Published online Feb 27, 2023. doi: 10.4254/wjh.v15.i2.311
Peer-review started: November 1, 2022
First decision: December 13, 2022
Revised: December 13, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: February 27, 2023
Processing time: 114 Days and 20.3 Hours
Autoimmune hepatitis consists of a chronic liver disease whose etiology is unknown. It is comprised of relevant immunological aspects and of immune-mediated liver injury. Eosinophilia may be a considerable feature, particularly happening in male patients.
We report here a Crohn´s disease patient presenting with de novo hypergammaglobulinemia, circulating autoantibodies and elevated transaminase levels. He also had significant peripheral eosinophilia and elevated immunoglobulin E levels at diagnosis. The pathology findings from liver biopsy were compatible with autoimmune hepatitis with eosinophilic infiltration.
This is the first report of autoimmune hepatitis with exuberant eosinophilic infiltration in the liver and bone marrow, described in a patient with Crohn’s disease.
Core Tip: There are very few reported cases of autoimmune hepatitis presenting with peripheral blood eosinophilia. This is the first report of autoimmune hepatitis with exuberant eosinophilic infiltration in the liver and bone marrow, described in a patient with Crohn’s disease.
- Citation: Garrido I, Lopes S, Fonseca E, Carneiro F, Macedo G. Autoimmune hepatitis and eosinophilia: A rare case report. World J Hepatol 2023; 15(2): 311-317
- URL: https://www.wjgnet.com/1948-5182/full/v15/i2/311.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i2.311
Peripheral blood eosinophilia is considered either a primary or a secondary phenomenon[1]. Primary eosinophilia often takes place within hematologic malignancies where cytogenetic or bone marrow histologic evidence regarding the clonal expansion of these cells can be found. On the other hand, causes of secondary eosinophilia include parasitosis, medications, malignancies and inflammatory or allergic conditions. Idiopathic eosinophilia consists of a diagnosis of exclusion, when no primary or secondary causes are detected.
Peripheral blood eosinophilia may be found in several hepatobiliary and gastrointestinal disorders. Indeed, some gastrointestinal diseases are eosinophil-mediated pathologies, such as eosinophilic gastroenteritis, inflammatory bowel disease, Helicobacter pylori infection, gastroesophageal reflux disease, collagenous colitis and celiac disease[2]. In addition, hepatic eosinophilia has been presented associated to primary biliary cirrhosis, sclerosing cholangitis, eosinophilic cholangitis and eosinophilic cholecystitis.
Currently, there are very few reported cases of autoimmune hepatitis presenting with peripheral blood eosinophilia, usually associated with other autoimmune conditions[3]. Hereafter we explore a case of autoimmune hepatitis associated with peripheral blood and tissue eosinophilia. This report aims at making physicians aware of that association in order to consider this diagnosis in a patient who presents elevated transaminases in concert with a high eosinophil count.
A 36-year-old Caucasian male with asthma and Crohn’s disease (Montreal classification A2L2B1), was under azathioprine until 4 years ago when it was discontinued due to clinical and endoscopic remission. His asthma was under control since childhood. He was not on medication and had no known drug allergies. The patient was asymptomatic.
In routine analysis, it was noticed a new-onset cytocholestasis (aspartate aminotransferase 86 U/L, alanine aminotransferase 240 U/L, gamma-glutamyl transferase 288 U/L, alkaline phosphatase 794/L) without hyperbilirubinemia or coagulopathy.
The patient denied any history that can suggest viral prodrome, sick contacts, recent travel, medication ingestion (comprising herbal or over-the-counter), exposure to well water, exposure to recreational drugs, tattoos, alcohol, high-risk sexual behavior or blood transfusions.
Normal.
Antinuclear antibody was positive (1:100, speckled pattern) as well as anti-smooth muscle. All other liver-related autoantibodies were negative (anti-mitochondrial, anti-liver-kidney microsomal, anti-soluble liver antigen and antineutrophil cytoplasmic). Immunoglobulin G (IgG) levels were elevated (3650 mg/dL). Serology for human immunodeficiency virus, hepatitis A virus, Epstein-Barr virus, cytomegalovirus, and herpes simplex virus type 1 and 2 were negative. Polymerase chain reaction considering hepatitis B, C and E viruses were negative, too. Alpha-1 antitrypsin, ceruloplasmin and iron tests were all normal. The same could be perceived for thyroid function.
Blood tests presented an absolute white blood cell count of 33.46 × 109/L. Differential count indicated 89.5% of eosinophilia, as well as an absolute eosinophil count of 30 × 109/L. In addition, immunoglobulin E (IgE) levels were elevated (8803 kU/L). Blood cultures and parasitological examination of the stools were negative. FIP1L1-PDGFRA fusion transcript was not detected.
The abdominal ultrasound showed a liver with a normal appearance and no intra-or extrahepatic biliary ductal dilation.
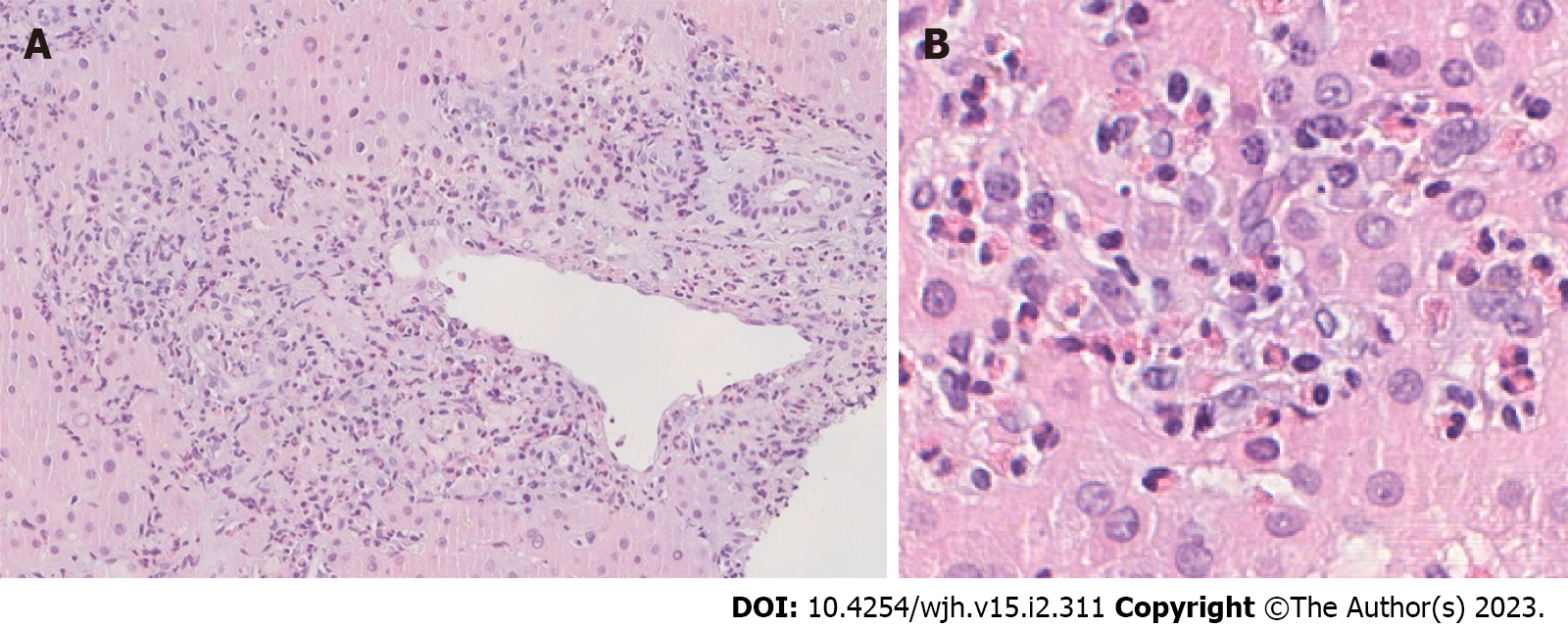
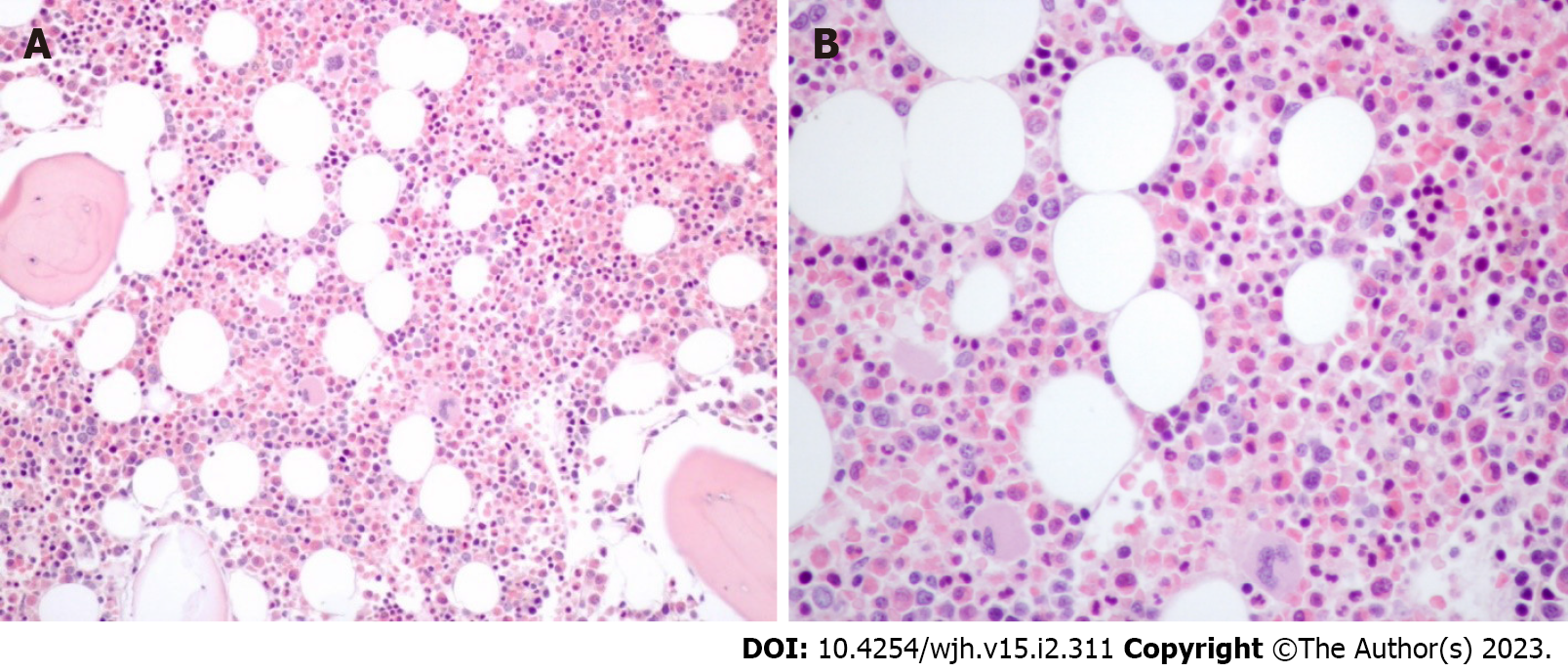
A liver biopsy was then performed, revealing infiltration of the portal tracts and intralobular hepatic parenchyma by numerous eosinophils (Figure 1). Lymphoplasmacytic portal tract inflammatory infiltrate with interface hepatitis was identified as well as small aggregates of plasma cells. The interlobular bile ducts appeared intact and iron and copper stains were negative. Furthermore, bone marrow biopsy showed marked eosinophilia, with normal maturation and absence of blasts (Figure 2).
The score regarding simplified diagnostic criteria of the International Autoimmune Hepatitis Group was 7 (likely diagnosis of autoimmune hepatitis)[4]. The score considering the revised original pretreatment scoring system of the International Autoimmune Hepatitis Group was 17 (definite diagnosis of autoimmune hepatitis)[5]. Due to lack of evidence for parasitic infection, the reactive bone marrow and the absence of other systemic conditions or drugs, the patient was diagnosed with autoimmune hepatitis with peripheral blood eosinophilia.
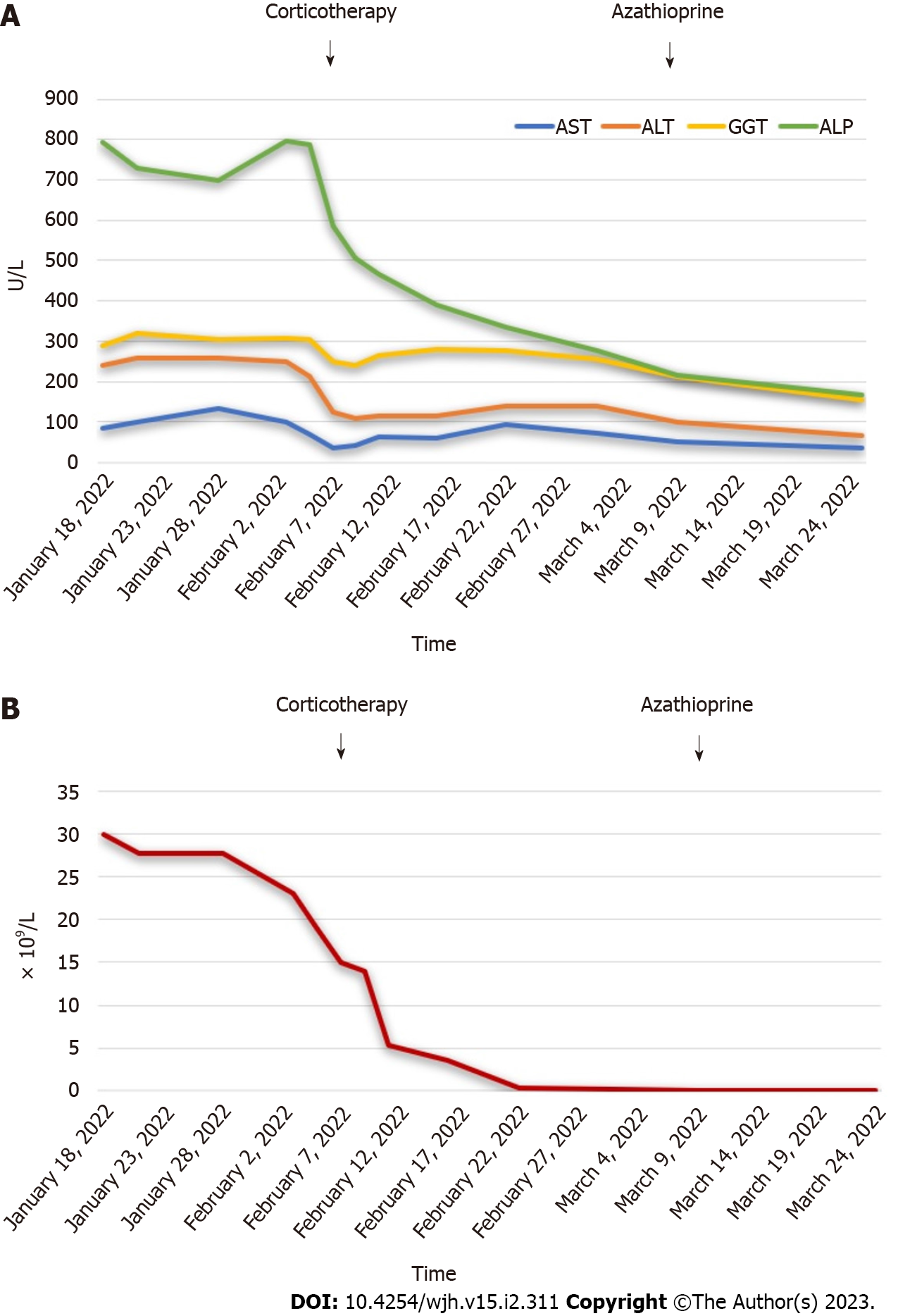
He started treatment with prednisone at 40 mg/d. Cytocholestasis (Figure 3A) and eosinophilia (Figure 3B) progressively improved. The corticosteroid dose was gradually titrated and azathioprine 2 mg/Kg was then added.
After a three-month treatment, follow-up tests showed a normal eosinophil count, liver IgG levels and function tests. These data supported the definite diagnosis of autoimmune hepatitis.
Autoimmune hepatitis is a chronic liver disease which is responsible for up to 20% of chronic hepatitis in Western countries. It has a mean annual incidence of 1.9 per 100.000 individuals and a prevalence of 16.9 in northern European population[6]. It is characterized by hypergammaglobulinemia, circulating autoantibodies and elevated transaminase levels[7]. Peripheral blood eosinophilia is present much less frequently. It has been described in a few cases, usually in association with other autoimmune conditions, such as Coombs positive hemolytic anemia, autoimmune thyroid disease, ulcerative colitis and arthritis[8-11]. As far as we know, this report is the first one concerning autoimmune hepatitis with peripheral blood eosinophilia described in a patient with Crohn’s disease. There are also some cases of blood eosinophilia associated with isolated autoimmune hepatitis, in the absence of other autoimmune conditions[3,12,13].
The development of hypereosinophilia when there is also ulcerative colitis and autoimmune hepatitis, which are two autoimmune conditions with a Th2 bias, suggests that a Th2-T-cell population is at the crossroads of the pathophysiology underlying these autoimmune diseases[10]. Other authors suggest that the concurrent existence of these processes mirrors related abnormal immunological events[8]. Another mechanism of eosinophilia can be the result of mast cell activation, which may take place in cholestatic liver disease in which mast cell-derived mediators cause activation and eosinophil chemotaxis[14].
It should be noted that, despite the fact that autoimmune hepatitis is most usually found in women (3:1 ratio), our report presents the case of a male patient[7]. Indeed, most cases described in the literature of autoimmune hepatitis with peripheral blood eosinophilia have also occurred in men (Table 1), which makes us ponder whether eosinophilia in autoimmune hepatitis can be considered a characteristic related to males.
| Ref. | Panush et al[8] | Kane et al[9] | Terrier et al[10] | Omata et al[13] | Chowdry et al[3] | Farani et al[11] | Makino et al[12] | Present case |
| Age | 14 yr old | 41 yr old | 16 yr old | 49 yr old | 18 yr old | 41 yr old | 7 yr old | 36 yr old |
| Sex | Male | Female | Male | Female | Male | Male | Male | Male |
| Transaminases | AST 700 U/L; ALT 1560 U/L | AST 200 U/L; ALT -- | AST 200 U/L; ALT 320 U/L | AST 1019 U/L; ALT 772 U/L | AST 955 U/L ALT 1194 U/L | AST 70 U/L; ALT 67 U/L | AST 419 U/L; ALT 306 U/L | AST 86 U/L; ALT 240 U/L |
| Eosinophil count | 7.437 × 109/L | 2.64 × 109/L | 63.2 × 109/L | 1.2 × 109/L | 3.3 × 109/L | 4.9 × 109/L | 9 × 109/L | 30 × 109/L |
| IgG level | 2300 mg/dL | 2600 mg/dL | -- | 1930 mg/dL | 2760 mg/dL | 3180 mg/dL | 5234 mg/dL | 3650 mg/dL |
| Positive antibodies | ANA, SMA | SMA | SMA | Negative | ANA, SMA | ANA | ANA | ANA, SMA |
| Hepatic eosinophilia | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Autoimmune disease associations | Coombs positive hemolytic anemia | Ulcerative colitis, autoimmune thyroid disease | Ulcerative colitis | None | None | Arthritis | None | Crohn’s disease |
Similar to Omata and colleagues, our patient also has a long history of asthma[13]. Nevertheless, the eosinophilia and elevated IgE levels were not associated with exacerbation of asthma but rather with elevated liver function tests. In fact, our patient had asthma under control for many years. In addition, other causes of eosinophilia, particularly immediate hypersensitivity to common allergens and parasitic infection, were excluded.
Liver biopsy is considered a prerequisite for the diagnosis of autoimmune hepatitis[15]. The classic histologic picture of autoimmune hepatitis includes interface hepatitis with dense plasma cell-rich lymphoplasmacytic infiltrates, emperipolesis, hepatocellular rosette formation, pycnotic necrosis and hepatocyte swelling. The discovery of hepatic eosinophils (even though it is not the predominant inflammatory cell type) enables the diagnosis of autoimmune hepatitis. In a study that describes the use of liver biopsy assessment in the discrimination of idiopathic autoimmune hepatitis vs drug-induced liver injury, Suzuki and colleagues discovered that intra-acinar eosinophils could be seen in 32.1% of times and portal tract eosinophils could be found in 60.7% of times regarding autoimmune hepatitis cases. Both of them were more usual than in cases of drug-induced liver injury[16].
In our case, the conjunction of plasma cells with interface hepatitis strongly supported the diagnosis of autoimmune hepatitis. However, the most striking aspect of this patient's disease was the exuberant hepatic eosinophilia. Similarly, the bone marrow aspirate showed a marked increase in normal-appearing cells of the eosinophil series. In this case, tissue eosinophilia was marked and blood eosinophilia was significant. In contrast, the other authors reported that none or only a few eosinophils were present in the liver biopsy[3].
With regard to treatment, it should be noted that in all cases there was an improvement in liver tests as well as in the eosinophil count. Our patient had a favorable response under treatment with corticosteroids and azathioprine. Other authors also used 6-mercaptopurine and mycophenolate mofetil with an equally favorable response[11,13]. There is a need for long-term cautious management so as to prevent the progression into liver failure or hepatic cirrhosis[17].
Pathophysiology of autoimmune disorders is incompletely understood. The coexistence of different diseases could suggest common pathogenic mechanisms. Herein we report a case of autoimmune hepatitis associated with peripheral blood eosinophilia and exuberant liver eosinophilia. It is our goal to emphasize this infrequent presentation of autoimmune hepatitis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nguyen TL, Vietnam; Rodrigues AT, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Curtis C, Ogbogu PU. Evaluation and Differential Diagnosis of Persistent Marked Eosinophilia. Immunol Allergy Clin North Am. 2015;35:387-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Zuo L, Rothenberg ME. Gastrointestinal eosinophilia. Immunol Allergy Clin North Am. 2007;27:443-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 3. | Chowdry S, Rubin E, Sass DA. Acute autoimmune hepatitis presenting with peripheral blood eosinophilia. Ann Hepatol. 2012;11:559-563. [PubMed] |
| 4. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1253] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 5. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Zeniya M. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1986] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 6. | Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 330] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 848] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 8. | Panush RS, Wilkinson LS, Fagin RR. Chronic active hepatitis associated with eosinophilia and Coombs'-positive hemolytic anemia. Gastroenterology. 1973;64:1015-1019. [PubMed] |
| 9. | Kane SP. Ulcerative colitis with chronic liver disease, eosinophilia and auto-immune thyroid disease. Postgrad Med J. 1977;53:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Terrier B, Fontaine H, Schmitz J, Perdu J, Hermine O, Varet B, Buzyn A, Suarez F. Coexistence and parallel evolution of hypereosinophilic syndrome, autoimmune hepatitis, and ulcerative colitis suggest common pathogenic features. Am J Gastroenterol. 2007;102:1132-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Farani JB, Albuquerque CB, de Oliveira JM, de Assis EA, de Oliveira Ayres Pinto E, de Lacerda Bonfante H. Arthritis, eosinophilia, and autoimmune liver disease: a diagnostic challenge. J Clin Rheumatol. 2015;21:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Makino S, Nishikado M, Awaguni H, Okumura K-i, Shinozuka J, Imashuku S. Autoimmune Hepatitis With Severe Hypergammaglobulinemia and Eosinophilia in a Child. Int J Clin Pediatr. 2020;9(2):50-54.. [DOI] [Full Text] |
| 13. | Omata F, Shibata M, Nakano M, Jacobs JL, Tokuda Y, Fukutake K, Takahashi O, Fukui T. Chronic hepatitis with eosinophilic infiltration associated with asthma. Intern Med. 2009;48:1945-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Yamazaki K, Suzuki K, Nakamura A, Sato S, Lindor KD, Batts KP, Tarara JE, Kephart GM, Kita H, Gleich GJ. Ursodeoxycholic acid inhibits eosinophil degranulation in patients with primary biliary cirrhosis. Hepatology. 1999;30:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Lohse AW, Sebode M, Bhathal PS, Clouston AD, Dienes HP, Jain D, Gouw ASH, Guindi M, Kakar S, Kleiner DE, Krech T, Lackner C, Longerich T, Saxena R, Terracciano L, Washington K, Weidemann S, Hübscher SG, Tiniakos D. Consensus recommendations for histological criteria of autoimmune hepatitis from the International AIH Pathology Group: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology. Liver Int. 2022;42:1058-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (2)] |
| 16. | Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, Lucena MI, Castiella A, Lindor K, Björnsson E. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Awadie H, Khoury J, Zohar Y, Yaccob A, Veitsman E, Saadi T. Long-term Follow-up of Severe Eosinophilic Hepatitis: A Rare Presentation of Hypereosinophilic Syndrome. Rambam Maimonides Med J. 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |