNA transcription is controlled by epigenetic machinery, mainly through cccDNA-bound histone 3 and histone 4 acetylation. DNA epigenetic modification could silence gene expression. Epigenetic modifications include cccDNA methylation and acetylation. HBV transcription and replication can be effectively restricted by protein arginine methyltransferase 5. HBV replication is limited by protein arginine methyltransferase 5 through cccDNA transcription suppression and pgRNA encapsidation inhibition[111-113]. Epigenetic editor studies are still preliminary for assessing its effect on HBV replication and sustainability[53].
A recent study revealed that non-histone host DNA-binding protein high mobility group box 1 targets the HBV cccDNA minichromosome and induces epigenetic silencing of viral transcription, which could be antagonized by the viral HBx protein[114]. Base editors are alternative gene editing tools that were engineered by fusing partially inactivated Cas nucleases to deaminases. They induce accurate mutations in the targeted sequences via deamination reactions, without promoting DSBs. This technique does not require donor DNA templates for gene correction[107]. The key to curing CHB infection is to eliminate or silence cccDNA within the hepatocyte nucleus. Therefore, there are several approaches in preclinical development.
The structural maintenance of chromosomes protein 5 and 6 (SMC5/6) complex binds to the episomal cccDNA templates. Through this binding, the SMC5/6 can block all HBV mRNA transcription except HBx mRNA transcription. In the cytoplasm, the HBx protein binds to the cullin 4 RING E3 ubiquitin ligase complex via its interaction with the cellular damaged DNA binding protein 1 (DDB1) forming the DDB1-CUL4 complex[115,116]. This complex is then transported to the nucleus targets to destroy the cccDNA-bound Smc5/6 complex, allowing cccDNA transcription[117]. In addition, it promotes development of HCC[118]. Accordingly, antiviral agents that interfere with HBx action can also lead to silencing of cccDNA transcription[119]. In cell culture models, nitazoxanide was found to restore SMC5/6 protein levels and block HBV transcription by inhibiting the HBx-DDB1 binding[120]. In a pilot study, nitazoxanide was given to nine treatment-naïve CHB patients for 48 wk. Results revealed undetectable HBV DNA in 89% of patients, and HBsAg disappeared in 33% of patients. HBeAg seroconversion occurred in the 2 HBeAg-positive patients. The drug was well tolerated with mild to moderate transient side effects that resolved during treatment. However, there was no follow-up for the responders[121].
Pevonedistat, a neuronal precursor cell expressed developmentally, downregulated protein 8 activating enzyme inhibitor and dicoumarol, an inhibitor of NAD(P)H quinone oxidoreductase 1, which was shown to reduce HBx expression[122,123], restore SMC5/6 levels, and suppress viral transcription in cultured hepatocytes[124] in a humanized mouse model. However, the major limitation to this approach may be the reactivation of cccDNA as soon as HBx becomes available again[122].
Viral RNA is the basis of the viral antigens and proteins. Prevention of viral replication and HBsAg production can be achieved through RNA inhibition of viral transcripts translation. This might restore the HBV-specific immune response and potentially lead to a functional cure. RNA interference (RNAi) through small-interfering RNA (siRNA) and anti-sense oligonucleotide (ASO) are new strategies for CHB treatment. RNAi targets post-transcriptional mRNAs and pgRNAs to decrease both HBV antigen production and viral replication. Reducing viral antigens may promote host immune reconstruction against HBV[125]. siRNA and ASO have different mechanisms of gene silencing. The double-stranded siRNA enters the cell cytoplasm, the duplex opens, and the antisense strand binds to the RNA-induced silencing complex (RISC). The antisense-RISC complex recognizes the mRNA. After binding, RISC induces gene silencing and mRNA degradation[126]. RISC is defined as a multiple turnover enzyme; a single siRNA could silence multiple mRNA transcripts after being induced by RISC[127]. However, single-stranded DNA oligomers (ASO) enter the cell and the nucleus, and they can bind to target mRNA alone. It binds to its corresponding site on the target viral RNA forming a DNA-RNA duplex. This allows HBV RNA degradation by ribonuclease H (a family of endonucleases), resulting in inhibition of the corresponding gene expression[128].
Small interfering RNAs (siRNAs) are designed to lower serum HBsAg through targeting specific viral mRNA sequences for all HBV RNA levels, even HBx mRNA[129]. Thus, siRNAs permit lowering viremia, antigenemia, and reducing the core and HBx proteins inside the hepatocyte. Several agents have been developed and reached phase II clinical trials, including the siRNAs VIR-2218 (also known as ALN HBV02), JNJ-3989 (ARO-HBV), RG6346 (DCR-HBVS), and JNJ-3989 (ARO-HBV) and the ASO GSK3228836 (IONIS-HBVRx)[130]. Studies are currently assessing if restoration of SMC5/6 complex induced by siRNA can lead to cccDNA silencing[119,131]. In a phase II study, a single dose siRNA ARC-520 in combination with entecavir resulted in a profound and durable decrease in serum HBV DNA and HBsAg levels[42]. In phase I/II clinical trials, re-designing siRNA to be able to target both integrated and cccDNA viral sequences resulted in an effective decrease in serum HBsAg levels in both HBeAg-positive and HBeAg-negative patients[132].
Several siRNA drugs are in phase II clinical trials. In 98% of the participants, NA co-administration with JNJ-3989 induced a decrease in HBsAg more than 1.0 Log10 IU/mL and a decrease of 1.93 Log10 IU/mL[133]. AB-729, Vir-2218, and RG6346 showed similar reductions in HBsAg titer[134]. An ASO phase II clinical study was conducted for GSK3228836. Each group of treatment-naïve and previously NA-treated received 300 mg six times. The serum HBsAg titer mean was reduced by more than 1.5 Log10 IU/mL[135]. The risk of post-treatment reactivation by the remaining cccDNA, the potential toxicity of the delivery vehicle, and the off-target toxicity are the main topics of interest for these agents.
The HBV core protein or HBcAg is essential for genome packaging, reverse transcription, and potentially for modulation of cccDNA. The HBc has recently emerged as a promising direct antiviral target that would affect multiple aspects of the viral life cycle. Several small molecules (chemical classes) are named core protein assembly modulators (CpAMs). There are two main classes of CpAMs. Class I are known as heteroaryldihydropyrimidines. They target the core protein and alter the kinetics of nucleocapsid formation resulting in deformed virus particles that would be noninfectious. Class II includes phenylpropenamides and sulfamoylbenzamide derivatives. They speed up capsid assembly to an extent that the HBV pgRNA could not enter inside the capsid resulting in morphologically normal capsids with no nucleic acid content and are therefore noninfectious[136,137]. Several CpAMs are in preclinical and clinical studies including morphothiadin, JNJ 56136379, and ABI-H0731[50]. They led to a significant decrease in HBV DNA but with smaller reductions of HBV RNA and HBsAg[138,139]. Thus, CpAM compounds directly revoke viral replication and post-infection spread[140].
In a preclinical study, adding GS-SBA-1 (a CpAM-E class, which is a potent inhibitor of extracellular HBV DNA in vitro) at the time of infection inhibited cccDNA establishment and the downstream viral products (HBsAg, HBeAg, and viral RNA). In vitro, GS-SBA-1 avoids creation of extracellular HBV RNA-containing viral particles. It is a potent CpAM that improved viral suppression when combined with an NA[141]. In clinical trials, the combination of NAs and CpAMs might lead to more potent suppression of viral replication, which could decrease the renewal of the cccDNA and inhibit its formation in newly infected cells[142]. However, withdrawal of the CpAM and NAs after short-term administration resulted in minimal changes in HBeAg and HBsAg levels coupled with a high rate of virologic relapse[143].
HBsAg enhances viral entry through binding of the preS1 region to the NTCP receptor. HBsAg is the most abundant HBV antigen, representing about 99.99% of circulating SVPs. This high circulating HBsAg contributes to the suppressive immune environment through interference with the signaling pathways of innate and adaptive immunity, contributing to chronicity of HBV[144]. Inhibition of HBsAg release is a potential step in preventing the release of enveloped HBV and the spread of infection and allowing improvement of the HBV-specific immune response. In addition, it clears circulating HBsAg in patients causing persistence control of HBV infection even after therapy withdrawal[124].
Nucleic acid polymers (NAPs) reduce the release of HBsAg through interrupting the assembly and secretion of HBV subviral particles. They are single-stranded nucleotides. They are either DNA-based NAPs (REP-2031 and REP- 2055) or RNA-based NAPs (REP-2165 and REP-2139). In the REP 102 study by Al-Mahtab et al[145], REP-2139-Ca monotherapy was given to 12 Bangladeshi patients; 9/12 patients showed 2.79–7.10 Log reduction of HBsAg. After a combination with PEG-INF-α or thymosin alpha 1 immunotherapy, there was a disappearance of HBsAg. Adverse effects occurred primarily during combination therapy. They were in the form of loss of appetite, dyspepsia, fever, and hair loss.
A phase II clinical trial of REP 2165 and REP 2139 revealed that the combination of either NAP with tenofovir TDF and PEG-IFN-α significantly decreased HBsAg levels in 15/20 patients, with seroconversion occurring in 11/20 patients with CHB in the first 24 wk. However, hepatitis flares were more common among patients receiving NAPs, especially those with undetectable HBsAg, suggesting that host-induced flares are the result of immune control of infection[146]. In clinical trials, a functional cure was developed after receiving REP 301, REP 301-LFT, and REP 401[147]. These effects of NAPs need to be confirmed in larger studies with monitoring the long-term safety and efficacy.
A monoclonal antibody (mAb) against HBsAg, mAb E6F6A, revealed a significant effect in a mouse model to overcome persistent HBV replication[148]. Another study reported restoration of anti-HBV T cell response with E6F6 immunotherapy in mice[149]. Recently, GC1102 mAb against HBsAg was investigated in a phase II clinical trial[80].
Like NAPs, S-antigen transport-inhibiting oligonucleotide polymers (STOPS) are single-stranded oligonucleotides that sequester cellular proteins necessary for HBsAg production and disrupting HBsAg secretion. STOPS were shown to be more potent than NAPs in vitro. STOPS could contribute to the functional cure of CHB as it inhibits the production of HBsAg and HBeAg and accelerates degradation of HBsAg. Antiviral compounds that act on cellular proteins will decrease the chance of the appearance of viral resistant mutations[71]. However, these molecules acting on cellular targets may exhibit toxicity. A phase I study evaluating the STOPS agent ALG-010133 demonstrated no reduction of HBsAg. Therefore, its further development has been discontinued[150].
To overcome acute HBV infection, the adaptive immune responses are the cornerstone for viral resolution through B and T viral specific cell function activation. Failure of the host immune response results in liver damage and CHB[151]. In CHB, the CD8+ T cells show functional impairment in both antigen-specific cytolytic activity and non-cytolytic functions, as the secretion of IFNγ and tumor necrosis factor-alpha are needed for intracellular viral elimination through killing the infected host cell[152]. High HBsAg load also leads to T cell functional exhaustion and apoptosis[153]. NK cells in the blood and liver have additional killing and depletion effects on HBV-specific CD8+ T cells[154]. In CHB, surviving HBV-specific CD8+ T cells are markedly dysfunctional compared to that in acute HBV-resolved patients[155].
Endosomal toll-like receptors (TLR) and cytosolic retinoic acid inducible gene I (RIG-I), melanoma differentiation antigen 5, or cyclic GMP-AMP synthase recognizes intracellular pathogenic nucleic acids leading to cytokine production and transcription of IFN-stimulated genes. HBV is able to evade and suppress the recognition of innate immunity[156]. An innovative immunotherapy approach is targeting functional restoration of T cell[151]. TLR agonists, RIG-1 agonists, checkpoint inhibitors, monoclonal antibodies, genetically engineered T cells, and therapeutic vaccines are being discovered.
The concept behind the therapeutic vaccines is the introduction of modified HBV antigens that will interact with antigen-presenting cells. The antigen-presenting cells stimulate HBV-specific T cells to produce antiviral cytokines such as IFN-γ[157]. Preclinical data in HBV mice models revealed good efficacy with therapeutic vaccines. However, in humans the results were unsatisfactory. This may be attributed to the high level of HBsAg in CHB patients, which interferes with the ability of the vaccine to induce HBV-specific T cells. GS-4774, a yeast-based T cell vaccine, was the first HBV therapeutic vaccine studied in HBV-infected humans. It was safe, well-tolerated, and increased cytokine production. However, there was no significant decline in HBsAg by week 48[158,159].
The therapeutic vaccine BRII-179 was evaluated in a phase I/IIb trial. It is a virus-like particle encoding for the three HBsAg proteins. BRII-179 produced a good humoral response in all vaccinated patients, and it increased the frequency of IFN-γ-producing T cells. However, there was no significant decrease in the level of HBsAg, HBV RNA, or HBcrAg[160]. To enhance the effectiveness of therapeutic vaccines, a combination with antiviral approaches that induce a satisfactory immune response are ongoing. This includes therapeutic vaccines with checkpoint inhibitors, TLR or RIG-I agonists, and/or other novel therapy to allow viral antigen clearance[80].
T cells of patients with CHB infection overexpress inhibitory receptors such as cytotoxic T lymphocyte associated antigen 4, programmed cell death protein 1 (PD-1), and T cell immunoglobulin and mucin containing 3. This may affect the T cell function resulting in decreasing the T cell immune response[153,161-163]. Blocking these checkpoint inhibitors may recover the exhausted T cells and restore their activity. T cell immunoglobulin and mucin containing 3 and cytotoxic T lymphocyte associated protein 4 checkpoint blockade were able to restore virus-specific CD8+ T cell responses in CHB patients[164,165]. An in vitro study was carried out on HBV-specific CD4+ T cells from CHB with HBeAg-negative infected persons. There was a functional boost of HBV-specific CD4+ T cells combined with programmed death ligand 1 blockade, leading to a marked increase in interleukin (IL)-21 and IFN-γ production. After blocking IL-10 receptors there was a reverse immune dysfunction in CHB infection[166]. Another in vitro study using anti-PD-1/anti-programmed death ligand 1 antibodies revealed only mild restoration of both peripheral and intrahepatic HBV-specific T cells[167]. However, in a preliminary clinical trial only 1 out of 12 patients achieved HBV cure after receiving minimal doses of anti-PD-1 antibodies[168].
Among the non-responder CHB patients, IL-2 therapy was able to significantly increase the frequency and function of HBV-specific CD8+ T cells, which was associated with an improvement in clinical outcomes and HBeAg seroconversion. It was concluded that the sequential IL-2 therapy has an effect on liberating the immune function in refractory CHB patients[169]. Another study revealed that HBV-specific T cells from immune-tolerant HBV-infected patients were liberated by IL-2 treatment, with high levels of IFN-γ, which was similar to immune-active patients. They concluded that a modified IL-2 molecule has the ability to reduce the toxic effects on T cells and are only directed towards CD8+ T cells[170].
However, the use of checkpoint inhibitors in the clinic has been constrained due to the death of hepatocytes causing acute liver failure and risk of autoimmunity[67]. The use of checkpoint blockade in CHB infection may be limited, despite their potential usefulness due to their safety and unpredictable response. Therefore, future safety studies are expected.
Several receptors, defined as pathogen recognition receptors, are required for effective function of the innate immune cells. The pathogen recognition receptors include TLRs, RIG-I-like receptors, formyl-methionyl-leucyl-phenylalanine, and others[171]. These receptors are the primary sensors of infection, and they represent the essential component for innate immune responses[12]. TLRs are the first line of defense against invading pathogens. They are accountable for detecting self and non-self-antigens, stimulating the maturation of dendritic cells, and initiating antigen-specific adaptive immune responses[172,173].
TLR agonists (TLR-7 and TLR-8) initiate the manufacture of endogenous IFNs, the induction of IFN-stimulated genes, and the activation of other signaling cascades such as the Janus kinase/signal transducer and activator of transcription signaling pathway, leading to HBV replication inhibition[174]. GS-9620, a TLR-7 agonist, increases the T cell and NK cell responses, while it reduces the capability of NK to suppress T cells[175]. GS-9620 achieved a durable suppression of HBV replication in preclinical studies. It allowed the induction of type I IFN[176]. In HBV-infected chimpanzees, oral GS-9620 induced a decrease in circulating levels of HBsAg and HBV DNA in the serum and hepatic cells[177] as well as in woodchucks[176]. In a phase II study, GS-9620 was given once weekly and was safe and well-tolerated in CHB patients who were virally suppressed by oral antiviral treatment. The study revealed a regular dose-dependent pharmacodynamic induction of IFN-stimulated gene mRNA expression. However, HBsAg levels did not significantly decline[178]. It does not produce antiviral effects despite the stimulation of host NK and HBV-specific T cell responses[175].
Adding to its ability for immunomodulation, TLR stimulation was found to directly stimulate T cell function through metabolic regulation[179]. TLRs play an important role in sensing the initial invasion and activation of the innate immune response. In a phase Ia and phase II trial, selgantolimod (GS-9688), an oral TLR-8 agonist, enhanced the production of antiviral cytokines in preclinical studies and was used with no significant decline of HBsAg in virally suppressed patients with CHB and viremic CHB[180,181].
In order to strengthen HBV-specific T cell responses (overcome HBV-specific T cell exhaustion), an in vitro genetically engineered T cell carrying a chimeric T cell receptor containing anti-HBs-specific antibody or an HBV-specific T cell receptor gene transferred through a vector have been developed. In animal models and in vitro studies these engineered T cells were able to recognize HBV-infected cells, carrying HBV proteins on their surfaces resulting in disappearance of HBV-infected cells and decreasing cccDNA[182,183]. They induced HBV infection clearance through cytokine secretion and cytotoxicity[184,185]. It has the advantage that the T-cell receptor (TCR)-T cells are not suppressed by the high levels of soluble antigens in the serum of CHB patients, as they are not recognized by them[186].
However, there are some concerns about the safety of this technology, as it carries the risk of going beyond immune control resulting in liver damage, and this limited its clinical use. Therefore, the addition of potent antiviral treatments to selective immune modulation may be a good strategy inducing functional cure for HBV, without causing severe liver damage progression[187]. TCR-reprogrammed non-lytic T cells have been developed to avoid the risk of severe liver damage. It can produce low amounts of perforin and granzyme B. However, in HBV-infected hepatocytes it is able to decrease viral infection by activation of the anti-viral cytidine deaminases APOBEC3[187,188]. Despite several studies currently addressing engineered T cell therapy challenges, its clinical application is still limited as it is technically difficult.
iC9 and HSV-TK were assessed as safety systems in the context of adoptive T cell transfer for the treatment of persistent CHB[189]. In vitro T cell cytotoxicity and cytokine production were abruptly stopped after HBV-specific TCR and S-chimeric antigen receptor T cells were infected with iC9 or HSV-TK. In vivo S-chimeric antigen receptor T cell induction of iC9 resulted in a rapid and significant decrease of transplanted T cells and prevented cytokine release and liver damage. However, it resulted in a decrease of antiviral effectiveness.
Preliminary evidence in an animal model demonstrated the reduction of HBsAg and HBV DNA levels without inducing significant liver damage. To prove the safety and efficacy of using genetically engineered T cells, an HBsAg-specific TCR or adoptive transfer of autologous T cells expressing HBV-specific TCR were demonstrated in patients with HCC caused by HBV[190,191]. To better understand the safety and efficacy of these novel approaches, results from clinical studies in non-HCC patients are necessary.
In theory, triple combinations of the three categories of therapy (reduction of viral antigen load, inhibition of viral replication, and immune stimulation) might be considered the ideal combination. To inhibit transcription and replication and boost immunity, vonafexor is a new agent. It is a farnesoid X receptor agonist that reduces HBV transcription. In a phase II open label study, HBeAg-negative patients with CHB were treated with TDF for 24 wk and randomly allocated to receive 24 wk of a NAP (REP 2139 or REP 2165) plus TDF and PEG-IFN or PEG-IFN plus TDF to reduce antigen burden, inhibit replication, and boost immunity[146].
The data available recommend combination therapy to achieve functional cure in CHB[192]. The best clinical evidence of the benefits of the alternative therapy is the combination of NAs and PEG-IFN, which were examined in a meta-analysis. Initial combination therapy vs initial NA monotherapy showed a nonsignificant increased relative risk of HBsAg loss of 1.44. While PEG-IFN add-on vs NA monotherapy showed a significantly improved relative risk of 4.52[193].
A recent study evaluated the combination of siRNA (JNJ-3989) with or without a CpAM (JNJ-6379) plus NA. It revealed that the triple regimen (siRNA plus CpAM plus NA) had the lowest rate of response compared with siRNA plus NA only[194]. This raises the possibility of an interaction between the CpAM and siRNA. Therefore, not all combinations will result in synergy.
Kuipery et al[195] treated infected HepG2-NTCP with TDF, TLR 7/8 agonists, or RNAi using siRNAs. They found that TDF and TLR7/8 agonists had no impact on T cell recognition. The administration of siHBVs, which inhibit viral replication and antigen expression, strongly suppressed HBV-specific CD8 T cell recognition. Similarly, we demonstrated that siHBV-mediated antigen reduction could negate the ability of TLR conditioned media to enhance antigen presentation to HBV-specific CD8 T cells. Understanding how CD8 T cell recognition is altered by these drug classes will help inform logical combination strategies for hepatitis B treatments. Immunomodulation and RNAi, but not NAs, alter the recognition of infected HepG2-NTCP by HBV-specific CD8 T cells. This could present a significant obstacle for combining immunotherapies and require careful immunological follow-up post-RNAi treatment.
The safety of new HBV therapies should always be compared with the well-proven safety of NAs. Increased additional risk should be weighed vs the predictable clinical outcomes. ALT flares are a major concern in HBV drug application, which may be induced either in relation to host immune responses or antiviral or drug-induced flares. Those related to the host immune response are associated with a decrease in serum HBV DNA and viral antigen levels. Virus-induced flares may be related to increased viral activity, lack of drug efficacy or resistance, or the side effects of antiviral agents[196]. Development of ALT flares necessitate close patient monitoring to identify the type of flare, whether to stop the antiviral, and whether it can be resumed. Even after ceasing treatment, close monitoring is mandatory, as flares may also occur due to viral reactivation, immune recovery, or delayed liver toxicity.
Hepatitis B elimination is achievable but needs greater financial support. It will not be attained without finding and treating all HBV-infected people. In developing countries, many people are unaware of their infection, and few are receiving antiviral therapy[197,198]. The goals are to reduce new infections by 90% by 2030, with a prevalence in children of no more than 0.1%, by improving the delivery of a birth dose of vaccine, which will increase from 39% in 2015 to 90% globally. In addition, all pregnant women will be tested for HBV, and new antiviral treatment-based interventions will be developed[199]. The vaccine is highly cost–effective, safe, and available as a combination with other vaccines in the Expanded Program on Immunization. Universal hepatitis B vaccination should be undertaken in all countries regardless of HBV endemicity. The hepatitis B vaccine is the first “anti-cancer” vaccine, as it prevents hepatitis B, the leading cause of HCC[200]. The introduction of HBV immunization in 189 countries by the end of 2018 resulted in an estimated 84% of the world’s population receiving three doses of the HBV vaccine. Nonetheless, the estimates are still insufficient (76%) in Africa, and just 11% of newborn babies in Africa received the HBV birth dosage, which is still a very low coverage rate (38%) internationally[199].
In Egypt, birth dose has been nationally added to the HBV vaccination program at the beginning of 2017[6]. After vaccination, anti-HBs levels decline over time[201]. Immunocompetent people who achieve an anti-HBs level ≥ 10 mIU/mL after receiving three doses of the hepatitis B vaccine remain protected, even if anti-HBs levels decline to < 10 mIU/mL due to persistent memory cells. Booster doses are not recommended for people with normal immune status who have been vaccinated[202-204]. Revaccination may be recommended for non-responders and certain high-risk populations[5,205].
Intense viral deactivation alone does not attain HBsAg reduction or loss. Combining NAs and immunotherapy reduces HBsAg levels and induces HBsAg loss in some patients, particularly those with low baseline HBsAg levels. Agents that are specifically prepared to decrease viral antigen load could not achieve constant HBsAg loss when used alone. Thus, the use of combinations of all three therapy types is recommended[142]. Moreover, combination therapy with the current anti-HBV therapy would be more effective in causing a drop in HBsAg levels and the eventual curing of HBV. To evaluate the synergistic effect of anti-HBV drugs and eradicate this global health issue, combination therapy with CpAM and entry inhibitors, siRNA, immunomodulators, or therapeutic vaccines to rebuild the immune system may be used[140].
HBV persistence can occur in an overt state with the presence of serum HBsAg due to induction and maintenance of a defective immune response, especially an adaptive immune response resulting from the interaction between viral and host factors. It can also persist in a long-term occult state with the absence of serum HBsAg, owing to the long life of the HBV genome, including cccDNA and integrated DNA within the nucleus of hepatocytes. Nine medications are now licensed for the treatment of CHB, including two IFN conventional and PEG-IFN formulations as well as seven NAs (lamivudine, telbivudine, adefovir, entecavir, TDF, tenofovir alafenamide +, and besifovir dipivoxil) (only in Korea). The therapeutic vaccines that have been studied to date are largely ineffective at significantly and permanently suppressing HBV in animal models or CHB patients, but they are quite successful at priming particular T and B cell responses to HBV antigens.
Although a functioning T cell response is necessary for the management of HBV, it is unquestionably insufficient for an effective immunotherapeutic strategy. Gene therapy including editing, silencing, and epigenetic alterations have been assessed to modify HBV. Most of them act through direct deactivation of cccDNA and HBV infection cure. Monotherapy of entry inhibitors is not expected to induce complete elimination of cccDNA due to a slow rate of hepatocyte turnover and cccDNA decay. In order to effectively treat CHB, a combination of NA-based antiviral therapy, intrahepatic innate immunity activation, stimulation of particular T cell responses, and activation of non-immune mechanisms for sustained HBV control without unfavorable side effects may be required. The combined use of potent antiviral treatments and selective immune modulation may be the best strategy to accomplish a functional cure for HBV, without inducing severe tissue damage and disease progression. To reach the goal of global elimination of HBV by 2030, the WHO published certain measures that include full childhood vaccination, preventing perinatal transmission, harm reduction, blood safety, and testing and treatment. Unfortunately, most countries are not on track to meet these targets by 2030.
The authors would like to acknowledge Eng. Salma El-Atroush for designing and drawing the figure presented in this review article and Omer A Kamal.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aygun S, Turkey; B Lankarani K, Iran; Chen S, China; Ielasi L, Italy; Rajeshwari K, India S-Editor: Liu XF L-Editor: Filipodia P-Editor: Cai YX
| 1. | World Health Organization (WHO). Hepatitis B. Nov 16, 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. |
| 2. | World Health Organization (WHO). Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. July 15, 2021. [cited 4 February 2023]. Available from: https://www.who.int/publications/i/item/9789240027077. |
| 3. | Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 4. | World Health Organization (WHO) (2022). Hepatitis B. March 5, 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b#:~:text=WHO%20estimates%20that%20296%20million,%20carcinoma%20(primary%20 Liver%20cancer). |
| 5. | Salama II, Sami SM, Salama SI, Rabah TM, El Etreby LA, Abdel Hamid AT, Elmosalami D, El Hariri H, Said ZN. Immune response to second vaccination series of hepatitis B virus among booster dose non-responders. Vaccine. 2016;34:1904-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Salama II, Sami SM, Said ZN, Salama SI, Rabah TM, Abdel-Latif GA, Elmosalami DM, Saleh RM, Abdel Mohsin AM, Metwally AM, Hassanin AI, Emam HM, Hemida SA, Elserougy SM, Shaaban FA, Fouad WA, Mohsen A, El-Sayed MH. Early and long term anamnestic response to HBV booster dose among fully vaccinated Egyptian children during infancy. Vaccine. 2018;36:2005-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | World Health Organization (WHO). Global Hepatitis Report 2017. [accessed 2020 April 5] 2017: 1-68 Available from: https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=6B2A9D244077971D8F7BDB1E60E30734?sequence=1. |
| 8. | Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim SG, Lu F, Penicaud MC, Tavis JE, Thimme R; Members of the ICE-HBV Working Groups; ICE-HBV Stakeholders Group Chairs; ICE-HBV Senior Advisors, Zoulim F. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;4:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 380] [Article Influence: 63.3] [Reference Citation Analysis (1)] |
| 9. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 967] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 10. | Centers for Disease Control and Prevention (CDC). Viral Hepatitis. United States, 2023. Available from: https://www.cdc.gov/hepatitis/hbv/index.htm. |
| 11. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 12. | Gehring AJ, Protzer U. Targeting Innate and Adaptive Immune Responses to Cure Chronic HBV Infection. Gastroenterology. 2019;156:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 13. | Lee HM, Banini BA. Updates on Chronic HBV: Current Challenges and Future Goals. Curr Treat Options Gastroenterol. 2019;17:271-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 540] [Article Influence: 77.1] [Reference Citation Analysis (1)] |
| 15. | Inoue T, Tanaka Y. Hepatitis B virus and its sexually transmitted infection - an update. Microb Cell. 2016;3:420-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Turton KL, Meier-Stephenson V, Badmalia MD, Coffin CS, Patel TR. Host Transcription Factors in Hepatitis B Virus RNA Synthesis. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 17. | Li H, Zhuang Q, Wang Y, Zhang T, Zhao J, Zhang Y, Zhang J, Lin Y, Yuan Q, Xia N, Han J. HBV life cycle is restricted in mouse hepatocytes expressing human NTCP. Cell Mol Immunol. 2014;11:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 939] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 19. | McNaughton AL, D'Arienzo V, Ansari MA, Lumley SF, Littlejohn M, Revill P, McKeating JA, Matthews PC. Insights From Deep Sequencing of the HBV Genome-Unique, Tiny, and Misunderstood. Gastroenterology. 2019;156:384-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, Serrecchia J, Blankenship S, Ward JW, Holtzman D; Centers for Disease Control and Prevention (CDC). Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64:453-458. [PubMed] |
| 21. | Farag MM, Peschel G, Müller M, Weigand K. Characterization Of The Interaction Between Subviral Particles Of Hepatitis B Virus And Dendritic Cells - In Vitro Study. Infect Drug Resist. 2019;12:3125-3135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Chai N, Chang HE, Nicolas E, Han Z, Jarnik M, Taylor J. Properties of subviral particles of hepatitis B virus. J Virol. 2008;82:7812-7817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Kuipery A, Gehring AJ, Isogawa M. Mechanisms of HBV immune evasion. Antiviral Res. 2020;179:104816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 24. | Li Y, Xia Y, Cheng X, Kleiner DE, Hewitt SM, Sproch J, Li T, Zhuang H, Liang TJ. Hepatitis B Surface Antigen Activates Unfolded Protein Response in Forming Ground Glass Hepatocytes of Chronic Hepatitis B. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Glebe D, Goldmann N, Lauber C, Seitz S. HBV evolution and genetic variability: Impact on prevention, treatment and development of antivirals. Antiviral Res. 2021;186:104973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Lee JM, Ahn SH. Quantification of HBsAg: basic virology for clinical practice. World J Gastroenterol. 2011;17:283-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Wu J, Yu Y, Dai Y, Zhang Y, Cheng J. Research Progress on the Mechanism of Persistent Low-Level HBsAg Expression in the Serum of Patients with Chronic HBV Infection. J Immunol Res. 2022;2022:1372705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Zhao F, Xie X, Tan X, Yu H, Tian M, Lv H, Qin C, Qi J, Zhu Q. The Functions of Hepatitis B Virus Encoding Proteins: Viral Persistence and Liver Pathogenesis. Front Immunol. 2021;12:691766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Weber B, Melchior W, Gehrke R, Doerr HW, Berger A, Rabenau H. Hepatitis B virus markers in anti-HBc only positive individuals. J Med Virol. 2001;64:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Said ZN, Sayed MH, Salama II, Aboel-Magd EK, Mahmoud MH, Setouhy ME, Mouftah F, Azzab MB, Goubran H, Bassili A, Esmat GE. Occult hepatitis B virus infection among Egyptian blood donors. World J Hepatol. 2013;5:64-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH; REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 32. | Lauterbach-Rivière L, Bergez M, Mönch S, Qu B, Riess M, Vondran FWR, Liese J, Hornung V, Urban S, König R. Hepatitis B Virus DNA is a Substrate for the cGAS/STING Pathway but is not Sensed in Infected Hepatocytes. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | Carey I, Gersch J, Bruce M, Wang B, Kuhns M, Cloherty G, Dusheiko G, Agarwal K. FRI-159- Can HBcrAg and pre-genomic HBV RNA predict the risk of ALT flares after nucleoside analogue therapy withdrawal: delineating the clinical utility of new biomarkers? J Hepatol. 2019;. [DOI] [Full Text] |
| 34. | Wang L, Cao X, Wang Z, Gao Y, Deng J, Liu X, Zhuang H. Correlation of HBcrAg with Intrahepatic Hepatitis B Virus Total DNA and Covalently Closed Circular DNA in HBeAg-Positive Chronic Hepatitis B Patients. J Clin Microbiol. 2019;57:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2020;26:261-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 36. | Carey I, Gersch J, Wang B, Moigboi C, Kuhns M, Cloherty G, Dusheiko G, Agarwal K. Pregenomic HBV RNA and Hepatitis B Core-Related Antigen Predict Outcomes in Hepatitis B e Antigen-Negative Chronic Hepatitis B Patients Suppressed on Nucleos(T)ide Analogue Therapy. Hepatology. 2020;72:42-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 37. | Gerlich WH. The enigma of concurrent hepatitis B surface antigen (HBsAg) and antibodies to HBsAg. Clin Infect Dis. 2007;44:1170-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Suzuki Y, Maekawa S, Komatsu N, Sato M, Tatsumi A, Miura M, Matsuda S, Muraoka M, Nakakuki N, Shindo H, Amemiya F, Takano S, Fukasawa M, Nakayama Y, Yamaguchi T, Inoue T, Sato T, Sakamoto M, Yamashita A, Moriishi K, Enomoto N. Hepatitis B virus (HBV)-infected patients with low hepatitis B surface antigen and high hepatitis B core-related antigen titers have a high risk of HBV-related hepatocellular carcinoma. Hepatol Res. 2019;49:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Mak LY, Seto WK, Fung J, Yuen MF. New Biomarkers of Chronic Hepatitis B. Gut Liver. 2019;13:589-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, Loglio A, Facchetti F, Lampertico P, Levrero M, Zoulim F. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 41. | Liu Y, Jiang M, Xue J, Yan H, Liang X. Serum HBV RNA quantification: useful for monitoring natural history of chronic hepatitis B infection. BMC Gastroenterol. 2019;19:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Wang J, Yu Y, Li G, Shen C, Meng Z, Zheng J, Jia Y, Chen S, Zhang X, Zhu M, Song Z, Wu J, Shao L, Qian P, Mao X, Wang X, Huang Y, Zhao C, Zhang J, Qiu C, Zhang W. Relationship between serum HBV-RNA levels and intrahepatic viral as well as histologic activity markers in entecavir-treated patients. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | Liu S, Deng R, Zhou B, Liang X, Liu Z, Peng J, Chen J, Zhou Y, Guo Y, Chen Y, Li W, Shen S, Lu X, Zhao S, Liao X, Liang H, Lan Y, Hou J, Fan R, Sun J. Association of Serum Hepatitis B Virus RNA With Hepatocellular Carcinoma Risk in Chronic Hepatitis B Patients Under Nucleos(t)ide Analogues Therapy. J Infect Dis. 2022;226:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Ding WB, Wang MC, Yu J, Huang G, Sun DP, Liu L, Zhang JN, Yang Y, Liu H, Zhou WP, Yang F, Yuan SX. HBV/Pregenomic RNA Increases the Stemness and Promotes the Development of HBV-Related HCC Through Reciprocal Regulation With Insulin-Like Growth Factor 2 mRNA-Binding Protein 3. Hepatology. 2021;74:1480-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Yang X, Li H, Sun H, Fan H, Hu Y, Liu M, Li X, Tang H. Hepatitis B Virus-Encoded MicroRNA Controls Viral Replication. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Gan W, Chen X, Wu Z, Zhu X, Liu J, Wang T, Gao Z. The relationship between serum exosome HBV-miR-3 and current virological markers and its dynamics in chronic hepatitis B patients on antiviral treatment. Ann Transl Med. 2022;10:536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Hu B, Wang R, Fu J, Su M, Du M, Liu Y, Li H, Wang H, Lu F, Jiang J. Integration of hepatitis B virus S gene impacts on hepatitis B surface antigen levels in patients with antiviral therapy. J Gastroenterol Hepatol. 2018;33:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Yang HC, Tsou HH, Pei SN, Chang CS, Chen JH, Yao M, Lin SJ, Lin J, Yuan Q, Xia N, Liu TW, Chen PJ, Cheng AL, Hsu C; Taiwan Cooperative Oncology Group. Quantification of HBV core antibodies may help predict HBV reactivation in patients with lymphoma and resolved HBV infection. J Hepatol. 2018;69:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 49. | Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, Piratvisuth T, Jia JD, Mizokami M, Cheng G, Chen GF, Liu ZW, Baatarkhuu O, Cheng AL, Ng WL, Lau P, Mok T, Chang JM, Hamid S, Dokmeci AK, Gani RA, Payawal DA, Chow P, Park JW, Strasser SI, Mohamed R, Win KM, Tawesak T, Sarin SK, Omata M. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. 2021;15:1031-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 50. | König A, Yang J, Jo E, Park KHP, Kim H, Than TT, Song X, Qi X, Dai X, Park S, Shum D, Ryu WS, Kim JH, Yoon SK, Park JY, Ahn SH, Han KH, Gerlich WH, Windisch MP. Efficient long-term amplification of hepatitis B virus isolates after infection of slow proliferating HepG2-NTCP cells. J Hepatol. 2019;71:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Xia Y, Protzer U. Control of Hepatitis B Virus by Cytokines. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 53. | Bloom K, Maepa MB, Ely A, Arbuthnot P. Gene Therapy for Chronic HBV-Can We Eliminate cccDNA? Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Chen J, Wang Y, Wu XJ, Li J, Hou FQ, Wang GQ. Pegylated interferon α-2b up-regulates specific CD8+ T cells in patients with chronic hepatitis B. World J Gastroenterol. 2010;16:6145-6150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 56. | Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B Infection: A Review. JAMA. 2018;319:1802-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 487] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 57. | Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB, Lai CL, Lau JYN, Schluep T, Xu Z, Lanford RE, Lewis DL. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 58. | van Bömmel F, Berg T. Stopping long-term treatment with nucleos(t)ide analogues is a favourable option for selected patients with HBeAg-negative chronic hepatitis B. Liver Int. 2018;38 Suppl 1:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Sonneveld MJ, Park JY, Kaewdech A, Seto WK, Tanaka Y, Carey I, Papatheodoridi M, van Bömmel F, Berg T, Zoulim F, Ahn SH, Dalekos GN, Erler NS, Höner Zu Siederdissen C, Wedemeyer H, Cornberg M, Yuen MF, Agarwal K, Boonstra A, Buti M, Piratvisuth T, Papatheodoridis G, Maasoumy B; CREATE Study Group. Prediction of Sustained Response After Nucleo(s)tide Analogue Cessation Using HBsAg and HBcrAg Levels: A Multicenter Study (CREATE). Clin Gastroenterol Hepatol. 2022;20:e784-e793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 60. | Sonneveld MJ, Chiu SM, Park JY, Brakenhoff SM, Kaewdech A, Seto WK, Tanaka Y, Carey I, Papatheodoridi M, van Bömmel F, Berg T, Zoulim F, Ahn SH, Dalekos GN, Erler NS, Höner Zu Siederdissen C, Wedemeyer H, Cornberg M, Yuen MF, Agarwal K, Boonstra A, Buti M, Piratvisuth T, Papatheodoridis G, Chen CH, Maasoumy B; CREATE study group. Probability of HBsAg loss after nucleo(s)tide analogue withdrawal depends on HBV genotype and viral antigen levels. J Hepatol. 2022;76:1042-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 61. | Marcellin P, Wong DK, Sievert W, Buggisch P, Petersen J, Flisiak R, Manns M, Kaita K, Krastev Z, Lee SS, Cathcart AL, Crans G, Op den Brouw M, Jump B, Gaggar A, Flaherty J, Buti M. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019;39:1868-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 62. | Squires KE, Mayers DL, Bluemling GR, Kolykhalov AA, Guthrie DB, Reddy P, Mitchell DG, Saindane MT, Sticher ZM, Edpuganti V, De La Rosa A. ATI-2173, a Novel Liver-Targeted Non-Chain-Terminating Nucleotide for Hepatitis B Virus Cure Regimens. Antimicrob Agents Chemother. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Mayers D, Jucov A, Anastasiy I, Ghicavii N, Ogilvie L, Squires K, De La Rosa A. Phase 1 Results for ATI- 2173, a novel active site polymerase inhibitor nucleotide (ASPIN), in HBV-infected subjects. J Hepatol 2021; 75: S743 Available from: https://www.antiostherapeutics.com/wp-content/uploads/2021/06/EASL-2021_Mayers_PO-1251_Poster.pdf. |
| 64. | Gao Y, Kong F, Song X, Shang J, Yao L, Xia J, Peng Y, Liu W, Gong H, Mu M, Cui H, Han T, Chen W, Wu X, Yang Y, Yan X, Jin Z, Wang P, Zhu Q, Chen L, Zhao C, Zhang D, Jin W, Wang D, Wen X, Liu C, Jia J, Mao Q, Ding Y, Jin X, Zhang Z, Li G, Niu J. Pradefovir Treatment in Patients With Chronic Hepatitis B: Week 24 Results From a Multicenter, Double-Blind, Randomized, Noninferiority, Phase 2 Trial. Clin Infect Dis. 2022;74:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Zhang H, Hu Y, Wu M, Liu J, Zhu X, Li X, Chen H, Li C, Liu C, Niu J, Ding Y. Randomised clinical trial: safety, efficacy and pharmacokinetics of HS-10234 vs tenofovir for the treatment of chronic hepatitis B infection. Aliment Pharmacol Ther. 2021;53:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 66. | Pierra Rouviere C, Dousson CB, Tavis JE. HBV replication inhibitors. Antiviral Res. 2020;179:104815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 67. | Yardeni D, Chang KM, Ghany MG. Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure. Gastroenterology. 2023;164:42-60.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 68. | AADSL. Treatment of chronic hepatitis B. [accessed 2023 Feb 5]. Available from: https://www.guidelinecentral.com/guideline/10618/#section-328315. 2022. |
| 69. | Zhu M, Wang H, Lou T, Xiong P, Zhang J, Li L, Sun Y, Wu Y. Current treatment of chronic hepatitis B: Clinical aspects and future directions. Front Microbiol. 2022;13:975584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 70. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2846] [Article Influence: 406.6] [Reference Citation Analysis (0)] |
| 71. | Kao CC, Nie Y, Ren S, De Costa NTTS, Pandey RK, Hong J, Smith DB, Symons JA, Beigelman L, Blatt LM. Mechanism of action of hepatitis B virus S antigen transport-inhibiting oligonucleotide polymer, STOPS, molecules. Mol Ther Nucleic Acids. 2022;27:335-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Martinez MG, Boyd A, Combe E, Testoni B, Zoulim F. Covalently closed circular DNA: The ultimate therapeutic target for curing HBV infections. J Hepatol. 2021;75:706-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 73. | Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: Stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76:1249-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 74. | Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. Hepatology. 2017;66:1296-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 75. | Xia Y, Liang TJ. Development of Direct-acting Antiviral and Host-targeting Agents for Treatment of Hepatitis B Virus Infection. Gastroenterology. 2019;156:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 76. | Ahn SH, Kim W, Jung YK, Yang JM, Jang JY, Kweon YO, Cho YK, Kim YJ, Hong GY, Kim DJ, Um SH, Sohn JH, Lee JW, Park SJ, Lee BS, Kim JH, Kim HS, Yoon SK, Kim MY, Yim HJ, Lee KS, Lim YS, Lee WS, Park NH, Jin SY, Kim KH, Choi W, Han KH. Efficacy and Safety of Besifovir Dipivoxil Maleate Compared With Tenofovir Disoproxil Fumarate in Treatment of Chronic Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. 2019;17:1850-1859.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Foster R, Conover M, Canizres C, Trepanier D, Ure D, Matkovits T, Mayo P. Pharmacokinetic-pharmacodynamic modeling of Tenofovir Exalidex in HBV subjects. J Hepatol. 2018;. [DOI] [Full Text] |
| 78. | Alexopoulou A, Vasilieva L, Karayiannis P. New Approaches to the Treatment of Chronic Hepatitis B. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 79. | Glebe D, Urban S, Knoop EV, Cag N, Krass P, Grün S, Bulavaite A, Sasnauskas K, Gerlich WH. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 Lipopeptides and tupaia hepatocytes. Gastroenterology. 2005;129:234-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 80. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 81. | Wedemeyer H, Schoneweis K, Bogomolov P, Natalia Voronkova, Chulanov V, Stepanova T, Bremer B, Allweiss L, Dandri M, Burhenne J, Haefeli WE, Ciesek S, Dittmer U, Alexandrov A, Urban S. GS-13-Final results of a multicenter, open-labelphase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferonAlpha 2a in patients with chronic HBV/HDV co-infection. J Hepatol. 2019;. [DOI] [Full Text] |
| 82. | Wedemeyer H, Schöneweis K, Bogomolov PO, Chulanov V, Stepanova T, Viacheslav M, Allweiss L, Dandri M, Ciesek S, Dittmer U, Haefeli WE, Alexandrov A, Urban S. AS072 - 48 weeks of high dose (10 mg) bulevirtide as monotherapy or with peginterferon alfa-2ain patientswith chronic HBV/HDV co-infection. J Hepatol. 2020;73:S52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 83. | Foti G, Scaglione V, Carlo Torti C. Novel Therapies in HBV Infection. Clinical Management Issues. 2019;13:53-60. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 84. | Blank A, Eidam A, Haag M, Hohmann N, Burhenne J, Schwab M, van de Graaf S, Meyer MR, Maurer HH, Meier K, Weiss J, Bruckner T, Alexandrov A, Urban S, Mikus G, Haefeli WE. The NTCP-inhibitor Myrcludex B: Effects on Bile Acid Disposition and Tenofovir Pharmacokinetics. Clin Pharmacol Ther. 2018;103:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 85. | Tang Y, Liang H, Zeng G, Shen S, Sun J. Advances in new antivirals for chronic hepatitis B. Chin Med J (Engl). 2022;135:571-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Kirstgen M, Lowjaga KAAT, Müller SF, Goldmann N, Lehmann F, Alakurtti S, Yli-Kauhaluoma J, Glebe D, Geyer J. Selective hepatitis B and D virus entry inhibitors from the group of pentacyclic lupane-type betulin-derived triterpenoids. Sci Rep. 2020;10:21772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Tu T, Urban S. Virus entry and its inhibition to prevent and treat hepatitis B and hepatitis D virus infections. Curr Opin Virol. 2018;30:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 88. | Javanbakht H, Mueller H, Walther J, Zhou X, Lopez A, Pattupara T, Blaising J, Pedersen L, Albæk N, Jackerott M, Shi T, Ploix C, Driessen W, Persson R, Ravn J, Young JAT, Ottosen S. Liver-Targeted Anti-HBV Single-Stranded Oligonucleotides with Locked Nucleic Acid Potently Reduce HBV Gene Expression In Vivo. Mol Ther Nucleic Acids. 2018;11:441-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 89. | Shim G, Kim D, Park GT, Jin H, Suh SK, Oh YK. Therapeutic gene editing: delivery and regulatory perspectives. Acta Pharmacol Sin. 2017;38:738-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 90. | Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14:8096-8106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 427] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 91. | Carroll D. Genome engineering with targetable nucleases. Annu Rev Biochem. 2014;83:409-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 92. | Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 949] [Cited by in RCA: 1096] [Article Influence: 219.2] [Reference Citation Analysis (0)] |
| 93. | Wang J, Xu ZW, Liu S, Zhang RY, Ding SL, Xie XM, Long L, Chen XM, Zhuang H, Lu FM. Dual gRNAs guided CRISPR/Cas9 system inhibits hepatitis B virus replication. World J Gastroenterol. 2015;21:9554-9565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 94. | Kostyushev D, Kostyusheva A, Brezgin S, Zarifyan D, Utkina A, Goptar I, Chulanov V. Suppressing the NHEJ pathway by DNA-PKcs inhibitor NU7026 prevents degradation of HBV cccDNA cleaved by CRISPR/Cas9. Sci Rep. 2019;9:1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 95. | Zhang X, Wang L, Wu Y, Li W, An J, Zhang F, Liu M. Knockout of Myostatin by Zinc-finger Nuclease in Sheep Fibroblasts and Embryos. Asian-Australas J Anim Sci. 2016;29:1500-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Saha SK, Saikot FK, Rahman MS, Jamal MAHM, Rahman SMK, Islam SMR, Kim KH. Programmable Molecular Scissors: Applications of a New Tool for Genome Editing in Biotech. Mol Ther Nucleic Acids. 2019;14:212-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Li D, He W, Liu X, Zheng S, Qi Y, Li H, Mao F, Liu J, Sun Y, Pan L, Du K, Ye K, Li W, Sui J. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 98. | Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, Voytas DF, Long CR, Whitelaw CB, Fahrenkrug SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012;109:17382-17387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 99. | Ramalingam S, Annaluru N, Kandavelou K, Chandrasegaran S. TALEN-mediated generation and genetic correction of disease-specific human induced pluripotent stem cells. Curr Gene Ther. 2014;14:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 100. | Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1079] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 101. | Xiao A, Wang Z, Hu Y, Wu Y, Luo Z, Yang Z, Zu Y, Li W, Huang P, Tong X, Zhu Z, Lin S, Zhang B. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res. 2013;41:e141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 102. | Anderson KR, Haeussler M, Watanabe C, Janakiraman V, Lund J, Modrusan Z, Stinson J, Bei Q, Buechler A, Yu C, Thamminana SR, Tam L, Sowick MA, Alcantar T, O'Neil N, Li J, Ta L, Lima L, Roose-Girma M, Rairdan X, Durinck S, Warming S. CRISPR off-target analysis in genetically engineered rats and mice. Nat Methods. 2018;15:512-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 103. | Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24:927-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 875] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 104. | Chen CC, Guan G, Qi X, Abulaiti A, Zhang T, Liu J, Lu F, Chen X. Pacbio Sequencing of PLC/PRF/5 Cell Line and Clearance of HBV Integration Through CRISPR/Cas-9 System. Front Mol Biosci. 2021;8:676957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Xiao-Jie L, Hui-Ying X, Zun-Ping K, Jin-Lian C, Li-Juan J. CRISPR-Cas9: a new and promising player in gene therapy. J Med Genet. 2015;52:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 106. | Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25:1234-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 810] [Cited by in RCA: 717] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 107. | Martinez MG, Smekalova E, Combe E, Gregoire F, Zoulim F, Testoni B. Gene Editing Technologies to Target HBV cccDNA. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 108. | Yang YC, Yang HC. Recent Progress and Future Prospective in HBV Cure by CRISPR/Cas. Viruses. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Seeger C, Sohn JA. Complete Spectrum of CRISPR/Cas9-induced Mutations on HBV cccDNA. Mol Ther. 2016;24:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 110. | Martinez MG, Inchauspe A, Delberghe E, Chapus F, Neveu G, Alam A, Carter K, Testoni B, Zoulim F. SAT376-Targeting hepatitis B virus with CRISPR/Cas9 approach. J Hepatol. 2020;73 Suppl 1:S841-S842. [DOI] [Full Text] |
| 111. | Murai K, Kodama T, Hikita H, Shimoda A, Fukuoka M, Fukutomi K, Tahata Y, Makino Y, Yamada R, Sakamori R, Tatsumi T. Novel Anti-HBV Therapies Using CRISPR/Cas9 Targeting HBV Genome Strongly Suppress HBV. Hepatology. 2020;72 Suppl 1: 61A-62A. Available from: https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/hep.31578. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 112. | Zhang W, Chen J, Wu M, Zhang X, Zhang M, Yue L, Li Y, Liu J, Li B, Shen F, Wang Y, Bai L, Protzer U, Levrero M, Yuan Z. PRMT5 restricts hepatitis B virus replication through epigenetic repression of covalently closed circular DNA transcription and interference with pregenomic RNA encapsidation. Hepatology. 2017;66:398-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 113. | Dandri M. Epigenetic modulation in chronic hepatitis B virus infection. Semin Immunopathol. 2020;42:173-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 114. | Kim ES, Zhou J, Zhang H, Marchetti A, van de Klundert M, Cai D, Yu X, Mitra B, Liu Y, Wang M, Protzer U, Guo H. Hepatitis B virus X protein counteracts high mobility group box 1 protein-mediated epigenetic silencing of covalently closed circular DNA. PLoS Pathog. 2022;18:e1010576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 115. | Sitterlin D, Lee TH, Prigent S, Tiollais P, Butel JS, Transy C. Interaction of the UV-damaged DNA-binding protein with hepatitis B virus X protein is conserved among mammalian hepadnaviruses and restricted to transactivation-proficient X-insertion mutants. J Virol. 1997;71:6194-6199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 116. | Li T, Robert EI, van Breugel PC, Strubin M, Zheng N. A promiscuous alpha-helical motif anchors viral hijackers and substrate receptors to the CUL4-DDB1 ubiquitin ligase machinery. Nat Struct Mol Biol. 2010;17:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 117. | Decorsière A, Mueller H, van Breugel PC, Abdul F, Gerossier L, Beran RK, Livingston CM, Niu C, Fletcher SP, Hantz O, Strubin M. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 118. | Minor MM, Hollinger FB, McNees AL, Jung SY, Jain A, Hyser JM, Bissig KD, Slagle BL. Hepatitis B Virus HBx Protein Mediates the Degradation of Host Restriction Factors through the Cullin 4 DDB1 E3 Ubiquitin Ligase Complex. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 119. | Dandri M, Petersen J. cccDNA Maintenance in Chronic Hepatitis B - Targeting the Matrix of Viral Replication. Infect Drug Resist. 2020;13:3873-3886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 120. | Han X, Zhou C, Jiang M, Wang Y, Wang J, Cheng Z, Wang M, Liu Y, Liang C, Wang Z, Weikert R, Lv W, Xie J, Yu X, Zhou X, Luangsay S, Shen HC, Mayweg AV, Javanbakht H, Yang S. Discovery of RG7834: The First-in-Class Selective and Orally Available Small Molecule Hepatitis B Virus Expression Inhibitor with Novel Mechanism of Action. J Med Chem. 2018;61:10619-10634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 121. | Rossignol JF, Bréchot C. A Pilot Clinical Trial of Nitazoxanide in the Treatment of Chronic Hepatitis B. Hepatol Commun. 2019;3:744-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Cheng ST, Hu JL, Ren JH, Yu HB, Zhong S, Wai Wong VK, Kwan Law BY, Chen WX, Xu HM, Zhang ZZ, Cai XF, Hu Y, Zhang WL, Long QX, Ren F, Zhou HZ, Huang AL, Chen J. Dicoumarol, an NQO1 inhibitor, blocks cccDNA transcription by promoting degradation of HBx. J Hepatol. 2021;74:522-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 123. | Sekiba K, Otsuka M, Ohno M, Yamagami M, Kishikawa T, Seimiya T, Suzuki T, Tanaka E, Ishibashi R, Funato K, Koike K. Pevonedistat, a Neuronal Precursor Cell-Expressed Developmentally Down-Regulated Protein 8-Activating Enzyme Inhibitor, Is a Potent Inhibitor of Hepatitis B Virus. Hepatology. 2019;69:1903-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 124. | Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, Remouchamps C, Chou WM, Thasler WE, Hüser N, Durantel D, Liang TJ, Münk C, Heim MH, Browning JL, Dejardin E, Dandri M, Schindler M, Heikenwalder M, Protzer U. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 125. | Hui RW, Mak LY, Seto WK, Yuen MF. RNA interference as a novel treatment strategy for chronic hepatitis B infection. Clin Mol Hepatol. 2022;28:408-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 126. | Gareri C, Polimeni A, Giordano S, Tammè L, Curcio A, Indolfi C. Antisense Oligonucleotides and Small Interfering RNA for the Treatment of Dyslipidemias. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 127. | Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nat Struct Mol Biol. 2004;11:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 409] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 128. | Liang XH, Sun H, Nichols JG, Crooke ST. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol Ther. 2017;25:2075-2092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 129. | Thi EP, Dhillon AP, Ardzinski A, Bidirici-Ertekin L, Cobarrubias KD, Cuconati A, Kondratowicz AS, Kwak K, Li AHL, Miller A, Pasetka C, Pei L, Phelps JR, Snead NM, Wang X, Ye X, Sofia MJ, Lee ACH. ARB-1740, a RNA Interference Therapeutic for Chronic Hepatitis B Infection. ACS Infect Dis. 2019;5:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 130. | Choi HSJ, Tonthat A, Janssen HLA, Terrault NA. Aiming for Functional Cure With Established and Novel Therapies for Chronic Hepatitis B. Hepatol Commun. 2022;6:935-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 131. | Martinez MG, Villeret F, Testoni B, Zoulim F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. 2020;40 Suppl 1:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 132. | Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2018;47:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 133. | Gane E, Locarnini S, Lim TH, Strasser S, Sievert W, Cheng W, Thompson A, Given B, Schluep T, Hamilton J, Biermer M, Kalmeijer R, Beumont M, Lenz O, Ridder FD, Cloherty G, Ka-Ho Wong D, Schwabe C, Jackson K, Ferrari C, Lai CL, Gish R, Yuen MF. Short interfering RNA JNJ-3989 combination therapy in chronic hepatitis B shows potent reduction of all viral markers but no correlate was identified for HBsAg reduction and baseline factors. J Hepatol. 2021;75: S2 Available from: https://www.natap.org/2021/EASL/EASL_39.htm. |
| 134. | Yuen MF, Lim YS, Cloutier D, Thanawala V, Shen L, Arizpe A, Tay C, Gupta S, Cathcart AL, Hwang C, Pang P, Gane E. Preliminary results from a phase 2 study evaluating VIR-2218 alone and in combination with pegylated interferon alfa-2a in participants with chronic hepatitis B infection. Hepatology. 2021;74: 63A. Available from: https://natap.org/2021/AASLD/AASLD_100.htm. |
| 135. | Yuen MF, Heo J, Jang JW, Yoon JH, Kweon YO, Park SJ, Tami Y, You S, Yates P, Tao Y, Cremer J, Campbell F, Elston R, Theodore D, Paff M, Bennett CF, Kwoh TJ. Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: a phase 2 randomized controlled trial. Nat Med. 2021;27:1725-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 136. | Berke JM, Dehertogh P, Vergauwen K, Mostmans W, Vandyck K, Raboisson P, Pauwels F. Antiviral Properties and Mechanism of Action Studies of the Hepatitis B Virus Capsid Assembly Modulator JNJ-56136379. Antimicrob Agents Chemother. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 137. | Tsounis EP, Tourkochristou E, Mouzaki A, Triantos C. Toward a new era of hepatitis B virus therapeutics: The pursuit of a functional cure. World J Gastroenterol. 2021;27:2727-2757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (3)] |
| 138. | Yuen MF, Agarwal K, Gane EJ, Schwabe C, Cheng W, Sievert W, Kim DJ, Ahn SH, Lim YS, Visvanathan K, Ruby E, Liaw S, Colonno R, Lopatin U. Interim safety, tolerability pharmacokinetics, and antiviral activity of ABI-H0731, a novel core protein allosteric modulator, in healthy volunteers and non-cirrhotic viremic subjects with chronic hepatitis B. InInternational Liver Congress (ILC), 2018 Elsevier BV. Available from: http://www.elsevier.com/locate/jhep.2018. [DOI] [Full Text] |
| 139. | Zoulim F, Yogaratnam J, Vandenbossche JJ, Lenz O, Talloen W, Vistuer C, Moscalu I, Streinu-Cercel A, Bourgeois S, Buti M, Crespo J. Safety, pharmakokinetics and antiviral activity of novel capsid assembly modulator (CAM) JNJ-56136379 (JNJ-6379) in treatmentnaive chronic hepatitis B (CHB) patients without cirrhosis. J Hepatol. 2018;68:S102. [DOI] [Full Text] |
| 140. | Taverniti V, Ligat G, Debing Y, Kum DB, Baumert TF, Verrier ER. Capsid Assembly Modulators as Antiviral Agents against HBV: Molecular Mechanisms and Clinical Perspectives. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 141. | Burdette D, Hyrina A, Song Z, Beran RK, Cheung T, Gilmore S, Kobayashi T, Li L, Liu Y, Niedziela-Majka A, Medley J, Mehra U, Morganelli P, Novikov N, Niu C, Tam D, Tang J, Wang J, Yue Q, Fletcher SP, Holdorf MM, Delaney WE 4th, Feierbach B, Lazerwith S. Characterization of a Novel Capsid Assembly Modulator for the Treatment of Chronic Hepatitis B Virus Infection. Antimicrob Agents Chemother. 2023;67:e0134822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 142. | Lim SG, Baumert TF, Boni C, Gane E, Levrero M, Lok AS, Maini MK, Terrault NA, Zoulim F. The scientific basis of combination therapy for chronic hepatitis B functional cure. Nat Rev Gastroenterol Hepatol. 2023;20:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 143. | Yuen MF, Ma X, Hassanein TI, Kwo PY, Ma J, Li L, Kitrinos K, Knox SJ, Stamm LM, Bae H, Sulkowski MS, Elkhashab M, Agarwal K. HBV pgRNA and DNA both rebound immediately following discontinuation of the core inhibitor vebicorvir despite continued NRTI treatment in patients with HBeAg positive chronic hepatitis B virus infection: findings from a phase 2 open label study. Hepatology. 2021;74: 65A. Available from: https://www.natap.org/2021/AASLD/AASLD_31.htm. |
| 144. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1700] [Cited by in RCA: 1712] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 145. | Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One. 2016;11:e0156667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 146. | Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology. 2020;158:2180-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 147. | Hershkovich LS, Shekhtman L, Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Cotler SJ, Vaillant A, Dahari H. HBsAg, anti-HBs and ALT kinetic characterization during NAP based combination therapy of HBeAg negative chronic hepatitis B infection. J Hepatol 2021; 75: S750. Available from: http://replicor.com/wp-content/uploads/2021/09/EASL2021_HBV-kinetics-under-NAP-based-therapy.pdf. |
| 148. | Zhang TY, Yuan Q, Zhao JH, Zhang YL, Yuan LZ, Lan Y, Lo YC, Sun CP, Wu CR, Zhang JF, Zhang Y, Cao JL, Guo XR, Liu X, Mo XB, Luo WX, Cheng T, Chen YX, Tao MH, Shih JW, Zhao QJ, Zhang J, Chen PJ, Yuan YA, Xia NS. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut. 2016;65:658-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 149. | Gao Y, Zhang TY, Yuan Q, Xia NS. Antibody-mediated immunotherapy against chronic hepatitis B virus infection. Hum Vaccin Immunother. 2017;13:1768-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 150. | Aligos Therapeutics. Aligos Halting Further Development of STOPSTM Drug Candidate, ALG-010133. [cited 2022 January 6]. Available from: https://investor.aligos.com/node/7291/pdf. |
| 151. | Barili V, Boni C, Rossi M, Vecchi A, Zecca A, Penna A, Missale G, Ferrari C, Fisicaro P. Metabolic regulation of the HBV-specific T cell function. Antiviral Res. 2021;185:104989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 152. | Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64:S71-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 153. | Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G, Maini MK. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 154. | Huang WC, Easom NJ, Tang XZ, Gill US, Singh H, Robertson F, Chang C, Trowsdale J, Davidson BR, Rosenberg WM, Fusai G, Toubert A, Kennedy PT, Peppa D, Maini MK. T Cells Infiltrating Diseased Liver Express Ligands for the NKG2D Stress Surveillance System. J Immunol. 2017;198:1172-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 155. | Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 156. | Walter AJ, van de Klundert MA, Jung S. Many Ways to Communicate-Crosstalk between the HBV-Infected Cell and Its Environment. Pathogens. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 157. | Hoogeveen RC, Boonstra A. Checkpoint Inhibitors and Therapeutic Vaccines for the Treatment of Chronic HBV Infection. Front Immunol. 2020;11:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 158. | Boni C, Janssen HLA, Rossi M, Yoon SK, Vecchi A, Barili V, Yoshida EM, Trinh H, Rodell TC, Laccabue D, Alfieri A, Brillo F, Fisicaro P, Acerbi G, Pedrazzi G, Andreone P, Cursaro C, Margotti M, Santoro R, Piazzolla V, Brunetto MR, Coco B, Cavallone D, Zhao Y, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Massetto B, Fung S, Ahn SH, Ma X, Mangia A, Ferrari C. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients With Chronic Hepatitis. Gastroenterology. 2019;157:227-241.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 159. | Boni C, Rossi M, Vecchi A, Laccabue D, Giuberti T, Alfieri A, Andreone P, Cursaro C, Margotti M, Mangia A, Santoro R, Piazzolla V, Brunetto MR, Coco B, Cavallone D, Lau A, Gaggar A, Ferrari C. PS-050 - combined GS-4774 and tenofovir therapy can improve HBV-specific T cell responses in patients with chronic active hepatitis B. J Hepatol. 2017;66:S29. [DOI] [Full Text] |
| 160. | Ma H, Lim TH, Leerapun A, Weltman M, Jia J, Lim YS, Tangkijvanich P, Sukeepaisarnjaroen W, Ji Y, Le Bert N, Li D, Zhang Y, Hamatake R, Tan N, Li C, Strasser SI, Ding H, Yoon JH, Stace NH, Ahmed T, Anderson DE, Yan L, Bertoletti A, Zhu Q, Yuen MF. Therapeutic vaccine BRII-179 restores HBV-specific immune responses in patients with chronic HBV in a phase Ib/IIa study. JHEP Rep. 2021;3:100361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 161. | Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 162. | Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A, Zachoval R, Wächtler M, Spannagl M, Haas J, Diepolder HM, Jung MC. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 163. | Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 900] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 164. | Kumar S, Naqvi RA, Khanna N, Pathak P, Rao DN. Th3 immune responses in the progression of leprosy via molecular cross-talks of TGF-β, CTLA-4 and Cbl-b. Clin Immunol. 2011;141:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z. Blockade of Tim-3 signaling restores the virus-specific CD8⁺ T-cell response in patients with chronic hepatitis B. Eur J Immunol. 2012;42:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 166. | Jacobi FJ, Wild K, Smits M, Zoldan K, Csernalabics B, Flecken T, Lang J, Ehrenmann P, Emmerich F, Hofmann M, Thimme R, Neumann-Haefelin C, Boettler T. OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection. J Hepatol. 2019;70:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 167. | Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G, Ferrari C. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682-693, 693.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 168. | Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 169. | Wang D, Fu B, Shen X, Guo C, Liu Y, Zhang J, Sun R, Ye Y, Li J, Tian Z, Wei H. Restoration of HBV-specific CD8(+) T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-α therapy. Signal Transduct Target Ther. 2021;6:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 170. | Bénéchet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, Fumagalli V, Lusito E, Moalli F, Bianchessi V, Andreata F, Zordan P, Bono E, Giustini L, Bonilla WV, Bleriot C, Kunasegaran K, Gonzalez-Aseguinolaza G, Pinschewer DD, Kennedy PTF, Naldini L, Kuka M, Ginhoux F, Cantore A, Bertoletti A, Ostuni R, Guidotti LG, Iannacone M. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature. 2019;574:200-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 171. | Nosratabadi R, Alavian SM, Zare-Bidaki M, Shahrokhi VM, Arababadi MK. Innate immunity related pathogen recognition receptors and chronic hepatitis B infection. Mol Immunol. 2017;90:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 172. | Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3474] [Cited by in RCA: 3513] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 173. | Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol. 2018;37:20-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 174. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1212] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 175. | Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, Lampertico P, Grossi G, Facchetti F, Brunetto MR, Coco B, Cavallone D, Mangia A, Santoro R, Piazzolla V, Lau A, Gaggar A, Subramanian GM, Ferrari C. TLR7 Agonist Increases Responses of Hepatitis B Virus-Specific T Cells and Natural Killer Cells in Patients With Chronic Hepatitis B Treated With Nucleos(T)Ide Analogues. Gastroenterology. 2018;154:1764-1777.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 176. | Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, Bellezza CA, Cote PJ, Zheng J, Halcomb R, Fosdick A, Fletcher SP, Daffis S, Li L, Yue P, Wolfgang GH, Tennant BC. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol. 2015;62:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 177. | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508-1517, 1517.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 178. | Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Yoshida EM, Ahn SH, Tsai NCS, Fung S, Gane EJ. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 179. | Li Q, Yan Y, Liu J, Huang X, Zhang X, Kirschning C, Xu HC, Lang PA, Dittmer U, Zhang E, Lu M. Toll-Like Receptor 7 Activation Enhances CD8+ T Cell Effector Functions by Promoting Cellular Glycolysis. Front Immunol. 2019;10:2191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 180. | Gane EJ, Kim HJ, Visvanathan K, Kim YJ, Nguyen AH, Wallin JJ, Chen DY, McDonald C, Arora P, Tan SK, Gaggar A, Roberts SK, Lim YS. Safety, Pharmacokinetics, and Pharmacodynamics of the Oral TLR8 Agonist Selgantolimod in Chronic Hepatitis B. Hepatology. 2021;74:1737-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 181. | Gane E, Dunbar PR, Brooks A, Zhao Y, Tan S, Lau A, Yang J, Gaggar A, Subramanian M, Kottilil S, Tang L. Efficacy and safety of 24 wk treatment with oral TLR8 agonist, selgantolimod, in virally-suppressed adult patients with chronic hepatitis B: a phase 2 study. J Hepatol. 2020;73:S52. [DOI] [Full Text] |
| 182. | Bertoletti A, Le Bert N. Immunotherapy for Chronic Hepatitis B Virus Infection. Gut Liver. 2018;12:497-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 183. | Krebs K, Böttinger N, Huang LR, Chmielewski M, Arzberger S, Gasteiger G, Jäger C, Schmitt E, Bohne F, Aichler M, Uckert W, Abken H, Heikenwalder M, Knolle P, Protzer U. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 184. | Bohne F, Chmielewski M, Ebert G, Wiegmann K, Kürschner T, Schulze A, Urban S, Krönke M, Abken H, Protzer U. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology. 2008;134:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 185. | Wisskirchen K, Kah J, Malo A, Asen T, Volz T, Allweiss L, Wettengel JM, Lütgehetmann M, Urban S, Bauer T, Dandri M, Protzer U. T cell receptor grafting allows virological control of Hepatitis B virus infection. J Clin Invest. 2019;129:2932-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 186. | Kah J, Koh S, Volz T, Ceccarello E, Allweiss L, Lütgehetmann M, Bertoletti A, Dandri M. Lymphocytes transiently expressing virus-specific T cell receptors reduce hepatitis B virus infection. J Clin Invest. 2017;127:3177-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 187. | Bertoletti A, Tan AT. HBV as a target for CAR or TCR-T cell therapy. Curr Opin Immunol. 2020;66:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 188. | Koh S, Kah J, Tham CYL, Yang N, Ceccarello E, Chia A, Chen M, Khakpoor A, Pavesi A, Tan AT, Dandri M, Bertoletti A. Nonlytic Lymphocytes Engineered to Express Virus-Specific T-Cell Receptors Limit HBV Infection by Activating APOBEC3. Gastroenterology. 2018;155:180-193.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 189. | Klopp A, Schreiber S, Kosinska AD, Pulé M, Protzer U, Wisskirchen K. Depletion of T cells via Inducible Caspase 9 Increases Safety of Adoptive T-Cell Therapy Against Chronic Hepatitis B. Front Immunol. 2021;12:734246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 190. | Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D, Moriconi F, Farzhenah F, Mazzoni A, Chan L, Morris E, Thrasher A, Maini MK, Bonino F, Stauss H, Bertoletti A. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 191. | Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, Wu J, Li Y, Shi L, Fu JL, Yu S, Shen Y, Liu L, Luan J, Shi M, Xie Y, Zhou CB, Wong RW, Lu-En W, Koh S, Bertoletti A, Wang T, Zhang JY, Wang FS. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int. 2021;15:1402-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 192. | Anderson RT, Lim SG, Mishra P, Josephson F, Donaldson E, Given B, Miller V. Challenges, Considerations, and Principles to Guide Trials of Combination Therapies for Chronic Hepatitis B Virus. Gastroenterology. 2019;156:529-533.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 193. | Liu J, Wang T, Zhang W, Cheng Y, He Q, Wang FS. Effect of combination treatment based on interferon and nucleos(t)ide analogues on functional cure of chronic hepatitis B: a systematic review and meta-analysis. Hepatol Int. 2020;14:958-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 194. | Yuen MF, Locarnini S, Lim TH, Strasser SI, Sievert W, Cheng W, Thompson AJ, Given BD, Schluep T, Hamilton J, Biermer M, Kalmeijer R, Beumont M, Lenz O, De Ridder F, Cloherty G, Ka-Ho Wong D, Schwabe C, Jackson K, Lai CL, Gish RG, Gane E. Combination treatments including the small-interfering RNA JNJ-3989 induce rapid and sometimes prolonged viral responses in patients with CHB. J Hepatol. 2022;77:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 195. | Kuipery A, Sanchez Vasquez JD, Mehrotra A, Feld JJ, Janssen HLA, Gehring AJ. Immunomodulation and RNA interference alter hepatitis B virus-specific CD8 T-cell recognition of infected HepG2-NTCP. Hepatology. 2022;75:1539-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 196. | Kullak-Ublick GA, Merz M, Griffel L, Kaplowitz N, Watkins PB. Liver safety assessment in special populations (hepatitis B, C, and oncology trials). Drug Saf. 2014;37 Suppl 1:S57-S62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 197. | Howell J, Pedrana A, Schroeder SE, Scott N, Aufegger L, Atun R, Baptista-Leite R, Hirnschall G, 't Hoen E, Hutchinson SJ, Lazarus JV, Olufunmilayo L, Peck R, Sharma M, Sohn AH, Thompson A, Thursz M, Wilson D, Hellard M. A global investment framework for the elimination of hepatitis B. J Hepatol. 2021;74:535-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 198. | Cox AL, El-Sayed MH, Kao JH, Lazarus JV, Lemoine M, Lok AS, Zoulim F. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17:533-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 199. | Moghadami M, Dadashpour N, Mokhtari AM, Ebrahimi M, Mirahmadizadeh A. The effectiveness of the national hepatitis B vaccination program 25 years after its introduction in Iran: a historical cohort study. Braz J Infect Dis. 2019;23:419-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 200. | Hepatitis B Foundation. Vaccine for Hepatitis B. 2020. Available from: https://www.hepb.org/prevention-and-diagnosis/vaccination/. |
| 201. | Centers for Disease Control and Prevention (CDC). Hepatitis B Questions and Answers for Health Professionals. Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, 2020. Available from: https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm#vaccFAQ. |
| 202. | Afifi S, Mahran MH, Said ZN, Salama II, and El-Khayat HA. Serum Level of Anti-hepatitis B Surface Antigen among Newborns and Fully Vaccinated Infants and Children Aged 6 to 11 Years. Australian Journal of Basic and Applied Sciences. 2009; 3: 3239-3245. Available from: https://www.cabdirect.org/cabdirect/abstract/20103359441. |
| 203. | Sami SM, Salama II, Abdel-Latif GA, El Etreby LA, Metwally AI, Abd El Haliem NF. Hepatitis B Seroprotection and the Response to a Challenging Dose among Vaccinated Children in Red Sea Governorate. Open Access Maced J Med Sci. 2016;4:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 204. | Salama II, Sami SM, Elserougy SM, Emam HM, Salama SI, Elhariri HM, Hemeda SA, Hassanain AI, Abdel Mohsen AM, Fouad WA, El Etreby LA, Said ZN. Humoral Immune Memory to Hepatitis B Vaccine after Primary Vaccination of Children and Adolescents in Assiut, Egypt. Oman Med J. 2020;35:e175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 205. | Centers for Disease Control and Prevention (CDC). Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger, United States, 2020. National Center for Immunization and Respiratory Diseases, 2020. Available from: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. |