Published online Apr 27, 2023. doi: 10.4254/wjh.v15.i4.477
Peer-review started: December 27, 2022
First decision: January 19, 2023
Revised: February 4, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: April 27, 2023
Processing time: 113 Days and 7.9 Hours
As a result of the obesity epidemic, Nonalcoholic fatty liver disease (NAFLD) and its complications have increased among millions of people. Consequently, a group of experts recommended changing the term NAFLD to an inclusive terminology more reflective of the underlying pathogenesis; metabolic-associated fatty liver disease (MAFLD). This new term of MAFLD has its own disease epidemiology and clinical outcomes prompting efforts in studying its differences from NAFLD. This article discusses the rationale behind the nomenclature change, the main differences, and its clinical implications.
Core Tip: A new nomenclature to represent the underlying pathophysiology of fatty liver disease has been created and labeled metabolic-associated fatty liver disease. This article discusses the rationale behind the nomenclature change, the main differences to nonalcoholic fatty liver disease, and its clinical implications.
- Citation: Alomari M, Rashid MU, Chadalavada P, Ragheb J, Zafar H, Suarez ZK, Khazaaleh S, Gonzalez AJ, Castro FJ. Comparison between metabolic-associated fatty liver disease and nonalcoholic fatty liver disease: From nomenclature to clinical outcomes. World J Hepatol 2023; 15(4): 477-496
- URL: https://www.wjgnet.com/1948-5182/full/v15/i4/477.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i4.477
Nonalcoholic fatty liver disease (NAFLD) is a well-established terminology that was first coined by Ludwig and colleagues in 1980[1] to describe fatty liver disease arising in the absence of significant alcohol intake. Over the last four decades, there has been a rapidly growing global burden of NAFLD and its subtype nonalcoholic steatohepatitis (NASH)[2] which has a potentially progressive course that can lead to cirrhosis, hepatocellular carcinoma, liver transplantation, and potential death[3]. Currently, it is one of the most common causes of liver disease worldwide affecting nearly a quarter of the population[4,5], with increased recognition and diagnosis in younger individuals[6].
The rising body of research on NAFLD/NASH has led to a better understanding of its underlying pathophysiology and its relationship with metabolic syndrome[7]. Indeed, obesity and diabetes are the strongest risk factors associated with NAFLD/NASH. The public health and economic impacts of fatty liver disease have provoked extensive clinical trial activity targeted toward finding treatments for NASH among patients, regulators, and the biotechnology and pharmaceutical industries[8]. Despite this rapidly evolving activity, NASH resolution, and fibrosis regression rates are only 20%-30%[9].
In an effort to recognize the importance of metabolic abnormalities in an inclusive rather than an exclusive diagnosis, a group of international experts suggested a change of the name from NAFLD to metabolic-associated fatty liver disease (MAFLD)[10-12]. The criteria to diagnose MAFLD are based on evidence of hepatic steatosis in addition to one of the following three criteria: overweight/obesity, presence of type 2 diabetes mellitus, or evidence of metabolic dysregulation. Hence, MAFLD is more reflective of the heterogeneous pathogenesis of metabolic fatty liver diseases than NAFLD.
Immediately after the reappraisal of the nomenclature, multiple studies have been carried out to better understand the epidemiologic impact of this new terminology and its differences from NAFLD. For example, in a US population-based study by Kim et al[13], it was found that MAFLD was associated with an increased risk of all-cause mortality after adjusting for metabolic risk factors, while NAFLD was not. Interestingly, insulin resistance and stage of fibrosis were predictors of increased liver mortality in NAFLD but not MAFLD whose liver-associated mortality is primarily driven by alcohol-associated liver disease[14]. However, an awareness of the differences between these conditions and their early recognition remains poor among general practitioners[15]. Given patients with fatty liver disease are usually asymptomatic, a high index of suspicion is required to make the diagnosis. Additionally, the clinical guidelines of MAFLD and NAFLD need to be updated on a rolling basis to keep up with the most recent management practices to prevent disease progression.
In this article, we will discuss the rationale and history behind the nomenclature change, as well as the core differences between MAFLD and NAFLD with respect to various clinical aspects in contemporary practice.
"Fatty liver" was first described by Thomas Addison in 1836, who noted alcohol-related steatotic changes in liver histology[16,17]. A couple of decades later in 1857, George Budd noted similar histology in inactive, obese patients with a high-fat diet and alcohol intake[18]. Subsequently, Austin Flint observed a correlation between high carbohydrate intake with worsening steatosis and interval cirrhosis[19].
By the early 1900s, fatty liver changes unrelated to alcohol were well established but the mechanism of injury remained unclear. The role of diabetes as a risk factor for fatty liver began to be recognized by Pfluger in 1905 who noted an increase in hepatic steatosis in dogs who developed diabetes following total pancreatectomy[20]. These findings were later extrapolated to humans in 1936 when diet and newly discovered insulin were suggested as treatments for hepatomegaly secondary to steatosis in patients with juvenile diabetes[21].
In 1979, Adler and Schaffner developed a schema for fatty liver disease in overweight non- and light drinkers which included "fatty liver", "fatty hepatitis", "fatty fibrosis", and "fatty cirrhosis"[22]. A year later, fatty hepatitis would be designated "nonalcoholic steatohepatitis" (NASH) when Dr. Ludwig coins the term during characterizations of 20 Liver biopsies in primarily female patients with obesity and/or diabetes harboring lobular hepatitis, focal necrosis, and Mallory bodies on histology[1]. Not long after in 1986, NASH was included in the spectrum of "non-alcoholic fatty liver disease" NAFLD by Schaffner and Thaler[23,24].
Because the histology of alcohol-related and NAFLD steatosis is nearly indistinguishable from each other, NAFLD is a diagnosis of exclusion. Despite its name, NAFLD ironically includes patients with alcohol consumption of less than 14 and 7 drinks per week for men and women, respectively[25]. Further blurring the lines between NAFLD and alcoholic liver disease are studies recognizing that heavy alcohol drinkers who are obese are more likely to develop cirrhosis than non-obese heavy drinkers[25,26].
As more studies on NAFLD arose in the 21st century, NASH reappeared consistently as part of a syndrome including obesity, arterial hypertension, insulin resistance, dyslipidemia, and/or cardiovascular disease[27,28]. To better capture this syndrome, terms like "metabolic syndrome", "Syndrome X", "Insulin Resistance syndrome", and "the deadly quartet" were used[29]. The need for a more unified definition was reiterated in 2005, considering that the name "NAFLD" in no way highlighted the underlying metabolic etiologies, associated risk factors, or the phenotypic heterogeneity of the disease[30]. This paved the way for the introduction of the term "metabolic-associated fatty liver disease" in 2011 and was subsequently adopted in 2020 by international consensus[10,12]. Whereas NAFLD is defined as the presence of steatosis in > 5% of hepatocytes in the absence of other liver disease etiologies, MAFLD is defined by hepatic steatosis and components of the metabolic syndrome. MAFLD recognizes the positive determinants of the disease rather than defining the disease as the absence of other diseases, akin to the transition from “Non-Hepatitis A/Hepatitis B” to formally recognizing that entity as “Hepatitis C”[17]. Just as simultaneous alcohol-related liver disease and viral hepatitis can vary in disease behavior and prognosis from either entity alone, MAFLD can analogously exist with other liver diseases, including alcohol-related liver disease as patients with concomitant liver disease causes may have different outcomes than those of either disease apart[31-33].
The adoption of the new inclusive nomenclature and diagnostic criteria for MAFLD[10] has called for multiple studies and[34-40] several meta-analyses estimating the prevalence of the disease under the new diagnostic criteria in the setting of rising numbers of patients with overweight, obesity and type 2 diabetes mellitus[41]. These studies have estimated a global prevalence of 24.2% to 39.22% for MAFLD, comprising half of the overweight and obese adults[42], compared to a 15.3% to 33.86% for NAFLD[43]. MAFLD and NAFLD patients share clinical and pathogenic features leading to similarities in their overall prevalence. However, there are differences based on the presence of other liver diseases (i.e., alcoholic liver disease) that would still meet the criteria for MAFLD but not for NAFLD.
Compared to NAFLD, MAFLD is more likely to be diagnosed in Europe and Asia[44,45]. In addition, a non-statistically significant trend toward increased MAFLD in Hispanic ethnicity was reported[13]. Male sex, higher body mass index, lower high-density lipoprotein, higher triglyceride levels, and elevated aminotransferases carry a more significant correlation with MAFLD. Additionally, patients with MAFLD are more likely to have hypertension, diabetes mellitus, and chronic kidney disease[45]. Higher aminotransferases and the presence of advanced liver fibrosis in ultrasound elastography and liver biopsy are more common in patients with MAFLD+/NAFLD- when compared to a similar population with MAFLD-/NAFLD+. Mild alcohol consumption has been noted to be associated with a higher prevalence of significant fibrosis in those patients[46].
An analysis of the National Health and Nutrition Examination Survey (NHANES) database of the United States from 2017 to March 2020 found that the sample of patients with MAFLD had a higher prevalence of malignancies, coronary artery disease, myocardial infarction, heart failure, chronic pulmonary diseases, and psychiatric disorders, including sleep problems and depression when compared to the sample of patients with NAFLD; with the majority of these conditions being present in patients with more significant liver fibrosis[47].
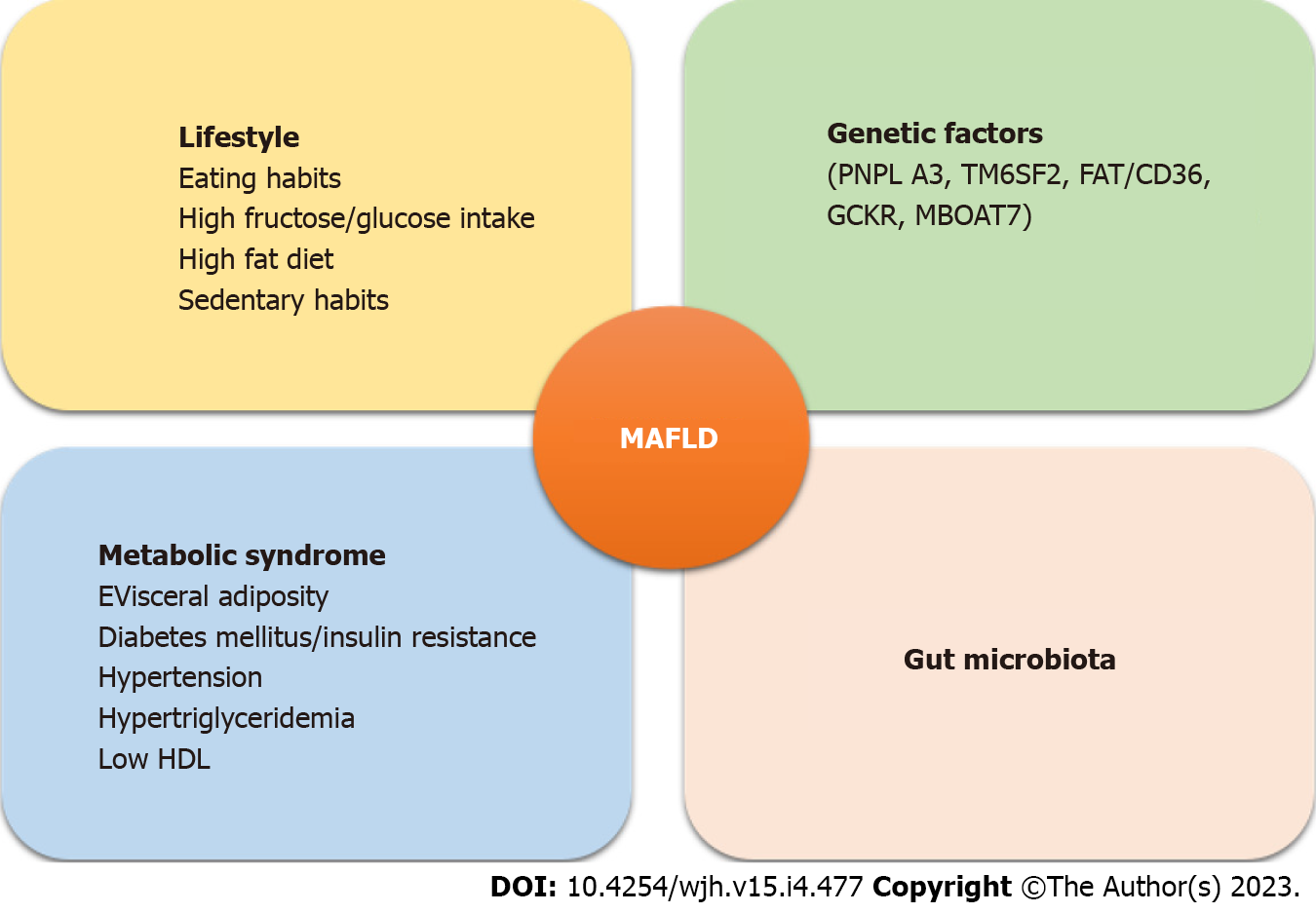
The exact pathophysiology of MAFLD\NAFLD remains largely unknown but a combination of genetic and environmental factors plays a crucial role (Figure 1). A contributing mechanism appears to be overnutrition and an increase in visceral adipose tissue[48]. Macrophages invade adipose tissue creating a pro-inflammatory state that promotes insulin resistance and excessive lipolysis enhancing the delivery of free fatty acids to the liver. An increased intrinsic lipogenesis overwhelms the liver's capacity to metabolize fatty acids resulting in dysfunction of the hepatocytes’ mitochondria and endoplasmic reticulum[49]. This dysfunction creates excessive oxidation of fatty acids and production of reactive oxygen species leading to hepatocyte death which promotes further inflammation and a vicious cycle[50].
Role of gut microbiota: Gut microbiotas affect pro-inflammatory and anti-inflammatory balance in the liver by their effect on gut barrier function. Increased fructose intake leads to increased permeability of enteric cells. Fructokinase is an enzyme in the liver that is also highly expressed in the gut. Metabolism of fructose by fructokinase in the intestine can result in increased permeability of enteric cells' tight junctions. This leads to increased absorption of endotoxins into the portal circulation[51]. Endotoxemia activates the innate immune system and the resulting inflammation has been shown to have a role in the transition from steatosis to steatohepatitis and cirrhosis[52] Co-occurrence of fatty liver/NASH and alteration of gut microbiota and disruption of epithelial barrier do not prove causation. The cause or consequence relationship between gut microbiota and NAFLD remains unclear. Some authors have hypothesized that liver damage might precede alteration in gut microbiota and permeability of tight junctions of enteric cells[53].
Gut microbiota also plays an important role in the metabolism of carbohydrates. Gut microbacteria can ferment dietary sugars into alcohol that can enter the portal circulation increasing oxidative stress and inflammation in the liver[54]. Studies have shown that in patients with MAFLD/NAFLD, there are significantly more gut bacteria associated with increased alcohol levels in the blood as compared to obese controls. Furthermore, some bacterial species like Escherichia, Enterobacter, Proteobacteria, and Bacteroides were found to be higher in NASH patients as compared to healthy controls[55-57].
Genetic factors: PNPLA3 (patatin-like phospholipase domain containing 3) genetic variation has been associated with MAFLD/NAFLD independent of metabolic syndrome. This gene codes for I148M that hydrolyzes triglycerides in adipose tissue resulting in increased delivery of free fatty acids to the liver[58].
TM6SF2 (transmembrane 6 superfamily member 2) encodes E167K which is a lipid transporter on the endoplasmic reticulum. Genetic variation in this protein causes loss of function and increased deposition of triglycerides in the liver[59].
Upregulation of genes coding for SREBP1c (sterol regulatory binding protein-1c), chREBP (car
Other genes associated with MAFLD/NAFLD, and NASH are GCKR, MBOAT7, FAT/CD36, IGFBP2, PGC1alpha, SIRT1, miR-122, and miR-34. HSD17B13 appears to have a protective role[61].
There is a close association between eating habits, obesity, and NAFLD. Increased consumption of refined carbohydrates, animal proteins, soft drinks, a high-fat diet, and fructose are closely associated with the development of MALFD/NAFLD[51]. Carbohydrates with high glycemic index led to increased liver glycogen and fat content and increased serum triglycerides. The predominant mechanism is increased de Novo Lipogenesis[62]. Saturated fatty acids and a diet high cholesterol in diet are associated with advanced fibrosis in MAFLD.
Patients with obesity and MAFLD/NAFLD have a more sedentary lifestyle[63,64]. Finally, another modifiable factor associated with advanced fibrosis in MAFLD/NAFLD is a smoking history of > 10 pack-years although the exact mechanism is unknown[65].
The presence of > 5% steatotic hepatocytes on liver biopsy is considered the minimum histologic criteria to diagnose fatty liver disease. Macrovesicular steatosis is the most common pattern although a mixed macro/microvesicular steatosis is seen in some cases as well. Pure microvesicular steatosis is not common. In MAFLD/NAFLD, small areas of lobular and portal inflammation and lipogranulomatous changes can be seen but features of hepatocyte injury and fibrosis are absent. Steatosis in adults has predilection to start in acinar zone 3 (perivenular)[66]. During the histological examination, the involvement of hepatocytes is assessed in percentages: 0%-33%-mild, 33%-66%-moderate, and > 66% hepatocyte involvement is categorized as severe steatosis[67].
On the other hand, histological features of NASH include steatosis and more severe inflammation than mentioned above along with hepatocyte injury and fibrosis. Ballooning, apoptosis, and/or necrosis are typical features of hepatocyte injury. Ballooning is of particular importance in NASH as its presence has been associated with a more aggressive disease and higher progression to cirrhosis[68]. However, the recognition of hepatocyte ballooning has significant inter-observer variation[69]. Hepatic apoptosis appears as acidophil bodies on liver biopsy. The Acidophil body index (acidophil bodies per mm2 of tissue) serves as further confirmation of NASH when the diagnosis is uncertain.
Fibrosis in NASH typically starts in zone 3 and has a "chicken wire" pattern which entails the deposition of fibrotic material along sinusoids of zone 3 and around hepatocytes. As the disease progresses, bridging fibrosis and features of macronodular or mixed cirrhosis are seen[70]. Other histological features that may be seen in the fatty liver include megamitochondria, iron deposition, glycogenated nuclei, and Mallory-Denk bodies[71].
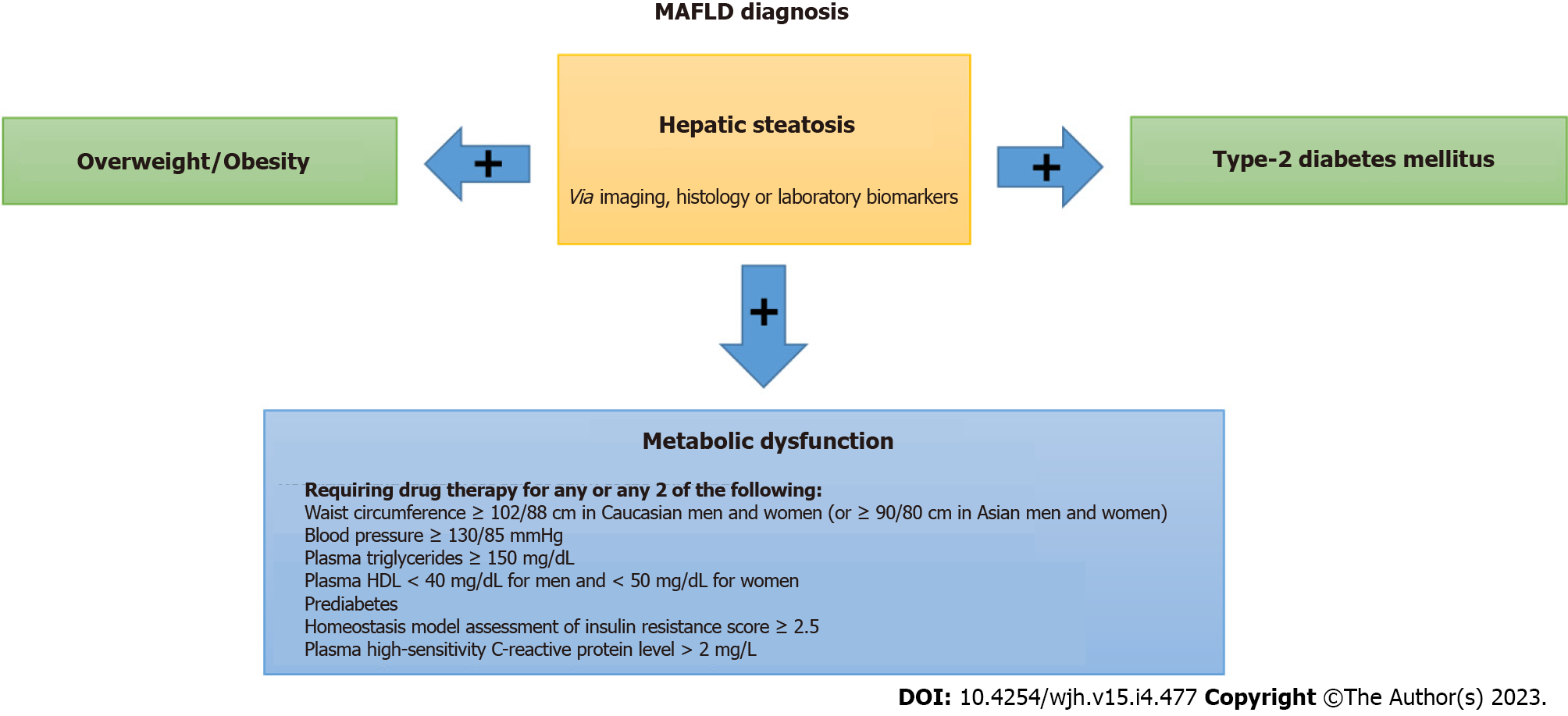
With recently published guidelines, experts have refined the criteria for the diagnosis of MAFLD. The new criterion involves the presence of hepatic steatosis in adults and the presence of one of the following: Type 2 diabetes mellitus, overweight/obesity, or metabolic dysregulation. Proposed diagnostic criteria for MAFLD are outlined in Figure 2.
Various modalities can be used to evaluate for the presence of hepatic steatosis including ultrasound, ultrasound elastography (i.e., transient elastography, acoustic radiation force impulse imaging, strain elastography), computed tomography, magnetic resonance imaging (MRI), magnetic resonance spectroscopy, MRI-derived proton density fat fraction, histology and serum biomarkers (i.e., fatty liver index, hepatic steatosis index, NAFLD liver fat score). Recently, ultrasound elastography has been increasingly used in clinical practice as compared to ultrasound. Ultrasound has limited sensitivity for detecting liver steatosis < 20% and performance may be suboptimal in patients with higher body mass index (BMI) > 40 kg/m2[72-74].
Patients with normal body weight can still develop MAFLD. It was demonstrated in a recent study that patients with BMI < 23 kg/m2 have the same disease severity on histology when compared to patients with BMI > 25 kg/m2[75]. It is also known that metabolically unhealthy patients who are not obese and have MAFLD are at increased risk for cardiovascular morbidity and liver damage as compared to metabolically healthy individuals[76]. Moreover, hepatic fat can be an early indicator of metabolic dysfunction. Therefore, in the new criteria in addition to diabetes mellitus and overweight/obesity, patients with lean/normal weight with metabolic dysregulation are included. In these patients, the presence of at least two metabolic risk factors is needed for diagnosis. The various risks include waist circumference ≥ 102/88 cm in caucasian men and women, blood pressure ≥ 130/85 mmHg, serum triglycerides ≥ 150 mg/dL, serum high-density lipoprotein cholesterol < 40 mg/dL for men, and < 50 mg/dL for women, prediabetes, serum high-sensitivity C-reactive protein level > 2 mg/L and homeostasis model assessment of insulin resistance score ≥ 2.5. Patients who meet the criteria for MAFLD and also have one or other chronic liver condition causing fatty liver should be classified as having dual etiology (or more) fatty liver disease.
The experts have also suggested that disease severity in MAFLD should be defined by grade of activity and fibrosis stage similar to NAFLD and NASH. Patients without typical histology of steatohepatitis but have cirrhosis can be defined as MAFLD-related cirrhosis if there is past or present evidence of metabolic dysregulation risk factors for MAFLD with at least one of the following: MAFLD on a previous liver biopsy or documentation of steatosis by hepatic imaging[77].
Despite the high worldwide prevalence, significant healthcare burden, and costs, there are currently no FDA-approved treatments for NAFLD. The 2018 AASLD practice guidelines on the treatment of NAFLD are based on the following four principles: (1) Effective diet and lifestyle modifications to achieve weight loss; (2) Identification and correction of underlying cardiometabolic risk factors; (3) Pharmacological therapy primarily aimed at improving hepatic steatosis/fibrosis; and (4) Close monitoring and prevention of complications of NAFLD[78].
Obesity and diabetes mellitus constitute the major pathophysiological risk factors for NAFLD, and drugs that modify or alter glucose metabolism and/or body weight comprise the current mainstay therapies focused on improving clinical outcomes, such as the degree of hepatic inflammation and/or fibrosis[3]. Over the past several years, multiple potential treatment options have been extensively investigated in this regard. These include insulin sensitizers and glucose-lowering drugs (such as pioglitazone, glucagon-like peptide-1 receptor agonists (GLP-1RA), sodium-glucose co-transporter-2 (SGLT-2) inhibitors), antioxidants (such as vitamin E), lipid-lowering drugs (statins), farnesoid X activated receptor (FXR) agonists and others.
The following section focuses on the different available treatment strategies for NAFLD highlighting our understanding of key elements regarding therapy.
Lifestyle modifications in the form of diet, exercise, and weight loss remain the cornerstone and first-line recommendation for treating patients with NAFLD/MAFLD[79]. Weight loss has by far the best evidence thus far as an independent predictor for improvement in histopathological features of NASH. The best likelihood for sustained weight loss appears to be a combination of a hypocaloric diet (decrease in caloric intake by 500-1000 kcal/d) and moderate-intensity exercise[80-82]. While weight loss of a minimum of 7%-10% of body weight is required to improve a majority of histopathological features of NASH, losing body weight by at least 3%-5% results in improved hepatic steatosis[83]. These findings were observed in a 12-mo prospective study that revealed a dose-response curve with significant improvement in histopathology with a greater degree of weight loss. Patients who achieved greater than 10% weight loss had improvement in all features of NASH, including portal inflammation and fibrosis. Importantly, patients who lost at least 5% of body weight stabilized or improved fibrosis in 94% of the cases[84]. Other lifestyle modifications may result in benefits as physical activity of more than 150 min/wk or an increase in activity level by more than 60 min/wk have been associated with a decrease in serum aminotransferases independent of weight loss[85]. On the other hand, a Mediterranean diet containing low saturated fat and high polyunsaturated and monounsaturated fats is found to be beneficial[86].
Pioglitazone, a peroxisome proliferator-activated receptor agonist, is a thiazolidinedione derivative that modulates glucose and lipid metabolism. It ameliorates insulin resistance in addition to creating positive effects on vascular biology, adipose tissue function, and inflammation[80]. These unique properties of pioglitazone led to immense interest among researchers in exploring its potential role in patients with NAFLD/MAFLD. A single-center clinical trial performed almost a decade ago suggested that pioglitazone at a dose of 45 mg daily in addition to a hypocaloric diet improved histological findings of steatosis, ballooning necrosis, and inflammation. However, the degree of fibrosis was no different from that of the placebo group[87,88]. More recently, in another study by Belfort et al[89], pioglitazone treatment not only improved the NAFLD activity score and metabolic parameters but also caused significant regression of liver fibrosis. These effects were observed after 36 mo of therapy with no significant difference in adverse events except for net weight gain of > 5.0 kg when compared to patients who did not receive pioglitazone.
Apart from the diabetic population, pioglitazone has also proven to be quite effective in NASH patients without diabetes mellitus (DM). In the Pioglitazone vs Vitamin E vs Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial, a substantially higher percentage of patients who received pioglitazone achieved resolution of NASH. However, the primary endpoint of at least ≥ 2-point improvement in NAFLD activity score did not reach predetermined statistical significance[90]. Hence, although pioglitazone is advocated for type 2 diabetes mellitus (T2DM) patients with biopsy-proven NASH, its use in the non-diabetic population still remains a debate among hepatologists.
A key mechanism of hepatocellular inflammation and injury in patients with NASH is oxidative stress. Vitamin E is an antioxidant that has been investigated in multiple studies as a potential treatment option for NAFLD. Initial studies demonstrated improved steatosis and inflammation with vitamin E administration but most of these were underpowered. Furthermore, it was challenging to compare data among the available studies largely due to significant heterogeneity regarding the dose and formulation of vitamin E, inclusion criteria, and concomitant use of other antioxidants or drugs.
More recently, the PIVENS clinical trial revealed that oral a-tocopherol, administered at a dose of 800 IU/d, results in considerable improvement in liver histology at 96 wk in non-diabetic biopsy-proven NASH patients[91]. These findings are substantiated by another US-based (TONIC) clinical trial that reported a significantly higher percentage of resolution of steatohepatitis in pediatric patients with NAFLD[92]. Despite similar convincing data from a few other studies, a high dose of vitamin E has been associated with increased all-cause mortality and a higher risk of prostate cancer with no improvement in fibrosis[93,94]. As such, the general consensus at this time includes consideration of vitamin E therapy in non-diabetic NAFLD/MAFLD patients following a patient-centered individualized approach. It is currently not recommended for treating NASH patients with DM or cirrhosis.
NAFLD/MAFLD are associated with metabolic syndrome that constitutes HTN, T2DM, dyslipidemia, and obesity. Current society guidelines recommend treating associated comorbidities in NAFLD patients in addition to treating the liver disease itself. Treatment with statins was associated with lower steatosis, inflammation, and fibrosis in NASH patients[95-98]. Moreover, patients who received statins had substantially lower cardiovascular mortality without any significant liver-related adverse events. A nationwide nested case-control study from South Korea including 11593409 patients from a nationwide database suggested that statins lower the risk of occurrence of NAFLD independent of accompanying T2DM[99]. In addition to the noted benefit in reducing the incidence of NAFLD, this study also highlighted that statin usage also prevented the progression to advanced fibrosis in patients with pre-existing fatty liver disease with an adjusted odds ratio of of 0.43; 95%CI 0.42–0.44). It is therefore reasonable to initiate anti-lipid therapy for patients that meet treatment criteria. While the concern for statin-induced hepatotoxicity prevails, severe liver injury from statins is extremely rare regardless of baseline elevation of transaminases or the presence of underlying chronic liver disease.
Glucagon-like peptide (GLP)-1 is a gut-derived incretin hormone secreted in response to oral food intake. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) constitute a novel class of well-established anti-diabetic drugs with encouraging data on the utility of these agents in treating obesity, preventing cardiovascular diseases, improving kidney function, and lowering mortality in patients with T2DM[99]. A growing body of evidence suggests that GLP-1RAs exert anti-NASH activity through several mechanisms such as increased insulin secretion, delayed gastric emptying, modulation of appetite, promoting fat redistribution, and reduced fat accumulation in the hepatocytes. A landmark UK-based 48-wk multicenter RCT (LEAN) revealed that NASH patients treated with liraglutide had a higher resolution of biopsy-proven steatohepatitis with no worsening of fibrosis when compared with placebo. Of those patients treated with liraglutide, 39% achieved resolution of NASH as opposed to only 9% in the placebo group. [Relative risk 4.3 (95%CI 1.0–17.7); P = 0.019]. These findings were supported by a Japanese pilot study (LEAN-J) where treatment with liraglutide was associated with significantly improved liver function and histological features in NASH patients with glucose intolerance[100].
Semaglutide, another GLP-1RA is currently approved for the treatment of T2DM and under extensive evaluation for weight loss[101]. Although semaglutide shares its mechanism of action with liraglutide, it has gained rapid recognition for its more pronounced metabolic effects with regard to improved glycemic control, reduced cardiovascular risk, and effective weight loss[102]. A placebo-controlled 72-wk trial suggested that once-daily subcutaneous semaglutide administered at a dose of 0.4 mg results in a higher likelihood of NASH resolution without worsening of underlying fibrosis in addition to a dose-dependent weight loss[103-105]. Despite these encouraging results, the rate of fibrosis regression was comparable between the treatment and placebo groups, and a higher number of patients treated with semaglutide experienced gastrointestinal side effects such as nausea and vomiting[106]. More refined data on histological outcomes and adverse effects will be required before the widespread use of semaglutide in routine clinical practice for the sole treatment of NAFLD/MAFLD.
Renal sodium-glucose co-transporter 2 (SGLT2) inhibitors are another class of anti-diabetic agents that work through the inhibition of glucose reabsorption in the kidneys in combination with enhanced urinary excretion of excess glucose. The consequent glucosuria results in lower blood glucose levels and eventual weight loss. A Malaysian open-label pilot study demonstrated that empagliflozin significantly improved steatosis, hepatocyte ballooning, and fibrosis in a small cohort of biopsy-proven NASH patients with T2DM. Interestingly, these effects were noted after a short duration of treatment (6 mo) and remained significant when compared with a historical placebo group at 48 wk[107]. Another meta-analysis of six randomized controlled trials including 309 patients by Xing et al[108] reported a positive effect of SGLT2 inhibitors in patients with NAFLD and T2DM. Patients treated with SGLT2 inhibitors achieved significant weight loss in addition to lower liver fat and improved alanine transaminase levels. Apart from the encouraging evidence on anti-NASH metabolic effects, several other studies have demonstrated improved cardiovascular outcomes in T2DM patients treated with SGLT2 inhibitors. Cardiovascular disease (CVD) currently remains the most common cause of death in patients with NAFLD, thus highlighting the added benefit of reduced cardiovascular deaths on the overall prognosis of NAFLD patients. Based on the aforementioned data, GLP-1 RAs, and SGLT2 inhibitors, either as monotherapy or combination therapy appear to be promising treatment options for NAFLD/MAFLD.
Dipeptidyl peptidase-4 (DPP-4) inhibitors such as sitagliptin, vildagliptin, and saxagliptin; slow the inactivation of incretin hormones such as GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) by selective inhibition of DPP-4 enzymes. As such, these agents indirectly increase insulin synthesis and lower glucagon levels through the prolonged action of GLP-1. Despite the observed benefit in early studies, these drugs have failed to significantly alter the histological profile in diabetic patients with NAFLD/MAFLD[109]. Possible explanations for the lack of clinical benefit postulated thus far in the literature include non-selective inhibition of both GLP-1 and GIP enzymes that could result in counterproductive effects on weight and fatty liver, questionable need for higher dosages in human models, and short study duration (3-6 mo). DPP-4 inhibitors are currently not recommended as therapies for patients with hepatic steatosis or steatohepatitis.
The Farnesoid X receptor is a ligand-activated nuclear receptor that is a key regulator for bile acid signaling and metabolism. In recent years, FXR has gained considerable interest as a potential therapeutic target for the treatment of NASH. FXR activation leads to the inhibition of bile acid synthesis and increased conjugation, transport, and excretion of bile acids. These changes ultimately result in decreased cholestasis with protection of the liver from the deleterious effect of bile accumulation[110].
Obeticholic acid (OCA), a synthetic 6α-ethyl derivative of natural human bile acid, chenodeoxycholic acid regulates lipid and glucose metabolism through stimulation and upregulation of FXR activity. The FLINT trial for the treatment of NASH revealed that OCA administered at a dose of 25 mg daily improved multiple liver histology parameters in nearly half (45%) of the patients at 72 wk follow-up[111]. These findings were noted despite the study including a significant proportion of patients with T2DM and vitamin E non-responders. This landmark study underscored the clinical relevance of FXR agonists in improving hepatic insulin sensitivity and inhibition of lipogenesis in the NASH population. These findings were later corroborated by another phase 3 study (REGENERATE) that evaluated the efficacy of OCA in 2400 patients with NASH including 2100 patients with stage 2 or 3 Liver fibrosis. This study revealed that a significantly higher proportion of patients treated with either 10 mg or 25 mg of OCA achieved regression of fibrosis compared to placebo but there was no difference between the groups in regards to complete resolution of steatohepatitis[112]. While the most common adverse event was mild to moderate pruritis, the incidence of serious adverse events was similar across the groups.
Tropifexor is a novel non-bile acid agonist of FXR that has demonstrated potent in vivo activity in animal models. This drug is believed to be highly efficacious in upregulating FXR target genes even at very low doses and has currently progressed into clinical development. Several phase 2 human clinical trials on the safety and efficacy of Tropifexor in patients with NASH are under evaluation[113-115].
Weight loss through lifestyle modifications is often challenging to achieve or sustain due to the substantial need for strict adherence and patient compliance. Bariatric surgery may be opted to achieve weight loss in select patients as it helps improve lipid metabolism and inflammatory pathways involved in the pathophysiology of NAFLD. National Institute of Health consensus criteria currently recommends bariatric surgery for patients with a BMI of 35 to 39.9 kg/m2 with any severe obesity-related comorbidity such as T2DM, HTN, NAFLD/MAFLD, and/or NASH.
A systematic review appraising 29 studies by Bower et al[116] showed significant improvement in several histological (steatosis, hepatocyte ballooning, lobular inflammation, and fibrosis) and biochemical parameters of NAFLD following bariatric surgery. A more recent meta-analysis spanning 21 studies with a total of 2374 patients reaffirmed the noted benefit of bariatric surgery in patients with NAFLD with almost 88% of patients achieving improvement in steatosis and 30% having an improvement or resolution in liver fibrosis.
Apart from the long-term sustained weight loss, bariatric surgery also ameliorates NAFLD/MAFLD through multiple other mechanisms including but not limited to enhanced secretion of satiety hormones, variation in dietary habits, improvement in T2DM, alterations in bile acid homeostasis, modification of gut microbiome[116]. Further longitudinal controlled studies are needed to delineate the benefits and type of bariatric surgery as a therapy for those with NAFLD/MAFLD.
Although a few case series on bariatric surgery suggest an acceptable safety profile of these procedures in patients with cirrhosis, a vast majority of these studies included patients with well-compensated cirrhosis. More recently, evolving data demonstrated a modest but nonnegligible risk of complications following bariatric surgery in patients with more advanced cirrhosis. The common complications reported in such patients undergoing bariatric surgery include anastomotic leak, prolonged hospital stay, prolonged intubation, ileus, higher need for blood transfusion, and less commonly sepsis and fulminant hepatic failure[117-120]. As such, bariatric surgery is currently preferred for patients with Child A cirrhosis due to the acceptable risk of complications from surgical and hepatic factors[121-123].
Several gut microbiota is known to interact with carbohydrate and lipid metabolism by regulating homeostasis, immunity, and several metabolic pathways. Gut microbiome dysbiosis increases gut permeability thereby increasing exposure of hepatocytes to endotoxins that eventually can lead to hepatocyte inflammation and fibrosis[124]. Studies from animal models suggest that oral administration of prebiotics, probiotics, and synbiotics improves lipid metabolism, dysbiosis, insulin resistance, and hypercholesterolemia associated with hepatic inflammation by altering multiple genes involved in B-oxidation and lipogenesis.
A randomized controlled trial by Vrieze et al[125] suggested that FMT improved insulin resistance in Caucasian males with metabolic syndrome thus raising a potential therapeutic option for NAFLD/MAFLD. Needless to say, there is a dire need for more improved and standardized methods of gut microbiome analysis along with a better understanding of interactions between diet, dysbiosis, and environmental factors and their impact on the gut-liver axis to help devise effective and targeted treatment options.
Multiple other studies recently evaluated anti-fibrotic and anti-apoptotic therapies as unique treatment options for patients with NAFLD[126,127]. Albeit the intriguing preliminary results, these studies failed to show considerable clinical benefit in phase 2/3 clinical trials largely due to the limited utility of these drugs in the early stages of NAFLD. The introduction of MAFLD and its diagnostic criteria appear promising as it would potentially allow the incorporation of pharmacotherapeutic agents used in the treatment of metabolic syndrome at an earlier stage in the development of NAFLD.
If left untreated, patients with NAFLD are at risk of advanced fibrosis. A meta-analysis encompassing 11 studies with 411 patients with biopsy-proven NASH showed that 33.6% of patients have eventual fibrosis progression. The annual fibrosis progression rate in patients with NAFLD and stage 0 fibrosis at baseline was 0.07 stages (95%CI, 0.02-0.11 stages), compared with 0.14 stages in patients with NASH (95%CI, 0.07-0.21 stages). These findings correspond to 1 stage of progression over 14.3 years for patients with NAFLD (95%CI, 9.1-50.0 y) and 7.1 years for patients with NASH (95%CI, 4.8-14.3 y)[128]. While baseline demographics such as BMI, age, history of alcohol use, and T2DM play a major role in determining progression to advanced fibrosis, disease-related risk factors such as elevated baseline transaminases, presence of necroinflammation, ballooning degeneration, and Mallory hyaline on histology are also known to contribute to disease progression[70,129-132].
Advanced fibrosis eventually leads to hepatic decompensation from the development of cirrhosis and end-stage liver disease (2.69 events per 100 person-years). Liver-related mortality is the third cause of death in patients with NAFLD[133].
Cirrhosis secondary to NAFLD/MAFLD does confer a higher risk of hepatocellular carcinoma (HCC) than those without cirrhosis[134]. Interestingly, the presence of fatty liver disease also confers a higher risk of HCC even in the absence of cirrhosis. Past data suggests that patients with non-cirrhotic NAFLD have a modest risk of HCC when compared to the general population[135,136]. This risk is notably higher in male patients > 65 years with a smoking history, co-existing DM, and/or baseline elevation of ALT[137-144]. As such, performing routine periodic surveillance of these patients with abdominal imaging and measuring alpha-fetoprotein levels could be considered.
Cardiovascular disease is the most common cause of death among NAFLD/MAFLD patients followed by extrahepatic malignancies[145]. MAFLD is the hepatic manifestation of metabolic syndrome; as such, a vast majority of these patients have additional cardiometabolic risk factors. Indeed, when compared to NAFLD patients a nationwide database study from China demonstrated that MAFLD patients had an increased medium/high 10-year CVD risk according to Framingham risk score [1064 (29.92%) vs 1022 (26.37%), P < 0.005][146]. While steatosis confers a lesser risk of CVD compared to steatohepatitis, the overall individual risk of CVD ultimately stems from the combination of the stage of fatty liver disease and the presence of other cardiometabolic risk factors. Patients with steatohepatitis and /or advanced fibrosis and those with co-existing T2DM are considered special risk groups for significant cardiovascular events and mortality[147].
In addition to coronary atherosclerosis, NAFLD/MAFLD patients are at a higher risk of cardiac arrhythmias such as atrial fibrillation and ventricular arrhythmias. Furthermore, many NAFLD patients have left ventricular systolic and/or diastolic dysfunction, aortic valve sclerosis, and mitral calcifications[148-153]. While several studies have revealed a higher burden of cardiorenal disease in patients with MAFLD, a recent meta-analysis by Wen et al[147] evaluating ten studies suggested a 1.95 times higher incidence of CVD or CVD-related mortality in the MAFLD patients than in the control group[146,154,155]. Therefore, a thoughtful risk assessment of CVD, evaluation for subclinical atherosclerosis, and presumptive diagnostic studies and interventions for high-risk patients are needed to lower the high global disease burden of CVD in NAFLD/MAFLD patients. Studies evaluating the association of NAFLD/MAFLD, and CVD are listed in Table 1 for further review.
| Study | Number of patients | Type of study | Outcome measure | Results |
| Liang et al[168] | 6873 | Cohort Study with a 4.6 yr follow up | Associations of MAFLD and NAFLD with DM, CKD, and CVD | MAFLD was associated with higher risks of CVD (hazard ratio 1.44; 95%CI, 1.15-1.81); Similar associations were observed for NAFLD, except for a higher incidence of DM in MAFLD patients with HBV infection and excess alcohol consumption |
| Wang et al[169] | 12183 | Cross-sectional study (SPECT – China) | Compare the cardiovascular and renal burden between MAFLD and NAFLD patients | The odds ratio of previous CVD was higher in patients with MAFLD. Male 1.50 (1.22,1.85) vs 1.35 (1.1, 1.66); female 1.58 (1.33,1.87) vs 1.45 (1.22, 1.72) |
| Zhang et al[170] | 19617 | Nationwide database study | The burden of CKD and CVD in adults with MAFLD and NAFLD | The cardiorenal burden may be greater for MAFLD than for NAFLD |
| Lee et al[171] | 8962813 | Cohort study with a 10.1 yr follow up | Association of MAFLD and NAFLD with CVD | MAFLD patients have a higher risk of CKD when compared to NAFLD [1.43 (1.41–1.45) vs 1.09 (1.03–1.15)] |
| Yoneda et al[172] | 2452949 | Nationwide database study | Association of MAFLD and NAFLD with CVD | The incidence rates of CVD were 2.82 (95%CI 2.64-3.01) per 1000 person-yr in the NAFLD groups and 2.69 (95%CI 2.55-2.83) per 1000 person-years in the MAFLD groups |
| Guerreiro et al[173] | 1233 | Retrospective cross-sectional study | Compare CVR and risk of CVD between patients with NAFLD and MAFLD | In patients with MAFLD and NAFLD, CVR was intermediate/high (36.4 and 25.7%, P = 0.209) and CVD occurred in 20.1 and 12.8% (P = 0.137) of the cases, respectively, with no influence of liver injury severity |
| Zhang et al[158] | 11673 | Retrospective study | Compare the risk of CVD between patients with NAFLD and MAFLD | MAFLD was more significant than NAFLD in medium/high 10-yr CVD risk (according to Framingham risk score) [1064 (29.92%) vs 1022 (26.37%), P < 0.005] |
| Wen et al[147] | - | Meta-analysis | Investigate the risk of CVD incidence or CVD-related mortality in patients diagnosed with MAFLD and NAFLD | The incidence of CVD or CVD mortality was 1.95 times higher in the MAFLD group than in the control group. The risk of CVD or death from CVD was significantly higher in the MAFLD-only group than in the NAFLD-only group, with an RR of 2.57 (95%CI 1.41–4.71; I2 = 78%, P = 0.002) |
| Guo et al[174] | 12794 | Cohort study | Study the relationship between MAFLD and incident CVD | The incidence of CVD in the patients with MAFLD was significantly higher than that in the non-MAFLD patients (18.38% vs 9.02%, P ≤ 0.001; aHR = 1.37, 95%CI = 1.20-1.56) |
| Moon et al[175] | 8919 | Cohort study | Effect of MAFLD on future mortality and CVD using a prospective community-based cohort study | T2DM in MAFLD increased the risk of both mortality (HR, 2.07; 95%CI, 1.52 to 2.81) and CVD (HR, 1.42; 95%CI, 1.09 to 1.85) |
| Zou et al[176] | 513 | Cross-sectional study | Prevalence of MAFLD and its relationship with CVD risks in RA patients | RA patients with MAFLD had a higher rate of CVD events (17.3% vs 9.2%) and a higher proportion of high estimated 10-yr CVD risk (55.5% vs 26.1%) than those without |
Chronic kidney disease (CKD) is a worldwide public health problem affecting up to 10%-15% of the general population. More recently, a strong association between NAFLD/MAFLD and CKD has been established independent of commonly co-existing diseases such as obesity, HTN, and T2DM. A meta-analysis by Targher et al[156] suggested that NAFLD patients had a considerably higher risk of incident CKD than those without NAFLD. (OR 1.87; 95%CI 1.3-4.1). Few other studies also demonstrated similar findings even after adjustment for potential confounders such as age, sex, BMI, and other well-established metabolic risk factors[157]. Currently postulated etiologies include upregulation of the renin-angiotensin system and impairment of antioxidant defense. Other associated factors that link NAFLD/MAFLD and CKD include metabolic syndrome, platelet activation, dysbiosis, unhealthy eating habits, and aging[158-160].
Since the advent of the new diagnostic criteria for MAFLD, numerous studies have suggested a significant association of MAFLD with CKD, particularly in patients with concomitant DM. A nationwide cohort study evaluating 268946 patients by Jung et al[161] revealed a higher adjusted hazard ratio (aHR) for incident CKD in MAFLD patients when compared to those with NAFLD (1.18 (95%CI, 1.01-1.39; P = 0.040). Studies evaluating the association of NAFLD/MAFLD, and CKD are listed in Table 2 for further review.
| Study | Number of patients | Type of Study | Outcome measure | Results |
| Sun et al[177] | 12571 | Cross-sectional study | Association between MAFLD and NALFD with CKD | MAFLD patients had lower GFR (74.96 ± 18.21) and higher prevalence of CKD (29.6%) |
| Liang et al[168] | 6873 | Cohort Study with a 4.6 yr follow up | Associations of MAFLD and NAFLD with T2DM, CKD, and CVD | MAFLD was associated with a higher risk of CKD (RR 1.64; 95%CI, 1.39-1.94). Similar associations were observed for NAFLD, except for a higher incidence of DM in MAFLD patients with HBV infection and excess alcohol consumption. |
| Deng et al[178] | 4869 | A cross-sectional study from the NHANES database 2017 – 2018 | Association between MAFLD and CKD | Higher prevalence of CKD in MAFLD subjects than in non-MALFD subjects (22.2% vs 19.1%, P = 0.048) |
| Wang et al[169] | 12183 | Cross-sectional study (SPECT – China) | Compare the cardiovascular and renal burden between MAFLD and NAFLD patients | OR of CKD was higher in males with NAFLD [CKD: 1.44 (1.05, 1.96) vs 1.56 (1.14, 2.12)] than those with MAFLD |
| Su et al[179] | 5594 | Cross-sectional study | Association between MAFLD and CKD | MAFLD was independently associated with an increased risk of CKD [odds ratio (OR): 1.35, 95%CI: 1.09-1.67]. MAFLD with T2DM had significant associations with increased risk of CKD (OR: 2.85, 95%CI: 2.24-3.63), as well as increased eGFR and UACR |
| Hu et al[180] | 15010 | Cross-sectional study | Association between MAFLD and CKD | MAFLD was significantly associated with a higher CKD prevalence (OR 1.715, 95%CI 1.389-2.117, P < 0.001). MAFLD alone was not an independent risk factor for CKD |
| Hashimoto et al[181] | 27371 | Cross-sectional study | Association between FLD and MAFLD with CKD | MAFLD was associated with the risk of incident CKD [adjusted hazard ratio 1.24 (1.14-1.36), P < 0.001], whereas FLD without MD was not [1.11 (0.85-1.41), P = 0.433] |
| Zhang et al[170] | 19617 | A retrospective nationwide cohort study | Renal burdens in adults with MAFLD and NAFLD | The cardiorenal burden may be greater for MAFLD than for NAFLD |
| Jung et al[161] | 268946 | A retrospective nationwide cohort study | Association between MAFLD and NALFD with CKD | The adjusted hazard ratio (aHR) for incident CKD in MAFLD was 1.18 (95%CI, 1.01-1.39; P = 0.040) compared to those with NAFLD |
| Tanaka et al[182] | 13159 | Retrospective single-center study | Associations of FL, NAFLD, and MAFLD with the development of CKD | MAFLD [HR (95%CI): 1.12 (1.02-1.26), P = 0.027], but not FL or NAFLD, was an independent risk factor for incident CKD |
Metabolic syndrome is a well-known risk factor for colorectal cancer. Consequently, several studies have suggested that NASH is independently associated with a heightened risk of colorectal adenomas and advanced colonic neoplasms. Of late, Fukunaga et al[162] suggested that MAFLD identifies colorectal adenomas more accurately than NAFLD (OR 3.191; 95%CI 1.494-7.070; P = 0.003), with a particularly high risk of colonic adenomas in individuals with non-obese MAFLD (OR 3.351; 95%CI 1.589-7.262; P ≤ 0.001). Whether NAFLD/MAFLD directly contributes to colon cancer or if colon cancer occurs due to shared metabolic risk factors remains unclear. Patients with NAFLD are believed to have lower adiponectin levels that result in lesser endothelial cell apoptosis and increased proliferation of neoplastic cells, supporting the former possibility of a direct carcinogenic effect of steatohepatitis. Moreover, increased risk of other extrahepatic malignancies such as gastric cancer[163], pancreatic cancer[164], esophageal cancer[165], breast cancer[166], and prostate cancer[167] are noted in patients with NAFLD.
Other commonly associated conditions in patients with NAFLD/MAFLD include gastroesophageal reflux disease, obstructive sleep apnea syndrome, psychological dysfunction, hypothyroidism, growth hormone deficiency, and polycystic ovarian syndrome. Despite the substantial research and evidence revealing an association between NAFLD/MAFLD and the aforementioned extrahepatic complications, there are no standardized screening recommendations for these conditions in these patient populations. Careful surveillance and proactive treatment of these extrahepatic complications might improve overall outcomes, morbidity, and mortality in patients with NAFLD/MAFLD.
The change in nomenclature has impacted the current understanding of fatty liver disease and stimulated more interest from the research community to better understand and treat this silent but deadly condition. As a limitation of our review, it only provides information about what is known and has been published to date as data on MAFLD is emerging. As new evidence becomes available, practitioners will be better able to tackle this disease and prevent its deleterious complications.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Italy; Ozlu T, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 2. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3785] [Article Influence: 540.7] [Reference Citation Analysis (2)] |
| 3. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4939] [Article Influence: 705.6] [Reference Citation Analysis (9)] |
| 4. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1315] [Article Influence: 219.2] [Reference Citation Analysis (0)] |
| 5. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 6. | Wiegand S, Keller KM, Röbl M, L'Allemand D, Reinehr T, Widhalm K, Holl RW; APV-Study Group and the German Competence Network Adipositas. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond). 2010;34:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Hamid O, Eltelbany A, Mohammed A, Alsabbagh Alchirazi K, Trakroo S, Asaad I. The epidemiology of non-alcoholic steatohepatitis (NASH) in the United States between 2010-2020: a population-based study. Ann Hepatol. 2022;27:100727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Drew L. Drug development: Sprint finish. Nature. 2017;551:S86-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2908] [Article Influence: 415.4] [Reference Citation Analysis (1)] |
| 10. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2200] [Article Influence: 440.0] [Reference Citation Analysis (1)] |
| 11. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2819] [Article Influence: 563.8] [Reference Citation Analysis (1)] |
| 12. | Balmer ML, Dufour JF. [Non-alcoholic steatohepatitis - from NAFLD to MAFLD]. Ther Umsch. 2011;68:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 305] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 14. | Younossi ZM, Paik JM, Al Shabeeb R, Golabi P, Younossi I, Henry L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology. 2022;76:1423-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 130] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 15. | Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Addison T. Observations on fatty degeneration of the liver. Guys Hosp Rep. 1836;1:476-85. |
| 17. | Lonardo A, Leoni S, Alswat KA, Fouad Y. History of Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2020;21:5888. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 18. | Budd G. On Diseases of the Liver. 3rd ed. Blanchard, Lea, editors. 1857. Available from: http://resource.nlm.nih.gov/65840330R. |
| 19. | A Treatise on the Principles and Practice of Medicine; Designed for the Use of Practitioners and Students of Medicine. Atlanta Med Surg J. 1867;7B:565-571. [PubMed] |
| 20. | Pfluger E. Glycogen and Its Relation to Diabetes. 2nd ed. von Martin Hager V, editor. 1905. |
| 21. | Connor CL. Fatty infiltration of the liver and the development of cirrhosis in diabetes and chronic alcoholism. Am J Pathol. 1938;14:347-364.9. [PubMed] |
| 22. | Adler M, Schaffner F. Fatty liver hepatitis and cirrhosis in obese patients. Am J Med. 1979;67:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 237] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986;8:283-298. [PubMed] |
| 24. | Ayonrinde OT. Historical narrative from fatty liver in the nineteenth century to contemporary NAFLD - Reconciling the present with the past. JHEP Rep. 2021;3:100261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Weng G, Dunn W, Gong W. Effect of alcohol consumption on nonalcoholic fatty liver disease. Transl Gastroenterol Hepatol. 2019;4:70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 436] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 27. | Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, Lurie Y, Bass DD. Fatty liver--an additional and treatable feature of the insulin resistance syndrome. QJM. 1999;92:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6:578-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 330] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 29. | Sarafidis PA, Nilsson PM. The metabolic syndrome: a glance at its history. J Hypertens. 2006;24:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Loria P, Lonardo A, Carulli N. Should nonalcoholic fatty liver disease be renamed? Dig Dis. 2005;23:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Tsui JI, Pletcher MJ, Vittinghoff E, Seal K, Gonzales R. Hepatitis C and hospital outcomes in patients admitted with alcohol-related problems. J Hepatol. 2006;44:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, Janssen HLA, Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 33. | Takahashi A, Arinaga-Hino T, Ohira H, Abe K, Torimura T, Zeniya M, Abe M, Yoshizawa K, Takaki A, Suzuki Y, Kang JH, Nakamoto N, Fujisawa T, Tanaka A, Takikawa H; Japan AIH Study Group (JAIHSG). Non-alcoholic fatty liver disease in patients with autoimmune hepatitis. JGH Open. 2018;2:54-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | García-Compeán D, Jiménez-Rodríguez AR. NAFLD VS MAFLD. The evidence-based debate has come. Time to change? Ann Hepatol. 2022;27:100765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Fouad Y, Elwakil R, Elsahhar M, Said E, Bazeed S, Ali Gomaa A, Hashim A, Kamal E, Mehrez M, Attia D. The NAFLD-MAFLD debate: Eminence vs evidence. Liver Int. 2021;41:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 36. | Ratziu V, Rinella M, Beuers U, Loomba R, Anstee QM, Harrison S, Francque S, Sanyal A, Newsome PN, Younossi Z. The times they are a-changin' (for NAFLD as well). J Hepatol. 2020;73:1307-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | van Kleef LA, de Knegt RJ. The transition from NAFLD to MAFLD: One size still does not fit all-Time for a tailored approach? Hepatology. 2022;76:1243-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Valenti L, Pelusi S. Redefining fatty liver disease classification in 2020. Liver Int. 2020;40:1016-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Wong VW, Lazarus JV. Prognosis of MAFLD vs. NAFLD and implications for a nomenclature change. J Hepatol. 2021;75:1267-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Mantovani A. MAFLD vs NAFLD: Where are we? Dig Liver Dis. 2021;53:1368-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1453] [Article Influence: 242.2] [Reference Citation Analysis (1)] |
| 42. | Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, Ma Z, Bruno MJ, de Knegt RJ, Cao W, Peppelenbosch MP, Ghanbari M, Li Z, Pan Q. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin Gastroenterol Hepatol. 2022;20:e573-e582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 43. | Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, Chien N, Trinh S, Henry L, Stave CD, Hosaka T, Cheung RC, Nguyen MH. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 44. | Xian YX, Weng JP, Xu F. MAFLD vs. NAFLD: shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin Med J (Engl). 2020;134:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 45. | Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, Yong JN, Xiao J, Lee CW, Chan M, Chew NW, Xuan Tan EX, Siddiqui MS, Huang D, Noureddin M, Sanyal AJ, Muthiah MD. An Observational Data Meta-analysis on the Differences in Prevalence and Risk Factors Between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21:619-629.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 144] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 46. | Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 47. | Theofilis P, Vordoni A, Kalaitzidis RG. Metabolic Dysfunction-Associated Fatty Liver Disease in the National Health and Nutrition Examination Survey 2017-2020: Epidemiology, Clinical Correlates, and the Role of Diagnostic Scores. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 49. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1676] [Article Influence: 419.0] [Reference Citation Analysis (33)] |
| 50. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2112] [Article Influence: 234.7] [Reference Citation Analysis (1)] |
| 51. | Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, Sanchez-Lozada LG, Rosen HR, Lanaspa MA, Diehl AM, Johnson RJ. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 636] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 52. | Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 53. | Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, Deshpande V, Singh G, Turner JR, Yarmush ML, Chung RT, Patel SJ. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. 2015;1:222-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 54. | Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 393] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 55. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1278] [Article Influence: 106.5] [Reference Citation Analysis (1)] |
| 56. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1049] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 57. | Fei N, Bruneau A, Zhang X, Wang R, Wang J, Rabot S, Gérard P, Zhao L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 58. | Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 739] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 59. | Longo M, Meroni M, Paolini E, Erconi V, Carli F, Fortunato F, Ronchi D, Piciotti R, Sabatini S, Macchi C, Alisi A, Miele L, Soardo G, Comi GP, Valenti L, Ruscica M, Fracanzani AL, Gastaldelli A, Dongiovanni P. TM6SF2/PNPLA3/MBOAT7 Loss-of-Function Genetic Variants Impact on NAFLD Development and Progression Both in Patients and in In Vitro Models. Cell Mol Gastroenterol Hepatol. 2022;13:759-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 60. | Fougerat A, Montagner A, Loiseau N, Guillou H, Wahli W. Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 61. | Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 62. | Hydes T, Alam U, Cuthbertson DJ. The Impact of Macronutrient Intake on Non-alcoholic Fatty Liver Disease (NAFLD): Too Much Fat, Too Much Carbohydrate, or Just Too Many Calories? Front Nutr. 2021;8:640557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 63. | Hallsworth K, Thoma C, Moore S, Ploetz T, Anstee QM, Taylor R, Day CP, Trenell MI. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 64. | Hamurcu Varol P, Kaya E, Alphan E, Yilmaz Y. Role of intensive dietary and lifestyle interventions in the treatment of lean nonalcoholic fatty liver disease patients. Eur J Gastroenterol Hepatol. 2020;32:1352-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 65. | Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:753-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 66. | Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:5286-5296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (2)] |
| 67. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1478] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 68. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2343] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 69. | Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 70. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 646] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 71. | Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 72. | Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O'Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Zh, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1610] [Cited by in RCA: 1744] [Article Influence: 193.8] [Reference Citation Analysis (0)] |
| 73. | Ampuero J, Aller R, Gallego-Durán R, Banales JM, Crespo J, García-Monzón C, Pareja MJ, Vilar-Gómez E, Caballería J, Escudero-García D, Gomez-Camarero J, Calleja JL, Latorre M, Albillos A, Salmeron J, Aspichueta P, Lo Iacono O, Francés R, Benlloch S, Fernández-Rodríguez C, García-Samaniego J, Estévez P, Andrade RJ, Turnes J, Romero-Gómez M; HEPAmet Registry. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48:1260-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 74. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1619] [Article Influence: 115.6] [Reference Citation Analysis (1)] |
| 75. | Arrese M, Barrera F, Triantafilo N, Arab JP. Concurrent nonalcoholic fatty liver disease and type 2 diabetes: diagnostic and therapeutic considerations. Expert Rev Gastroenterol Hepatol. 2019;13:849-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 76. | Rastogi A, Shasthry SM, Agarwal A, Bihari C, Jain P, Jindal A, Sarin S. Non-alcoholic fatty liver disease - histological scoring systems: a large cohort single-center, evaluation study. APMIS. 2017;125:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 77. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 78. | Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 558] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 79. | Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 397] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 80. | St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 81. | Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB; NASH CRN Research Group. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460-8; quiz 469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 318] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 82. | Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 83. | Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 84. | Sung KC, Ryu S, Lee JY, Kim JY, Wild SH, Byrne CD. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol. 2016;65:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 85. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1622] [Article Influence: 162.2] [Reference Citation Analysis (1)] |
| 86. | Guveli H, Kenger EB, Ozlu T, Kaya E, Yilmaz Y. Macro- and micronutrients in metabolic (dysfunction) associated fatty liver disease: association between advanced fibrosis and high dietary intake of cholesterol/saturated fatty acids. Eur J Gastroenterol Hepatol. 2021;33:e390-e394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 88. | Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711-725.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 659] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 89. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 90. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 91. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2467] [Article Influence: 164.5] [Reference Citation Analysis (2)] |
| 92. | Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 93. | Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa Y, Yoneda M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 94. | Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between vitamin E and non-alcoholic steatohepatitis: a meta-analysis. Int J Clin Exp Med. 2015;8:3924-3934. [PubMed] |
| 95. | Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1820] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 96. | Gerss J, Köpcke W. The questionable association of vitamin E supplementation and mortality--inconsistent results of different meta-analytic approaches. Cell Mol Biol (Noisy-le-grand). 2009;55 Suppl:OL1111-OL1120. [PubMed] [DOI] [Full Text] |
| 97. | Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4:158-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 98. | Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C. SELECT: the Selenium and Vitamin E Cancer Prevention Trial: rationale and design. Prostate Cancer Prostatic Dis. 2000;3:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, Maggioni M, Kakela P, Wiklund O, Mozzi E, Grimaudo S, Kaminska D, Rametta R, Craxi A, Fargion S, Nobili V, Romeo S, Pihlajamaki J, Valenti L. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 100. | Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1058] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 101. | Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, Araki N, Tanaka K, Yamaguchi M, Matsuda Y, Ide Y, Otsuka T, Ozaki I, Ono N, Eguchi T, Anzai K; Japan Study Group for NAFLD (JSG-NAFLD). Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res. 2015;45:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 102. | Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, Lingvay I, Thomsen M, Wadden TA, Wharton S, Wilding JPH, Rubino D. Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity (Silver Spring). 2020;28:1050-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 103. | Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, Vergès B, Marre M. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 104. | O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 505] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 105. | Nauck MA, Meier JJ. MANAGEMENT OF ENDOCRINE DISEASE: Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181:R211-R234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 106. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1199] [Article Influence: 299.8] [Reference Citation Analysis (0)] |
| 107. | Lai LL, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Empagliflozin for the Treatment of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes Mellitus. Dig Dis Sci. 2020;65:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 108. | Xing B, Zhao Y, Dong B, Zhou Y, Lv W, Zhao W. Effects of sodium-glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. J Diabetes Investig. 2020;11:1238-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 109. | Wang X, Zhao B, Sun H, You H, Qu S. Effects of sitagliptin on intrahepatic lipid content in patients with non-alcoholic fatty liver disease. Front Endocrinol (Lausanne). 2022;13:866189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 110. | Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 373] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 111. | Xi Y, Li H. Role of farnesoid X receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed Pharmacother. 2020;121:109609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 112. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1793] [Article Influence: 179.3] [Reference Citation Analysis (3)] |
| 113. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 922] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 114. | Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB, Mutnick D, Bursulaya B, Schmeits J, Wu X, Bao D, Zoll J, Kim Y, Groessl T, McNamara P, Seidel HM, Molteni V, Liu B, Phimister A, Joseph SB, Laffitte B. Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH). J Med Chem. 2017;60:9960-9973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 115. | Jean Lucas K, Lopez P, Lawitz E. Safety and efficacy of tropifexor in patients with fibrotic nonalcoholic steatohepatitis: 48-week results from part c of the phase 2 flight-fxr study [Abstract]. Hepatology 2020; 72. Available from: https://www.natap.org/2020/AASLD/AASLD_118.htm. |
| 116. | Bower G, Toma T, Harling L, Jiao LR, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: a Systematic Review of Liver Biochemistry and Histology. Obes Surg. 2015;25:2280-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 117. | Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 118. | Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, le Roux CW. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 119. | Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux CW, Goldstone AP. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63:891-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 120. | Morales-Marroquin E, Hanson B, Greathouse L, de la Cruz-Munoz N, Messiah SE. Comparison of methodological approaches to human gut microbiota changes in response to metabolic and bariatric surgery: A systematic review. Obes Rev. 2020;21:e13025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 121. | Chauhan M, Singh K, Thuluvath PJ. Bariatric Surgery in NAFLD. Dig Dis Sci. 2022;67:408-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 122. | Dallal RM, Mattar SG, Lord JL, Watson AR, Cottam DR, Eid GM, Hamad G, Rabinovitz M, Schauer PR. Results of laparoscopic gastric bypass in patients with cirrhosis. Obes Surg. 2004;14:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 123. | Takata MC, Campos GM, Ciovica R, Rabl C, Rogers SJ, Cello JP, Ascher NL, Posselt AM. Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surg Obes Relat Dis. 2008;4:159-64; discussion 164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 124. | Brolin RE, Bradley LJ, Taliwal RV. Unsuspected cirrhosis discovered during elective obesity operations. Arch Surg. 1998;133:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2011] [Article Influence: 154.7] [Reference Citation Analysis (0)] |
| 126. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 742] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 127. | Yin X, Guo X, Liu Z, Wang J. Advances in the diagnosis and treatment of non-alcoholic fatty liver disease. International Journal of Molecular Sciences. 24(3):2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 128. | Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, Kohli A, Sarin S, Caldwell SH, Alkhouri N, Shiffman ML, Camargo M, Li G, Kersey K, Jia C, Zhu Y, Djedjos CS, Subramanian GM, Myers RP, Gunn N, Sheikh A, Anstee QM, Romero-Gomez M, Trauner M, Goodman Z, Lawitz EJ, Younossi Z; STELLAR-3 and STELLAR-4 Investigators. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 129. | Noureddin M, Anstee QM, Loomba R. Review article: emerging anti-fibrotic therapies in the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2016;43:1109-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 130. | Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, Younossi Z. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224-1229, 1229.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 131. | Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, Merriman R, Hameed B, Doo E, Kleiner DE, Behling C, Loomba R; NASH CRN. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 132. | Francque SM, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, Michielsen P, Van Gaal L. Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin Gastroenterol Hepatol. 2012;10:1162-8; quiz e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 133. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1235] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 134. | Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 135. | Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, Chalasani N, Kowdley K, Hameed B, Wilson LA, Yates KP, Belt P, Lazo M, Kleiner DE, Behling C, Tonascia J; NASH Clinical Research Network (CRN). Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 701] [Article Influence: 175.3] [Reference Citation Analysis (0)] |
| 136. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 574] [Article Influence: 44.2] [Reference Citation Analysis (2)] |
| 137. | Thomas JA, Kendall BJ, Dalais C, Macdonald GA, Thrift AP. Hepatocellular and extrahepatic cancers in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur J Cancer. 2022;173:250-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 138. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 464] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 139. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 140. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, Kawata S, Uto H, Takami S, Sumida Y, Takamura T, Kawanaka M, Okanoue T; Japan NASH Study Group, Ministry of Health, Labour, and Welfare of Japan. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-33; quiz e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 141. | Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, Hanouneh IA, Alhaddad R, Alkhouri N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 142. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-31.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 490] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 143. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 569] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 144. | Bengtsson B, Stål P, Wahlin S, Björkström NK, Hagström H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019;39:1098-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 145. | Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, Ratziu V. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017;46:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 146. | Myers S, Neyroud-Caspar I, Spahr L, Gkouvatsos K, Fournier E, Giostra E, Magini G, Frossard JL, Bascaron ME, Vernaz N, Zampaglione L, Negro F, Goossens N. NAFLD and MAFLD as emerging causes of HCC: A populational study. JHEP Rep. 2021;3:100231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 147. | Wen W, Li H, Wang C, Chen C, Tang J, Zhou M, Hong X, Cheng Y, Wu Q, Zhang X, Feng Z, Wang M. Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: A meta-analysis. Front Endocrinol (Lausanne). 2022;13:934225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 148. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 149. | Sinn DH, Kang D, Chang Y, Ryu S, Gu S, Kim H, Seong D, Cho SJ, Yi BK, Park HD, Paik SW, Song YB, Lazo M, Lima JA, Guallar E, Cho J, Gwak GY. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017;66:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 150. | Mantovani A. Nonalcoholic Fatty Liver Disease (NAFLD) and Risk of Cardiac Arrhythmias: A New Aspect of the Liver-heart Axis. J Clin Transl Hepatol. 2017;5:134-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 151. | Mantovani A, Rigamonti A, Bonapace S, Bolzan B, Pernigo M, Morani G, Franceschini L, Bergamini C, Bertolini L, Valbusa F, Rigolon R, Pichiri I, Zoppini G, Bonora E, Violi F, Targher G. Nonalcoholic Fatty Liver Disease Is Associated With Ventricular Arrhythmias in Patients With Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care. 2016;39:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 152. | Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Rodella S, Zoppini G, Mantovani W, Barbieri E, Byrne CD. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8:e57183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 153. | Käräjämäki AJ, Pätsi OP, Savolainen M, Kesäniemi YA, Huikuri H, Ukkola O. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study). PLoS One. 2015;10:e0142937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 154. | Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15:425-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 155. | Bonapace S, Valbusa F, Bertolini L, Pichiri I, Mantovani A, Rossi A, Zenari L, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with aortic valve sclerosis in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e88371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 156. | Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 157. | Mantovani A, Pernigo M, Bergamini C, Bonapace S, Lipari P, Valbusa F, Bertolini L, Zenari L, Pichiri I, Dauriz M, Zoppini G, Barbieri E, Byrne CD, Bonora E, Targher G. Heart valve calcification in patients with type 2 diabetes and nonalcoholic fatty liver disease. Metabolism. 2015;64:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 158. | Zhang P, Dong X, Zhang W, Wang S, Chen C, Tang J, You Y, Hu S, Zhang S, Wang C, Wen W, Zhou M, Tan T, Qi G, Li L, Wang M. Metabolic-associated fatty liver disease and the risk of cardiovascular disease. Clin Res Hepatol Gastroenterol. 2023;47:102063. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 159. | Önnerhag K, Hartman H, Nilsson PM, Lindgren S. Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD). Scand J Gastroenterol. 2019;54:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 160. | Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, George J, Barrera F, Hafliðadóttir S, Björnsson ES, Armstrong MJ, Hopkins LJ, Gao X, Francque S, Verrijken A, Yilmaz Y, Lindor KD, Charlton M, Haring R, Lerch MM, Rettig R, Völzke H, Ryu S, Li G, Wong LL, Machado M, Cortez-Pinto H, Yasui K, Cassader M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 522] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 161. | Jung CY, Koh HB, Park KH, Joo YS, Kim HW, Ahn SH, Park JT, Kim SU. Metabolic dysfunction-associated fatty liver disease and risk of incident chronic kidney disease: A nationwide cohort study. Diabetes Metab. 2022;48:101344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 162. | Fukunaga S, Nakano D, Kawaguchi T, Eslam M, Ouchi A, Nagata T, et al. Non-obese MAFLD is associated with colorectal adenoma in health check examinees: A Multicenter Retrospective Study. International Journal of Molecular Sciences. 2021;22:5462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 163. | Hamaguchi M, Hashimoto Y, Obora A, Kojima T, Fukui M. Non-alcoholic fatty liver disease with obesity as an independent predictor for incident gastric and colorectal cancer: a population-based longitudinal study. BMJ Open Gastroenterol. 2019;6:e000295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 164. | Chang CF, Tseng YC, Huang HH, Shih YL, Hsieh TY, Lin HH. Exploring the relationship between nonalcoholic fatty liver disease and pancreatic cancer by computed tomographic survey. Intern Emerg Med. 2018;13:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 165. | Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - A longitudinal cohort study. J Hepatol. 2019;71:1229-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 166. | Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, Bae IY, Kim HK, An J, Shim JH, Kim KM, Lim YS. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 167. | Zhao S, Wang Y, Wu W, Yang S, Feng L, Tao F, Ge W, Shen M, Xu W. Nonalcoholic fatty liver disease and risk of prostatic diseases: Roles of insulin resistance. Andrologia. 2021;53:e14060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 168. | Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, Yang C, Zong G, Wu J, Jia W. Association of MAFLD With Diabetes, Chronic Kidney Disease, and Cardiovascular Disease: A 4.6-Year Cohort Study in China. J Clin Endocrinol Metab. 2022;107:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 169. | Wang Y, Yu Y, Zhang H, Chen C, Wan H, Chen Y, Xia F, Yu S, Wang N, Ye L, Lu Y. Cardiovascular and renal burdens among patients with MAFLD and NAFLD in China. Front Endocrinol (Lausanne). 2022;13:968766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 170. | Zhang HJ, Wang YY, Chen C, Lu YL, Wang NJ. Cardiovascular and renal burdens of metabolic associated fatty liver disease from serial US national surveys, 1999-2016. Chin Med J (Engl). 2021;134:1593-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 171. | Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2021;19:2138-2147.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 314] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 172. | Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, Kobayashi T, Nogami A, Higurashi T, Kato S, Hosono K, Saito S, Nakajima A. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. 2021;56:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 173. | Guerreiro GTS, Longo L, Fonseca MA, de Souza VEG, Álvares-da-Silva MR. Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD? Hepatol Int. 2021;15:380-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 174. | Guo Y, Yang J, Ma R, Zhang X, Guo H, He J, Wang X, Cao B, Maimaitijiang R, Li Y, Peng X, Zhang S, Guo S. Metabolic Dysfunction-Associated Fatty Liver Disease Is Associated with the Risk of Incident Cardiovascular Disease: A Prospective Cohort Study in Xinjiang. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 175. | Moon JH, Kim W, Koo BK, Cho NH; Innovative Target Exploration of NAFLD (ITEN) consortium. Metabolic Dysfunction-Associated Fatty Liver Disease Predicts Long-term Mortality and Cardiovascular Disease. Gut Liver. 2022;16:433-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 176. | Zou YW, Li QH, Gao JW, Pan J, Ma JD, Chen LF, Lin JZ, Mo YQ, Zhang XP, Liu PM, Dai L. Association Between Metabolic Dysfunction-Associated Fatty Liver Disease and Cardiovascular Risk in Patients With Rheumatoid Arthritis: A Cross-Sectional Study of Chinese Cohort. Front Cardiovasc Med. 2022;9:884636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 177. | Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, Targher G, Byrne CD, Yuan WJ, Zheng MH. MAFLD and risk of CKD. Metabolism. 2021;115:154433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 178. | Deng Y, Zhao Q, Gong R. Association Between Metabolic Associated Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study from NHANES 2017-2018. Diabetes Metab Syndr Obes. 2021;14:1751-1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 179. | Su W, Chen M, Xiao L, Du S, Xue L, Feng R, Ye W. Association of metabolic dysfunction-associated fatty liver disease, type 2 diabetes mellitus, and metabolic goal achievement with risk of chronic kidney disease. Front Public Health. 2022;10:1047794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 180. | Hu Q, Chen Y, Bao T, Huang Y. Association of metabolic dysfunction-associated fatty liver disease with chronic kidney disease: a Chinese population-based study. Ren Fail. 2022;44:1996-2005. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 181. | Hashimoto Y, Hamaguchi M, Okamura T, Nakanishi N, Obora A, Kojima T, Fukui M. Metabolic associated fatty liver disease is a risk factor for chronic kidney disease. J Diabetes Investig. 2022;13:308-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 182. | Tanaka M, Mori K, Takahashi S, Higashiura Y, Ohnishi H, Hanawa N, Furuhashi M. Metabolic dysfunction-associated fatty liver disease predicts new onset of chronic kidney disease better than fatty liver or nonalcoholic fatty liver disease. Nephrol Dial Transplant. 2023;38:700-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |