Published online Sep 27, 2022. doi: 10.4254/wjh.v14.i9.1790
Peer-review started: April 7, 2022
First decision: May 29, 2022
Revised: June 24, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: September 27, 2022
Processing time: 168 Days and 20.8 Hours
Although stereotactic body radiation therapy (SBRT) is increasingly used, its application has not yet been regulated by the main international guidelines, leaving the decision to multidisciplinary teams.
To assess magnetic resonance imaging (MRI) features of hepatocellular carcinoma (HCC) treated with SBRT, highlighting the efficacy of the treatment and the main aspects of the lesion before and after the procedure.
As part of a retrospective study, 49 patients who underwent SBRT for HCC between January 2013 and November 2019 were recruited. Each patient under
Signal hyperintensity in the T2-weighted sequences showed a statistically significant reduction in the number of lesions at the post-SBRT first control (P = 0.0006). Signal hyperintensity in diffusion-weighted imaging-weighted sequences was decreased at MRI first control (P < 0.0001). A statistically significant increase of apparent diffusion coefficient values from a median of 1.01 to 1.38 at the first post-control was found (P < 0.0001). Capsular retraction was increased at the late evaluation (P = 0.006). Band-like signal hypointensity in the hepatobiliary phase was present in 94% at the late control (P = 0.006). The study of the risk of outfield progression vs infield pro
The efficacy of SBRT should be evaluated not in the first 6 mo, but at least 9 mo post-SBRT, when infield progression persists at very low rates while the risk of outfield progression increases significantly.
Core Tip: As part of a retrospective study, 49 patients who underwent stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma between January 2013 and November 2019 were recruited. Each patient underwent a pre-treatment magnetic resonance imaging examination with a hepatospecific contrast agent and a similar follow-up examination within 6 mo of therapy. In addition, 22 patients underwent a second follow-up examination after the first 6 mo. The study results show that the efficacy of SBRT should be evaluated not in the first 6 mo, but at least 9 mo post-SBRT, when infield progression persists at very low rates while the risk of outfield progression increases significantly.
- Citation: Serafini A, Ruggeri V, Inchingolo R, Gatti M, Guarneri A, Maino C, Ippolito D, Grazioli L, Ricardi U, Faletti R. Liver magnetic resonance imaging for evaluation of response to treatment after stereotactic body radiation therapy of hepatocellular carcinoma. World J Hepatol 2022; 14(9): 1790-1803
- URL: https://www.wjgnet.com/1948-5182/full/v14/i9/1790.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i9.1790
Although stereotactic body radiation therapy (SBRT) is increasingly used, its application has not yet been regulated by the main international guidelines, leaving the decision to multidisciplinary teams. Lack of diffusion and standardization of treatment indications makes the radiological definition of outcome particularly complex and not completely concordant with the main therapy evaluation systems (modified Response Evaluation Criteria in Solid Tumors [mRECIST] and European Association for the Study of the Liver [EASL]). By analysing the magnetic resonance imaging (MRI) semeiological characteristics of the hepatocellular carcinoma (HCC) lesions treated by SBRT and the remaining liver pare
In addition, search of possible correlations between MRI findings and clinical, laboratory, and radiotherapy data could help to prevent radio-induced liver damage and to implement customized treatment planning. This has made it possible to use this treatment in different stages of HCC, both in patients with early-stage unifocal disease and in patients not eligible for other loco-regional therapies and for palliative purposes[2,3].
From a technical point of view, the number of target lesions as well as their location within the liver are potential limitations in treatment planning and dose distribution. A minimum distance of at least 5 mm of the target HCCs from adjacent hollow viscera is recommended, otherwise the dose has to be reduced to match tolerance of neighboring organs.
Semeiotic characteristics of SBRT-treated lesions differ from the imaging of other locoregional therapies: Whereas the latter results in immediate devascularization of tumor, radiotherapy leads to histological changes in the lesion and surrounding parenchyma that are gradual over time. Reflecting evolution of histological changes, an acute, subacute, or chronic stage can also be distinguished[4-10].
The main aim of the study was to analyse MRI features of HCC lesions treated by SBRT and the remaining liver parenchyma, to monitor how these properties evolve over time and how these aspects may modify subsequent diagnostic course of therapy.
A retrospective observational study was conducted in 49 patients (mean age 64.44 years, range 48.71-84.51), 22 females and 27 males, undergoing radiotherapy between January 2013 and November 2019.
In 29 (59%) patients, SBRT was chosen as the first treatment option, and in 20 (41%) patients, it was in combination with previous locoregional treatments on the same lesion. Six (12%) of these patients subsequently underwent liver transplantation (bridge therapy).
Sixty-one lesions were treated; among those, 42 were newly diagnosed HCCs and 19 were focal lesions that had already undergone previous treatment and were therefore attributable to persistent or recurrent disease.
In the period between January 2013 and June 2020, each patient underwent an MRI examination with a hepatospecific contrast agent prior to treatment, and a similar follow-up examination within 6 mo of therapy (mean time 4 mo). In addition, 22 patients (a total of 36 lesions) underwent a second follow-up after the first 6 mo (mean time 9 mo).
All MRI examinations were performed with 1.5 T equipment (Philips Achieva), with a hepatospecific contrast agent (Primovist 0.25 mmol/mL, Bayer Schering Pharma, Berlin, Germany) infused at a dose of 0.1 mL/kg with a flow rate of 2 mL/sec.
The acquired images were re-evaluated using the PACS system (Synapse PACS, Tokyo, Japan) by a radiologist with 15 years of experience in abdominal MRI.
For each lesion, the following characteristics were analysed and catalogued: Newly diagnosed HCC or previously treated HCC (persistence or recurrence of disease); Liver segment; Centroparenchymal or subcapsular location (distance from glissonian surface ≤ 10 mm); Diameter of hypervascularised tissue in the arterial phase; Diameter of the lesion in basal (T1-weighted in-phase and out-of-phase) sequences; Diameter in hepatobiliary excretory phase sequences; Average lesion diameter; Presence or absence of signal hyperintensity in the T2-weighted sequences, in relation to the surrounding parenchyma; Presence or absence of signal enhancement on diffusion-weighted imaging (DWI); and apparent diffusion coefficient (ADC) value (obtained by manually positioned region of interest [ROI]).
All lesions that did not show an increase in size or contrast-enhancement intensity in the arterial phase were considered free of progressing disease, and therefore treatment was assessed as effective. Tumor staging was performed according to the BCLC system. Pre-treatment Child-Pugh score, pretreatment ALBI, occurrence of RILD, Child-Pugh score variation after treatment, ALBI change after treatment, total liver volume, and planning target volume (PTV) were also assessed.
The following features were analysed in the post-treatment MRI examinations: Features analysed on pre-treatment MRI examination; Presence or absence of infield progression: Signs of disease progression within the treated field (increase in size and/or increase in intensity of arterial contrast-enhancement); Presence or absence of outfield progression: Signs of disease progression outside the radio-treated field according to mRECIST criteria; Presence or absence of ring-like enhancement (altered vascularity of the parenchyma adjacent to the treated lesion); Presence or absence of signal hyperintensity in T2-weighted sequences in the perilesional parenchyma; Presence or absence of capsular retraction; Presence or absence of "band" signal hypointensity in T1-weighted gradient echo fat saturated sequences obtained in the perilesional parenchyma; T1-weighted sequences obtained during hepatobiliary excretion of irradiated parenchyma; Calculation of the volume of "band" area in T1-weighted gradient echo fat saturated sequences acquired during hepatobiliary excretion by manual segmentation using polygonal ROIs, using the OsiriX DICOM Viewer software. A retrospective analysis was performed comparing pre-treatment MRI characteristics with subsequent follow-ups.
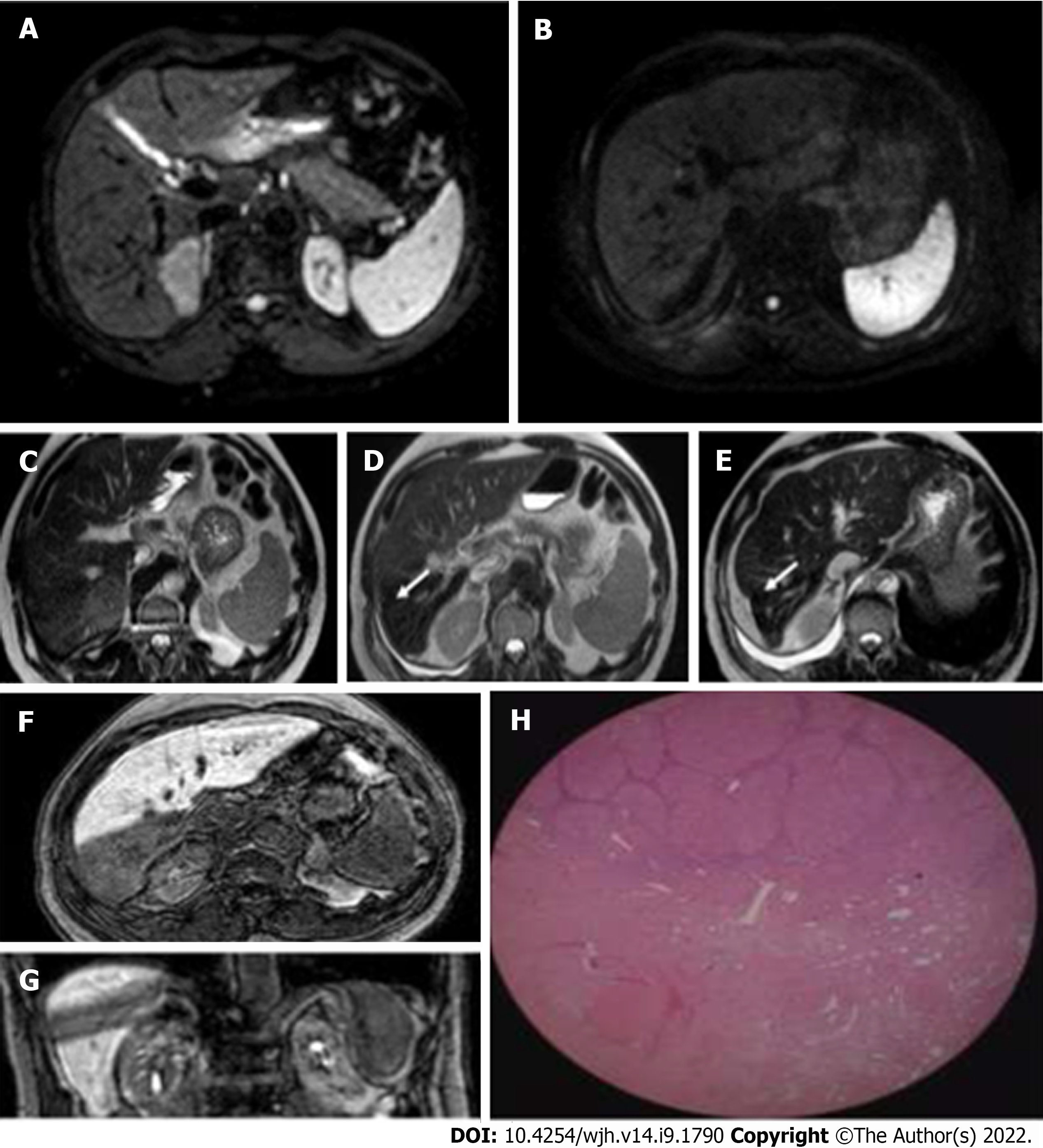
Almost 55.7% of the treated lesions had a subcapsular location; the distribution in the different hepatic segments was as follows: 3 in S1, 4 in S2, 2 in S3, 8 in S4, 10 in S5, 5 in S6, 9 in S7, and 20 in S8 (Figures 1-5).
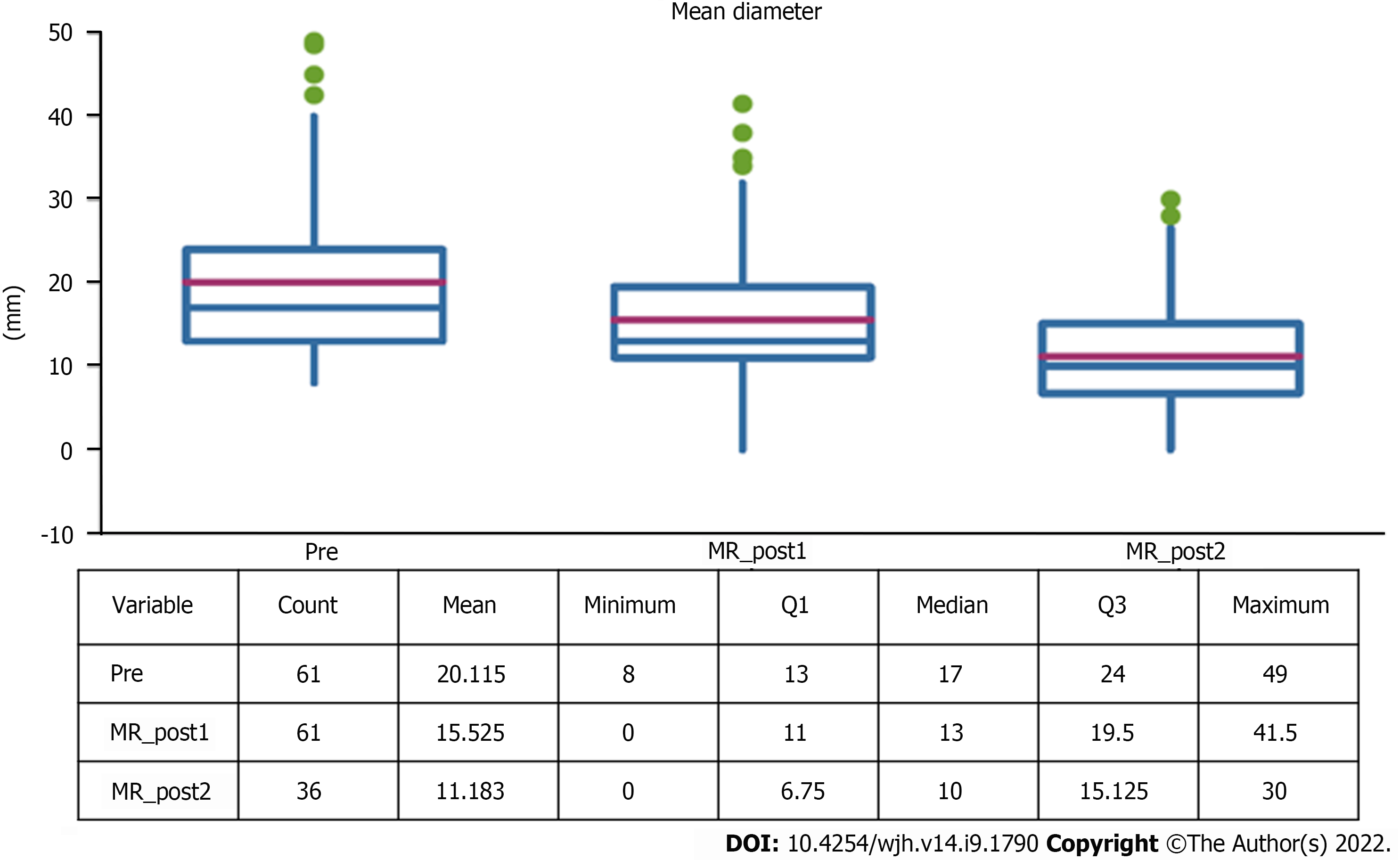
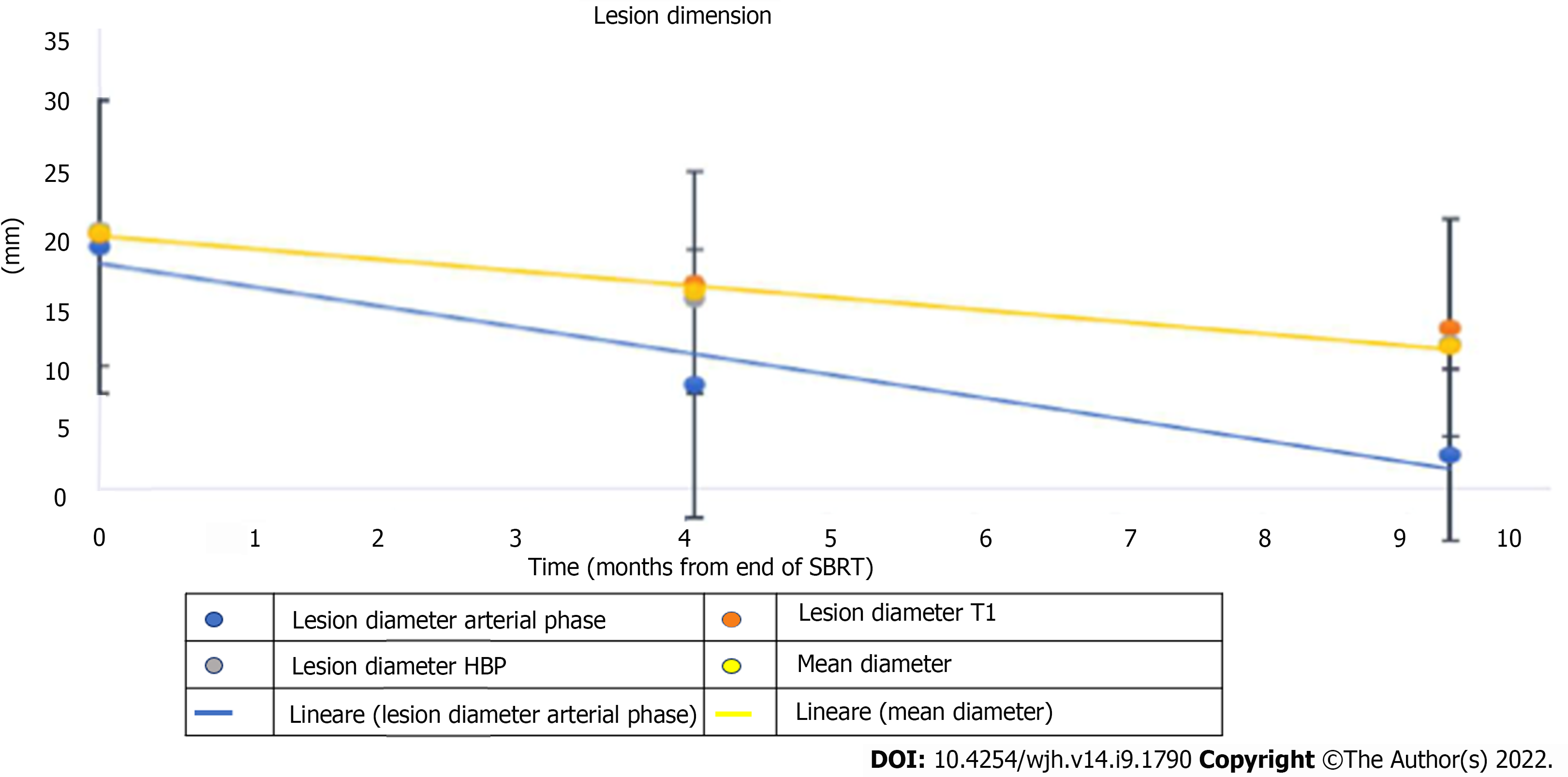
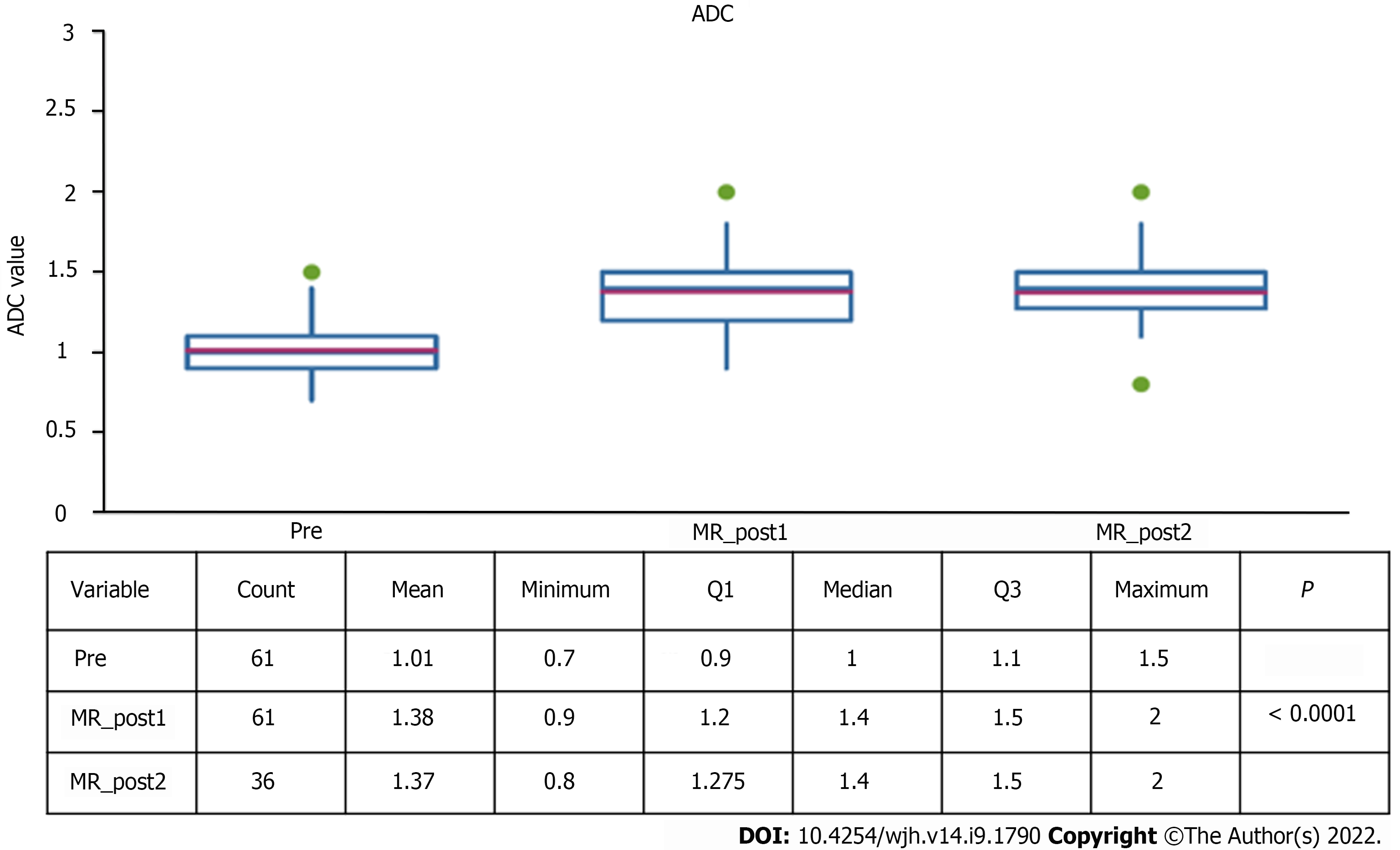
The average diameter of the lesions was 17 mm (SD 13-24mm), a value that was significantly reduced both at the first control (10 mm, SD 11-20mm) and at the second follow-up (10 mm, SD 7-15mm) (Table 1).
| End of SBRT | 1° MRI FU | (Pre vs 1°FU) | 2° MRI FU | (1° vs 2°FU) | |
| T (mo) | 0 | 4.1 (3.1- 6.7) | < 0.0001 | 9.3 (6.2-12.3) | |
| Arterious D | 17 (12-24) | 0 (0-16) | < 0.0001 | 0 (0-0) | < 0.005 |
| T1-weighted D | 17 (12-23) | 13 (11-21) | < 0.0001 | 11 (8-17) | < 0.005 |
| HBP D | 17 (13-24) | 13 (10-19) | < 0.0001 | 10 (7-16) | < 0.005 |
| Average D | 17 (13-24) | 13 (11-20) | 10 (7-15) | < 0.009 | |
| ADC | 1.0 | 1.4 | < 0.0006 | 1.4 | 0.19 |
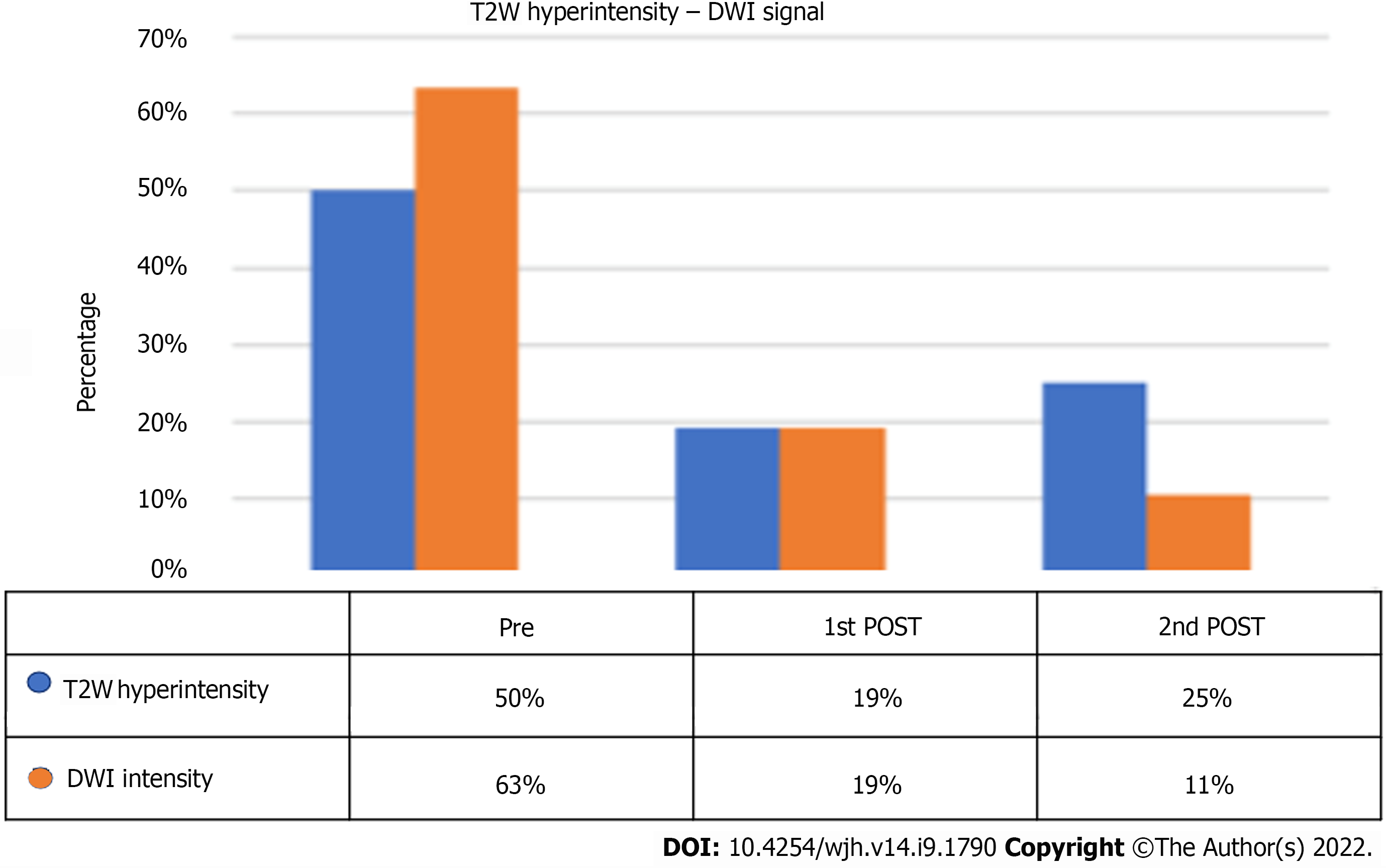
| T2-weighted hyperintensity | 38 (62%) | 18 (30%) | < 0.0001 | 9 (25%) | 0.81 |
| DWI | 41 (68%) | 11 (18%) | 3 (9%) | 0.34 | |
| Ring enhancement | 50 (82%) | 25 (69%) | 0.24 | ||
| Perilesional T2 hyp | 51 (84%) | 27 (75%) | 0.44 | ||
| HBP band-like | 58 (95%) | 34 (94%) | 0.74 | ||
| Capsular retraction | 20 (33%) | 23 (64%) | 0.006 | ||
| Infield progression | 3 (5%) | 1 (3%) | |||
| Outfield progression | 11 (18%) | 10 (28%) |
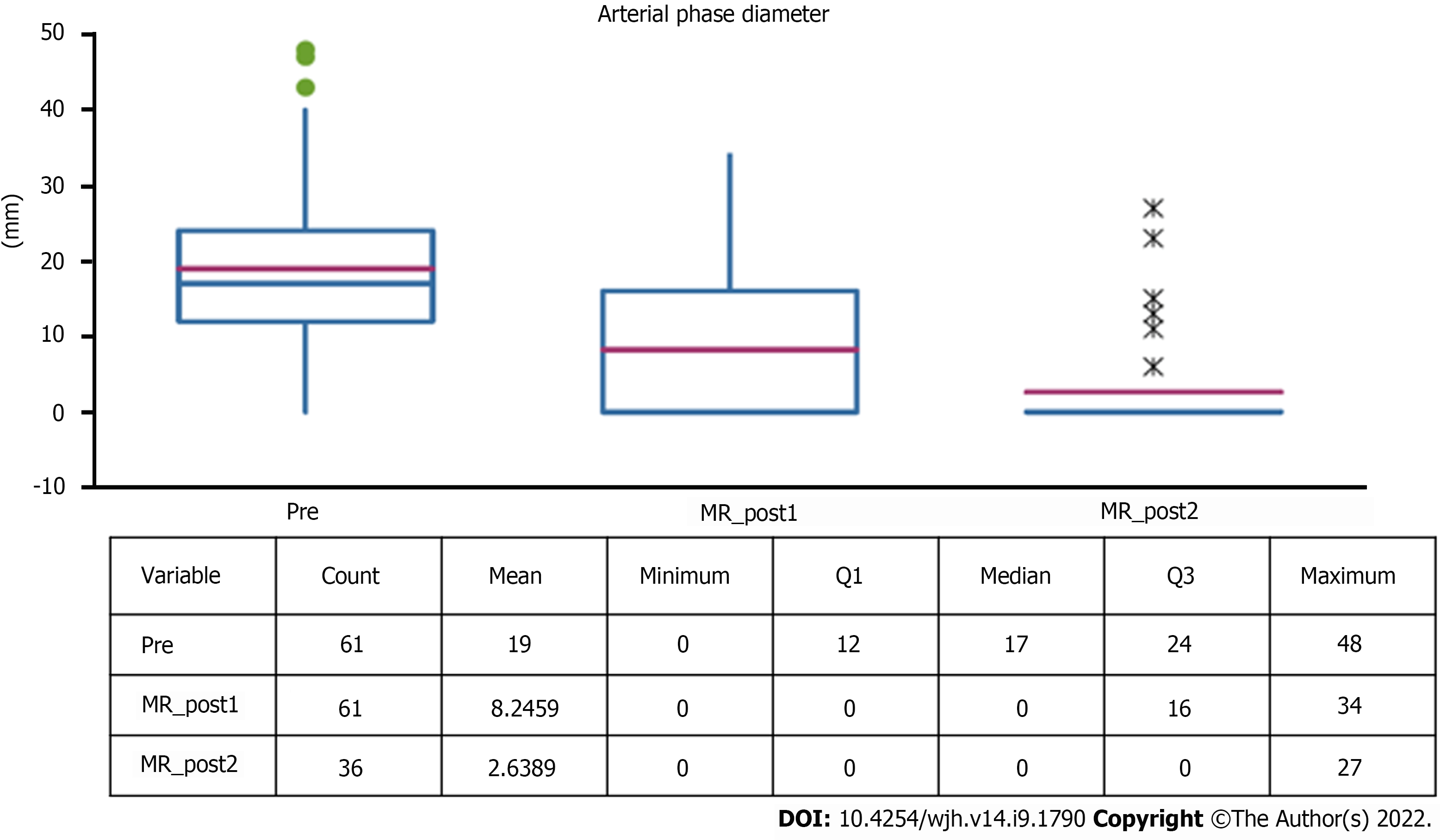
Five (8.2%) out of 61 lesions were hypovascular HCC. The remaining lesions showed typical post-contrast features with a mean diameter of 17 mm (range 12-24 mm), with a statistically significant reduction at follow-up (Figure 6).
Both the diameter during the hepatobiliary phase and in basal T1 weighted sequences underwent a size reduction at both controls (Figure 7).
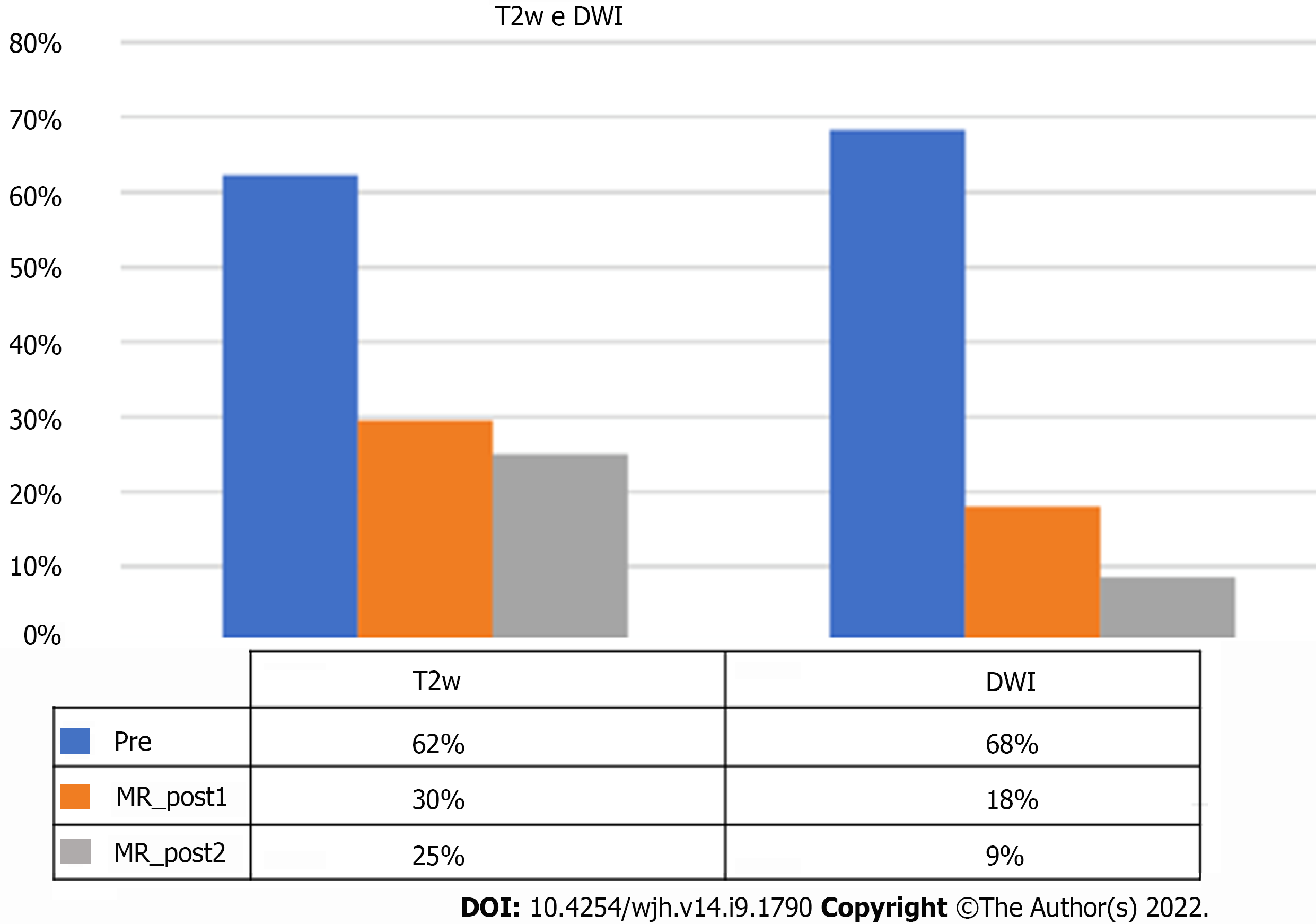
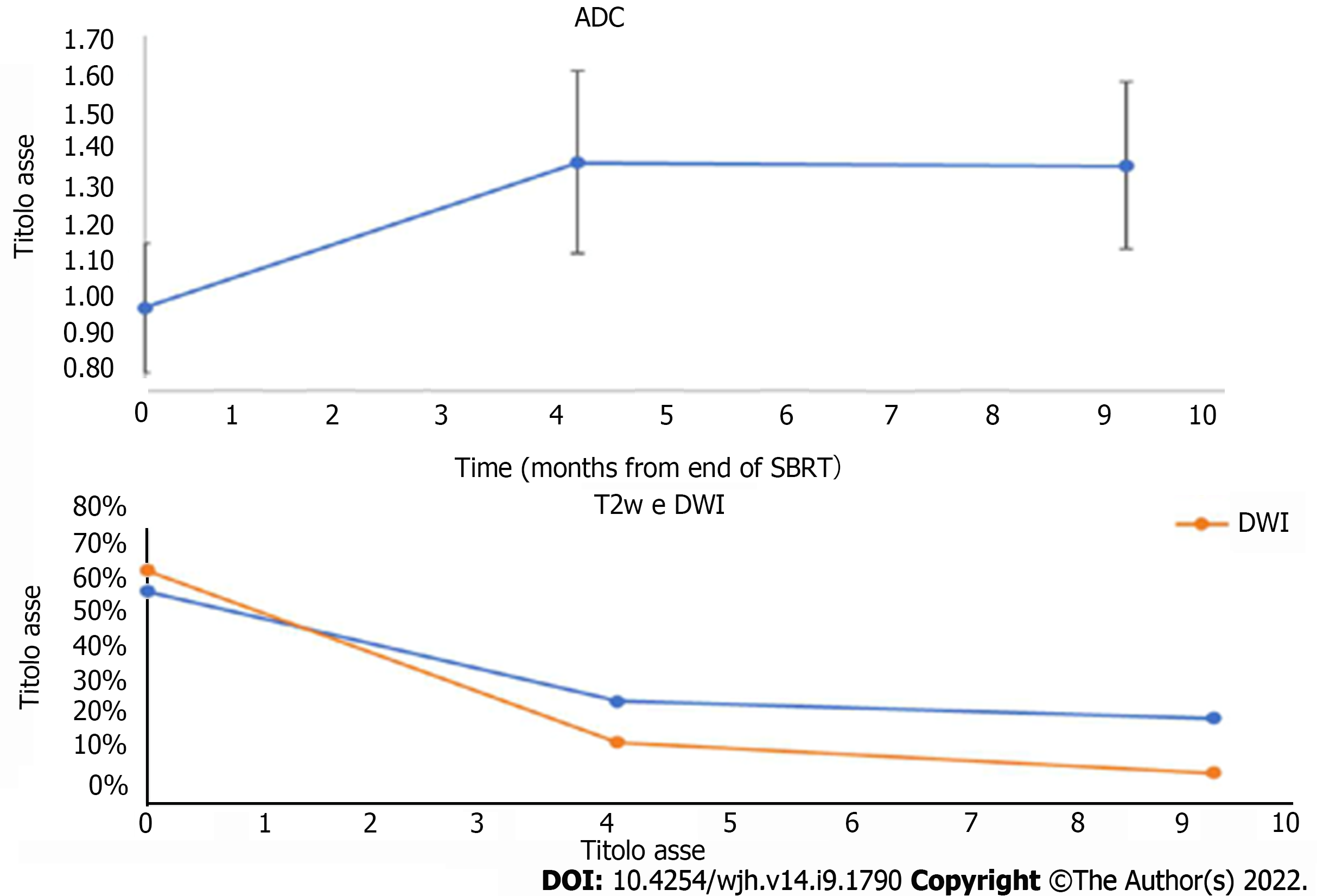
On pre-treatment MRI, signal hyperintensity in the T2-weighted sequences was found in 62% of lesions, but it was only 30% at the post-SBRT first control, with a statistically significant reduction in the number of lesions (P = 0.0006).
Signal hyperintensity in DWI-weighted sequences was found in 68% of lesions and in only 18% at MRI first control (P < 0.0001). For both T2 and DWI variations, no statistically significant changes were found between first and second MRI controls (Figure 8).
These variations were associated with a statistically significant increase of ADC values, which increased from a median of 1.01 at the pre-treatment examination to 1.38 at the first post-control (P < 0.0001).
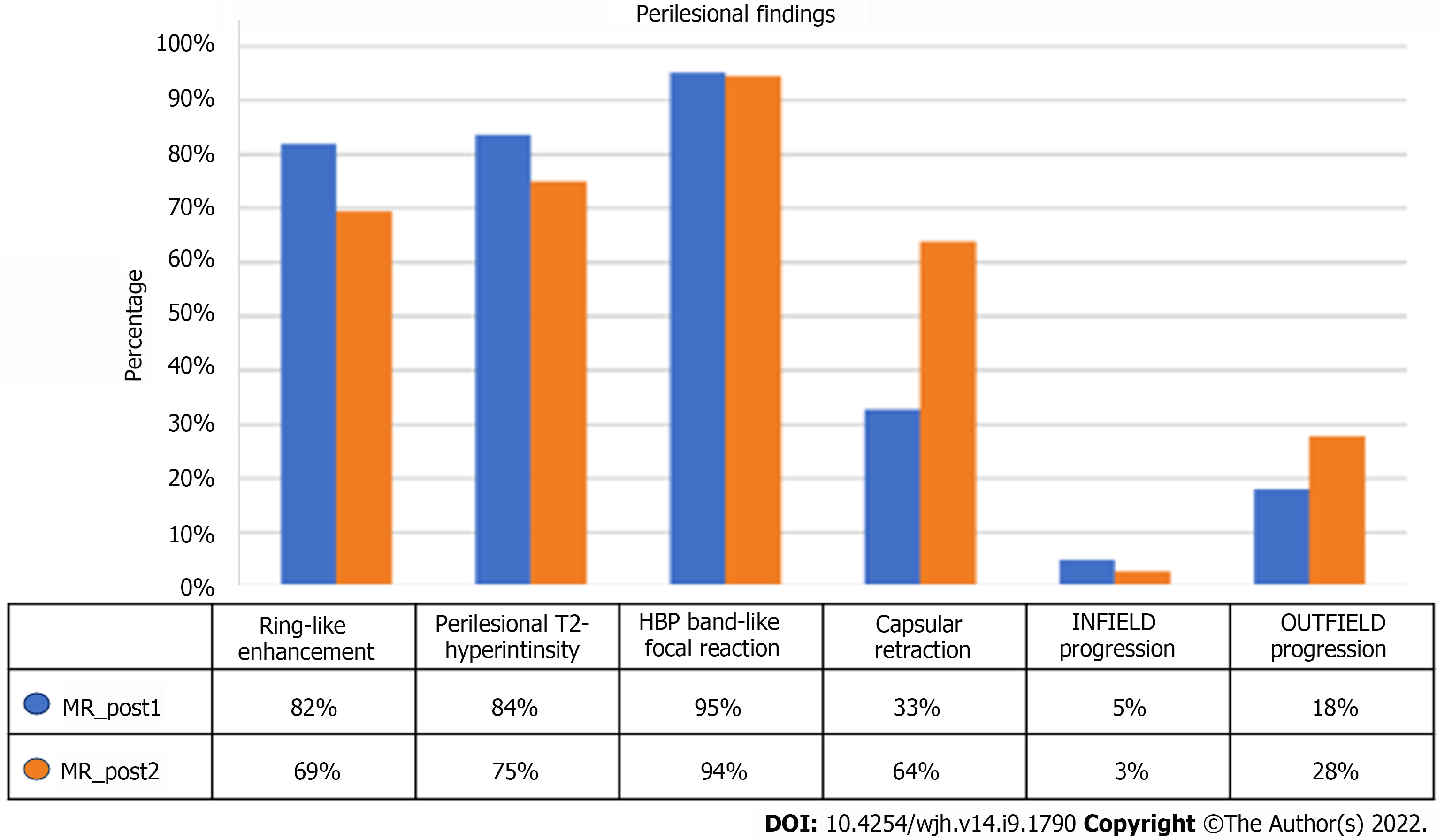
In most of the lesions, the typical characteristics of the action of SBRT were identified. In particular, at the first MRI examination, 82% of the lesions showed ring-like enhancement and 84% perilesional hyperintensity in T2-weighted sequences. These percentages tended to decrease at the second MRI examination (69% and 75%, respectively) (Figure 9).
Capsular retraction was evident in 33% of cases at the first control, a features that significantly increased to 64% at the late evaluation (P = 0.006).
Band-like signal hypointensity in the hepatobiliary phase was present at the first control in 95% of cases and in 94% at the late control (P = 0.006). The mean volume of the area of hypointensity calculated by manual segmentation was 85.47 cm3 (range 15.21-248.16) (Figure 10).
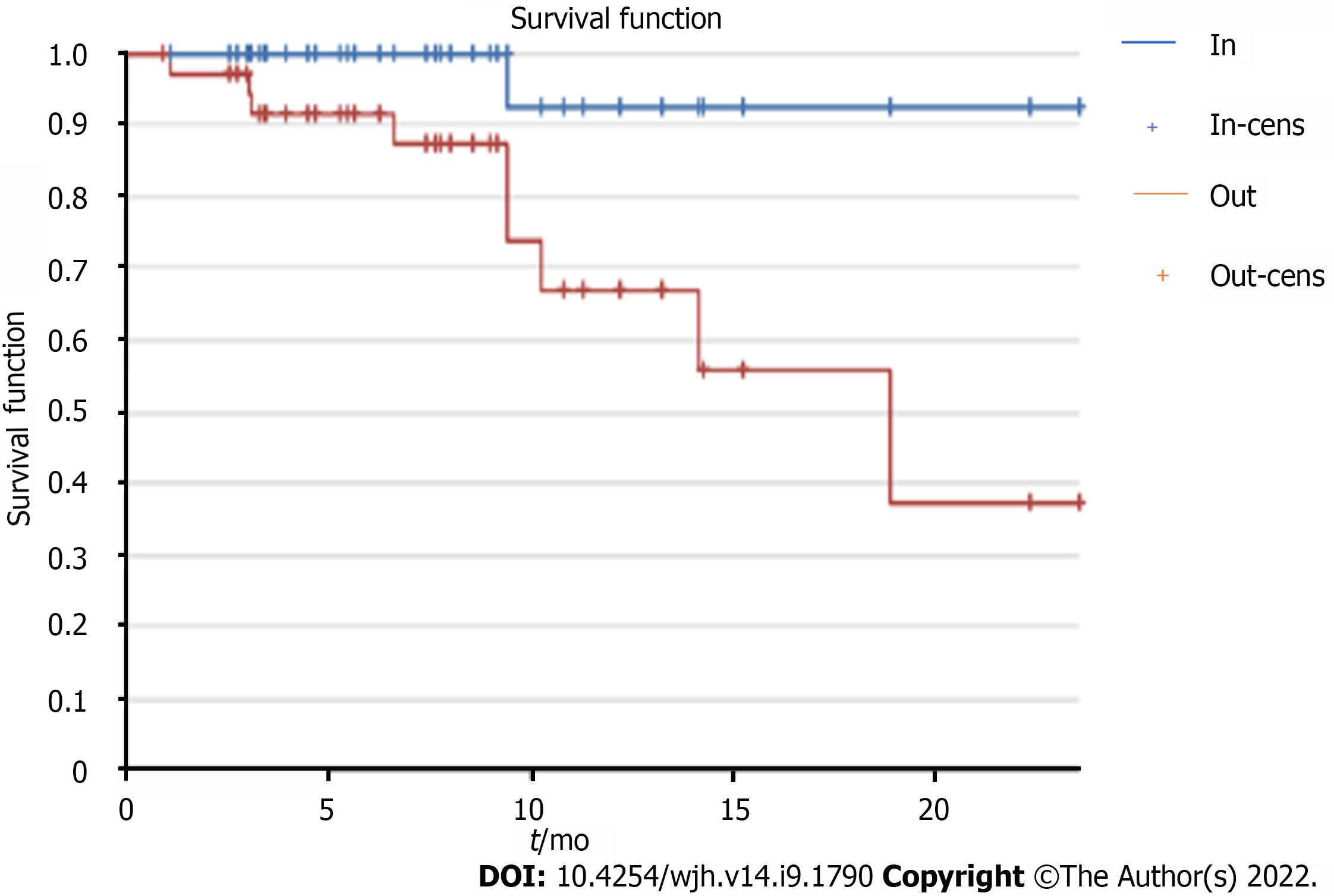
Considering the mRECIST criteria for evaluation of response to therapy, at the first examination signs of infield progression were observed in 5% of cases (3 lesions), while it was 18% for outfield progression signs (Figure 11).
At the second MRI check-up, only one (3%) case of infield progression and 19 (28%) cases of outfield progression were observed (Figure 12).
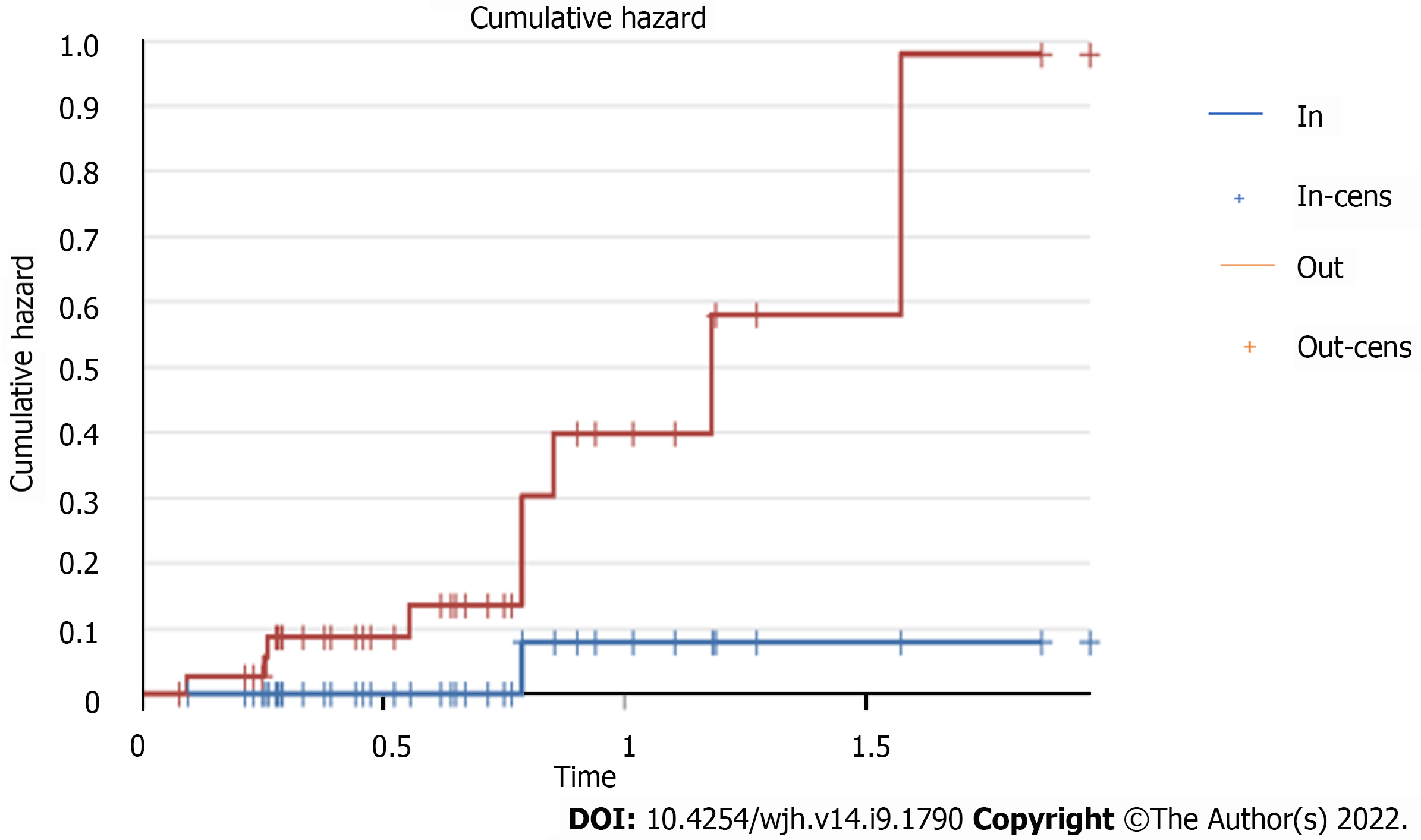
The study of the risk of outfield progression vs infield progression revealed a hazard ratio of 9.
The risk increased as time progressed, with a sharp increase in the cumulative outfield progression hazard of around 9 after the end of therapy, as shown by the Kaplan-Meier-curve study (Figure 13).
The time free from progression through the Kaplan-Meier curve showed a plateau of onset of infield progression around 9 mo, an interval in which outfield progression tended to increase.
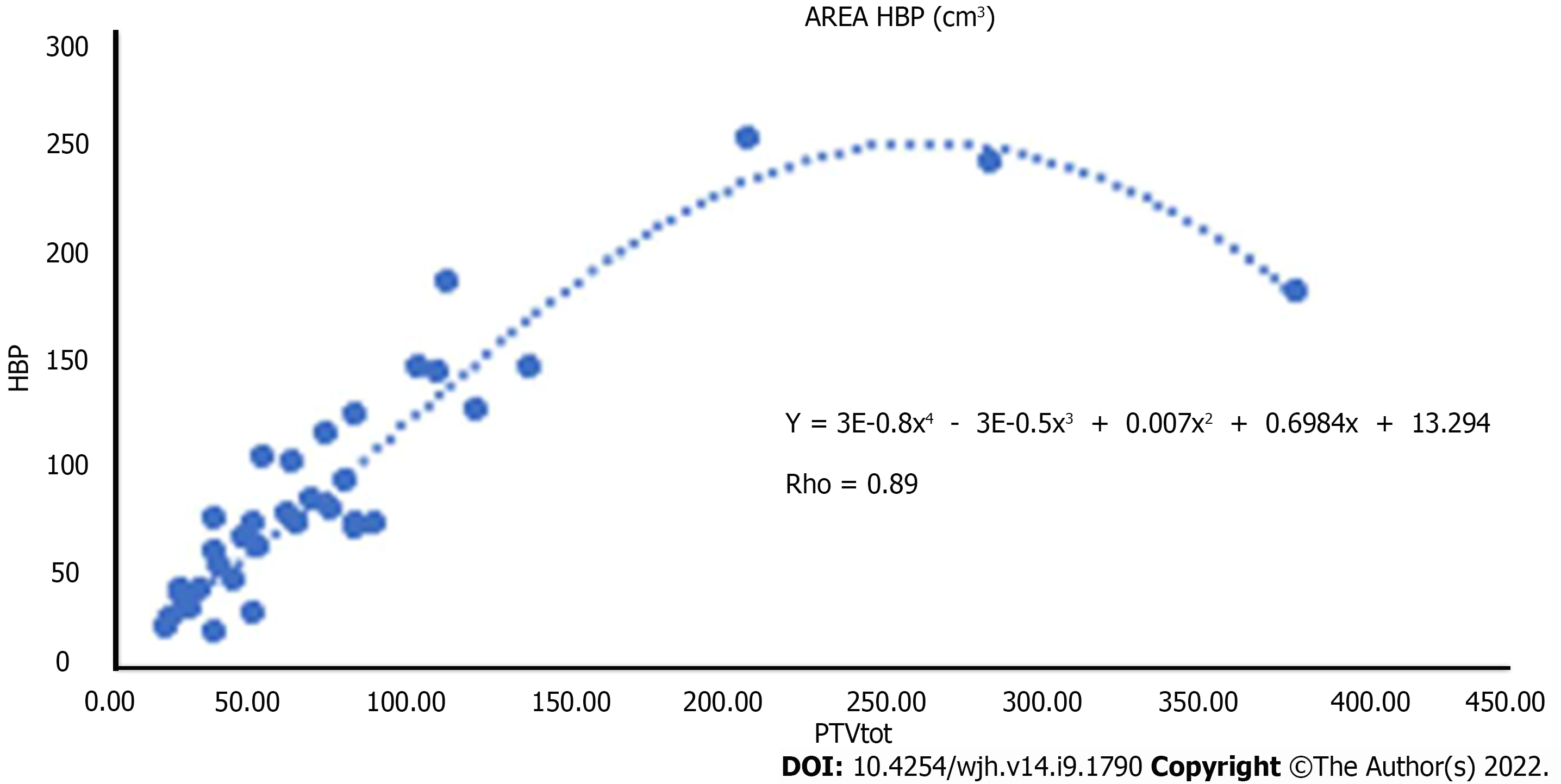
A direct relationship was also found between the area of band hypointensity during hepatobiliary excretion calculated by segmentation and PTV.
The two volumes were linked by a parabolic correlation: Up to certain volumes of PTV, the area of hypointensity also increased in a directly proportional manner. For particularly high PTV values (greater than 300 cm3), the hepatic reaction area remained at significantly lower values.
No further statistically significant correlations were found between the available clinical data, the radiotherapy data obtained, and the radiological findings.
In the acute stage (1-3 mo post-SBRT), typical peripheral hyperarterization can be seen, which persists or subsides in the subsequent post-contrast phases, referred to as ring-like enhancement. These changes imply the phenomena of venous congestion and reactive hyperemia in the treated area[10].
In the subacute stage (3-6 mo post-SBRT), the parenchyma involved shows relative hypoattenuation in basal and portal acquisitions, with progressive enhancement in the late phase, related to the occlusion of the centrolobular veins and reduced intravenous contrast clearance[6].
In the chronic stage (more than 6 mo after treatment), imaging will reveal changes caused by radio-induced fibrosis[6,11].
The study showed the effectiveness of the treatment in controlling local disease, in particular, as already described in the literature, the reduction in the diameters of the lesion assessed (arterial, hepatobiliary, and basal sequences) also becomed increasingly marked at the controls following the first[5,6].
This phenomenon is attributable to microscopic phenomena due to venocclusion that leads to fibrosis and collapse of the liver lobules at a late stage, and a reduced nutrient supply to the lesion and a progressive volumetric reduction of the whole radio-treated parenchyma[12,13].
In addition to the analysis of the classical criteria for locoregional treatment, the study focused on the analysis of signal intensities in the T2- and DWI-weighted sequences. Both sequences provide information about the nature of the tissue and the cellularity of the lesion and are therefore useful "sentinel" parameters of treatment outcome.
Signal hyperintensity both in the long TR and DWI sequences tended to decrease at the first control, remaining stable at subsequent controls, showing isointensisty to the surrounding liver parenchyma[6,10].
There is a statistically significant increase in the ADC values measured before and after SBRT, probably due to a reduction in the cellularity of the lesions due to necrosis.
As already described by Oldrini et al[10], persistence of arterial enhancement after stereotactic radiotherapy is common. In particular, arterial enhancement persisted in our population, but its diameter tended to decrease over time, probably due to progressive and slow necrosis and intralesional gigantocellular reaction. If this hypervascularisation, especially in a short-distance follow-up, was to be assessed in the same way as other locoregional therapies according to mRECIST criteria, it would be considered as a persistence of viable tissue and evaluated as ineffective[9,10]. This is in contradiction with what was reported in the literature, attesting to a percentage of complete response to SBRT that tends to progressively increase up to 90% at 12-24 mo after treatment, a figure confirmed by our study in which infield progression at the second control was 3%.
For a correct interpretation of post-procedural imaging, it is essential to recognize features in the peri-injury parenchyma and how they change over time: Peripheral hypervascularisation, signal hyperintensity in long TR sequences, and band hypointensity in the hepatobiliary excretion phase.
In the context of computed tomography (CT), the imaging characteristics of focal hepatic reaction have been well described. In the immediate post-treatment, hyperdensity occurs in early vascular phase as a consequence of sinusoidal congestion and reduced venous drainage, which gradually subsides in the portal and late phases. This may make it difficult to distinguish any persistence of hypervascularised lesional tissue in the arterial phase.
Over time, as fibrosis sets in, there will be contrastographic impregnation of the closely packed parenchyma closely contiguous to the lesion, included in the field of irradiation in the late stages[14].
The above contrastographic features, defined as "ring-like" enhancement, were similarly present in the MRI survey of our population.
Associated with this aspect is the signal hyperintensity of the treated areas in the acquisitions with T2-weighted and fat saturated T2-weighted sequences, which is also an indicator of radio-induced venoocclusive damage, which in the earliest phases is due to oedema and hyperemia, and with time to fibrosis[15].
This latter factor is particularly evident in the phenomenon of capsular retraction, present in 64% of cases at the late follow-up.
All these described elements confirm the data available in the literature on the capability of multiparametric MRI in the evaluation of locoregional hepatic therapy both in terms of post-procedural control and follow-up.
The use of liver specific contrast agents, based on the functional alteration of the hepatocytes, allows precise delineation of the irradiated field, which will appear hypointense during excretion, with a typical "band" configuration[7,8,16]. This alteration further highlights how radiotherapy-induced structural changes in the liver through veno-venous disease can have a negative impact on the liver's immune system.
We have also found a direct correlation between the focal hepatic reaction volume calculated by segmentation and the PTV programmed by radiotherapy of the parabolic type.
The fact that beyond a certain PTV there is not an equal increase in the volume of focal hepatic reaction area could be explained by two factors, one of which is closely linked to the MRI method, which does not allow adequate quantification of the parenchyma. The other explanation could be due to the fibrotic response of the liver: Greater fibrosis in absolute terms results in a greater reduction in liver volume, thus negatively affecting the quantification of the area of hypointensity. According to some authors, this association could be exploited from a clinical-radiotherapeutic point of view both to assess the accuracy of centering and possibly modify it by reducing radio-induced damage, and to quantify "in vivo" radiation-induced liver damage and use it as a quantitative biomarker of hepatotoxicity[17-20] .
Nevertheless, the integration of liver function parameters and MRI-quantifiable liver damage might in the future allow further customised dose delivery or provide additional information to the radiologist in the post-therapeutic evaluation, so as to identify possible biomarkers predictive of liver damage.
However, this finding, which can be obtained from the earliest post-treatment controls, underlines the technical accuracy of the procedure.
In our population, no correlation was found between the occurrence of toxicity, the change in blood values, and the radiological findings described.
The stability of these characteristics of good response to treatment and frequency of infield progression is concomitant with the rise of the frequency curve of outfield progression, which from the 9th month onwards is 9 times more frequent than local progression. This finding could lay a basis for different follow-up timing in patients treated by SBRT.
It is in fact known that histological changes cause long-term radiological effects, therefore delaying the first follow-up in selected patients beyond the usual 6 mo (all too often not respected) would allow radiologist to express more confidence in the treatment region and at the same time have a greater chance of detecting new liver lesions, thus allowing a better correct staging.
This study showed the effectiveness of treatment in controlling local disease; indeed, while infield progression decreased from 5% to 3% of the population at subsequent controls, outfield progression tended to increase (from 18% to 28%). However, although it is increasingly used in clinical practice today, the assessment of its effects by MRI is still lacking.
Limitations of this work are undoubtedly its retrospective nature. This leads both to a lack of systematic planning of the diagnostic procedure of the patients, which sometimes results in an incorrect and non-standardised timing, and to a low population size, since many patients, especially in the follow-ups after the first one, undergo CT scans. Moreover, given the highly differentiated indications, patients undergoing SBRT are a particularly heterogeneous population including individuals with very different lesion sizes and stages. It is therefore clear that this does not allow an indication of the impact of the treatment on survival.
In addition, as a treatment is particularly effective in controlling local disease, it is not possible to create two comparative subpopulations in order to identify any prognostic or predictive indicators of response to treatment.
In conclusion, our study emphasized the role of liver MRI after SBRT for HCC: A multiparametric approach using a liver specific contrast agent provides more information about lesion and liver parenchyma changes compared to conventional CT studies. The direct correlation between the area of hypointensity in the hepatobiliary phase and the PTV is indicative of the accuracy of the radiotherapy treatment and useful to define the infield and outfield progression of the disease.
Although stereotactic body radiation therapy (SBRT) is increasingly used, its application has not yet been regulated by the main international guidelines, leaving the decision to multidisciplinary teams.
Literature is lacking in works assessing the role of liver magnetic resonance imaging (MRI) in the evaluation of hepatocellular carcinoma (HCC) treated by SBRT.
To analyse MRI features of HCC lesions treated by SBRT and monitor how these properties evolve over time and how these aspects may modify subsequent diagnostic course of therapy.
A retrospective observational study was conducted in 49 patients (mean age 64.44 years, range 48.71-84.51), 22 females and 27 males, undergoing radiotherapy between January 2013 and November 2019.
The most significant results came from the evaluation of infield and outfield progression. The risk increased as time progressed, with a sharp increase in the cumulative outfield progression hazard of around 9 after the end of therapy, as shown by the Kaplan-Meier curve study.
A multiparametric approach using a liver specific contrast agent provides more information about lesion and liver parenchyma changes compared to conventional computed tomography studies. The direct correlation between the area of hypointensity in the hepatobiliary phase and the PTV is useful to define the infield and outfield progression of the disease.
Future studies should enlarge the sample of patients and perform further follow-ups for the patients who have already undergone the first two checks.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao X, China; Sahin TT, Turkey S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Cai YX
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 3. | Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, Russo M, Beecroft R, Ghanekar A, Bhat M, Brierley J, Greig PD, Knox JJ, Dawson LA, Grant DR. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 4. | Yip C, Cook GJR, Owczarczyk K, Goh V. Challenges in imaging assessment following liver stereotactic body radiotherapy: pitfalls to avoid in clinical practice. Chin Clin Oncol. 2017;6:S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Wong TC, Lee VH, Law AL, Pang HH, Lam KO, Lau V, Cui TY, Fong AS, Lee SW, Wong EC, Dai JW, Chan AC, Cheung TT, Fung JY, Yeung RM, Luk MY, Leung TW, Lo CM. Prospective Study of Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma on Waitlist for Liver Transplant. Hepatology. 2021;74:2580-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Haddad MM, Merrell KW, Hallemeier CL, Johnson GB, Mounajjed T, Olivier KR, Fidler JL, Venkatesh SK. Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol (NY). 2016;41:2061-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Mendiratta-Lala M, Gu E, Owen D, Cuneo KC, Bazzi L, Lawrence TS, Hussain HK, Davenport MS. Imaging Findings Within the First 12 Months of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2018;102:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Mendiratta-Lala M, Masch W, Shankar PR, Hartman HE, Davenport MS, Schipper MJ, Maurino C, Cuneo KC, Lawrence TS, Owen D. Magnetic Resonance Imaging Evaluation of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy: Long Term Imaging Follow-Up. Int J Radiat Oncol Biol Phys. 2019;103:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Sheth N, Osborn V, Lee A, Schreiber D. Stereotactic Ablative Radiotherapy Fractionation for Hepatocellular Carcinoma in the United States. Cureus. 2020;12:e8675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Oldrini G, Huertas A, Renard-Oldrini S, Taste-George H, Vogin G, Laurent V, Salleron J, Henrot P. Tumor response assessment by MRI following stereotactic body radiation therapy for hepatocellular carcinoma. PLoS One. 2017;12:e0176118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Lee S, Kim H, Ji Y, Cho B, Kim SS, Jung J, Kwak J, Park JH, Lee SW, Kim JH, Yoon SM. Evaluation of Hepatic Toxicity after Repeated Stereotactic Body Radiation Therapy for Recurrent Hepatocellular Carcinoma using Deformable Image Registration. Sci Rep. 2018;8:16224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Miften M, Vinogradskiy Y, Moiseenko V, Grimm J, Yorke E, Jackson A, Tomé WA, Ten Haken RK, Ohri N, Méndez Romero A, Goodman KA, Marks LB, Kavanagh B, Dawson LA. Radiation Dose-Volume Effects for Liver SBRT. Int J Radiat Oncol Biol Phys. 2021;110:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Fajardo LF, Colby TV. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med. 1980;104:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Kokabi N, Camacho JC, Xing M, Qiu D, Kitajima H, Mittal PK, Kim HS. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39:969-978. [PubMed] [DOI] [Full Text] |
| 15. | Mastrocostas K. Imaging post-stereotactic body radiation therapy responses for hepatocellular carcinoma: typical imaging patterns and pitfalls. Abdom. Radiol. 2019;44:1795-1807. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Fukugawa Y, Namimoto T, Toya R, Saito T, Yuki H, Matsuyama T, Ikeda O, Yamashita Y, Oya N. Radiation-induced Liver Injury after 3D-conformal Radiotherapy for Hepatocellular Carcinoma: Quantitative Assessment Using Gd-EOB-DTPA-enhanced MRI. Acta Med Okayama. 2017;71:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Khandelwal SR, Scarboro SB. Giving Radiologists and Other Clinicians the Tools to Identify Radiation Effects on Imaging Studies. Radiol Imaging Cancer. 2021;3: e210001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Boda-Heggemann J, Jahnke A, Chan MKH, Ghaderi Ardekani LS, Hunold P, Schäfer JP, Huttenlocher S, Wurster S, Rades D, Hildebrandt G, Lohr F, Dunst J, Wenz F, Blanck O. Direct dose correlation of MRI morphologic alterations of healthy liver tissue after robotic liver SBRT. Strahlenther Onkol. 2018;194:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Jung SH, Yu JI, Park HC, Lim DH, Han Y. A feasibility study evaluating the relationship between dose and focal liver reaction in stereotactic ablative radiotherapy for liver cancer based on intensity change of Gd-EOB-DTPA-enhanced magnetic resonance images. Radiat Oncol J. 2016;34:64-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Sanuki N, Takeda A, Oku Y, Eriguchi T, Nishimura S, Aoki Y, Mizuno T, Iwabuchi S, Kunieda E. Threshold doses for focal liver reaction after stereotactic ablative body radiation therapy for small hepatocellular carcinoma depend on liver function: evaluation on magnetic resonance imaging with Gd-EOB-DTPA. Int J Radiat Oncol Biol Phys. 2014;88:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |