Published online Sep 27, 2022. doi: 10.4254/wjh.v14.i9.1830
Peer-review started: June 1, 2022
First decision: June 23, 2022
Revised: July 15, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: September 27, 2022
Processing time: 113 Days and 11.6 Hours
Primary hepatic leiomyosarcoma (PHL) is a rare tumor with a very low incidence of about 0.2%.
A 48-year-old diabetic, hypertensive, and morbidly obese female patient pre
This case report highlights the rare malignant mesenchymal hepatic tumor. To confirm PHL diagnosis, one requires peculiar histopathological findings with ancillary IHC confirmation. Management options include adequate/complete surgical resection followed by chemotherapy and/or radiotherapy.
Core Tip: Primary hepatic leiomyosarcoma is an extremely rare tumor among all primary hepatic malignancies, with approximately 70 cases worldwide, including our case. The rare nature of the disease has precluded its underlying pathogenesis. Clinical manifestations are usually nonspecific, and tumors are generally asymptomatic until they significantly increase in size. They have a relatively poor prognosis and aggressive metastatic potential. The preferred type of treatment is surgical excision, which is sometimes combined with adjuvant chemotherapy and radiotherapy; however, little is known about their effectiveness because of the disease rarity. In-depth studies are needed to shed light on this uncommon clinical entity.
- Citation: Ahmed H, Bari H, Nisar Sheikh U, Basheer MI. Primary hepatic leiomyosarcoma: A case report and literature review . World J Hepatol 2022; 14(9): 1830-1839
- URL: https://www.wjgnet.com/1948-5182/full/v14/i9/1830.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i9.1830
Primary hepatic leiomyosarcoma (PHL) is a rare mesenchymal hepatic tumor whose clinical manifestations are often nonspecific and remain asymptomatic until there is a significant increase in tumor size causing a mass effect. This manuscript presents a case report of a 48-year-old woman with PHL.
A 48-year-old woman presented to the outpatient clinic with complaints of abdominal pain associated with weight loss.
The patient’s symptoms started 2 mo prior with recurrent episodes of abdominal pain without any specific aggravating or relieving factor(s).
She had no documented history of fever, jaundice, liver disease(s), blood transfusion, tattooing, or alcohol abuse.
Her co-morbidities included diabetes, hypertension, and obesity. She had no family history of carcinoma.
On examination, there was a palpable mass in the epigastrium. The rest of the clinical examination was unremarkable, without any signs of disease elsewhere in the body.
Complete blood count, serum biochemical profile, coagulation profile, and carbohydrate antigen 19-9 were normal. Viral serology was nonreactive.
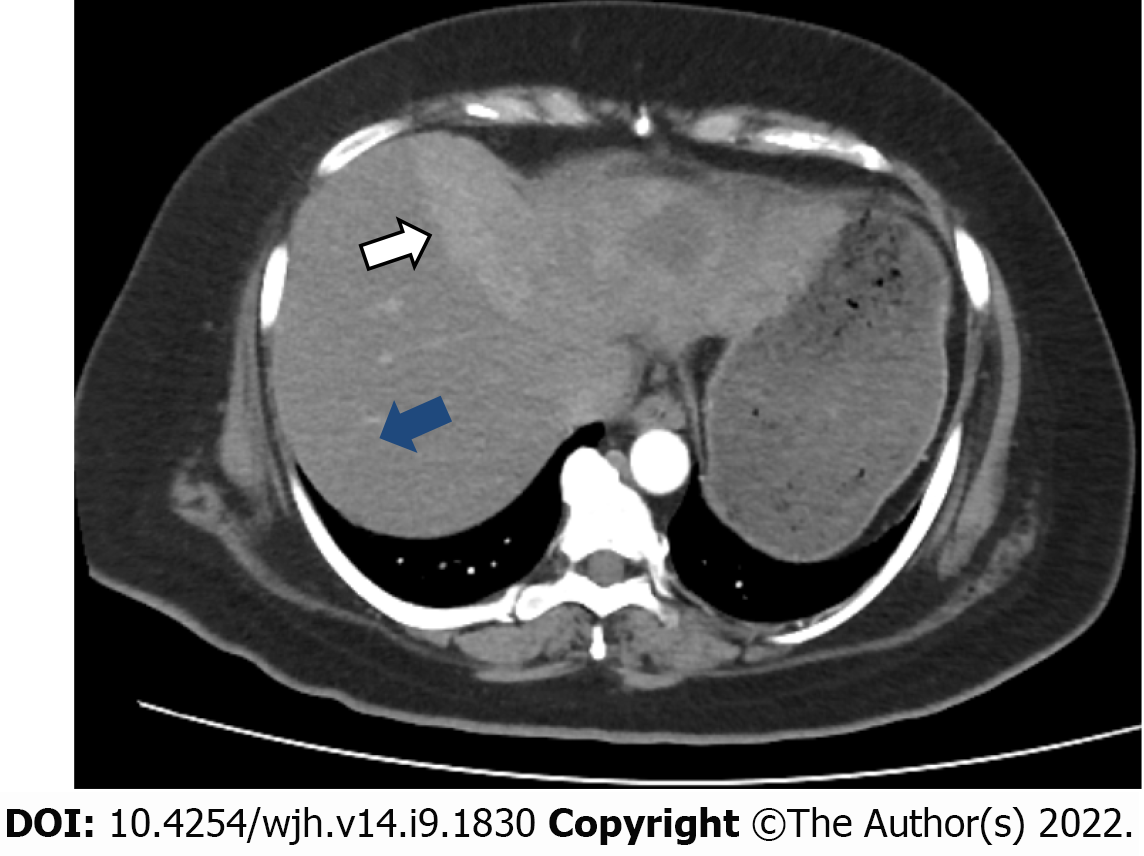
An initial imaging evaluation with a contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed an ill-defined heterogeneous contrast-enhancing and hyper vascular mass in the left lobe of the liver with extension up to the middle hepatic vein and compression effect on the left hepatic vein. The approximate measurement of the mass was 9.9 cm × 7.8 cm with central necrosis in the mass and partial wash-out on delayed images. The tumor encased the left portal vein branch at its point of bifurcation; however, the main portal vein and its right branch were not involved. No signs of hepatic cirrhosis were identified on imaging studies (Figure 1).
Positron emission tomography-CT (PET-CT) scan showed a maximum standardized uptake value (SUV) of the left hepatic mass of 6.4. The remaining liver had a standard baseline hepatic SUV (hepatic parenchymal baseline reference activity was up to 2.96 SUV).
No significant abnormality was seen above or below the diaphragm. In addition, the lungs, gastr
Ultrasound-guided biopsy was done, which showed cores of liver tissue with a proliferation of tumor cells having spindled-shaped nuclei with features of pleomorphism, up to 10 mitosis/10 high-power fields (HPFs), and necrosis. These findings suggested a spindle cell neoplasm raising a differential diagnosis of GI stromal tumor (GIST) and leiomyosarcoma. A panel of Immunohistochemical stains were applied that depicted a desmin-positive/caldesmon-negative/discovered on GIST1-negative (DOG1-)/cluster of differentiation 117-negative (CD117-) profile, thereby supporting the definitive diagnosis of PHL.
The case was discussed for management discussion in GI and hepatobiliary multidisciplinary team (MDT) meetings. According to MDT recommendations, surgical resection of the liver mass was planned, as there was no clinical and radiological evidence of metastatic disease or unknown primary disease elsewhere in the body.
The definitive preoperative diagnosis of the presented case was PHL in the absence of clinical and radiological evidence of metastatic disease or unknown primary disease elsewhere in the body.
A formal extended left hepatectomy was performed. Staging laparoscopy was negative for metastasis, followed by a traditional laparotomy using a Mercedes Benz incision. The total operating time was 540 min with a blood loss of 300 milliliters.
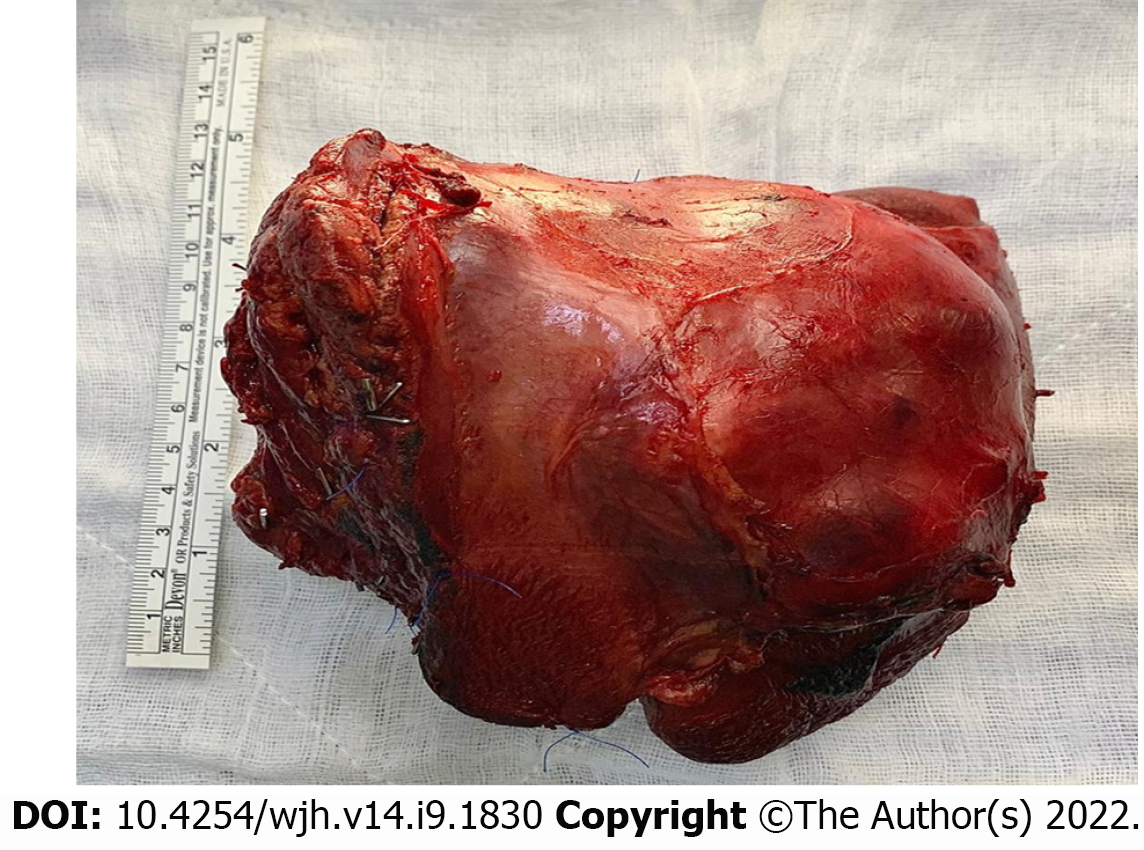
Intraoperatively, the left lobe of the liver was involved by a large tumor extending inferiorly to the caudate lobe (left side only) and superiorly up to the middle hepatic vein (MHV). MHV and its branches were sacrificed. An en bloc resection was achieved with an intact capsule (Figure 2). Resection margins were confirmed to be free of the tumor with the help of intraoperative ultrasound. Enlarged hilar nodes (hepatic artery lymph node) and periportal lymph node were also removed separately. No distant metastasis or aortocaval nodes were found per operatively.
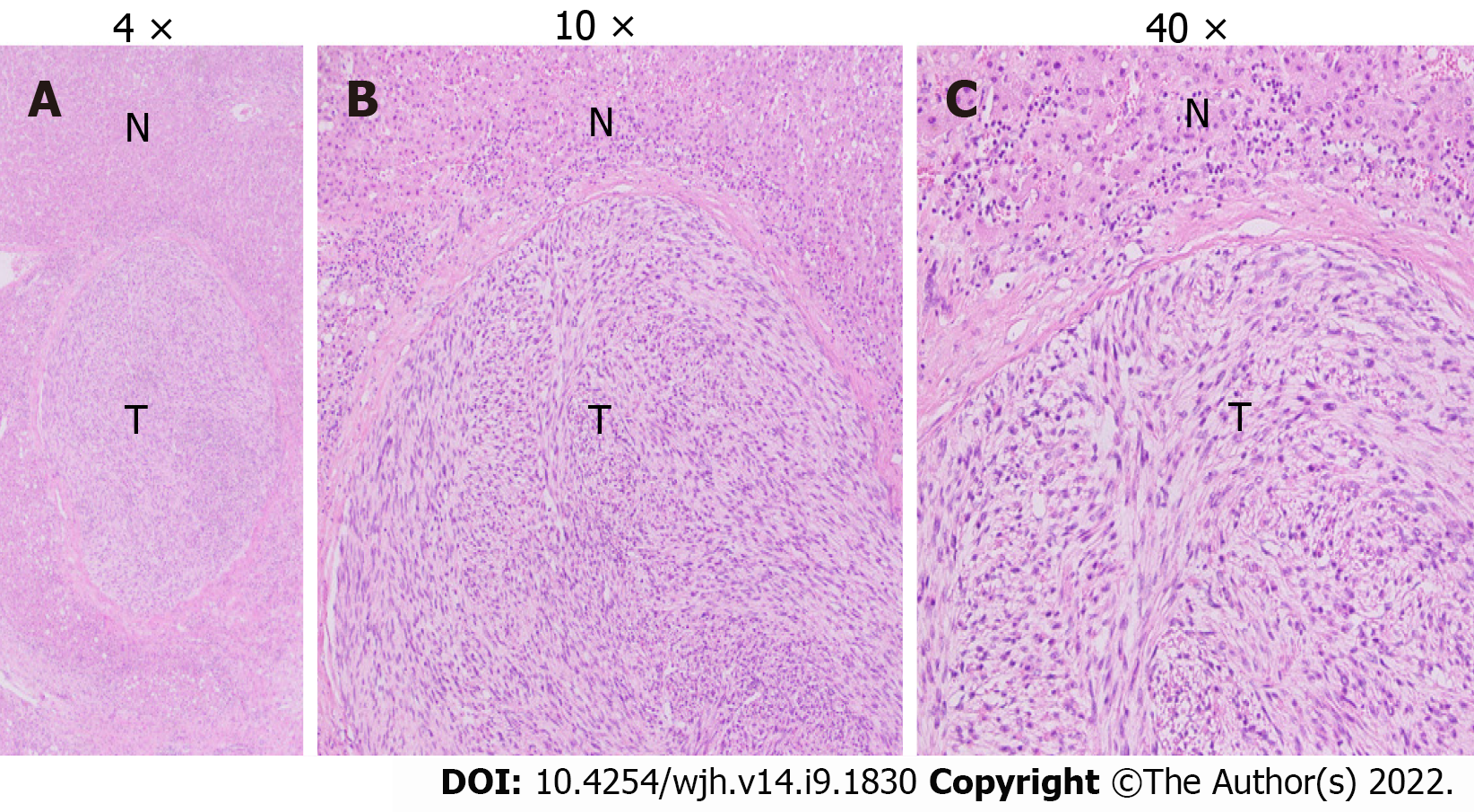
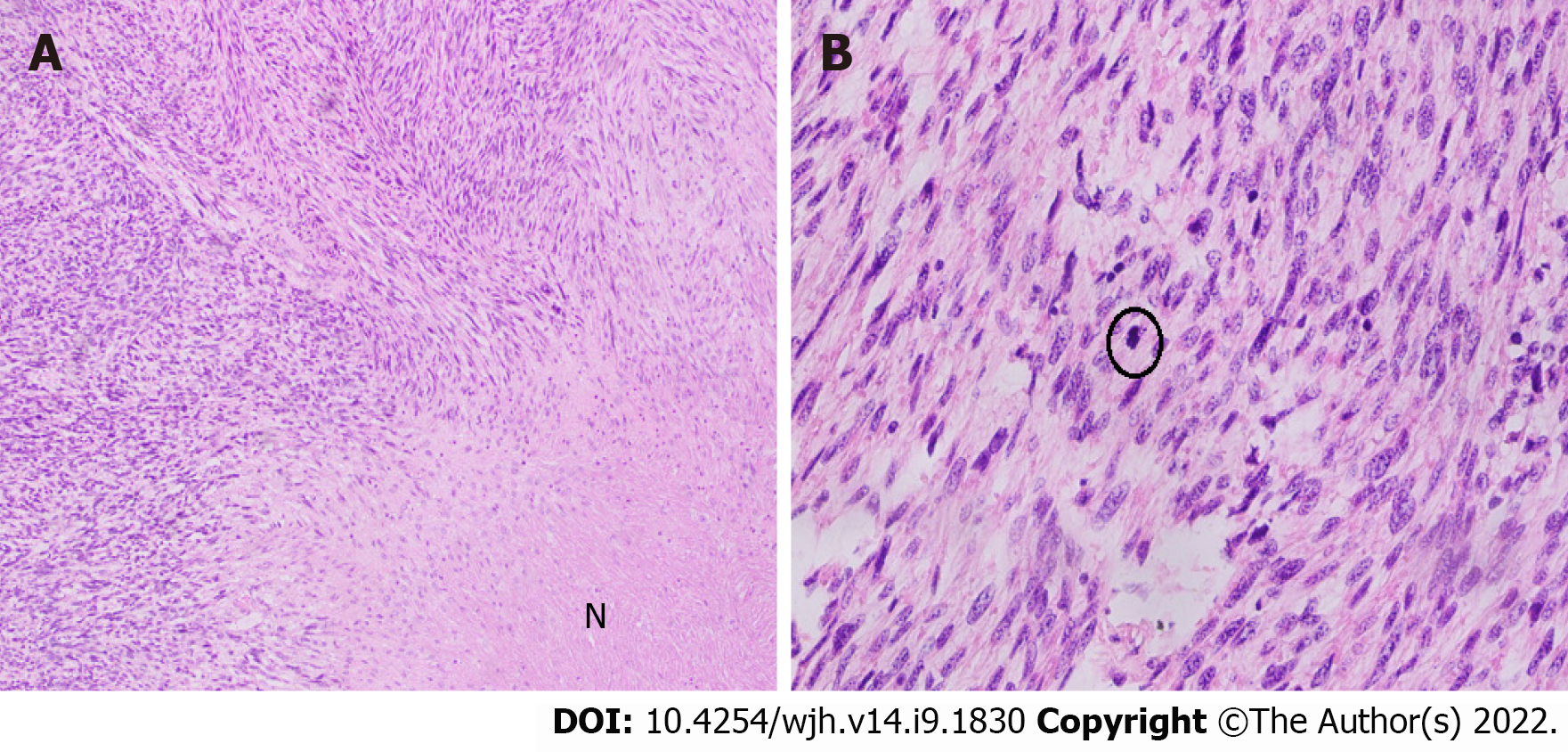
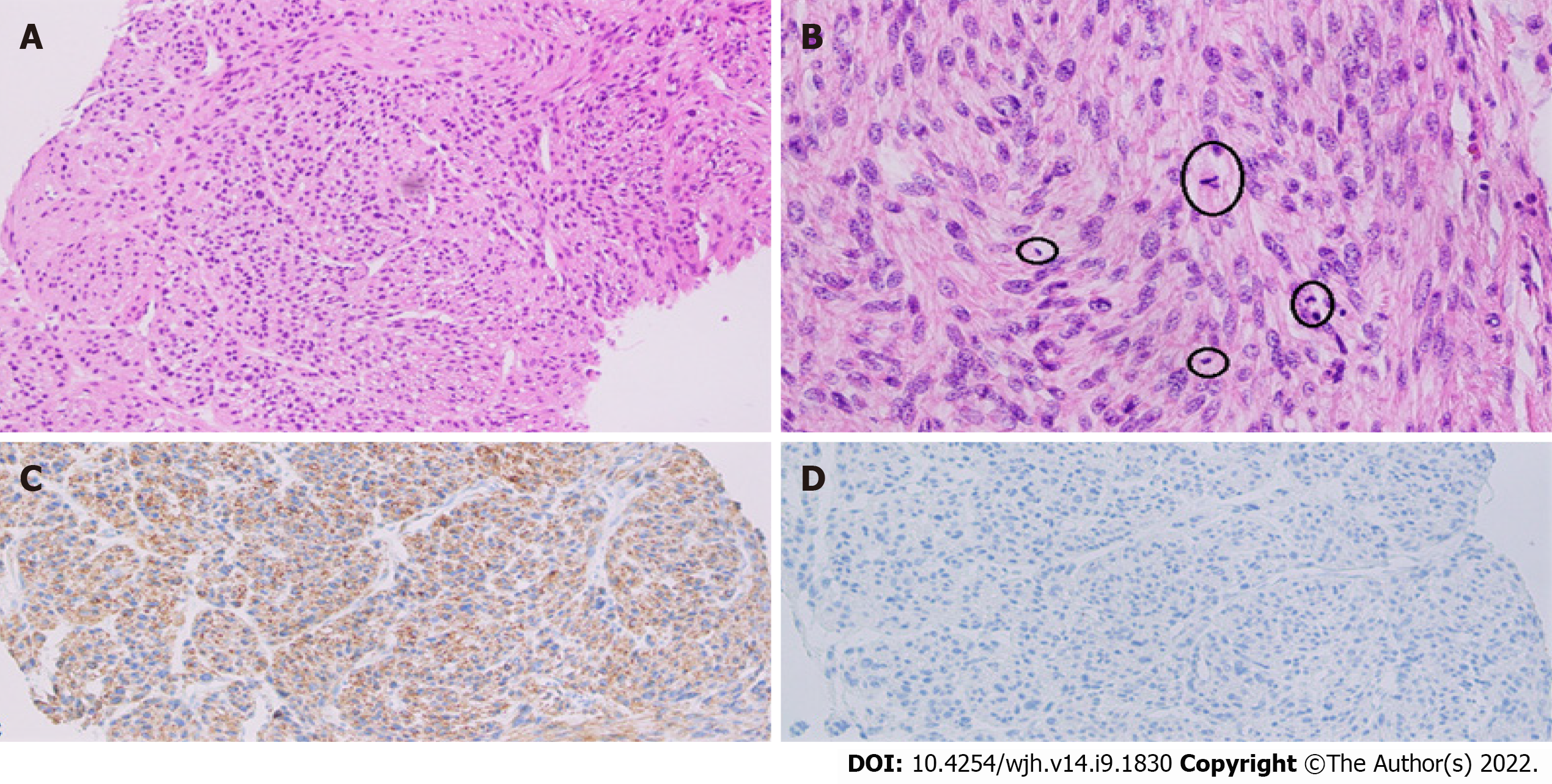
Grossly, the left hepatectomy specimen measured 18 cm × 12.5 cm × 9.5 cm. Serial slicing of the specimen revealed a tumor (10.5 cm × 7.5 cm) with a nodular and whorled white appearance. The hepatic capsule was intact without any evident perforation. The microscopic evaluation revealed a cellular tumor with a fascicular array of tumor cells having spindle-shaped nuclei with a moderate-to-severe degree of cellular pleomorphism and more than 20 mitoses per 10 HPFs. Necrosis was focally seen. Lympho vascular invasion was not identified (Figures 3 and 4). All three regional lymph nodes (hepatic artery, periportal, and gallbladder neck) were negative for metastasis.
Desmin (clone DE-R11, Ventana; Roche Diagnostics, Indianapolis, IN, USA), a marker of smooth muscle differentiation, was positive in tumor cells; and caldesmon (clone E-89; Cell Marque Co., Rocklin, CA, USA) was negative in tumor cells. In addition, CD117 (clone EP-10; Leica, Wetzlar, Germany) and DOG1 (clone SP31; Cell Marque) were also negative in tumor cells, ruling out the possibility of the closest differential diagnosis of GIST (Figure 5).
The postoperative recovery of the patient was uneventful. She was discharged on the 6th postoperative day. The patient remained asymptomatic until the follow-up visit after 6 mo. No short- or long-term postoperative complications were reported. CT and PET-CT scans were performed during this follow-up visit, which showed no evidence of recurrence and metastases. The patient was later presented in the MDT of Sarcoma, where it was decided to keep the patient under bi-annual surveillance, and no adjuvant treatment was recommended.
Sarcomas from the liver are rare, constituting only 1%-2% of all primary hepatic malignant tumors[1,2]. Leiomyosarcoma accounts for 8%-10% of all hepatic sarcomas, whereas other hepatic sarcomas including angiosarcoma, fibrosarcoma, liposarcoma, embryonal sarcoma, malignant fibrous histiocytoma, carcinosarcoma, and epithelioid hemangioendothelioma collectively comprise nearly 70%-80% of hepatic sarcomas[3]. Hepatic leiomyosarcomas are generally metastatic, arising from the GI tract, uterus, retroperitoneum, or lungs, requiring careful staging[2]. To the best of our knowledge, only about 70 cases, including case series, have been published internationally, including our patient (Tables 1 and 2)[1].
| Ref. | Patient age in yr | Sex | Risk factor/s | Management | Follow-up/ outcome |
| Jeong et al[12], 2008 | 49 | F | Wedge resection | Died at 18 mo | |
| Cioffi et al[2], 1996 | 62 | M | Died at 20 mo | ||
| O'Leary et al[27], 1982 | 69 | M | Alive at 24 mo | ||
| Baur et al[28], 1993 | 69 | F | Surgery | Recurrence after 10 yr | |
| Sato et al[29], 2000 | 62 | F | Diagnosis at autopsy | ||
| Iordanidis et al[30] , 2002 | 25 | M | Surgery | Death at three mo | |
| Lee et al[31], 2002 | 64 | F | Right lobectomy + wedge resection of the left lobe | No evidence of disease at 24 mo | |
| Shivathirthan et al[32], 2011 | 78 | M | Chemotherapy, radiation | Death at 10 mo | |
| Muranushi et al[33], 2019 | 62 | M | Gastric gist post resection | Left hepatectomy+ resection of 3 other lesions | No evidence of disease |
| Watanabe et al[34], 1991 | 62 | M | Lobectomy | No evidence of disease at 5 mo | |
| Esposito et al[35], 2020 | 78 | M | Left hepatectomy | No evidence of disease at 18 mo | |
| Yoshikawa et al[36], 1977 | 58 | F | Wedge resection | Death, 11th postoperative day | |
| Bloustein[37], 1978 | 12 | F | Trisegmentectomy, chemotherapy | No evidence of disease at 6 yr | |
| Maki et al[6], 1987 | 86 | F | Surgery | No evidence of disease at 5 mo | |
| Holloway et al[15], 1996 | 63 | M | Conservative | ||
| Soyer et al[19], 1996 | 67 | F | Surgery | ||
| Tsuji et al[10], 2000 | 68 | M | Hepatitis c | Diagnosis at autopsy | |
| Fujita et al[9], 2002 | 33 | F | Prior renal transplant | Right posterior segmentectomy | No evidence of disease at 24 mo |
| Giuliante et al[11], 2009 | 26 | M | Hodgkin's lymphoma | Right lobectomy + wedge resection of segment id | Death at 25 mo |
| Liang et al[21], 2009 | 44 | F | Liver transplant | Death at 34 mo | |
| Shivathirthan et al[32], 2011 | 67 | M | Extended left hepatectomy + partial resection of segment 6 | No evidence of disease at 9 mo | |
| Tsai et al[5], 2013 | Five mo | M | Chemotherapy + partial hepatectomy (segment vi) + adjuvant chemotherapy | No evidence of disease at 48 mo | |
| Feretis et al[24], 2019 | 68 | F | Hepatitis b | Left hepatectomy+ cholecystectomy, chemotherapy, target therapy, redo surgery for recurrence | Recurrence at 18 mo 2nd recurrence at 21 mo died at 37 mo |
| Zhu et al[25], 2019 | 63 | M | Unresectable tumor TACE (2011) | No evidence of disease at 82 mo | |
| Vella et al[1], 2020 | 77 | F | Right hepatectomy | No evidence of disease at 8 mo |
| Ref. | Patient age in yr | Sex | Treatment | Follow up |
| Almogy et al[22], 2004 | 58 | F | Surgery + chemotherapy | Death at 4 mo |
| 63 | F | Surgery + chemotherapy | Death at 12 mo | |
| Watanabe et al[34], 2008 | 63 | F | Diagnosis at autopsy | |
| 49 | F | Diagnosis at autopsy | ||
| Matthaei et al[14], 2009 | 19 | F | Liver transplant | Death at 73 mo |
| 64 | F | Surgery | No evidence of disease 181 mo | |
| 53 | F | Surgery | Death at 21 mo | |
| 55 | M | Surgery | No evidence of disease at 133 mo | |
| 51 | M | Liver transplant | No evidence of disease at 144 mo | |
| 59 | M | Surgery | Death at 45 mo | |
| 63 | F | Surgery | No evidence of disease at 133 mo | |
| Shamseddine et al[23], 2010 | 25 | F | Right lobectomy | Death at 22 mo |
| 39 | M | Extended right lobectomy | Death at 19 d | |
| 30 | M | Right lobectomy + chemotherapy | No evidence of disease at 12 mo | |
| Esposito et al[35] ,2020 | 78 | M | Left hepatectomy | No evidence of disease at 18 mo |
| 53 | M | Right extended hepatectomy | Lung metastasis at seven mo death at 14 mo |
Leiomyosarcoma potentially originates from the smooth muscle cells in the round ligament, intrahepatic blood vessels, and bile ducts. Tumors arising from intrahepatic veins have a worse prognosis because they tend to progress to Budd-Chiari syndrome. The differentiation between benign leiomyoma and low-grade leiomyosarcoma is based on mitotic figures per HPF, although variation occurs according to the site of origin[3,4].
There is no apparent sex predilection with an approximate male-to-female ratio of 1:1 in the literature review of cases reported to date including our patient. Age at the time of presentation is variably reported with a range of 5 mo to 86 years (mean age of 51.3 years)[5,6].
No specific pathological causes of PHL have been identified to date, although the literature review shows an association with multiple etiological factors, including immunosuppression due to acquired immunodeficiency syndrome. Epstein-Barr virus infection is reported in two cases and a previous history of immunosuppression postrenal transplant in 1 case. The other reported associations include hepatitis C virus infection, exposure to thorotrast, and previously treated Hodgkin’s lymphoma[7-11].
PHL usually pursues an indolent course being asymptomatic initially until they enlarge in size causing non-specific symptoms such as abdominal pain or discomfort, a palpable mass, fever, jaundice, anorexia, nausea or vomiting, and weight loss. The literature review revealed a single patient presenting as an emergency due to acute intraabdominal hemorrhage following tumor rupture. There is usually a single mass, although there are cases with multiple masses. Tumor size varies significantly ranging from 0.6 cm to 30 cm in the largest dimension (mean and median diameters of 10.3 cm and 9.1 cm, respectively). In our case, the tumor size was 10 cm in its largest dimension. The distribution of PHL within the liver segments and lobes also differs, with 2/3rd cases involving the right lobe and 1/3rd cases involving the left lobe. The case presented here involved the left lobe of the liver[6,11-16].
Histological examination usually shows intersecting bundles of spindle-shaped cells with hype
Ultrasonography usually shows hypoechoic or heterogeneous echogenic mass. CT findings generally describe a hypodense and sometimes heterogeneous mass with inhomogeneous and often peripheral enhancement after intravenous contrast administration, which may show regions of cystic degeneration, demonstrated in our case[17]. Ferrozzi et al[18] reported that hemorrhagic necrosis, along with cystic degeneration and necrosis, are likely to be secondary to tumor growth. Magnetic resonance imaging characteristically displays homogeneous or heterogeneous hypointense T1-weighted images and hyperintense T2-weighted images[19]. PET-CT scan SUV max can be correlated with the tumor size, tumor-node-metastasis staging, and histology subtype[20]. In our case, the SUV max of the primary tumor was 6.4, with a baseline hepatic parenchymal SUV of 2.96.
Therapeutic options vary depending upon the tumor size and/or stage on initial presentation. Hepatic resection (wedge resection, segmentectomy, lobectomy, or extended hepatectomy with the intention of R0 resection) remains the only potentially curative surgical option for non-metastatic including our case. However, 4 patients with tumors confined within the liver had liver transplantation[14,21].
Some authors have also reported that adjuvant chemotherapy consisted of various drugs, including doxorubicin and ifosfamide, which help to attain prolonged survival after complete resection. In addition, three cases have been treated with radiotherapy as part of a combined adjuvant treatment along with chemotherapy[5,22-24].
Transarterial chemoembolization and transarterial infusion of epirubicin and carboplatin were also reported in individual cases as treatment modalities for PHL[25].
The median survival is 37.5 mo with a 5-year survival rate of about 40%; two large case series reported 67% disease-specific survival at 5 years after R0 resection. Matthaei et al[14] in a case series, reported more than 10 years of survival after hepatectomy. Chi et al reported a median overall survival of 19 mo with 1-, 2-, and 5-year survival rates of 61.2%, 41.1%, and 14.5% respectively[26].
Age is another crucial predictive factor for prognosis, with patients below 50 years achieving better survival. Currently, no effective therapeutic options have been reported for unresectable PHL. Fujita et al[9] reported a patient with metastatic leiomyosarcoma surviving 3 mo after diagnosis, who received only palliative and conservative therapy.
In conclusion, PHL is a rare malignant disease with often delayed presentation, relatively poor prognosis, and aggressive metastatic potential. The most preferred management options include surgical excision combined with chemotherapy and radiotherapy. However, very little is known about the efficacy of available therapeutic options because of the rarity of the disease. Therefore, in-depth studies are required to assess its causative, prognostic, and predictive parameters.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao L, China; Wang P, China S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Vella S, Cortis K, Pisani D, Pocock J, Aldrighetti L. Case of primary hepatic leiomyosarcoma successfully treated with laparoscopic right hepatectomy. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Cioffi U, Quattrone P, De Simone M, Bonavina L, Segalin A, Masini T, Montorsi M. Primary multiple epithelioid leiomyosarcoma of the liver. Hepatogastroenterology. 1996;43:1603-1605. [PubMed] |
| 3. | Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D'Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH, Dematteo RP. Management of primary liver sarcomas. Cancer. 2007;109:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Mitra S, Rathi S, Debi U, Dhiman RK, Das A. Primary Hepatic Leiomyosarcoma: Histopathologist's Perspective of a Rare Case. J Clin Exp Hepatol. 2018;8:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Tsai PS, Yeh TC, Shih SL. Primary hepatic leiomyosarcoma in a 5-mo-old female infant. Acta Radiol Short Rep. 2013;2:2047981613498722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Maki HS, Hubert BC, Sajjad SM, Kirchner JP, Kuehner ME. Primary hepatic leiomyosarcoma. Arch Surg. 1987;122:1193-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Chelimilla H, Badipatla K, Ihimoyan A, Niazi M. A rare occurrence of primary hepatic leiomyosarcoma associated with epstein barr virus infection in an AIDS patient. Case Rep Gastrointest Med. 2013;2013:691862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Metta H, Corti M, Trione N, Masini D, Monestes J, Rizzolo M, Carballido M. Primary hepatic leiomyosarcoma--a rare neoplasm in an adult patient with AIDS: second case report and literature review. J Gastrointest Cancer. 2014;45 Suppl 1:36-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Fujita H, Kiriyama M, Kawamura T, Ii T, Takegawa S, Dohba S, Kojima Y, Yoshimura M, Kobayashi A, Ozaki S, Watanabe K. Primary hepatic leiomyosarcoma in a woman after renal transplantation: report of a case. Surg Today. 2002;32:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Tsuji M, Takenaka R, Kashihara T, Hadama T, Terada N, Mori H. Primary hepatic leiomyosarcoma in a patient with hepatitis C virus-related liver cirrhosis. Pathol Int. 2000;50:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Giuliante F, Sarno G, Ardito F, Pierconti F. Primary hepatic leiomyosarcoma in a young man after Hodgkin's disease: diagnostic pitfalls and therapeutic challenge. Tumori. 2009;95:374-377. [PubMed] |
| 12. | Jeong TY, Kim YS, Park KJ, Lee JS, Huh JG, Ryu SH, Lee JH, Moon JS. [A case of primary leiomyosarcoma of the liver presenting with acute bleeding]. Korean J Gastroenterol. 2008;51:194-198. [PubMed] |
| 13. | Majumder S, Dedania B, Rezaizadeh H, Joyal T, Einstein M. Tumor rupture as the initial manifestation of primary hepatic leiomyosarcoma. Gastrointest Cancer Res. 2014;7:33-34. [PubMed] |
| 14. | Matthaei H, Krieg A, Schmelzle M, Boelke E, Poremba C, Rogiers X, Knoefel WT, Peiper M. Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg. 2009;144:339-44; discussion 344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Holloway H, Walsh CB, Thomas R, Fielding J. Primary hepatic leiomyosarcoma. J Clin Gastroenterol. 1996;23:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Lin YH, Lin CC, Concejero AM, Yong CC, Kuo FY, Wang CC. Surgical experience of adult primary hepatic sarcomas. World J Surg Oncol. 2015;13:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Yu RS, Chen Y, Jiang B, Wang LH, Xu XF. Primary hepatic sarcomas: CT findings. Eur Radiol. 2008;18:2196-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Ferrozzi F, Bova D, Zangrandi A, Garlaschi G. Primary liver leiomyosarcoma: CT appearance. Abdom Imaging. 1996;21:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Soyer P, Blanc F, Vissuzaine C, Marmuse JP, Menu Y. Primary leiomyosarcoma of the liver MR findings. Clin Imaging. 1996;20:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Yoh T, Seo S, Morino K, Fuji H, Ikeno Y, Ishii T, Taura K, Nakamoto Y, Higashi T, Kaido T, Uemoto S. Reappraisal of Prognostic Impact of Tumor SUVmax by 18F-FDG-PET/CT in Intrahepatic Cholangiocarcinoma. World J Surg. 2019;43:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Liang X, Xiao-Min S, Jiang-Ping X, Jie-Yu Y, Xiao-Jun Z, Zhi-Ren F, Guo-Shan D, Rui-Dong L. Liver transplantation for primary hepatic leiomyosarcoma: a case report and review of the literatures. Med Oncol. 2010;27:1269-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Almogy G, Lieberman S, Gips M, Pappo O, Edden Y, Jurim O, Simon Slasky B, Uzieli B, Eid A. Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol. 2004;30:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Shamseddine A, Faraj W, Mukherji D, El Majzoub N, Khalife M, Soubra A, Shamseddine A. Unusually young age distribution of primary hepatic leiomyosarcoma: case series and review of the adult literature. World J Surg Oncol. 2010;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Feretis T, Kostakis ID, Damaskos C, Garmpis N, Mantas D, Nonni A, Kouraklis G, Dimitroulis D. Primary Hepatic Leiomyosarcoma: a Case Report and Review of the Literature. Acta Medica (Hradec Kralove). 2018;61:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Zhu KL, Cai XJ. Primary hepatic leiomyosarcoma successfully treated by transcatheter arterial chemoembolization: A case report. World J Clin Cases. 2019;7:525-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Chi M, Dudek AZ, Wind KP. Primary hepatic leiomyosarcoma in adults: analysis of prognostic factors. Onkologie. 2012;35:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | O'Leary MR, Hill RB, Levine RA. Peritoneoscopic diagnosis of primary leiomyosarcoma of liver. Hum Pathol. 1982;13:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Baur M, Pötzi R, Lochs H, Neuhold N, Walgram M, Gangl A. Primary leiomyosarcoma of the liver--a case report. Z Gastroenterol. 1993;31:20-23. [PubMed] |
| 29. | Sato S, Hosoi K, Kagawa T. A primary leiomyosarcoma of the liver: an autopsy report. Elec J Pathol Histol. 2000;6:8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Iordanidis F, Hytiroglou P, Drevelegas A, Kodonas F, Ioannidis I, Nenopoulou H, Papadimitriou CS. A 25-year-old man with a large hepatic tumor and multiple nodular lesions. Semin Liver Dis. 2002;22:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Lee HJ, Lee JL, Choi WH. A case of primary myxoid leiomyosarcoma of the liver. Korean J Intern Med. 2002;17:278-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Shivathirthan N, Kita J, Iso Y, Hachiya H, Kyunghwa P, Sawada T, Kubota K. Primary hepatic leiomyosarcoma: Case report and literature review. World J Gastrointest Oncol. 2011;3:148-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 33. | Muranushi R, Hoshino K, Hagiwara K, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A, Kubo N, Araki K, Harimoto N, Ikota H, Shibuya K, Miyazaki M, Shirabe K. Hepatic pleomorphic leiomyosarcoma after surgery for gastric gastrointestinal stromal tumor: a case report. Surg Case Rep. 2019;5:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Watanabe K, Saito A, Wakabayashi H, Kawaguchi T, Suzuki T. Two autopsy cases of primary leiomyosarcoma of the liver. Superiority of muscle-specific actin immunoreactivity in diagnosis. Acta Pathol Jpn. 1991;41:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Esposito F, Lim C, Baranes L, Salloum C, Feray C, Calderaro J, Azoulay D. Primary leiomyosarcoma of the liver: Two new cases and a systematic review. Ann Hepatobiliary Pancreat Surg. 2020;24:63-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Yoshikawa K, Satake K, Kinoshita H, Umeyama K, Hojo K. Primary leiomyosarcoma of the liver. Clin Oncol. 1977;3:197-202. [PubMed] |
| 37. | Bloustein PA. Hepatic leiomyosarcoma: ultrastructural study and review of the differential diagnosis. Hum Pathol. 1978;9:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |