Published online Sep 27, 2022. doi: 10.4254/wjh.v14.i9.1757
Peer-review started: May 5, 2022
First decision: June 8, 2022
Revised: June 17, 2022
Accepted: September 8, 2022
Article in press: September 8, 2022
Published online: September 27, 2022
Processing time: 140 Days and 22.3 Hours
There is a nationwide shortage of organs available for liver transplantation. Living donors help meet this growing demand. Not uncommonly, donors will have posi
To analyze the significance of positive autoantibodies in donors on post-tran
We performed a retrospective review of living liver donors who had undergone liver transplantation between January 1, 2012 and August 31, 2021. Demographic characteristics and pre-transplant data including antinuclear antibodies (ANA) and anti-smooth muscle antibody titers were collected in donors. Outcomes of interest were post-transplantation complications including mortality, biliary stri
172 living donor liver transplantations were performed during the study period, of which 115 patients met inclusion criteria. 37 (32%) living donors were auto
Isolated pre-transplant autoantibody positivity is not correlated to worse post-transplant outcomes in living liver donor transplants.
Core Tip: This was a retrospective study designed to analyze the significance of positive autoantibodies in donors on post-transplant outcomes in recipients in living donor liver transplantations. Post-transplantation rates of complications including mortality (P value = 1), infections (P value = 0.66), anastomotic strictures (P value = 0.07), and rejection (P value = 0.30) were found not to be statistically significant between the autoantibody positive and negative groups. These results suggest that isolated pre-transplant autoantibody positivity is not correlated to worse post-transplant outcomes in living liver donor transplants and should not preclude donors from donating.
- Citation: Loh J, Hashimoto K, Kwon CHD, Fujiki M, Modaresi Esfeh J. Positive autoantibodies in living liver donors. World J Hepatol 2022; 14(9): 1757-1766
- URL: https://www.wjgnet.com/1948-5182/full/v14/i9/1757.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i9.1757
There is a nationwide shortage of organs available for liver transplantation with more than 1700 patients dying annually while on the waitlist. Living donors help meet this growing demand. Of the 10109 liver transplantations performed in the United States in 2021, 568 were living-donor liver transplantations (OPTN, 2022).
Donors often undergo extensive screening prior to being approved. Transplant centers vary in their specific protocols for donor evaluation and selection, but general principles include ensuring that the donor has normal liver function and structure and is not a risk to the recipient with respect to disease transmission. It has been estimated that less than half of the candidates who complete transplant evaluation will be accepted for donation[1].
In the work-up of potential donors, positive autoantibodies are common and have been reported in up to 25% of healthy and asymptomatic individuals in the general population[2]. Positive auto
In the liver transplantation literature, few studies have evaluated the effect of pre-transplant positive autoantibodies on graft outcomes. While traditionally graft rejection has been associated with antibodies specific to organ donor HLA, there is mounting evidence supporting the association of non-HLA antibodies with rejection and decreased graft survival in kidney, heart, and lung transplantations[12,13]. It is hypothesized that tissue damage associated with ischemia-reperfusion, vascular injury, and rejection creates permissive conditions for autoantigens and allows for autoantibodies to bind to anti
However, it is unknown if donor autoantibody positivity in liver transplants predisposes to increased rates of post-transplantation complications and whether it should preclude donors from donating. In our retrospective study, we aimed to analyze the significance of positive donor autoantibodies on recipient post-transplant outcomes in living liver donor transplantations.
This was a retrospective study designed to compare post-transplantation outcomes between recipients who received transplants from positive autoantibody donors to those who received transplants from negative autoantibody donors. Data was collected by chart review. The study population consisted of patients that underwent a living liver donor transplantation between January 1, 2012 and August 31, 2021. Transplantations in recipients less than 18 years old and in those who received a transplant from a donor that did not have autoimmune markers checked during the pre-transplantation evaluation were excluded. Serum autoantibodies including antinuclear antibodies (ANA), antimitochondrial antibody (AMA), and anti-smooth muscle antibody (ASMA) were detected by indirect immunofluorescence. A positive antibody screen was defined by an ANA titer greater than or equal to 1:40 or anti-smooth muscle antibody greater than 1:20. Post-transplantation complications including rejection, biliary strictures, infection, post-transplantation lymphoproliferative disorder, bleeding, thrombosis, and portal vein stenosis were collected manually with chart review. Rejection was confirmed with a liver biopsy. The study was approved by our site’s Institutional Review Board.
Differences among post-transplant liver recipient outcomes between positive and negative autoantibody groups were assessed by a Fisher’s exact test to compare the dichotomous variables. Continuous variables were compared using independent unpaired t-tests. A Mantel-Haenszel chi-squared test with continuity correction was used to check for interaction between the cause of transplantation and post-transplant outcomes. Data analysis was performed with R statistical package, version 3.6.2.
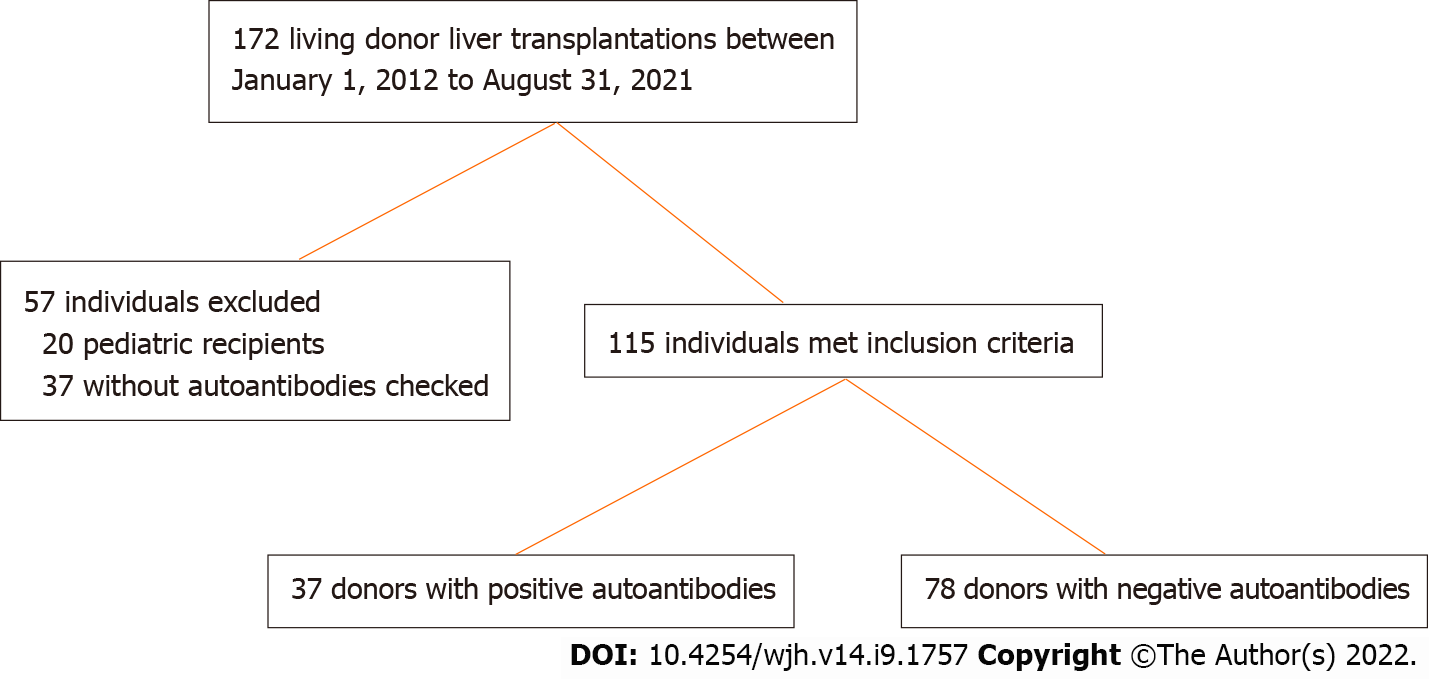
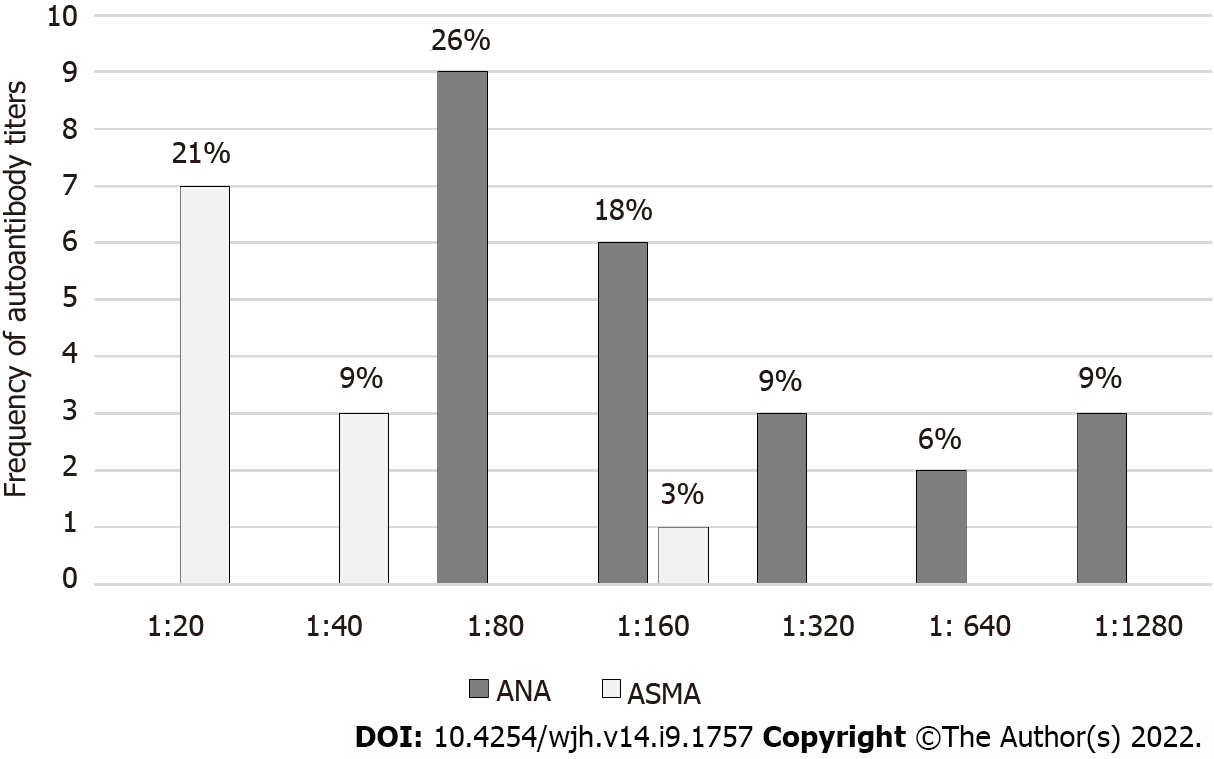
During the study period, a total of 172 living donor liver transplantations were performed at our center (Figure 1). There were 20 pediatric recipients and 37 did not have donor autoantibodies checked during the pre-transplantation evaluation and were excluded. Of the remaining 115 individuals, 78 (68%) donors had no detectable autoantibody titers while 37 (32%) donors had a positive autoantibody titer including 23 with a positive ANA, 11 with a positive ASMA, and 3 donors with both a positive ANA and ASMA. As shown in Figure 2, the median ANA titer was 1:160 (range 1:80 to 1:1280). The median ASMA was 1:40 (range 1:20 to 1:160). No donors were found to have a positive AMA.
Baseline characteristics were similar between the patients in both groups (Table 1). 59 (51%) were female. 103 (90%) of the recipients were Caucasian, 6 (5%) were Hispanic, 4 (3%) were Black, and 2 (2%) were Asian. The average age of recipients was 51 ± 15 years. The average recipient body mass index at time of transplantation was 28 ± 6 and the average listed model for end-stage liver disease sodium of the recipients at time of transplantation was 14 ± 5.
| Recipients of transplants from autoantibody positive donors (n = 37), No. (%) | Recipients of transplants from autoantibody negative donors (n = 78), No. (%) | P value | ||
| Race/Ethnicity | 0.61 | |||
| Caucasian | 33 (89) | 70 (90) | ||
| Hispanic | 3 (8) | 3 (4) | ||
| Black | 0 (0) | 4 (5) | ||
| Asian | 1 (3) | 1 (1) | ||
| Gender | 0.23 | |||
| Male | 15 (41) | 39 (50) | ||
| Female | 22 (59) | 39 (50) | ||
| Cause for transplantation | 0.11 | |||
| Alcoholic cirrhosis | 6 (16) | 8 (10) | ||
| Non-alcoholic steatohepatitis | 16 (43) | 19 (24) | ||
| Hepatitis B | 0 (0) | 1 (1) | ||
| Hepatitis C | 4 (11) | 4 (5) | ||
| PSC | 8 (22) | 13 (17) | ||
| PBC | 1 (3) | 3 (4) | ||
| AIH | 1 (3) | 5 (6) | ||
| With PBC | 0 | 1 | ||
| With PSC | 1 | 2 | ||
| AIH only | 0 | 2 | ||
| Malignancy | 0 (0) | 7 (9) | ||
| Other1 | 1 (3) | 17 (22) | ||
| Age at transplantation | 55 ± 15 | 50 ± 14 | 0.13 | |
| Average BMI | 28 ± 6 | 27 ± 6 | 0.22 | |
| Average MELD at transplantation | 15 ± 5 | 14 ± 5 | 0.71 | |
Indications for transplantation were similar between groups and included malignancy 8 (7%), alcoholic cirrhosis 14 (12%), viral hepatitis 9 (8%), nonalcoholic steatohepatitis 36 (31%), autoimmune hepatitis 6 (5%), primary biliary cholangitis 4 (3%), and primary sclerosing cholangitis 21 (18%). Of the 6 (5%) recipients that had autoimmune hepatitis, 3 (3%) also had overlapping primary sclerosing cholangitis, and 1 (1%) had overlapping primary biliary cholangitis. The types of malignancies included metastatic colon cancer 3 (3%), cholangiocarcinoma 2 (2%), metastatic rectal cancer 1 (1%), hepatocellular cancer 1 (1%), and metastatic neuroendocrine cancer 1 (1%). Other less common causes for transplantation included biliary atresia 2 (2%), congenital hepatic fibrosis 2 (2%), cryptogenic 6 (5%), common variable immunodeficiency 1 (1%), cystic fibrosis 1 (1%), polycystic liver disease 2 (2%), sarcoidosis 1 (1%), telomere syndrome 1 (1%), and portal vein thrombosis 1 (1%). One recipient underwent a simultaneous liver-kidney transplant.
Initial immunosuppressive regimens were similar for both groups. The standard immunosuppression protocol consisted of induction with thymoglobulin followed by the initiation of tacrolimus, mycophenolate, and prednisone. Mycophenolate was not started in 12 patients due to the development of bacteremia following transplant. Five were started on cyclosporine instead of tacrolimus due to a history of seizures or witnessed neurological changes after starting tacrolimus. One was switched from tacrolimus to cyclosporine after developing acute tubular necrosis requiring the initiation of hemo
As seen in Table 2, a total of 21 (18%) donors developed complications related to liver donation with the most common being symptomatic incisional hernias (6) and wound infections (6). Other less common complications of donation included chronic abdominal or incisional pain (3), portal vein stenosis requiring balloon angioplasty or an exploratory laparotomy (3), duodenal ulceration (1), pneumothorax requiring chest tube placement (1), and wound hematoma requiring exploratory laparotomy (1).
| Autoantibody positive (n = 37), No. (%) | Autoantibody negative (n = 78), No. (%) | |
| Chronic abdominal or incisional pain | 2 (5) | 1 (1) |
| Diaphragmatic or incisional hernia | 6 (8) | |
| Duodenal ulcer | 1 (1) | |
| Pneumothorax requiring chest tube | 1 (1) | |
| Portal vein stenosis | 1 (3) | 2 (3) |
| Wound hematoma requiring exploration | 1 (1) | |
| Wound infection | 2 (8) | 4 (5) |
| Total | 5 (14) | 16 (21) |
Recipients were followed on average for 2.6 years with a standard deviation of 1.8 years. Acute rejection occurred in 21 (18%) individuals. Two (2%) developed plasma cell rich rejection. The remaining 19 (17%) developed acute cellular rejection. 15 individuals had a single episode of rejection, 5 had 2 episodes, and 1 had 3 episodes. Most of the episodes of rejection were mild (15) with fewer cases of mild-moderate (2), moderate (4), or severe (2). Acute cellular rejection occurred on average 7.3 mo after transplantation with a standard deviation of 10.1 mo and range of 6 days to 13.8 mo. Two had developed allograft rejection in the setting of medication non-adherence. All patients were admitted for episodes of rejection. Of the 21 patients that developed rejection, 17 were treated with 1000 mg of IV methylprednisolone followed by an oral prednisone taper and adjustment in their long-term immunosuppression regimen. One patient was treated with a prednisone and immunosuppression dose increase, two were treated with lower doses of IV methylprednisolone, and one was switched to different immunosuppression agents. No patients included within the study timeframe developed chronic rejection.
Anastomotic biliary strictures developed in 48 patients (42%). Twenty-six (23%) recipients had strictures that resolved with serial ERCPs and stent placement. Sixteen (14%) underwent percutaneous transhepatic cholangiograms (PTHC). Six (5%) patients had recurrent episodes of biliary strictures despite PTHC placement and required surgical revision of the biliary anastomosis with a Roux-en-Y heptaticojejunostomy. Four (3%) had cholangitis and 20 (17%) developed bile leaks.
Infections post-transplantation occurred in 32 (28%) of individuals. Twelve had developed cytomegalovirus and were treated with valganciclovir, 13 developed bacterial infections requiring intravenous antibiotics, 4 developed fungal infections, and 3 developed coronavirus disease 2019 (COVID-19) pneumonia.
Twelve (10%) recipients died following transplantation. Causes of death included septic shock (4), COVID-19 pneumonia (1), recurrent cirrhosis in the setting of medication non-adherence and pneumocystis jiroveii pneumonia (1), malignancy (3), intracranial hemorrhage (1), massive PE (1), and unknown (1). Of these, two recipients died during the index admission. The median length of survival of those who died was 1.3 years (range 4 d to 4.9 years).
A total of 26 recipients experienced other complications as shown in Table 3. These included gastric or bowel perforation (2), kidney rupture (1), and diaphragmatic hernia requiring urgent exploratory laparotomy (1), hematoma (2), splenic artery bleeding (1), splenic artery aneurysm (1), hepatic artery or portal vein thrombosis (9), hepatic artery or portal vein stenosis (4), portal steal syndrome (2), small for size syndrome (1), and post-transplant lymphoproliferative disorder (1). Four recipients required re-transplantation. Two had developed hepatic artery thrombosis, one developed portal vein thrombosis, and one developed ischemia.
| Number of recipient complications in positive autoantibodies group, n = 37 (%) | Number of recipient complications in negative autoantibodies group, n = 78 (%) | |
| Bowel perforation | 0 | 1 (1) |
| Gastric perforation | 1 (3) | 0 |
| Kidney rupture | 0 | 1 (1) |
| Diaphragmatic hernia requiring urgent exploratory laparotomy | 1 (3) | 0 |
| Intraabdominal hematoma | 0 | 1 (1) |
| Retroperitoneal hematoma | 1 (3) | 0 |
| Splenic artery bleeding | 0 | 1 (1) |
| Splenic artery aneurysm s/p embolization | 1 (3) | 0 |
| Hepatic and splenic vein thrombosis | 1 (3) | 0 |
| Hepatic artery thrombosis | 1 (3) | 2 (3) |
| Hepatic artery stenosis | 2 (5) | 1 (1) |
| Hepatic artery-portal vein fistula s/p embolization | 0 | 1 (1) |
| Portomesenteric thrombosis | 0 | 1 (1) |
| Portal vein thrombosis | 1 (3) | 2 (3) |
| Portal vein stenosis | 0 | 1 (1) |
| Portal vein thrombosis and stenosis, bleeding from exploratory laparotomy | 0 | 1 (1) |
| Portal steal syndrome | 1 (3) | 1 (1) |
| Small for size syndrome | 0 | 1 (1) |
| Post-transplant lymphoproliferative disorder | 1 (3) | 0 |
| Total | 11 (30) | 15 (19) |
Post-transplantation rates of death (P value = 1), infections (P value = 0.66), and any post-transplantation complication (P value = 0.52) were similar between the autoantibody positive and negative groups (Table 4). Higher incidences of anastomotic strictures (P value = 0.07) and rejection (P value = 0.30) were observed in the positive autoantibody group; however, these differences were not statistically significant.
| Positive autoantibody (n = 37) | Negative autoantibody (n = 78) | Odds ratio (95%CI) | P value | |
| Death | 4 (11) | 8 (10) | 1.06 (0.22-4.31) | 1 |
| Strictures | 20 (54) | 28 (36) | 2.09 (0.88-5.02) | 0.07 |
| Rejection | 9 (24) | 12 (15) | 1.76 (0.58-5.16) | 0.30 |
| Infection | 9 (24) | 23 (29) | 0.77 (0.28-2.02) | 0.66 |
| Other complications | 11 (30) | 15 (19) | 1.77 (0.64-4.77) | 0.24 |
The principal findings of this study are that positive autoantibodies commonly associated with liver disease in donors are not correlated to higher rates of complications including rejection or stricture development.
In lung, kidney, and heart transplants, various non-HLA antibodies have been associated to worse graft outcomes. Proposed mechanisms of injury include the induction of cell lysis via activation of the complement cascade upon antibody binding and tissue damage associated with ischemia-reperfusion, vascular injury, and rejection creates permissive conditions for autoantigens and allows for auto
In a single center study in a pediatric population, ANA, ASMA, and angiotensin II receptor type-1 (AT1R) positivity was not associated with increased risk of fibrosis[15]. In a larger population consisting of adults, O׳Leary et al[16] evaluated autoantibodies that had been previously correlated to worse outcomes in renal transplants including AT1R and endothelin type A receptor autoantibodies and found that these patients did not have an increased risk of rejection or fibrosis progression. In an autoimmune hepatitis population, Dbouk et al[9] found no difference in post-transplant outcomes between those with high and low antibody titers. Autoantibodies in living donor liver transplantations have also been studied and observed post-transplantation with several studies finding a high prevalence of autoantibody titers post-transplantation. It has been proposed that the development of autoantibodies post-transplantation represents a nonspecific marker of liver injury rather than a predictor of post-transplant outcomes[8,10,11]. Fewer studies have examined the effect of pre-transplant autoantibody titers in adults on post-transplant outcomes.
Our results corroborate and expand upon the existing body of literature. We did not find any significant difference in rates of mortality, post-transplantation infection, or overall rates of post-transplantation complications among the autoantibody positive and negative groups. Mortality rates following transplantation were low in both groups and largely did not appear to be related to graft dysfunction. Interestingly, higher incidences of anastomotic strictures (P value = 0.07) and rejection (P value = 0.30) were observed in the positive autoantibody group though these differences were not statistically significant. Overall, there was also no significant difference in rates of complications (P value > 0.05) when comparing higher and lower titers of autoantibody positivity suggesting that isolated autoantibody positivity in asymptomatic donor is not correlated to an increased rate of post-transplantation complications.
There is some data suggesting that autoantibodies are correlated to the development of de novo autoimmune hepatitis or plasma cell rich rejection. Autoimmune hepatitis has been estimated to recur in 17%-42% of patients post transplantation with a median time to recurrence of approximately 4.6 years[17]. In our experience, the 2 (2%) individuals that developed plasma cell rich rejection received livers from autoantibody negative donors. No recipients developed a reoccurrence of autoimmune hepatitis. Re-transplantation indications in our study were predominately related to thrombotic events.
The positive autoantibody group in this study consisted of those with positive ANA and ASMA. ANA is a nonspecific marker with estimated sensitivity and specificity of 0.65 and 0.75 for autoimmune hepatitis[18]. Up to 75% of ANA-positive individuals have no identifiable disease and ASMA can be present in up to 43% of normal healthy individuals, whereas AMA is estimated to be present in less than 1%17. None of the donors in our study were found to have a positive AMA, which would have been a more specific marker of disease. The presence of autoantibodies in healthy individuals is common with an estimated prevalence of 25%-28% in the general population[2,20]; however, the presence of an autoantibody does not necessarily indicate the presence of an autoimmune disease or its severity. The prevalence of pre-transplant autoantibodies in donors in our study was 34%, similar to that of the general population. In disease, autoantibodies are considered pathological although the mechanism in which they result in disease is poorly understood[19]. It remains unclear whether they are primary or secondary consequences of the underlying process. As none of the donors with positive autoantibodies in this study were found to have liver disease, it is possible that the autoantibodies in these individuals are not pathogenic in of themselves.
Several limitations of our study must be acknowledged. This study was retrospective in nature and included a single center allowing a risk of type II error. Whether the results would be generalizable to a broader population would require a multi-center prospective study. Furthermore, due to the timeframe of the study, it is possible that some patients might develop strictures, rejection, or other complications that were not yet diagnosed over the duration of this study. The mean time to recurrence of autoimmune hepatitis has been reported to be 4.5 years, but may occur as early as 45 d after liver transplantation with the rate increasing with postsurgical interval. On average, patients were followed for 2.6 years following transplantation. In their study, Dbouk et al[9] examined the impact of age, race, sex, and autoimmune titer levels on recurrence rates or death, and found that African Americans were at a higher risk of, recurrence and death compared to other ethnic groups. Due to the predominantly Caucasian patient population skew in our cohort, we were unable to factor in race into our analysis. We also acknowledge that some donors were excluded due to lack of measurement of pre-transplant autoantibody titers. Despite this limitation, we believe our results provide a foundation for subsequent prospective multicenter studies.
In conclusion, we presented data on post-transplantation outcomes for 115 patients who received living liver donor transplants at our center. Patients were followed for an average of 2.6 years with patient survival of 90%. We found that patients who received transplants from autoantibody positive donors had similar rates of complications including strictures, death, and rejection to patients who received transplants from autoantibody negative donors. Our results expand upon existing literature suggesting that autoantibody positivity in asymptomatic donors is not correlated to worse transplant outcomes and should not preclude donation. Larger prospective studies with longer lengths of follow-up are needed to identify whether these results can be broadly applied to a wider population and whether other factors such as ethnicity or socioeconomic status may play a role in long-term transplantation outcomes.
Positive pre-transplant autoantibodies in donors are common and of unclear significance. There is a lack of data on the significance of positive donor autoantibodies on post-transplant outcomes in living liver donor transplantations.
The donor pool for liver transplantations remains limited and living liver donors help bridge the gap. It is therefore important to know whether positive autoantibodies in living donors have an effect on post-transplant outcomes and whether they should pose a barrier to transplantation.
The objective of this study was to analyze the significance of positive autoantibodies in donors on post-transplant outcomes and complications in recipients including rates of mortality, mortality, biliary strictures, biliary leaks, infection, and rejection.
This retrospective study included all patients above the age of 18 who underwent living liver donor transplantations at our center over a nine-year period (2012-20201). Demographic data and auto
Positive autoantibodies commonly associated with liver disease in donors were not correlated to higher rates of post-transplantation complications.
Our results expand upon existing literature suggesting that autoantibody positivity in asymptomatic donors is not correlated to worse transplant outcomes and should not preclude donation in living donor liver transplantations.
Larger prospective studies with longer lengths of follow-up are needed to identify whether these results can be broadly applied to a wider population and whether other factors such as ethnicity or soci
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casanova Rituerto D, Spain; He D, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Trotter JF, Wisniewski KA, Terrault NA, Everhart JE, Kinkhabwala M, Weinrieb RM, Fair JH, Fisher RA, Koffron AJ, Saab S, Merion RM; A2ALL Study Group. Outcomes of donor evaluation in adult-to-adult living donor liver transplantation. Hepatology. 2007;46:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Li QZ, Karp DR, Quan J, Branch VK, Zhou J, Lian Y, Chong BF, Wakeland EK, Olsen NJ. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13:R38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Foschi A, Zavaglia CA, Fanti D, Mazzarelli C, Perricone G, Vangeli M, Viganò R, Belli LS. Autoimmunity after liver transplantation: a frequent event but a rare clinical problem. Clin Transplant. 2015;29:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Pellegrini L, Parrilli G, Santonicola A, Cinquanta L, Caputo C, Ciacci C, Zingone F. Lack of Clinical Relevance of ANA and ASMA Positivity in Patients with Liver Transplantation without a History of Autoimmune Diseases. Biomed Res Int. 2017;2017:2456916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Avitzur Y, Ngan BY, Lao M, Fecteau A, Ng VL. Prospective evaluation of the prevalence and clinical significance of positive autoantibodies after pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2007;45:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Richter A, Grabhorn E, Helmke K, Manns MP, Ganschow R, Burdelski M. Clinical relevance of autoantibodies after pediatric liver transplantation. Clin Transplant. 2007;21:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Chen CY, Ho MC, Wu JF, Jeng YM, Chen HL, Chang MH, Lee PH, Hu RH, Ni YH. Development of autoantibodies after pediatric liver transplantation. Pediatr Transplant. 2013;17:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Riva S, Sonzogni A, Bravi M, Bertani A, Alessio MG, Candusso M, Stroppa P, Melzi ML, Spada M, Gridelli B, Colledan M, Torre G. Late graft dysfunction and autoantibodies after liver transplantation in children: preliminary results of an Italian experience. Liver Transpl. 2006;12:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Dbouk N, Parekh S. Impact of pretransplant antinuclear antibody and antismooth muscle antibody titers on disease recurrence and graft survival following liver transplantation in autoimmune hepatitis patients. J Gastroenterol Hepatol. 2013;28:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Dubel L, Farges O, Johanet C, Sebagh M, Bismuth H. High incidence of antitissue antibodies in patients experiencing chronic liver allograft rejection. Transplantation. 1998;65:1072-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Evans HM, Kelly DA, McKiernan PJ, Hübscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Cardinal H, Dieudé M, Hébert MJ. The Emerging Importance of Non-HLA Autoantibodies in Kidney Transplant Complications. J Am Soc Nephrol. 2017;28:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Reed EF. The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol. 2016;12:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Ekong UD, Antala S, Bow L, Sese D, Morotti R, Rodriguez-Davalos M, Gan G, Deng Y, Emre SH. HLA, Non-HLA Antibodies, and Eplet Mismatches in Pediatric Liver Transplantation: Observations From a Small, Single-Center Cohort. Exp Clin Transplant. 2019;17:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | OʼLeary JG, Demetris AJ, Philippe A, Freeman R, Cai J, Heidecke H, Smith C, Hart B, Jennings LW, Catar R, Everly M, Klintmalm GB, Dragun D. Non-HLA Antibodies Impact on C4d Staining, Stellate Cell Activation and Fibrosis in Liver Allografts. Transplantation. 2017;101:2399-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | González-Koch A, Czaja AJ, Carpenter HA, Roberts SK, Charlton MR, Porayko MK, Rosen CB, Wiesner RH. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transpl. 2001;7:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Zhang WC, Zhao FR, Chen J, Chen WX. Meta-analysis: diagnostic accuracy of antinuclear antibodies, smooth muscle antibodies and antibodies to a soluble liver antigen/liver pancreas in autoimmune hepatitis. PLoS One. 2014;9:e92267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Zeman MV, Hirschfield GM. Autoantibodies and liver disease: uses and abuses. Can J Gastroenterol. 2010;24:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst AM, Reimold A, Karp D, Wakeland EK, Olsen NJ. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |