Published online Feb 27, 2023. doi: 10.4254/wjh.v15.i2.255
Peer-review started: November 23, 2022
First decision: December 9, 2022
Revised: December 17, 2023
Accepted: January 31, 2023
Article in press: January 31, 2023
Published online: February 27, 2023
Processing time: 92 Days and 20.7 Hours
Despite its association with higher postoperative morbidity and mortality, the use of extended criteria donor (ECD) livers for transplantation has increased globally due to the high demand for the procedure.
To investigate the prevalence of ECD in donation after brain death (DBD) and its impact on organ acceptance for transplantation.
Retrospective analysis of DBD organ offers for liver transplantation between 2017 and 2020 in a high-volume transplant centre. The incidence of the Eurotransplant risk factors to define an ECD (ET-ECD) among DBD donors and the likelihood of organ acceptance over the years were analysed. The relationship between organ refusal for transplantation, the occurrence, and the number of ET-ECD was assessed by simple and multiple logistic regression adjustment.
A total of 1619 organ donors were evaluated. Of these, 78.31% (n = 1268) had at least one ET-ECD criterion. There was an increase in the acceptance of ECD DBD organs for transplantation (1 criterion: from 23.40% to 31.60%; 2 criteria: from 13.10% to 27.70%; 3 criteria: From 6.30% to 13.60%). For each addition of one ET-ECD variable, the estimated chance of organ refusal was 64.4% higher (OR 1.644, 95%CI 1.469-1.839, P < 0.001). Except for the donor serum sodium > 165 mmol/L (P = 0.310), all ET-ECD criteria increased the estimated chance of organ refusal for transplantation.
A high prevalence of ECD DBD was observed. Despite the increase in their utilisation, the pre
Core Tip: To suffice the demand of patients on the waiting list, the use of extended criteria donor (ECD) organs for transplantation has become a global need. This large retrospective analysis of 1619 donations after brain death (DBD) donor offers to a transplant centre in Brazil applied the Eurotransplant manual criteria to indicate an ECD. The prevalence of ECD was 78.31%. Whilst there was an increase in ECD-DBD liver transplantation over the years. Still, the presence and number of extended donor criteria were associated with an increased chance of donor organ rejection for transplantation.
- Citation: Braga VS, Boteon APCS, Paglione HB, Pecora RAA, Boteon YL. Extended criteria brain-dead organ donors: Prevalence and impact on the utilisation of livers for transplantation in Brazil. World J Hepatol 2023; 15(2): 255-264
- URL: https://www.wjgnet.com/1948-5182/full/v15/i2/255.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i2.255
Currently, organ shortage is a major limitation in transplantation. Although Brazil is the second country in the absolute number of liver transplants performed worldwide, it still needs to increase its figures. According to the Brazilian Transplant Registry, although 2245 liver transplants were performed in 2019, in that year, the waiting list had yet more 1213 people waiting for an organ[1]. In addition, the same report showed a progressive change in the demographic profile of organ donors, with an increase in the incidence of cerebrovascular diseases as the cause of death-in spite of trauma-and an increase in the proportion of donors older than 60 years old[1].
Although there is still no precise definition by the transplant community, donors who present, among other risk factors, older age, hypernatremia, prolonged time in the intensive care unit (ICU), abnormal liver enzymes, and moderate or severe steatosis are known as extended criteria donors (ECD)[2]. In addition, ECD allografts are associated with an increased risk of delayed graft function, primary nonfunction, and postoperative complications[2-4].
The first international study involving a large sample of patients promoted by the European Liver Intestine Transplant Association (ELITA) and the Eurotransplant Liver Intestine Advisory Committee (ELIAC) reports the following donor risk factors in liver transplantation: Age, ICU time, high body mass index (BMI), steatosis, hypernatremia, elevated alanine aminotransferase (ALT), elevated aspartate aminotransferase (AST), and raised total bilirubin levels[5]. Therefore, the donor is considered an ECD if one of these criteria is present.
To meet the demand of patients on the waiting list, using ECD organs for transplantation has become a global need[3,4]. For example, in the United States of America, from 2000 to 2005, the number of liver transplants increased by 21%[6]. Another study at the same centre reported a growth in the number of transplants with ECD organs (4.5% in 2008 compared to 0.5% in 1999)[7]. Furthermore, organ characteristics, such as ischaemia time and the use of partial grafts, negatively impact postoperative outcomes[3,8].
Despite the relevance of this topic and the numbers described above suggesting a demographic change in the organ donor population, data on ECD prevalence among organ donors and their utilisation rate are scarce. Therefore, this study aimed to evaluate the prevalence of ECD allografts in donation after brain death (DBD) liver transplantation and the likelihood of organ acceptance over the years.
The study involved a retrospective analysis of data obtained from liver donor offers for the Solid Organ Transplant Program of the Hospital Israelita Albert Einstein, São Paulo, Brazil, between June 2017 and December 2020. All liver allograft donors offered to our transplant centre over the study period were analysed. There were no exclusion criteria in the study. The study was reviewed and approved by the Research Ethics Committee of Hospital Israelita Albert Einstein with opinion 4.696.905, CAAE: 39704520.0.0000.0071.
As previously defined by the Eurotransplant[9], an ECD was defined as the presence of one or more of the following donor characteristics reflective of a high chance of post-transplant complications such as primary nonfunction and early allograft dysfunction (ET-ECD): Age > 65 years old, ICU stay > 7 d, BMI > 30 Kg/m2, liver steatosis > 40%, serum sodium > 165 mmol/L, ALT > 105 U/L, AST > 90 U/L, and total serum bilirubin > 3 mg/dL. Hepatic steatosis was evaluated by an experienced retrieval surgeon and reported as present when an estimation of more than 40% was observed.
In addition to the donor data described above, other variables were collected. This collection included the donor's place of origin (local: Donor in the city of São Paulo; regional: Donor in the state of São Paulo; national: Donor in another Brazilian State), gender, blood type (ABO system), race, cause of death (cerebrovascular accident, trauma, hypoxia, and others), history of alcoholism, and presence of cardiorespiratory arrest among donors. All information was obtained from a retrospective institutional database prospectively maintained by the hospital liver transplantation program management team. This information was delivered anonymised to the researchers.
The occurrence of the following outcomes over the years was assessed dichotomously (Yes vs No): (1) Organ offers acceptance for transplantation; and (2) transplantation of the donor organ. All these variables were considered only once, regardless of the number of times the organ was offered to different recipients of the transplant program.
Quantitative variables were described by medians and quartiles, given the distance between mean and median and asymmetry observed in the variables through histograms and normality tests. Categorical variables were described by absolute frequencies and percentages. Simple logistic regression models assessed the relationship between the occurrence and the number of ET-ECD criteria over the years. In addition, the simple Poisson regression adjustment was used to assess the year. The relationship between organ refusal for transplantation, the occurrence, and the number of ET-ECD criteria was also evaluated by simple and multiple logistic regression adjustment. Depending on the expected frequency per category, other associations between qualitative variables were assessed using Fisher's exact or Chi-squared tests. Finally, the nonparametric Mann-Whitney test was used to compare quantitative measures between groups, depending on the distribution of numerical measures. The SPSS statistical program version 22 (IBM Corp, Armonk, NY, United States) was used for analyses, and the significance level adopted was 5%.
A total of 1619 DBD liver donors were evaluated. The distribution of organ donor offers was proportionally similar during the studied period [2017 (6 mo): n = 251 (15.50%); 2018: n = 463 (28.60%); 2019: n = 455 (28.10%); 2020: n = 450 (27.79%)]. The mean donor age was 49.70 years old [standard deviation (SD) 14.74] and the mean donor BMI was 26.66 kg/m2 (SD 4.68). A detailed descriptive analysis of the donor characteristics by year is presented in Table 1.
| Variable (year) | 2017 | 2018 | 2019 | 2020 | Total |
| Donor's place of origin | |||||
| Local | 121 (48.21%) | 180 (38.88%) | 261 (57.36%) | 257 (57.11%) | 819 (50.59%) |
| Regional | 67 (26.69%) | 149 (32.18%) | 128 (28.13%) | 131 (29.11%) | 475 (29.34%) |
| Nacional | 63 (25.10%) | 134 (28.94%) | 66 (14.51%) | 62 (13.78%) | 325 (20.07%) |
| Gender, female | 119 (47.60%) | 193 (41.87%) | 190 (41.76%) | 174 (38.75%) | 676 (41.86%) |
| Blood type (ABO system) | |||||
| A | 82 (32.93%) | 150 (32.47%) | 157 (34.58%) | 168 (37.42%) | 557 (34.51%) |
| B | 38 (15.26%) | 52 (11.26%) | 48 (10.57%) | 36 (8.02%) | 174 (10.78%) |
| AB | 11 (4.42%) | 16 (3.46%) | 25 (5.51%) | 7 (1.56%) | 59 (3.66%) |
| O | 118 (47.39%) | 244 (52.81%) | 224 (49.34%) | 238 (53.01%) | 824 (51.05%) |
| Race | |||||
| Black | 39 (15.54%) | 42 (9.07%) | 57 (12.53%) | 46 (10.22%) | 184 (11.37%) |
| Mixed-race | 80 (31.87%) | 164 (35.42%) | 159 (34.95%) | 178 (39.56%) | 581 (35.89%) |
| White | 123 (49.00%) | 253 (54.64%) | 235 (51.65%) | 223 (49.56%) | 834 (51.51%) |
| Others | 9 (3.59%) | 4 (0.86%) | 4 (0.88%) | 3 (0.67%) | 20 (1.24%) |
| Age (categories) | |||||
| < 40 yr | 48 (19.12%) | 90 (19.44%) | 104 (22.86%) | 115 (25.56%) | 357 (22.05%) |
| 40 yr to 49 yr | 58 (23.11%) | 102 (22.03%) | 91 (20.00%) | 106 (23.56%) | 357 (22.05%) |
| 50 yr to 59 yr | 77 (30.68%) | 134 (28.94%) | 136 (29.89%) | 123 (27.33%) | 470 (29.03%) |
| 60 yr to 69 yr | 53 (21.12%) | 99 (21.38%) | 105 (23.08%) | 82 (18.22%) | 339 (20.94%) |
| ≥ 70 yr | 15 (5.98%) | 38 (8.21%) | 19 (4.18%) | 24 (5.33%) | 96 (5.93%) |
| Age (yr) | 50.50 (14.52) | 50.77 (15.07) | 50.00 (14.09) | 47.85 (15.03) | 49.70 (14.74) |
| ICU stay > 5 d | 105 (41.83%) | 187 (40.39%) | 178 (39.12%) | 200 (44.44%) | 670 (41.38%) |
| Cause of death | |||||
| Cerebrovascular accident | 175 (70.00%) | 294 (63.50%) | 298 (65.49%) | 277 (61.56%) | 1044 (64.52%) |
| Trauma | 43 (17.20%) | 113 (24.41%) | 101 (22.20%) | 121 (26.89%) | 378 (23.36%) |
| Hypoxia | 24 (9.60%) | 45 (9.72%) | 40 (8.79%) | 41 (9.11%) | 150 (9.27%) |
| Others | 8 (3.20%) | 11 (2.38%) | 16 (3.52%) | 11 (2.44%) | 46 (2.84%) |
| BMI (kg/m2) | 27.28 (5.04) | 26.65 (5.00) | 26.69 (4.57) | 26.31 (4.20) | 26.66 (4.68) |
| Alcoholism | 75 (29.88%) | 148 (31.97%) | 115 (25.27%) | 103 (22.89%) | 441 (27.24%) |
| Cardiorespiratory arrest | 61 (24.30%) | 99 (21.38%) | 82 (18.02%) | 77 (17.11%) | 319 (19.70%) |
| Vasoactive drugs in the donor | 221 (88.05%) | 422 (91.14%) | 408 (89.67%) | 405 (90.00%) | 1456 (89.93%) |
| AST (U/L)1 | 56.00 (32.00; 102.00) | 64.50 (37.00; 125.00) | 74.00 (39.00; 151.00) | 74.00 (39.40; 141.00) | 68.00 (38.00; 132.00) |
| ALT (U/L)1 | 47.00 (24.00; 93.00) | 49.00 (29.00; 96.00) | 45.70 (26.00; 103.00) | 47.00 (28.00; 89.00) | 47.00 (27.00; 96.00) |
| GGT (U/L)1 | 84.00 (34.00; 182.00) | 94.50 (38.55; 200.00) | 83.50 (39.00; 207.00) | 84.00 (36.00; 197.50) | 87.00 (37.00; 198.00) |
| Total bilirubin (mg/dL)1 | 0.50 (0.32; 0.91) | 0.50 (0.30; 0.91) | 0.55 (0.35; 0.97) | 0.52 (0.35; 0.90) | 0.52 (0.33; 0.92) |
There were 351 (21.68%) donor offers without ET-ECD criteria and 1268 (78.32%) with at least one ET-ECD criterion from 2017 to 2020. The frequency of ECD was similar across years [2017 (6 mo): n = 197 (78.49%); 2018: n = 367 (79.27%); 2019: n = 349 (76.70%); 2020: n = 355 (78.89%)]. Of the ECD offers, 57.96% (n = 735) had two or more ET-ECD criteria. A descriptive analysis of the prevalence of ET-ECD features over the years is described in Table 2.
| Variable (year) | 2017 | 2018 | 2019 | 2020 | Total |
| Macroscopic assessment of steatosis in the organ | 5 (1.99) | 0 (0.00) | 3 (0.66) | 4 (0.89) | 12 (0.74) |
| Age > 65 yr | 35 (14.00) | 69 (14.90) | 67 (14.73) | 52 (11.56) | 223 (13.78) |
| ICU > 7 d | 81 (32.27) | 143 (30.89) | 145 (31.87) | 153 (34.00) | 522 (32.24) |
| BMI > 30 kg/m2 | 61 (24.30) | 82 (17.71) | 90 (19.78) | 80 (17.78) | 313 (19.33) |
| Serum sodium > 165 mmol/L | 39 (15.54) | 82 (17.71) | 73 (16.04) | 64 (14.22) | 258 (15.94) |
| AST > 90 U/L | 72 (28.69) | 172 (37.23) | 184 (40.44) | 187 (41.65) | 615 (38.03) |
| ALT > 105 U/L | 54 (21.51) | 108 (23.38) | 111 (24.45) | 93 (20.71) | 366 (22.65) |
| Total bilirubin > 3 mg/dL | 6 (2.39) | 13 (2.83) | 25 (5.51) | 18 (4.01) | 62 (3.84) |
| Number of variables to classify a donor as an extended criteria donor | |||||
| 0 | 54 (21.51) | 96 (20.73) | 106 (23.30) | 95 (21.11) | 351 (21.68) |
| 1 | 94 (37.45) | 159 (34.34) | 122 (26.81) | 158 (35.11) | 533 (32.92) |
| 2 | 61 (24.30) | 133 (28.73) | 133 (29.23) | 119 (26.44) | 446 (27.55) |
| 3 | 32 (12.75) | 58 (12.53) | 68 (14.95) | 59 (13.11) | 217 (13.40) |
| 4 | 9 (3.59) | 15 (3.24) | 24 (5.27) | 17 (3.78) | 65 (4.01) |
| 5 | 1 (0.40) | 2 (0.43) | 2 (0.44) | 2 (0.44) | 7 (0.43) |
Every year after 2017, the estimated chance of a donor to be presenting with AST higher than 90 U/L is 17.7% greater [odds ratio (OR) 1.177, 95% confidence interval (CI) 1.068-1.298, P = 0.001]. There was no significant relationship between the year of offering and other ET-ECD risk factors. The results of the simple logistic regression model for the eight ET-ECD variables and the variable indicating the occurrence of at least one ET-ECD criterion are shown in Table 3. There was no significant relationship between the change in the number of ET-ECD characteristics by year of offering (estimated ratio of means 1.012, 95%CI 0.973-1.052, P = 0.551).
| Variable | Odds ratio (95%CI) | P value |
| Absence of macroscopic steatosis in the donor organ | 0.845 (0.491; 1.455) | 0.545 |
| Donor age > 65 yr | 0.925 (0.808; 1.059) | 0.259 |
| ICU stay > 7 d | 1.037 (0.938; 1.147) | 0.473 |
| BMI > 30 kg/m2 | 0.915 (0.813; 1.030) | 0.140 |
| Serum sodium > 165 mmol/L | 0.943 (0.830; 1.071) | 0.364 |
| AST > 90 U/L | 1.177 (1.068; 1.298) | 0.001 |
| ALT > 105 U/L | 0.979 (0.876; 1.095) | 0.717 |
| Total bilirubin > 3 mg/dL | 1.223 (0.952; 1.572) | 0.116 |
| ≥ 1 donor extended criteria | 0.991 (0.885; 1.110) | 0.878 |
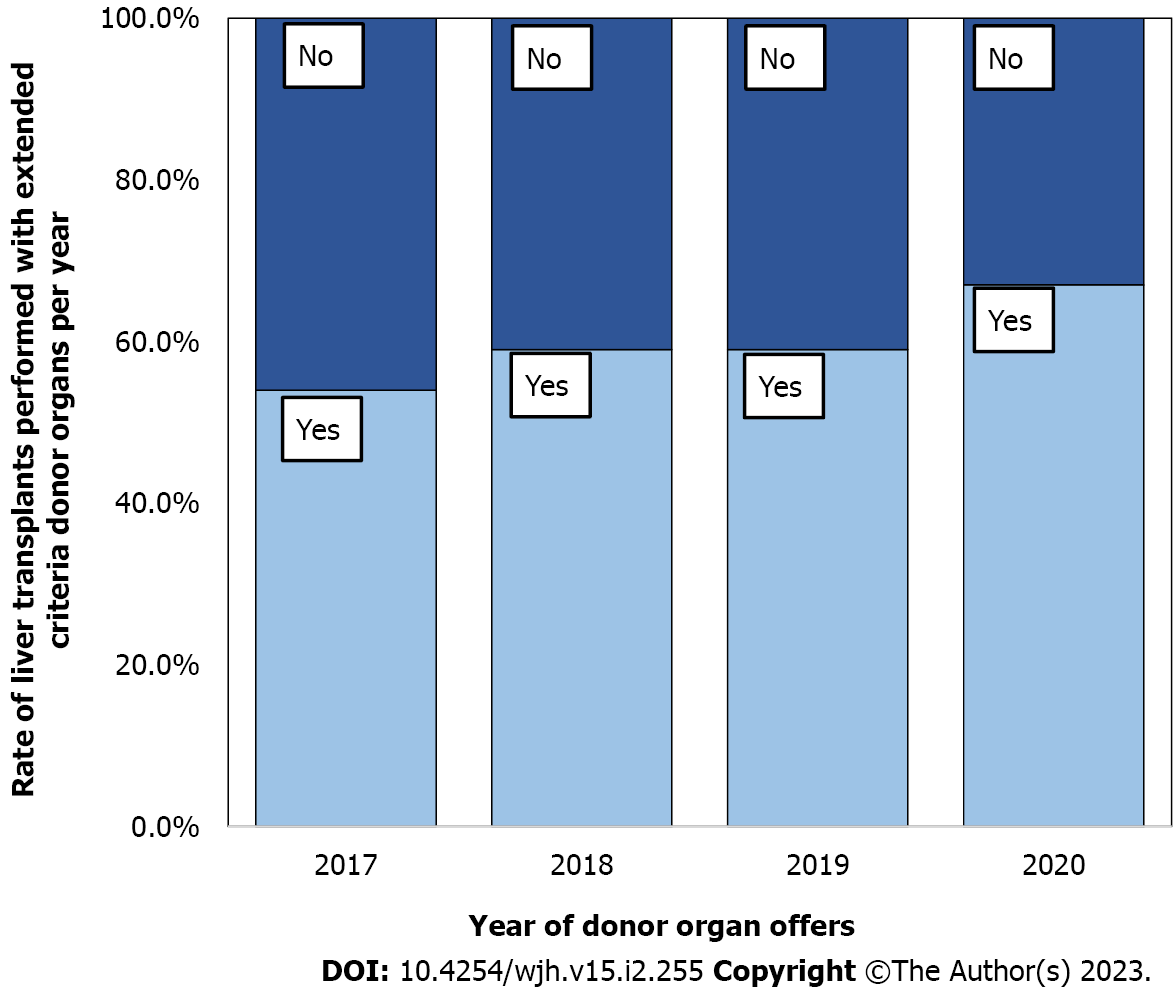
There was a reduction in the likelihood of donor organ refusal for transplantation during the studied period [2017 (6 mo): n = 193 (76.89%); 2018: n = 360 (77.75%); 2019: n = 310 (68.13%); 2020: n = 319 (70.89%)]. This reduction was due to the increased acceptance of ECD liver allografts for transplantation. As a result, there was an increase from 23.40% to 31.60% for 1 ET-ECD variable, from 13.10% to 27.70% for 2 ET-ECD variables, and from 6.30% to 13.60% for 3 ET-ECD variables. This growth in using ECD-DBD organs is reflected in the prevalence of ECD per year among the transplants performed, as demonstrated in Figure 1.
For each addition of one ET-ECD criterion, the estimated chance of organ refusal for transplantation was 64.4% greater (OR 1.644, 95%CI 1.469-1.839, P < 0.001). The results of the logistic regression analysis showed that all ET-ECD variables increased the estimated chance of refusing the organ for transplantation (Tables 4 and 5), except for donor serum sodium > 165 mmol/L (OR 1.173, 95%CI 0.862-1.596, P = 0.310).
| Logistic regression for donor organ refusal for transplantation (n = 1619) | ||
| Variable | Odds ratio for refusal (95%CI) | P value |
| Absence of macroscopic steatosis in the donor organ | 0.072 (0.016; 0.332) | < 0.001 |
| Donor age > 65 yr | 1.814 (1.264; 2.603) | 0.001 |
| ICU stay > 7 d | 1.810 (1.408; 2.328) | < 0.001 |
| BMI > 30 kg/m2 | 2.215 (1.601; 3.065) | < 0.001 |
| Serum sodium > 165 mmol/L | 1.173 (0.862; 1.596) | 0.310 |
| AST > 90 U/L | 1.713 (1.352; 2.171) | < 0.001 |
| ALT > 105 U/L | 2.007 (1.493; 2.697) | < 0.001 |
| Total bilirubin > 3 mg/dL | 3.011 (1.361; 6.664) | 0.007 |
| Multiple logistic regression model for donor organ refusal for transplantation (n = 1619) | |||
| Variable | Estimated proportionof refusal (95%CI) | Odds ratio for refusal (95%CI) | P value |
| Absence of macroscopic steatosis in the donor organ | 41.10% (12.04%; 78.06%) | 0.064 (0.013; 0.307) | < 0.001 |
| Donor age > 65 yr | 79.54% (59.84%; 91.02%) | 1.973 (1.360; 2.861) | < 0.001 |
| ICU stay > 7 d | 79.18% (60.23%; 90.52%) | 1.888 (1.455; 2.450) | < 0.001 |
| BMI > 30 kg/m2 | 80.69% (62.19%; 91.39%) | 2.279 (1.628; 3.190) | < 0.001 |
| AST > 90 U/L | 76.57% (56.61%; 89.11%) | 1.394 (1.051; 1.848) | 0.021 |
| ALT > 105 U/L | 78.44% (58.88%; 90.24%) | 1.729 (1.217; 2.456) | 0.002 |
| Total bilirubin > 3 mg/dL | 82.44% (60.00%; 93.63%) | 2.877 (1.282; 6.454) | 0.010 |
All significant variables in this analysis were included in a multiple logistic regression model to assess the relationship between organ refusal for transplantation and the occurrence of ET-ECD criteria. A significant association was identified between all measures considered in the model and organ refusal. The results are presented in Tables 4 and 5, along with each category's estimated proportions of refusal. They were evaluated in an adjusted manner in relation to the other variables in the model.
Estimating ECD prevalence among DBD donors is critical to developing strategies to expand the use of these higher-risk organs safely. This large retrospective analysis of 1619 DBD organ donors identified a high prevalence of ET-ECD criteria. In addition, the ECD rate remained constant over the studied period. Although an increase in the rate of ECD organ transplantation was identified, the occurrence of these criteria was associated with their refusal for transplantation.
Using ECD organs for transplantation is necessary, even if associated with higher morbidity and mortality[10]. This risk is continuous and progressively more significant with the accumulation of adverse donor and organ characteristics. Several studies have described donor variables associated with an increased risk of graft failure after transplantation, e.g., age, race, height, cerebrovascular accident as a cause of death, and split grafts[11].
By applying the ET-ECD criteria in the Eurotransplant region (Austria, Belgium, Croatia, Germany, Luxembourg, Netherlands, and Slovenia), at least one was present in more than 50% of liver donors[10]. Despite criticism regarding validating the prognostic value of these criteria, they are the only ones applied at the international level[12,13]. In the population investigated in our study, we found that almost 80% of DBD organ donors had at least one of these criteria to be considered an ECD.
Studies applying other criteria to classify an organ donor as an ECD have described their frequencies from approximately 50%[14] to 68%[15] in the United States of America and from 52.8%[16] to 59.9%[17] in Canada. In Brazil, a recent study applying different ECD indicative criteria described the transplantation of 56 ECD livers, representing 51% of the studied sample[18]. Previously, another study conducted in Brazil with data from 178 liver allografts reported an ECD rate of 76.97%[19]. Although these numbers support the high prevalence of ECD found in our study, the diversity of indicative criteria used in each study is a limiting factor for properly interpreting data.
The process of accepting a donor organ for transplantation considers characteristics of the recipient, such as the severity of the liver disease and their comorbidities, and factors of the donor and the donor organ. Non-transplanted livers are often from old donors, those with higher BMIs, viral hepatitis (B and C viruses), and a more significant number of comorbidities[20]. Still, findings in the biopsy are highlighted as a cause for discarding organs for transplantation[20,21]. In Brazil, a recent study reported that problems related to the donor organ (macroscopic pathological changes, visible organ damage, and inappropriate size) were the most common cause for donor organs not being used for transplantation[22].
The present study evaluated a significant sample of DBD organs over three years and a half. Although, probably because of the time interval studied, evolutionary changes in donor characteristics were not identified. The high prevalence of ECD was sustained during the study period. This diagnosis is concerning, especially considering the need to increase the number of transplants to meet the demand for the procedure. Therefore, implementing strategies to use ECD organs safely is necessary.
The routine application of the concept of donor-recipient risk balance (use of organs from higher-risk donors for recipients with lower severity of liver disease and fewer comorbidities) should underpin ECD organ transplantation[23,24]. However, alternative preservation methods may potentially be needed because of the inability of traditional static cold storage to maintain ECD organs effectively[25]. The application of dynamic organ preservation (the machine perfusion of the liver) in this setting is progressively more reported in the literature[3]. Machine perfusion aims to offer superior organ preservation, mitigate ischaemia-reperfusion injury in these highly vulnerable organs, assess their functional capacity, and potentially improve their quality before transplantation[26-29].
There are some limitations to this study. Firstly, this is a retrospective, single-centre study; drawing absolute conclusions based on this methodology may oversimplify the complexities of evaluating a donor organ offer for transplantation. In addition, although policies and the local culture of organ acceptance impact the decision of their use for transplantation, this effect is mitigated by their constancy during the study period. Furthermore, the reasons for discarding the offers were unavailable in our database. Consequently, some of these organs may have been initially declined for the first recipient of the program due to inappropriate size or logistical reasons, and another transplantation team may have subsequently accepted them, therefore, not returning to a recipient at our institution. However, this effect is random across all subjects and may impact all donors equally-regardless of whether ECD. It is also important to note that due to the Model for End-Stage Liver Disease score-based system of donor organ allocation in Brazil, through a single list according to the severity of liver disease, the refusal rate of ECD organs in our service does not necessarily reflect the percentage of use of these organs for transplantation in the country.
This study evaluated a large sample of DBD organ donors and found a high and sustained prevalence of ECD in Brazil, which surpassed the numbers reported in other countries. An increase in the use of these higher-risk organs for transplantation was noticed during the study period, possibly due to the high demand for the procedure. Despite this fact, the refusal rate of DBD organs for transplantation remains high, and the presence and the addition of ET-ECD criteria were associated with an increased chance of them being refused. Therefore, implementing strategies to safely extend the use of ECD organs is critical and demands attention from the transplant community to benefit as many patients waiting for transplantation as possible.
The use of extended criteria donor (ECD) organs for transplantation has become a global need due to the lack of donor organs to attend to the high demand for the procedure.
Knowing the real prevalence of ECD in donation after brain death (DBD) donor organs can pave the way for future research to understand better how to improve their use safely.
To determine the prevalence of ECD allografts in DBD liver transplantation and the likelihood of organ acceptance over the years.
This is a retrospective, single-centre study. Liver donor offers for the Solid Organ Transplant Program of the Hospital Israelita Albert Einstein, Sao Paulo, Brazil, were included between June 2017 and December 2020. Multivariate analysis was performed to determine if any Eurotransplant ECD criteria (ET-ECD) were independent risk factors for organ refusal for transplantation.
The prevalence of ECD among a total of 1619 organ donors analysed was 78.31%. There was an increase in the acceptance of ECD DBD organs for transplantation along the studied period. Despite that, for each addition of one ET-ECD criterion, the estimated chance of organ refusal was 64.4% higher (OR 1.644, 95%CI 1.469-1.839, P < 0.001).
There was a high prevalence of ECD DBD even though an increase in the utilisation rate of these higher-risk organs was noticed. The presence and the number of extended donor criteria were risk factors for their refusal for transplantation.
Further research is needed to develop more general accepted criteria to indicate ECD donor organs. This must guarantee more reliable data for comparison between countries. Furthermore, based on this diagnosis, strategies to increase ECD liver transplantation safely are urgently needed to attend to the demand for the procedure.
This paper presents independent research supported by the Brazilian Ministry of Health via the Support Program for Organizational Development of the SUS (PROADI-SUS) at the Hospital Israelita Albert Einstein. The views expressed are those of the author(s) and not necessarily those of the Ministry of Health, the PROADI-SUS, or the Hospital Israelita Albert Einstein.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Liver Transplantation Society; American College of Surgeons; The Transplantation Society; Associação Brasileira de Transplante de Órgãos; Academia Nacional de Medicina.
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li HL, China; Yamamoto T, United States S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Órgãos A. Dimensionamento dos transplantes no Brasil e em cada estado. Registro Brasileiro de Transplantes. 2020. Available from: www.abto.org.br/abtov03/Upload/file/RBT/2015/anual-n-associado.pdf. |
| 2. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1489] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 3. | Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S, Baimakhanov Z, Piros L, Eguchi S. Extended-criteria donors in liver transplantation Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. 2016;10:841-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Vodkin I, Kuo A. Extended Criteria Donors in Liver Transplantation. Clin Liver Dis. 2017;21:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (35)] |
| 5. | Blok JJ, Braat AE, Adam R, Burroughs AK, Putter H, Kooreman NG, Rahmel AO, Porte RJ, Rogiers X, Ringers J; European Liver Intestine Transplant Association Eurotransplant Liver Intestine Advisory Committee; Eurotransplant Liver Intestine Advisory Committee. Validation of the donor risk index in orthotopic liver transplantation within the Eurotransplant region. Liver Transpl. 2012;18:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Neto O. O doador limítrofe no transplante hepático. Brasília Med. 2011;48. |
| 7. | Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Lué A, Solanas E, Baptista P, Lorente S, Araiz JJ, Garcia-Gil A, Serrano MT. How important is donor age in liver transplantation? World J Gastroenterol. 2016;22:4966-4976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Oosterlee A, Rahmel A. Eurotransplant International Foundation Annual Report 2008. 2011. [cited 10 January 2023]. Available from: http://www.eurotransplant.org/cms/mediaobject.php? file= ar_2008.pdf. |
| 10. | Durand F, Renz JF, Alkofer B, Burra P, Clavien PA, Porte RJ, Freeman RB, Belghiti J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008;14:1694-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Feng S, Lai JC. Expanded criteria donors. Clin Liver Dis. 2014;18:633-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Bruzzone P, Giannarelli D, Adam R; European Liver and Intestine Transplant Association; European Liver Transplant Registry. A preliminary European Liver and Intestine Transplant Association-European Liver Transplant Registry study on informed recipient consent and extended criteria liver donation. Transplant Proc. 2013;45:2613-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S, Baimakhanov Z, Piros L, Eguchi S. Extended criteria donors in liver transplantation Part I: reviewing the impact of determining factors. Expert Rev Gastroenterol Hepatol. 2016;10:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, Zimmerman M, Hong J, Collins TE, Gornbein J, Amersi F, Weaver M, Cao C, Chen T, Hiatt JR, Busuttil RW. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1000 liver transplants. Ann Surg. 2006;243:748-53; discussion 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Tector AJ, Mangus RS, Chestovich P, Vianna R, Fridell JA, Milgrom ML, Sanders C, Kwo PY. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. 2006;244:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Schemmer P, Nickkholgh A, Hinz U, Gerling T, Mehrabi A, Sauer P, Encke J, Friess H, Weitz J, Büchler MW, Schmidt J. Extended donor criteria have no negative impact on early outcome after liver transplantation: a single-center multivariate analysis. Transplant Proc. 2007;39:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Pandya K, Sastry V, Panlilio MT, Yip TCF, Salimi S, West C, Virtue S, Wells M, Crawford M, Pulitano C, Strasser SI, McCaughan GW, Majumdar A, Liu K. Differential Impact of Extended Criteria Donors After Brain Death or Circulatory Death in Adult Liver Transplantation. Liver Transpl. 2020;26:1603-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Lugon Ferreira-Jr AC, Miguel GPS, Moscon I, Abreu IW, Aguiar JBOS, Vecci TRDS. Comparison of results on the use of extended criteria liver doners for transplants in Espírito Santo. Rev Col Bras Cir. 2021;48:e20202492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Fonseca-Neto OCL. O doador marginal: experiência de um centro de transplante de fígado. ABCD Arquivos Brasileiros de Cirurgia Digestiva. 2008;21:5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Carpenter DJ, Chiles MC, Verna EC, Halazun KJ, Emond JC, Ratner LE, Mohan S. Deceased Brain Dead Donor Liver Transplantation and Utilization in the United States: Nighttime and Weekend Effects. Transplantation. 2019;103:1392-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Desai C, Khan K, Girlanda R, Hawksworth J, Serrano P, Island E. UNOS Data Analysis of Discarded Liver-Grafts After Procurement. Transplantation. 2014;98:11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Bicudo de Oliveira L, Riccetto E, Boin IFSF. Prevalence and Profile of Discarded Liver Donors in a Tertiary Health Service in Brazil From 2015 to 2018. Transplant Proc. 2020;52:1251-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Briceño J, Ciria R, de la Mata M, Rufián S, López-Cillero P. Prediction of graft dysfunction based on extended criteria donors in the model for end-stage liver disease score era. Transplantation. 2010;90:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (35)] |
| 24. | Silberhumer GR, Pokorny H, Hetz H, Herkner H, Rasoul-Rockenschaub S, Soliman T, Wekerle T, Berlakovich GA, Steininger R, Muehlbacher F. Combination of extended donor criteria and changes in the Model for End-Stage Liver Disease score predict patient survival and primary dysfunction in liver transplantation: a retrospective analysis. Transplantation. 2007;83:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Vekemans K, Liu Q, Pirenne J, Monbaliu D. Artificial circulation of the liver: machine perfusion as a preservation method in liver transplantation. Anat Rec (Hoboken). 2008;291:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, Barton D, Curbishley S, Wilkhu M, Neil DAH, Hübscher SG, Muiesan P, Isaac JR, Roberts KJ, Abradelo M, Schlegel A, Ferguson J, Cilliers H, Bion J, Adams DH, Morris C, Friend PJ, Yap C, Afford SC, Mirza DF. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 27. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 856] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 28. | van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, Erdmann JI, Gilbo N, de Haas RJ, Heaton N, van Hoek B, Huurman VAL, Jochmans I, van Leeuwen OB, de Meijer VE, Monbaliu D, Polak WG, Slangen JJG, Troisi RI, Vanlander A, de Jonge J, Porte RJ; DHOPE-DCD Trial Investigators. Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med. 2021;384:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 389] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 29. | Boteon YL, Laing RW, Schlegel A, Wallace L, Smith A, Attard J, Bhogal RH, Neil DAH, Hübscher S, Perera MTPR, Mirza DF, Afford SC, Mergental H. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018;24:1699-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |