Published online Jan 27, 2023. doi: 10.4254/wjh.v15.i1.68
Peer-review started: September 13, 2022
First decision: October 20, 2022
Revised: October 25, 2022
Accepted: November 14, 2022
Article in press: November 14, 2022
Published online: January 27, 2023
Processing time: 124 Days and 13.4 Hours
Patients with autoimmune hepatitis (AIH) require life-long immunosuppressive agents that may increase the risk of poor coronavirus disease 2019 (COVID-19) outcomes. There is a paucity of large data at the population level to assess whe
To evaluate the impact of pre-existing AIH on the clinical outcomes of patients with COVID-19.
We conducted a population-based, multicenter, propensity score-matched cohort study with consecutive adult patients (≥ 18 years) diagnosed with COVID-19 using the TriNeTx research network platform. The outcomes of patients with AIH (main group) were compared to a propensity score-matched cohort of patients: (1) Without chronic liver disease (CLD); and (2) Patients with CLD except AIH (non-AIH CLD) control groups. Each patient in the main group was matched to a pa
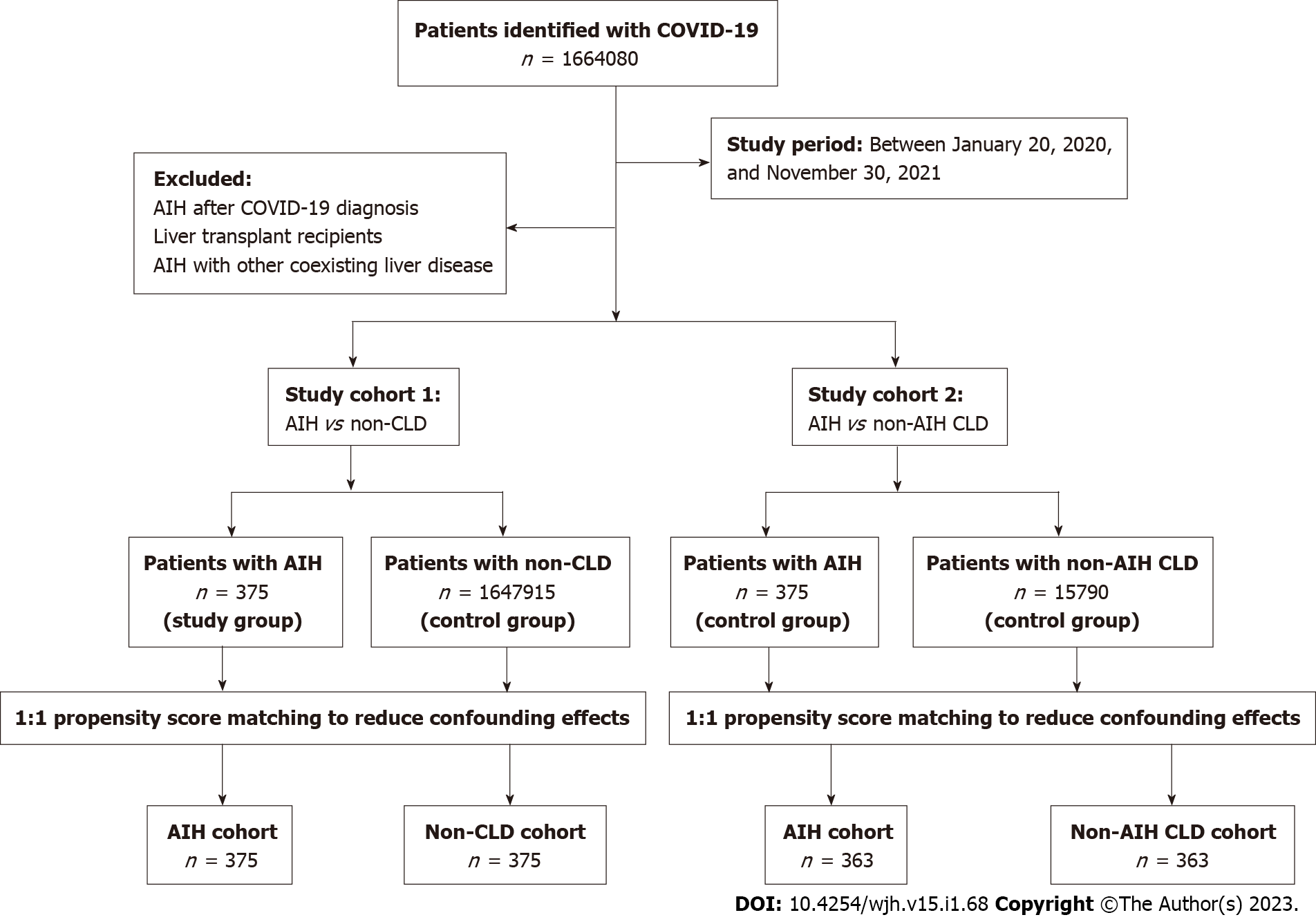
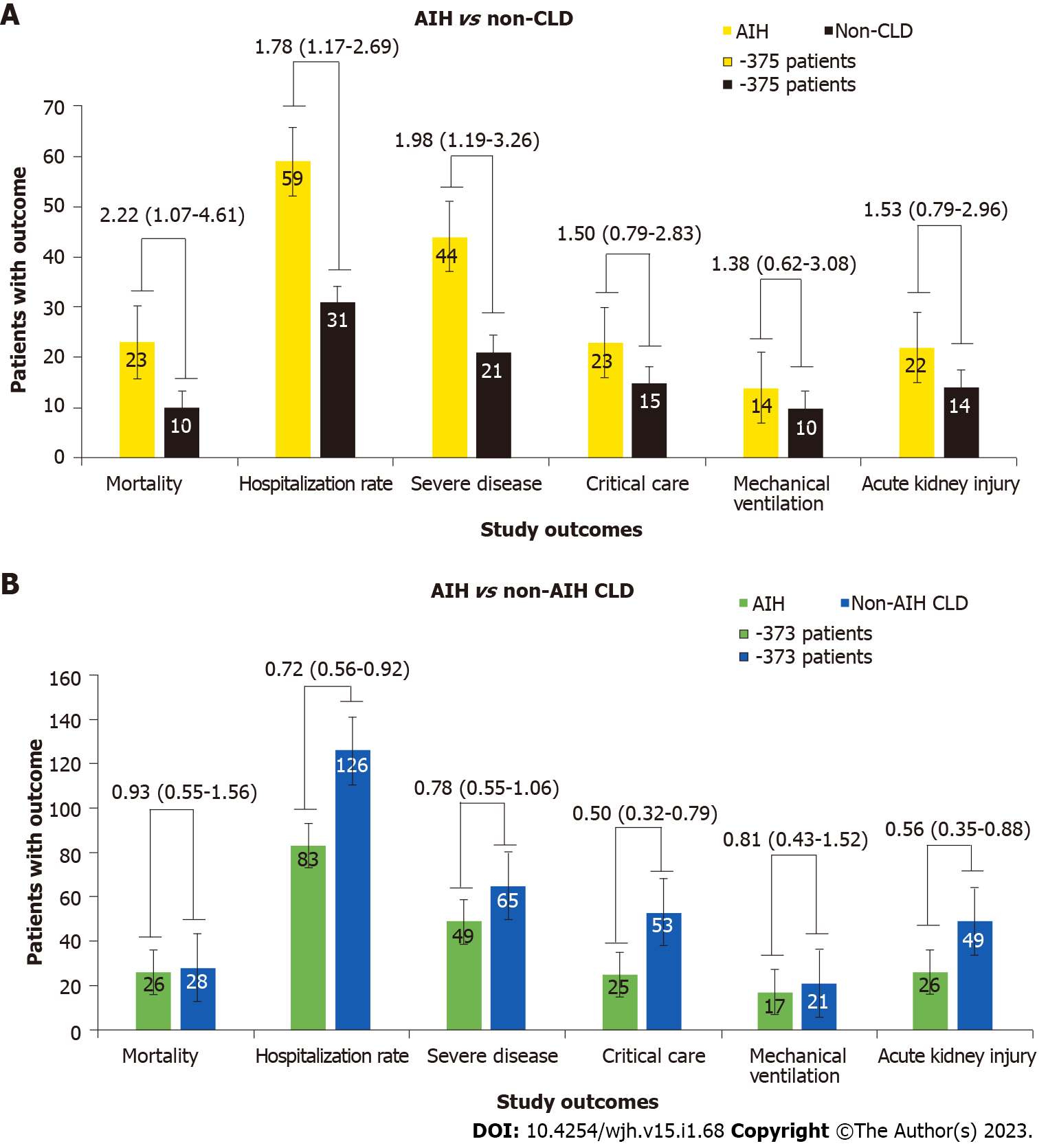
We identified 375 patients with AIH, 1647915 patients with non-CLD, and 15790 patients with non-AIH CLD with COVID-19 infection. Compared to non-CLD patients, the AIH cohort had an increased risk of all-cause mortality (RR = 2.22; 95%CI: 1.07-4.61), hospitalization rate (RR = 1.78; 95%CI: 1.17-2.69), and severe disease (RR = 1.98; 95%CI: 1.19-3.26). The AIH cohort had a lower risk of hospitalization rate (RR = 0.72; 95%CI: 0.56-0.92), critical care (RR = 0.50; 95%CI: 0.32-0.79), and AKI (RR = 0.56; 95%CI: 0.35-0.88) compared to the non-AIH CLD patients.
Patients with AIH are associated with increased hospitalization risk, severe disease, and all-cause mortality compared to patients without pre-existing CLD from the diagnosis of COVID-19. However, patients with AIH were not at risk for worse outcomes with COVID-19 than other cau
Core Tip: Autoimmune hepatitis (AIH) is a chronic inflammatory disease of the liver of unknown etiology. Patients with AIH may be at increased risk of severe illness from coronavirus disease 2019 (COVID-19) and have poor outcomes due to underlying chronic liver disease (CLD) and ongoing pre-existing immunosuppression therapies. Patients with AIH are associated with increased hospitalization risk, severe disease, and all-cause mortality compared to patients without pre-existing CLDs from the diagnosis of COVID-19. Patients with AIH had a lower risk of several outcomes, including hospitalization, a necessity for critical care, and acute kidney injury, compared to patients with pre-existing CLDs other than AIH.
- Citation: Krishnan A, Patel RA, Hadi YB, Mukherjee D, Shabih S, Thakkar S, Singh S, Woreta TA, Alqahtani SA. Clinical characteristics and outcomes of COVID-19 in patients with autoimmune hepatitis: A population-based matched cohort study. World J Hepatol 2023; 15(1): 68-78
- URL: https://www.wjgnet.com/1948-5182/full/v15/i1/68.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i1.68
As coronavirus disease 2019 (COVID-19) cases increase in the United States and globally, investigators continue to identify risk factors for adverse outcomes resulting from COVID-19 infection. The known risk factors for severe disease include older age, male gender, and comorbidities such as hypertension, diabetes, chronic obstructive pulmonary disease, and chronic liver diseases (CLDs)[1-3]. In addition, studies have shown that COVID-19 affects the liver, with over one-third of hospitalized COVID-19 patients presenting with abnormal liver function, which was also associated with a longer hospital stay[4,5]. In contrast, a higher risk of mortality and hospitalization rates have been reported in COVID-19 patients with pre-existing liver disease compared to those without liver disease[6].
Autoimmune hepatitis (AIH) is a genetically predisposed CLD. The exact mechanism regarding its immune dysfunction has yet to be elucidated; however, an imbalance between effector and regulatory immunity and molecular mimicry may play a role in its pathogenesis[7]. The prevalence rate of AIH in the United States is estimated to be 31.2/100000, which is similar to the prevalence rates reported in Europe[8]. The association between severe acute respiratory disease coronavirus 2 (SARS-CoV-2) and autoimmune diseases is very complex and only partially understood. In addition, understanding the outcomes of COVID-19 infections in AIH patients is particularly important due to the fact that patients with AIH require lifelong immunosuppressive agents to prevent cirrhosis and end-stage liver disease, which may increase the risk of viral and bacterial infections[9,10]. The clinical impact of the pre-existing use of immunosuppression in patients with COVID-19 remains complex and not clearly defined. Evidence is mixed regarding the impact of immunosuppressive agents on COVID-19 outcomes. Hence, existing data are controversial on the outcome following COVID-19 infection in patients with auto
On the other hand, increasing vaccine uptake and other public health safety measures have helped reduce the pandemic’s burden. However, considerable morbidity and mortality continue to accrue among non-immune and unvaccinated individuals. Furthermore, previous AIH literature has used small samples of patients with AIH. To address these research gaps, we performed a large population-based retrospective cohort study using data from the multicenter research network. Our analysis focused on evaluating AIH as an independent risk factor associated with severe diseases of SARS-CoV-2 and all-cause mortality.
This population-based, multicenter, retrospective cohort study was conducted using TriNetX (Cambridge, MA, United States), a federated health research network data set. TriNetX is a multi-institutional health research network that provides de-identified electronic medical records systems (EHRs) from the included healthcare organizations. Clinical variables (referred to as “facts” on the network) are derived directly through EHRs. Robust quality assurance on the network is achieved at the time of extraction before inclusion. The platform only provides aggregate patient counts and statistical summaries to ensure de-identification at all levels of retrieval and dissemination of patient data. TriNetX received a waiver from the Western institutional review board as a federated network since only aggregated counts and statistical summaries of de-identified information are included. No protected health information was obtained, and no study-specific activities were performed in the retrospective analyses. Details of the data source and quality checks are described in the supplementary data.
All adult patients (age ≥ 18 years) with AIH and confirmed COVID-19 infection between January 20, 2020, and November 30, 2021, were included. The search criteria for potential patients with COVID-19 were based on specific COVID-19 diagnosis codes or positive laboratory confirmation of COVID-19.
To determine the clinical impact of AIH on clinical outcomes of COVID-19, we compared AIH patients to a control group of patients without any pre-existing CLD, including AIH (non-CLD) and COVID-19. To assess the impact of AIH compared to other liver diseases, we compared AIH patients to a control group of patients with other pre-existing CLD (non-AIH CLD) and COVID-19. Details of the search criteria and diagnosis codes used for patient selection are described in the supplementary data.
Each patient in the main group was matched to a patient in the control group using 1:1 propensity score matching (PSM) to reduce confounding effects[15]. Covariates in the propensity score model were adjusted for a priori-identified potential confounders: Age, sex, race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or non-Hispanic other), body mass index (BMI), nicotine dependence, and comorbidities that are listed in Table 1. Logistic regression on these input matrices was used to obtain propensity scores for each patient in both cohorts. Logistic regression was performed in Python 3.6.5 (Python Software Foundation) using standard libraries NumPy and Sklearn. The same analyses were also performed in R 3.4.4 software (R Foundation for Statistical Computing, Vienna, Austria) to ensure outputs match. After calculating propensity scores, matching was performed using a greedy nearest-neighbor matching algorithm with a caliper of 0.1 pooled standard deviations. The order of the rows in the covariate matrix can affect the nearest neighbor matching; therefore, the order of the rows in the matrix was randomized to eliminate this bias.
| Variables | Before propensity score matching | After propensity score matching | ||||
| AIH (n = 375) | Non-CLD (n = 1647915) | P value | AIH (n = 375) | Non-CLD (n = 375) | P value | |
| Age, yr, mean ± SD | 53.1 ± 18.4 | 46.4 ± 18.8 | < 0.01 | 53.1 ± 8.4 | 53.2 ± 8.2 | 0.97 |
| Sex, n (%) | ||||||
| Female | 271 (72.2) | 893768 (54.2) | < 0.01 | 271 (72.2) | 256 (68.2) | 0.23 |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 37 (9.8) | 144535 (8.7) | 0.45 | 37 (9.8) | 36 (9.6) | 0.90 |
| Race, n (%) | ||||||
| White | 238 (63.4) | 1028824 (62.4) | 0.68 | 238 (63.4) | 228 (60.8) | 0.45 |
| Black or African American | 64 (17.1) | 245820 (14.9) | 0.24 | 64 (17.1) | 69 (18.4) | 0.63 |
| Other | 65 (17.3) | 325638 (19.7) | 0.24 | 65 (17.3) | 69 (18.4) | 0.70 |
| Nicotine dependence, n (%) | 76 (20.2) | 181270 (10.9) | < 0.01 | 72 (19.2) | 74 (19.7) | 0.67 |
| BMI (kg/m2), mean ± SD | 28.9 ± 7.05 | 29.9 ± 7.45 | 0.07 | 28.9 ± 7.05 | 30.8 ± 7.65 | 0.01 |
| Comorbidities, n (%) | ||||||
| Hypertension | 163 (43.4) | 384968 (23.3) | < 0.01 | 163 (43.4) | 159 (42.4) | 0.77 |
| Ischemic heart diseases | 59 (15.7) | 119162 (7.2) | < 0.01 | 59 (15.7) | 57 (15.2) | 0.84 |
| Heart failure | 38 (10.1) | 65245 (3.9) | < 0.01 | 38 (10.1) | 37 (9.8) | 0.90 |
| Diabetes | 74 (19.7) | 171727 (10.4) | < 0.01 | 74 (19.7) | 68 (18.1) | 0.58 |
| Chronic lower respiratory diseases | 82 (21.8) | 235406 (14.2) | < 0.01 | 82 (21.8) | 81 (21.6) | 0.93 |
| Cerebrovascular diseases | 37 (9.8) | 73209 (4.4) | < 0.01 | 37 (9.8) | 39 (10.4) | 0.81 |
| CKD of any stage | 46 (12.2) | 78265 (4.7) | < 0.01 | 46 (12.2) | 37 (9.8) | 0.29 |
| Neoplasms | 125 (33.3) | 293362 (17.8) | < 0.01 | 125 (33.3) | 132 (35.2) | 0.59 |
The primary study outcome was all-cause mortality from index events within 60 d. The index event was defined as either the time of COVID-19 diagnosis or the first COVID-19 positive test result date, whichever occurred first. Secondary outcomes were hospitalization, severe diseases, acute kidney injury (AKI), and intensive care (requiring extracorporeal membrane oxygenation or mechanical ventilation) in the 30 d from COVID-19 diagnosis. Severe disease was operationalized and defined as a composite outcome requiring intensive care or death within 30 d of COVID-19 diagnosis.
All statistical analyses were performed in real-time using the TriNetX platform. Continuous variables are expressed as means ± SD. Categorical variables were defined as frequency and percentage. For each outcome, the risk ratio (RR) and confidence intervals (CI) were calculated to compare the association of the AIH with the outcome. Numbers were then validated by comparing them with the output from SAS version 9.4. A-priori-defined two-sided alpha of less than ≤ 0.05 was used for statistical significance, and all statistical data analyses were performed utilizing the form of the limitation in real-time.
We identified 15790 non-AIH CLD and 1647915 non-CLD patients during the study period (Figure 1). Major etiologies of non-AIH CLD included alcoholic liver disease (n = 4159, 26.3%), NAFLD (n = 3085, 19.5%), viral hepatitis (n = 1093, 6.9%), and other diseases of the liver (n = 3456, 21.8%) (Supp
Baseline characteristics of the non-AIH CLD patients are described in Table 2. 6517 (41.2%) patients were female, and 1831 (11.5%) patients had a history of nicotine dependence. Compared to the AIH cohort, non-AIH CLD patients had higher rates of comorbidities, including hypertension (n = 11469, 72.6%), neoplasms (n = 8400, 53.1%), diabetes (n = 7882, 49.9%), and chronic lower respiratory diseases (n = 5843, 37.0%).
| Variables | Before propensity score matching | After propensity score matching | ||||
| AIH (n = 375) | Non-AIH CLD (n = 15790) | P value | AIH (n = 363) | Non-AIH CLD (n = 363) | P value | |
| Age, yr, mean ± SD | 53.1 ± 18.5 | 60.3 ± 12.1 | < 0.01 | 53.9 ± 18.1 | 54.3 ± 15.5 | 0.76 |
| Sex, n (%) | ||||||
| Female | 267 (71.2) | 6517 (41.2) | < 0.01 | 262 (72.1) | 266 (73.2) | 0.74 |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 82 (21.8) | 1579 (10) | < 0.01 | 82 (22.5) | 67 (18.4) | 0.19 |
| Race, n (%) | ||||||
| White | 236 (62.9) | 11132 (70.5) | 0.02 | 235 (64.7) | 218 (60.1) | 0.19 |
| Black or African American | 65 (17.3) | 2462 (15.5) | 0.41 | 58 (15.9) | 72 (19.8) | 0.17 |
| Other | 66 (17.6) | 1824 (11.5) | 0.04 | 62 (17.1) | 61 (16.8) | 0.92 |
| Nicotine dependence, n (%) | 87 (23.2) | 1831 (11.5) | < 0.01 | 89 (24.5) | 87 (23.9) | 0.94 |
| BMI (kg/m2), mean ± SD | 28.9 ± 7.1 | 30.4 ± 7.48 | 0.09 | 29 ± 7.11 | 28.7 ± 7.79 | 0.72 |
| Comorbidities, n (%) | ||||||
| Hypertension | 165 (44) | 11469 (72.6) | < 0.01 | 161 (44.3) | 200 (55.1) | 0.03 |
| Ischemic heart diseases | 62 (16.5) | 5472 (34.6) | < 0.01 | 59 (16.2) | 56 (15.4) | 0.76 |
| Heart failure | 39 (10.4) | 4196 (26.5) | < 0.01 | 38 (10.4) | 48 (13.2) | 0.25 |
| Diabetes | 74 (19.7) | 7882 (49.9) | 0.01 | 74 (20.4) | 66 (18.1) | 0.45 |
| Chronic lower respiratory diseases | 84 (22.4) | 5843 (37.0) | < 0.01 | 82 (22.5) | 79 (21.7) | 0.79 |
| Cerebrovascular diseases | 37 (9.8) | 3079 (19.4) | < 0.01 | 37 (10.1) | 39 (10.7) | 0.81 |
| CKD of any stage | 49 (13.1) | 5102 (32.3) | < 0.01 | 46 (12.6) | 55 (15.1) | 0.33 |
| Neoplasms | 131 (34.9) | 8400 (53.1) | < 0.01 | 124 (34.1) | 131 (36.1) | 0.59 |
During the same study period, 375 patients with AIH were identified. Baseline characteristics are described in Tables 1 and 2. A majority (n = 271, 72.2%) of the patients were female, and 76 (20.2%) patients had a history of nicotine dependence. Common comorbidities were hypertension (n = 163, 43.4%), neoplasms (n = 125, 33.3%), chronic lower respiratory diseases (n = 82, 21.8%), and diabetes (n = 74, 19.7%). Coexistence of other immune-mediated disorders in the AIH cohort occurred most often with systemic lupus erythematosus (n = 61, 16.2%), rheumatoid arthritis (n = 57, 15.2%), Sjögren syndrome (n = 45, 12.0%), and ulcerative colitis (n = 27, 7.2%) (Supplementary Table 2). The most common immunosuppressive agents used in the AIH cohort were prednisone (n = 313, 83.4%), azathioprine (n = 170, 45.3%), and budesonide (n = 80, 21.3%) (Supplementary Table 2).
Patients with AIH compared to non-CLD: Results of laboratory vitals, symptoms, and laboratory findings between AIH and non-CLD cohorts are presented in Supplementary Table 3. All liver function tests were significantly different between the AIH and non-CLD patients (P < 0.01). After a propensity score-matched analysis, all liver function results remained significantly different between the cohorts: The mean alanine aminotransferase (ALT) (65.3 vs 23.6 U/L; P < 0.01), aspartate aminotransferase (54.9 vs 23.7 U/L; P = 0.01), total bilirubin (0.86 vs 0.53 mg/dL; P = 0.01), and alkaline phosphatase (ALP) (105.0 vs 88.3 U/L; P = 0.01), and serum albumin (3.88 vs 4.04 g/dL; P = 0.04) (Supplementary Table 3).
Compared to non-CLD patients, AIH patients had higher ferritin levels (336 vs 192 ng/mL; P = 0.01) and lower fibrinogen levels (303 vs 389 mg/dL; P = 0.04). After propensity matching, the higher ferritin level (336 vs 118 ng/mL; P = 0.01) and lower fibrinogen level (303 vs 436 mg/dL; P = 0.03) remained. All other inflammatory markers were measured with no significant differences (Supplementary Table 3).
Patients with AIH compared to non-AIH CLD: When compared to non-AIH CLD patients, AIH patients had higher ALT levels (65.6 vs 38.3 U/L; P < 0.01), lower total bilirubin (0.87 vs 1.58 mg/dL; P = 0.01), lower ALP (106 vs 129 U/L; P = 0.04), and higher serum albumin (3.87 vs 3.61 g/dL; P < 0.01). After PSM, the differences in total bilirubin (0.87 vs 2.09 mg/dL; P < 0.01), ALP (105 vs 133 U/L; P = 0.04), and serum albumin (3.87 vs 3.71 g/dL; P = 0.01) remained significant. Before PSM, AIH patients had higher C-reactive protein levels (15.4 vs 28.4 mg/L; P = 0.01) and lower erythrocyte sedimentation rate (24.3 vs 34.0 mm/h; P < 0.01). After PSM, the AIH group had higher fibrinogen levels (307 vs 234 mg/dL; P = 0.01) (Supplementary Table 4).
Patients with AIH compared to non-CLD: Before PSM, there were significant differences between the AIH and non-CLD cohorts in the rates of all hospitalization-related outcomes. AIH patients had a significantly higher risk of all-cause mortality (RR = 2.33; 95%CI: 1.66-3.28), hospitalization rate (RR = 2.14; 95%CI: 1.75-2.60), critical care (RR = 1.94; 95%CI: 1.35-2.79), severe disease (RR = 2.01; 95%CI: 1.55-2.59), need for mechanical ventilation (RR = 1.99; 95%CI: 1.21-3.27), and AKI (RR = 1.94; 95%CI: 1.38-2.72).
After PSM, the increased risk of all-cause mortality (RR = 2.22; 95%CI: 1.07-4.61), hospitalization rate (RR = 1.78; 95%CI: 1.17-2.69), and severe disease (RR = 1.98; 95%CI: 1.19-3.26), persisted in the AIH group. However, there were no significant differences in the rates of critical care (RR = 1.50; 95%CI: 0.79-2.83), need for mechanical ventilation (RR = 1.38; 95%CI: 0.62-3.08), and AKI (RR = 1.53; 95%CI: 0.79-2.96) (Figure 2A).
Patients with AIH compared to non-AIH CLD: When compared to the non-AIH CLD cohort, the AIH group had a lower risk of all-cause mortality (RR = 0.49; 95%CI: 0.34-0.68), critical care (RR = 0.43; 95%CI: 0.30-0.62), severe disease (RR = 0.57; 95%CI: 0.45-0.74), need for mechanical ventilation (RR = 0.41; 95%CI: 0.24-0.69), and AKI (RR = 0.38; 95%CI: 0.27-0.53), There were no significant differences between the groups in hospitalization rate (RR = 0.93; 95%CI: 0.78-1.10).
After PSM, lower risk persisted for hospitalization rate (RR = 0.72; 95%CI: 0.56-0.92), critical care (RR = 0.50; 95%CI: 0.32-0.79), and AKI (RR = 0.56; 95%CI: 0.35-0.88) among patients with AIH. However, there were no significant differences in all-cause mortality (RR = 0.93; 95%CI: 0.55-1.56), severe disease (RR = 0.78; 95%CI: 0.55-1.06), and need for mechanical ventilation (RR = 0.81; 95%CI: 0.43-1.52) between these groups (Figure 2B).
Although the number of COVID-19-related cases, rates of hospitalizations, and deaths are decreasing in the United States, the COVID-19 pandemic is still ongoing worldwide, and significant questions remain. Our data showed that the patients with AIH had a higher risk of hospitalization, severe COVID-19, and all-cause mortality than those without liver disease. Notably, a lower survival probability was also noted for AIH patients. On the other hand, compared to non-AIH CLD, AIH patients had a lower risk of hospitalization, critical care, and AKI without any difference in survival probability between the groups.
The present study is the largest United States-based study investigating severe COVID-19 in AIH patients. Our results showed an increased all-cause mortality risk in AIH patients compared to non-CLD, but no difference in all-cause mortality risk compared to non-AIH CLD. Marjot et al[16] demonstrated a similar significant difference in mortality risk between AIH and non-CLD patients; however, their findings showed a similar mortality risk between AIH and non-CLD patients. Efe et al[17] compared rates of adverse outcomes of COVID-19 between AIH patients and non-AIH CLD. The AIH cohort included 34 United States patients and 110 total AIH patients[14]. No differences were found in the risk of severe outcomes[17]. Although outcomes such as mortality, severe COVID-19, need for supplemental oxygen, and hospitalization was addressed in the study[14], the need for intensive care or mechanical ventilation was not. Marjot et al[16] also conducted an international retrospective study comparing 70 AIH to non-AIH CLD and non-CLD patients. There was an increased hospitalization risk for AIH patients compared to non-CLD patients[12]; however, there were no differences in the risk of severe outcomes between the AIH group and patients with other causes of CLD[13,14]. Outcomes assessed in this study included hospitalization, ICU requirement, ICU admission, the new requirement for renal replacement therapy, the need for invasive ventilation, and mortality. In AIH patients, age and advanced liver disease, but not immunosuppression, were found to be factors associated with mortality[13]. While both studies investigated hospitalization and mortality, neither study included AKI as an adverse outcome. Additionally, a small case series demonstrated a clinical course of COVID-19 in 10 AIH patients that were similar to the general population[15]. Our study addresses these gaps in knowledge while building on previous work with a focus on a larger cohort of United States-based AIH patients.
Our results may differ from previous studies due to the covariables included in our propensity score-matched analysis. Strengths of the present study include an expansion of clinical outcomes addressed in previous studies and an adjustment for confounders such as BMI, race, ethnicity, chronic kidney disease, neoplasms, and obstructive sleep apnea. The chronic, low-grade inflammation that is characteristic of obesity causes immune dysregulation, and obesity has been established as an independent risk factor for severe COVID-19 disease[14]. The inclusion of race and ethnicity as covariables are also important, as minority groups, including African American, Hispanic, and Asian American individuals, have higher rates of comorbidities that are associated with an increased risk of severe COVID-19 disease[18,19].
Autoimmune disease diagnosis has been associated with more severe COVID-19 disease[20,21]. The immunosuppressive agents used to treat AIH may increase the risk of viral and bacterial infections and delay viral clearance[22]. Our contrasting finding that AIH patients had a lower risk of several adverse outcomes compared to non-AIH CLD patients may be explained by the immunosuppressive agents that are used in its treatment. Other investigators have found a decreased risk of severe COVID-19 outcomes such as mechanical ventilation, death, and severe acute respiratory distress syndrome[23]. In contrast, a different study found a higher risk of severe COVID-19 in AIH patients who were on thiopurine or glucocorticoid therapy prior to COVID-19 infection[24]. Further investigation is needed to clarify the relationship between immunosuppression and COVID-19 outcomes.
Of note, we found that AIH patients had higher fibrinogen levels than the non-CLD group but lower than the non-AIH CLD group. This is consistent with the literature, as higher fibrinogen levels have been associated with disease severity and ICU admission in COVID-19 patients[25], which may be explained by the role of the cytokine storm that follows COVID-19 infection in disseminated in
Our study has several strengths. Firstly, our study is the first to examine severe COVID-19 outcomes in a large cohort of United States-based AIH patients. While other studies have also compared AIH COVID-19 outcomes to non-CLD and non-AIH CLD groups, our study includes a larger sample size of 375 patients with AIH. Additionally, we included AKI as an adverse outcome. AKI is a common complication of COVID-19, reported in approximately 29% of hospitalized patients and 78% of patients that require intubation[27]. The investigation of AKI in COVID-19 is important, as there may be differences in pathophysiology between AKI related to COVID-19 and non-COVID sepsis-associated AKI[25]. Secondly, we included a robust control and adjustment for baseline and potential confounders. Thirdly, the large sample in the propensity-matched analyses resulted in narrow confidence intervals. It allowed us to capture a significant number of outcomes, which lends strength to the conclusions that we have derived. Lastly, our cohort was derived from a multicenter database, increasing the generalizability of our findings within the United States.
The study had some notable limitations. First, the data derived from an EHRs-based database is susceptible to errors in coding or data entry when patient information is translated into the diagnosis and procedure codes. However, care was taken to use standardized measures to identify cases to minimize documentation errors. Second, even though we adjusted our analyses, it is still possible that there is some residual confounding we did not account for. Third, patients who were asymptomatic throughout the course of infection and who did not undergo COVID-19 testing were not captured in the study. Fourth, our data were not able to include COVID-19 vaccines or SARS-CoV-2 variants to assess the impact on accuracy in patients with AIH. Another limitation includes the absence of cirrhosis prevalence and Child-Pugh scores in our cohort. Our study did not examine the changes made in immunosuppressive therapy after COVID-19 diagnosis in AIH patients. Finally, we could not obtain long-term outcomes due to a comparatively short observation period.
In conclusion, in this cohort, we found that AIH patients have an increased hospitalization risk, severe COVID-19, and all-cause mortality compared to non-CLD patients. Compared to the large group of patients with non-AIH CLD, AIH patients had a lower risk of several outcomes, including hospitalization, a necessity for critical care, and AKI. These results confirm that many patients with existing AIH are at high risk and should continue to follow recommended preventive measures against SARS-CoV-2 exposure.
Severe illness and clinical outcomes can directly correlate with the underlying comorbidities of patients infected with coronavirus disease 2019 (COVID-19), including patients with autoimmune diseases. However, the clinical course of COVID-19 in patients with autoimmune hepatitis (AIH) is still not well studied.
AIH is a chronic inflammatory liver disease of unknown etiology in which autoimmune-mediated factors against hepatocytes are thought to play a key role. Patients with AIH may be at increased risk of severe illness from COVID-19 and have poor outcomes due to underlying chronic liver disease (CLD) and ongoing pre-existing immunosuppression therapies. Notably, there is a wide research gap in the perceived impact of COVID-19 on patients with AIH due to a high degree of heterogeneity in the existing literature.
This study aimed to evaluate the impact of pre-existing AIH on the clinical outcomes of patients with COVID-19.
A population-based, multicenter, propensity score-matched cohort study included 375 patients with AIH, 1647915 patients with non-CLD, and 15790 patients with non-AIH CLD with COVID-19 infection. To reduce confounding effects, we performed a 1:1 propensity score matching with each patient in the main group to a patient in the control group. The primary outcome was all-cause mortality at 60 d, and secondary outcomes were hospitalization rate, need for critical care, severe disease, mechanical ventilation, and acute kidney injury (AKI) at 30 d.
Patients with AIH had an increased risk of all-cause mortality [risk ratio (RR) = 2.22; 95% confidence interval (CI): 1.07-4.61], hospitalization rate (RR = 1.78), and severe disease (RR = 1.98) compared to the non-CLD controls. However, compared to the non-AIH CLD group, patients in the AIH cohort had a lower risk of hospitalization rate (RR = 0.72), critical care (RR = 0.50), and AKI (RR = 0.56).
This multicenter, propensity score-matched cohort study reveals that patients with AIH are at risk of worse COVID-19 outcomes than those without pre-existing CLD. However, patients with AIH were not at increased risk of COVID-19 adverse outcomes compared to matched patients with other causes of CLD.
Further studies with long-term follow-up of these patients are needed to understand the long-term impact of COVID-19 on the liver and elucidate the pathogenic mechanisms among patients with AIH.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moriya K, Japan; Rodrigues AT, Brazil S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W, Liang HL, Cao YY, Yan Q, Cao C, Gao HY, Brüggen MC, van de Veen W, Sokolowska M, Akdis M, Akdis CA. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 885] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 2. | Krishnan A, Hamilton JP, Alqahtani SA, A Woreta T. A narrative review of coronavirus disease 2019 (COVID-19): clinical, epidemiological characteristics, and systemic manifestations. Intern Emerg Med. 2021;16:815-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Krishnan A, Hamilton JP, Alqahtani SA, Woreta TA. COVID-19: An overview and a clinical update. World J Clin Cases. 2021;9:8-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 4. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 5. | Krishnan A, Prichett L, Tao X, Alqahtani SA, Hamilton JP, Mezey E, Strauss AT, Kim A, Potter JJ, Chen PH, Woreta TA. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J Gastroenterol. 2022;28:570-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 7. | Sucher E, Sucher R, Gradistanac T, Brandacher G, Schneeberger S, Berg T. Autoimmune Hepatitis-Immunologically Triggered Liver Pathogenesis-Diagnostic and Therapeutic Strategies. J Immunol Res. 2019;2019:9437043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Tunio NA, Mansoor E, Sheriff MZ, Cooper GS, Sclair SN, Cohen SM. Epidemiology of Autoimmune Hepatitis (AIH) in the United States Between 2014 and 2019: A Population-based National Study. J Clin Gastroenterol. 2021;55:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Pape S, Schramm C, Gevers TJ. Clinical management of autoimmune hepatitis. United European Gastroenterol J. 2019;7:1156-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 572] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 11. | Velayos FS, Dusendang JR, Schmittdiel JA. Prior Immunosuppressive Therapy and Severe Illness Among Patients Diagnosed with SARS-CoV-2: a Community-Based Study. J Gen Intern Med. 2021;36:3794-3801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Ruemmele FM, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481-491.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 580] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 13. | Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of Severe Coronavirus Disease 2019 in Patients With Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:1575-1578.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Andersen KM, Bates BA, Rashidi ES, Olex AL, Mannon RB, Patel RC, Singh J, Sun J, Auwaerter PG, Ng DK, Segal JB, Garibaldi BT, Mehta HB, Alexander GC; National COVID Cohort Collaborative Consortium. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022;4:e33-e41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 15. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 7513] [Article Influence: 536.6] [Reference Citation Analysis (0)] |
| 16. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Efe C, Dhanasekaran R, Lammert C, Ebik B, Higuera-de la Tijera F, Aloman C, Rıza Calışkan A, Peralta M, Gerussi A, Massoumi H, Catana AM, Torgutalp M, Purnak T, Rigamonti C, Gomez Aldana AJ, Khakoo N, Kacmaz H, Nazal L, Frager S, Demir N, Irak K, Ellik ZM, Balaban Y, Atay K, Eren F, Cristoferi L, Batıbay E, Urzua Á, Snijders R, Kıyıcı M, Akyıldız M, Ekin N, Carr RM, Harputluoğlu M, Hatemi I, Mendizabal M, Silva M, Idilman R, Silveira M, Drenth JPH, Assis DN, Björnsson E, Boyer JL, Invernizzi P, Levy C, Schiano TD, Ridruejo E, Wahlin S. Outcome of COVID-19 in Patients With Autoimmune Hepatitis: An International Multicenter Study. Hepatology. 2021;73:2099-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 18. | Gerussi A, Rigamonti C, Elia C, Cazzagon N, Floreani A, Pozzi R, Pozzoni P, Claar E, Pasulo L, Fagiuoli S, Cristoferi L, Carbone M, Invernizzi P. Coronavirus Disease 2019 in Autoimmune Hepatitis: A Lesson From Immunosuppressed Patients. Hepatol Commun. 2020;4:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Magesh S, John D, Li WT, Li Y, Mattingly-App A, Jain S, Chang EY, Ongkeko WM. Disparities in COVID-19 Outcomes by Race, Ethnicity, and Socioeconomic Status: A Systematic-Review and Meta-analysis. JAMA Netw Open. 2021;4:e2134147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 508] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 20. | Bertoglio IM, Valim JML, Daffre D, Aikawa NE, Silva CA, Bonfá E, Ugolini-Lopes MR. Poor Prognosis of COVID-19 Acute Respiratory Distress Syndrome in Lupus Erythematosus: Nationwide Cross-Sectional Population Study Of 252 119 Patients. ACR Open Rheumatol. 2021;3:804-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Wang F, Ma Y, Xu S, Liu H, Chen Y, Yang H, Shao M, Xu W, Kong J, Chen L, Shuai Z, Pan F. Prevalence and risk of COVID-19 in patients with rheumatic diseases: a systematic review and meta-analysis. Clin Rheumatol. 2022;41:2213-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Madhu D, Sharma S, Agarwal A, Saraya A. Special Considerations in the Management of Autoimmune Hepatitis in COVID-19 Hotspots: A Review. J Clin Transl Hepatol. 2021;9:568-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Monreal E, Maza S S, Gullón P, Natera-Villalba E, Chico-García JL, Beltrán-Corbellini Á, Martínez-Sanz J, García-Barragán N, Buisán J, Toledano R, Alonso-Canovas A, Pérez-Torre P, Matute-Lozano MC, López-Sendón JL, García-Ribas G, Corral Í, Fortún J, Montero-Errasquín B, Manzano L, Máiz-Carro L, Costa-Frossard L, Masjuan J; COVID-HRC Group. Non-severe immunosuppression might be associated with a lower risk of moderate-severe acute respiratory distress syndrome in COVID-19: A pilot study. J Med Virol. 2021;93:2243-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Efe C, Lammert C, Taşçılar K, Dhanasekaran R, Ebik B, Higuera-de la Tijera F, Calışkan AR, Peralta M, Gerussi A, Massoumi H, Catana AM, Purnak T, Rigamonti C, Aldana AJG, Khakoo N, Nazal L, Frager S, Demir N, Irak K, Melekoğlu-Ellik Z, Kacmaz H, Balaban Y, Atay K, Eren F, Alvares-da-Silva MR, Cristoferi L, Urzua Á, Eşkazan T, Magro B, Snijders R, Barutçu S, Lytvyak E, Zazueta GM, Demirezer-Bolat A, Aydın M, Heurgue-Berlot A, De Martin E, Ekin N, Yıldırım S, Yavuz A, Bıyık M, Narro GC, Kıyıcı M, Akyıldız M, Kahramanoğlu-Aksoy E, Vincent M, Carr RM, Günşar F, Reyes EC, Harputluoğlu M, Aloman C, Gatselis NK, Üstündağ Y, Brahm J, Vargas NCE, Güzelbulut F, Garcia SR, Aguirre J, Anders M, Ratusnu N, Hatemi I, Mendizabal M, Floreani A, Fagiuoli S, Silva M, Idilman R, Satapathy SK, Silveira M, Drenth JPH, Dalekos GN, N Assis D, Björnsson E, Boyer JL, Yoshida EM, Invernizzi P, Levy C, Montano-Loza AJ, Schiano TD, Ridruejo E, Wahlin S. Effects of immunosuppressive drugs on COVID-19 severity in patients with autoimmune hepatitis. Liver Int. 2022;42:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Sui J, Noubouossie DF, Gandotra S, Cao L. Elevated Plasma Fibrinogen Is Associated With Excessive Inflammation and Disease Severity in COVID-19 Patients. Front Cell Infect Microbiol. 2021;11:734005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 26. | Martinez MA, Franco S. Impact of COVID-19 in Liver Disease Progression. Hepatol Commun. 2021;5:1138-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, Cantaluppi V. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17:751-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 313] [Article Influence: 78.3] [Reference Citation Analysis (0)] |