Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1633
Peer-review started: February 17, 2022
First decision: April 5, 2022
Revised: April 15, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: August 27, 2022
Processing time: 189 Days and 15.7 Hours
The definition of metabolic-dysfunction-associated fatty liver disease (MAFLD) allows identification of metabolically complicated patients. Fibrosis risk scores are related to cardiovascular risk (CVR) scores and could be useful for the identification of patients at risk of systemic complications.
To evaluate the relationship between MAFLD and CVR using the Framingham risk score in a group of Mexican patients.
Cross-sectional, observational and descriptive study carried out in a cohort of 585 volunteers in the state of Veracruz with MAFLD criteria. The risk of liver fibrosis was calculated with aspartate aminotransferase-to-platelet ratio index, nonal
One hundred and twenty-five participants (21.4%) with MAFLD criteria were evaluated, average age 54.4 years, 63.2% were women, body mass index 32.3 kg/m2. The Framingham CVR was high in 43 patients (33.9%). Transient elastography was performed in 55.2% of volunteers; 39.1% with high CVR and predominance in advanced fibrosis (F3–F4). The logistic regression analysis showed that liver fibrosis, diabetes and hypertension independently increased CVR.
One of every three patients with MAFLD had a high CVR, and in those with high fibrosis risk, the CVR risk was even greater.
Core Tip: Metabolic-dysfunction-associated fatty liver disease (MAFLD) allows identification of metabolically complicated patients. Evaluation of the relationship between hepatic fibrosis and cardiovascular risk (CVR) in patients with MAFLD using the Framingham risk score allows us to identify which patients with MAFLD and liver fibrosis have a higher CVR than patients without fibrosis.
- Citation: Salgado Alvarez GA, Pinto Galvez SM, Garcia Mora U, Cano Contreras AD, Durán Rosas C, Priego-Parra BA, Triana Romero A, Amieva Balmori M, Roesch Dietlen F, Martinez Vazquez SE, Mendez Guerrero IO, Chi-Cervera LA, Bernal Reyes R, Martinez Roriguez LA, Icaza Chavez ME, Remes Troche JM. Higher cardiovascular risk scores and liver fibrosis risk estimated by biomarkers in patients with metabolic-dysfunction-associated fatty liver disease. World J Hepatol 2022; 14(8): 1633-1642
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1633.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1633
Nonalcoholic fatty liver disease (NAFLD) is characterized by steatosis > 5% in the absence of alcohol consumption and other causes of liver disease[1]. Due to its close relationship with the components of the metabolic syndrome, a consensus of international experts proposed a change of name, resulting in the concept of metabolic-dysfunction-associated fatty liver disease (MAFLD) as the new terminology[2-4].
The presence of diabetes mellitus (DM), obesity and metabolic dysregulation to establish the diagnosis of MAFLD can help identify patients with metabolically complicated fatty liver disease and consequently with higher cardiovascular risk (CVR). We consider that the Framingham score can be a useful tool in the evaluation of CVR in patients with MAFLD because it independently assesses the presence of diabetes as a CVR factor[5,6].
The Hepatic Fibrosis Scoring System is related to CVR scores in patients with MAFLD and can be useful for identifying the risk of systemic complications[7]. However, we do not have Mexican cohorts that consider the new definition of MAFLD and CVR[8]. Therefore, the objective of our work was to evaluate the relationship between MAFLD and CVR in a group of Mexican volunteers using the Framingham scale.
This was a cross-sectional, observational and descriptive study carried out in the population of a cross-sectional sample evaluated at the Instituto de Investigaciones Medico Biologicas and Centro de Servicios en Salud of the Universidad Veracruzana during February to March 2020. Residents of the State of Veracruz aged > 18 years were invited to participate. After signed informed consent, a medical evaluation was performed, which consisted of anthropometry measurements [weight, height, body mass index (BMI), waist and hip circumference, waist–hip index], biochemical studies [hematic biometry, glucose, creatinine, uric acid, lipids, aspartate aminotransferase (AST), alanine aminotransferase, alkaline phosphatase (AP), bilirubin, albumin and insulin] and liver ultrasound. In addition, blood pressure, personal history of DM, systemic arterial hypertension, dyslipidemia, cardiovascular events, and tobacco use were recorded.
From the studied population, patients older than 40 years with diagnostic criteria for MAFLD were included. Patients with cancer, terminal disease, history of cardiovascular events and pregnant women were excluded.
The risk of liver fibrosis was determined with aspartate aminotransferase-to-platelet ratio index (APRI), NAFLD and fibrosis-4 (FIB-4) scores. The APRI was at high risk of significant fibrosis with a score of > 1.5, indeterminate 0.5–1.5, and unlikely or absent < 0.5; NAFLD score was considered high risk with > 0.675, indeterminate 1.455 to 0.675, and absent < 1.455; FIB-4 score was considered high risk with > 3.25, indeterminate 1.45–3.25 and absent < 1.45[9-11]. Transient elastography (TE) with Fibroscan® was performed in patients with undetermined and high risk of liver fibrosis[12,13]. The CVR was calculated with the Framingham system which evaluates: age, sex, total cholesterol, high-density lipoprotein cholesterol, blood pressure, use of antihypertensive drugs, tobacco consumption, DM, history of vascular disease; classifying patients as low, moderate or high CVR[14,15].
The project was carried out in accordance with the principles of Good Clinical Practices and prior approval of the Ethics Committee with number IIMB-UV 2020/03.
The analysis of the results, elaboration of figures and tables was carried out with the IBM SPSS® Statistics version 22.0 . Nominal and ordinal variables were described with frequencies and percentages, continuous and discrete variables with measures of central tendency and dispersion according to their distribution. The comparison between groups was carried out with the χ2 test and analysis of variance. Nonparametric statistics with Spearman’s correlation test were used in the relationship between CVR and fibrosis. Statistical significance was considered when the P value was < 0.05.
Of the 585 volunteers, 125 (21.4%) who met the inclusion criteria were studied, 79 (63.2%) were women, average age 54.4 ± 8.8 years, BMI 32.3 ± 5.3 kg/m2.
According to the Framingham score, 46 patients (36.2%) had mild CVR, 36 (28.3%) moderate and 43 (33.9%) high. No differences were found by sex or BMI between the CVR categories. The patients’ age with high CVR was 59 ± 8.4 years; higher than in mild and moderate CVR (P = 0.028). The presence of DM, hypertension and tobacco use was significantly higher in patients with high CVR. The concentration of glucose and insulin was higher in patients with high CVR; therefore, the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index showed a value > 3.0 compatible with insulin resistance (P = 0.000) compared to patients with low and moderate CVR (HOMA-IR 1.96–3). The rest of the biochemical parameters evaluated did not show significant differences (Table 1). Fibrosis scores showed an increasing trend in patients with high CVR; however, this difference was not significant (0.094).
| Cardiovascular risk1 | ||||
| n (%) / mean ± SD | Mild, n = 46 | Moderate, n = 36 | High, n = 43 | P value |
| Sex | ||||
| Female | 35 (76.1) | 23 (63.9) | 21 (48.8) | 0.028 |
| Male | 11 (23.9) | 13 (36.1) | 22 (51.2) | |
| Age | 50.1 ± 8.1 | 54.6 ± 7.6 | 59 ± 8.4 | 0.0002 |
| BMI (average) | 32.5 ± 5.8 | 32.3 ± 5.9 | 32.1 ± 4.1 | 0.964 |
| Normal, n (%) | - | 2 (5.6) | 1 (2.3) | - |
| Overweight, n (%) | 16 (34.8) | 13 (36.1) | 12 (27.9) | 0.480 |
| Obesity, n (%) | 30 (65.2) | 21 (58.3) | 30 (69.8) | |
| Comorbidities | ||||
| Smoking, n (%) | 10 (21.7) | 16 (44.4) | 21 (48.8) | 0.018 |
| DM, n (%) | - | - | 28 (65.1) | 0.0002 |
| Hypertension, n (%) | 11 (23.9) | 16 (44.4) | 27 (62.8) | 0.0002 |
| SBP | 120 ± 12 | 127 ± 13 | 131 ± 16 | 0.0012 |
| Biochemistry | ||||
| PLQ (x 103/mm3) | 249 ± 82 | 229 ± 60 | 229 ± 68 | 0.335 |
| Glucose (mg/dL) | 93 ± 14 | 95 ± 16 | 147 ± 74 | 0.0002 |
| TB (mg/dL) | 0.62 ± 0.20 | 0.65 ± 0.26 | 0.75 ± 0.4 | 0.126 |
| AST (UI) | 34.0 ± 15.4 | 36.1 ± 13 | 37.2 ± 20.4 | 0.660 |
| ALT (UI) | 39.7 ± 28.1 | 37.6 ± 18.6 | 40.7 ± 35.1 | 0.888 |
| AP (UI) | 82.1 ± 19.2 | 96.5 ± 33.2 | 97.7 ± 36.6 | 0.0302 |
| Albumin (g/dL) | 4.0 ± 0.25 | 4.1 ± 0.32 | 4.0 ± 0.23 | 0.626 |
| Insulin | 8.9 ± 4.7 | 8.3 ± 4.3 | 12.8 ± 9.1 | 0.0042 |
| HOMA-IR | 2.0 ± 1.19 | 1.9 ± 1.0 | 4.4 ± 3.5 | 0.0002 |
| Creatinine (mg/dL) | 0.7 ± 0.1 | 0.9 ± 0.5 | 0.9 ± 0.3 | 0.289 |
| Uric acid (mg/dL) | 6.0 ± 1.3 | 5.9 ± 1.6 | 6.1 ± 1.6 | 0.813 |
| TC (mg/dL) | 205.9 ± 38.2 | 201.4 ± 36.4 | 192.5 ± 38.4 | 0.240 |
| LLD (mg/dL) | 114.9 ± 32.7 | 115.1 ± 27.9 | 104.3 ± 40.4 | 0.261 |
| HDL (mg/dL) | 52.7 ± 12.5 | 53.09 ± 18.3 | 48.6 ± 12.8 | 0.296 |
| TG (mg/dL) | 225.1 ± 332.6 | 167.6 ± 58.8 | 197.6 ± 96.6 | 0.477 |
| Fibrosis markers | ||||
| FIB-4 | 1.286 ± 0.772 | 1.569 ± 0.836 | 1.851 ± 1.744 | 0.094 |
| APRI | 0.345 ± 0.241 | 0.376 ± 0.215 | 0.419 ± 0.419 | 0.526 |
| NAFLD score | -1.166 ± 1.180 | -1.151 ± 1.060 | -0.677 ± 1.333 | 0.110 |
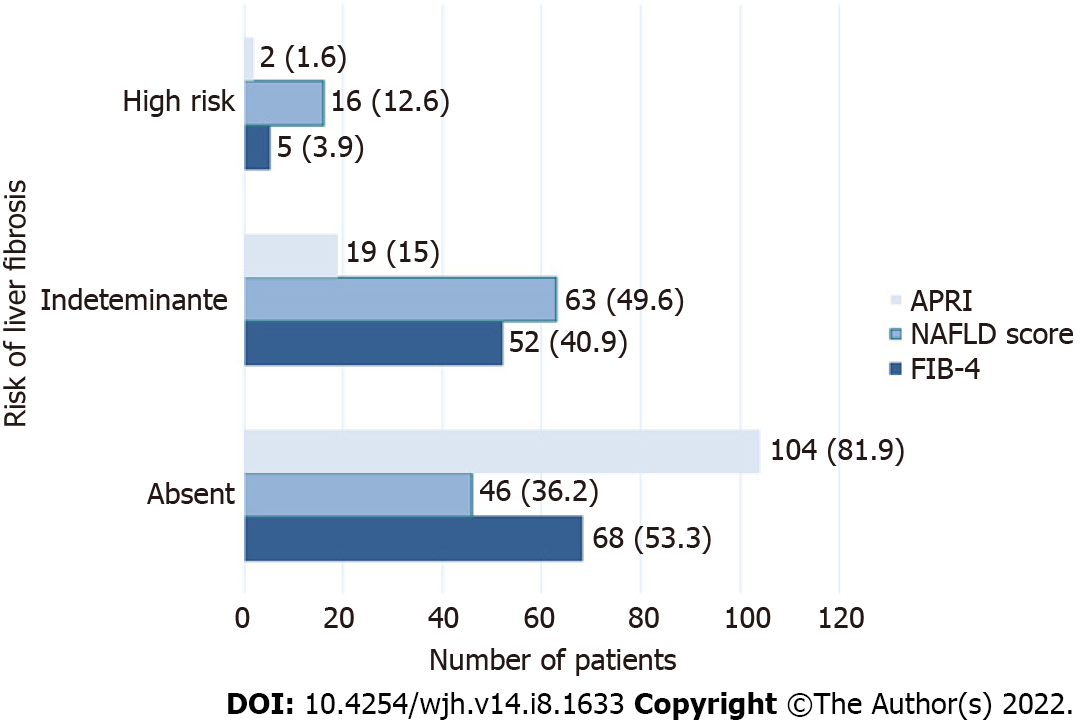
The distribution between the fibrosis risk stages by FIB-4, NAFLD and APRI scores is shown in Figure 1. Fifty-two patients (44.8%) with indeterminate and high risk of fibrosis were identified according to FIB-4, 79 (62.2%) according to NAFLD score and 21 (15.6%) with APRI.
The study was performed in 69 patients (55.2%) with indeterminate or high risk of fibrosis. Patients were identified as follows, F0: 19 (27.5%), F1: 12 (17.4%), F2: 11 (15.9%), F3: 13 (18.8%) and F4: 14 (20.3%). In the evaluation of hepatic steatosis by controlled attenuation parameter (CAP) the results were the following, S0: 13 patients (18.8%), S1: 7 (5.5%), S2: 3 (2.4%) and S3: 46 (36.2%).
The age distribution showed a significant difference between the risk of fibrosis due to FIB-4, as it was higher in the group with indeterminate fibrosis and lower in patients with absence of fibrosis (P = 0.000). The BMI and the comorbidities evaluated did not show significant differences between the risk groups. Patients with high risk of fibrosis had decreased platelet and albumin counts, as well as significantly elevated levels of total bilirubin, AST, and phase angle compared to patients without fibrosis or indeterminate risk of fibrosis (P = 0.000).
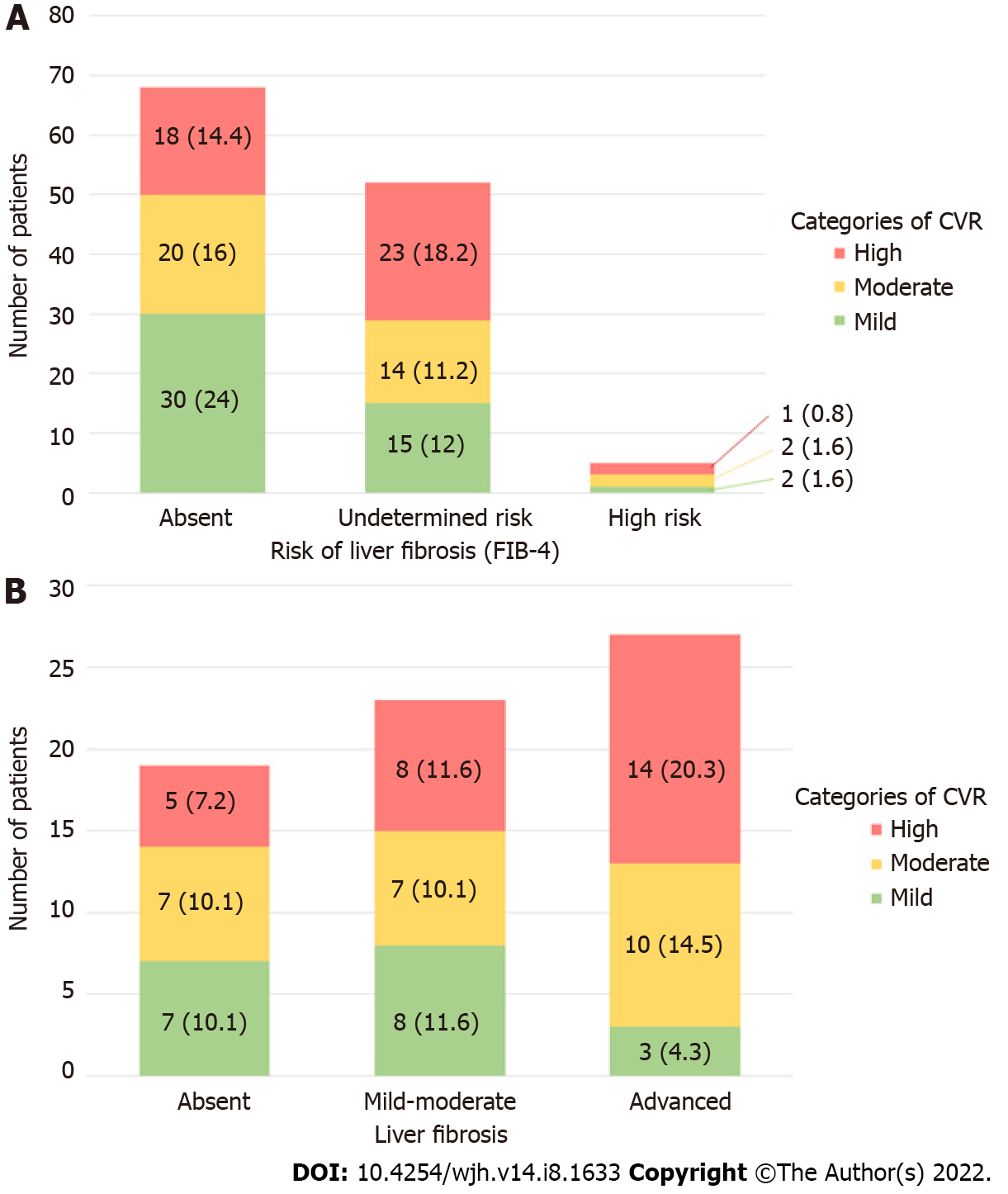
The correlation of liver fibrosis risk by FIB-4 and CVR according to the Framingham system showed that 33.4% of patients with MAFLD had a high CVR, predominantly in patients with indeterminate risk of fibrosis (18.2%). 14.4% of the patients with no fibrosis had a high CVR versus 19.8% of the patients with an indeterminate or high risk of fibrosis. The risk of fibrosis did not show a significant correlation with the severity of CVR (P = 0.257). The categories of CVR and risk of fibrosis by FIB-4 can be observed in Figure 2A.
In 69 patients evaluated with TE, 18 patients (26.1%) had mild CVR according to the Framingham system, 24 (34.7%) with moderate and 27 (39.1%) with high risk. It was observed that the group with the greatest number of patients with high CVR were those with advanced fibrosis. 39.1% of patients with MAFLD had a high CVR at the time of diagnosis with a predominance of advanced fibrosis (F3–F4). In relation to fibrosis severity, it was noted that in advanced fibrosis, 34.8% had moderate to high CVR, predominantly observing the correlation between advanced fibrosis and high CVR. A statistically significant relationship was reported between the presence of fibrosis and the severity of CVR (P = 0.026). The distribution of the different risk categories in patients with absent or mild-moderate fibrosis was heterogeneous as shown in Figure 2B.
The correlation between CAP and CVR showed that 28.9% of the patients with S3 had a high CVR. Although most of our patients were found in S3, the severity of the CAP did not show a significant relationship with the severity of the CVR (P = 0.254). Table 2 shows the correlation between CVR and CAP. In logistic regression analysis, the presence of fibrosis P = 0.007 [95% confidence interval (CI): 0.157–35.376], DM P = 0.000 (95%CI: 0.791–43.555) and hypertension P = 0.035 (95%CI: 0.085–5.228) were independently and significantly associated with the CVR, but not with the presence of steatosis P = 0.220 (95%CI: 0.144–22.921).
| Steatosis (CAP), n (%) | |||||
| S0, n = 13 | S1, n = 7 | S2, n = 3 | S3, n = 46 | P value | |
| Framingham | |||||
| Mild (n = 18) | 3 (4.3) | 2 (2.9) | 0 | 13 (18.8) | 0.254 |
| Moderate (n = 24) | 5 (7.2) | 5 (7.2) | 1 (1.4) | 13 (18.8) | |
| High (n =27) | 5 (7.2) | 0 | 2 (2.9) | 20 (28.9) | |
MAFLD is currently the most common chronic liver disease worldwide, present in 25% to 30% of the population. Although the severity of the disease criteria has not been established, it is described that the presence of fibrosis is the most important prognostic marker of mortality, independent of the severity of the fatty infiltration[16,17]. Our study, carried out in a Mexican population, showed that one out of every three patients with MAFLD had a high CVR, and the higher the fibrosis the greater the CVR. We show novel results as it is one of the first studies to use the new definition of MAFLD in association with CVR[18].
The change in diagnostic criteria from NAFLD to MAFLD was made recently. Therefore, in Mexico we have few prevalence studies with the new definition. The study carried out by Bellentani et al[19] in 585 healthy volunteers published as a summary showed a prevalence of MAFLD of 42.1; high if we compare it with the prevalence of NAFLD estimated worldwide. In our cohort, we found that 21.4% of the population over 40 years of age had MAFLD, like the prevalence of NAFLD reported in the adult population of various western countries. Although the prevalence of DM in patients with NAFLD is 50% to 70%, in our population, it was lower (22.4%). We found a high prevalence of overweight and obesity (97.6%), like that observed in other populations where between 80% and 90% has been reported. We consider that the differences may be because previously conducted studies consider the NAFLD criteria[20,21].
Liver biopsy is the gold standard in the evaluation of fibrosis, but it carries the risk of complications coupled with high cost. For this reason, the use of noninvasive systems such as serum markers, TE or magnetic resonance imaging is recommended. TE with FibroScan® is the most widely used and validated elastography worldwide with good sensitivity and specificity to diagnose F4 stage (both 92%). The FIB-4 index has been shown to be superior to other noninvasive markers in the identification of advanced fibrosis in patients with MAFLD; therefore, it is the marker of choice in the two-step algorithms[22]. Shah et al[23] compared the diagnostic performance of noninvasive fibrosis markers and concluded that the FIB-4 index is better at identifying advanced fibrosis in patients with MAFLD. The diagnostic performance of the markers used in our study showed similar results, with a higher correlation of advanced fibrosis by FIB-4 with TE.
The main cause of death in patients with MAFLD is cardiovascular disease, and the secondary causes are related to the liver. Cardiovascular diseases most frequently observed in patients with MAFLD are left ventricular dysfunction, atherosclerotic disease, disturbances in the cardiac conduction system, and cerebral ischemic events. These observations establish the close relationship between the severity of liver disease and the risk of fatal and nonfatal cardiovascular events[24]. Despite current evidence, in daily clinical practice, MAFLD is considered a benign entity. For this reason, our study focused on the identification of patients with MAFLD and a high risk of cardiovascular events and its relationship with hepatic fibrosis markers. Our purpose was to recommend early identification mechanisms to prevent complications and decrease mortality.
Fatty liver is associated with increased CVR regardless of the presence of DM, dyslipidemia, and hypertension. Therefore, early identification is important to reduce cardiovascular mortality[25]. Our results show that the majority of the population with MAFLD has mild CVR according to the Framingham system. However, more patients with higher CVR were identified at indeterminate risk of fibrosis due to FIB-4 (18.2%). Our results showed that most of the patients with high CVR have advanced fibrosis (F3–F4). In addition to this, these patients had a higher frequency of DM and hypertension. These results reflect that the higher the CVR, the greater the risk of liver fibrosis, which allows the early identification of patients with compensated advanced liver disease.
Cardiovascular disease and arteriosclerosis are the result of endothelial damage, dyslipidemia, and oxidative stress reported more frequently in patients with MAFLD. However, as reported in the literature, the severity of hepatic fatty infiltration did not demonstrate a relationship with CVR[5,26].
Various studies have reported that the FIB-4 index ≥ 2.67 is independently associated with coronary atherosclerosis and cardiovascular events; therefore, with an increase in CVR[27,28]. In our study, results similar to those published in previous clinical trials were observed, exhibiting a correlation between FIB-4 and the CVR systems compared to NAFLD and APRI (P < 0.05). Another limitation was that it was not a nationally representative population, as it only included volunteers from the State of Veracruz.
It is important to mention and recognize that our study had limitations that must be considered. One of them was that the prevalence of MAFLD was calculated in volunteers older than 40 years and in different clinical trials the population older than 18 years was included; therefore, the prevalence could be underestimated in our cohort. Finally, it is recognized that the gold standard for evaluating fibrosis and steatosis in patients with MAFLD is liver biopsy. However, due to the risks of this procedure, we performed the evaluation of fibrosis and steatosis with only biochemical markers and transient elastography.
One of every three patients with MAFLD had a high CVR and the greater severity of fibrosis correlated with a greater CVR. According to our results, the early identification of CVR in patients with MAFLD will allow establishment of preventive actions and timely treatment to reduce the risk of mortality in this population.
The investigation was carried out in an open population without a diagnosis of metabolic-dysfunction-associated fatty liver disease (MAFLD).
To identify patients with MAFLD and establish their cardiovascular risk (CVR).
The objective is to evaluate the relationship between fibrosis and steatosis measured by transition elastography in patients with MAFLD and CVR scores in Mexican patients.
Identification of patients with MAFLD in the open population. Subsequently, determination of the risk of fibrosis by noninvasive methods. Finally, CVR was determined by the Framingham risk scale and was related to the presence of fibrosis and steatosis.
21.4% of the study population met MAFLD criteria. The severity of CVR was related to the presence of fibrosis, but not with the severity of steatosis.
Patients with MAFLD and liver fibrosis have a higher CVR compared to patients without fibrosis, regardless of the severity of steatosis.
Prospective research is required to determine the best CVR score in patients with MAFLD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Gao Y, China; Hua J, China S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Ma YJ
| 1. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 198] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Lindenmeyer CC, McCullough AJ. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin Liver Dis. 2018;22:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 4. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2212] [Article Influence: 442.4] [Reference Citation Analysis (1)] |
| 5. | Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2021;19:2138-2147.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 6. | Takahashi Y, Kurosaki M, Tamaki N, Yasui Y, Hosokawa T, Tsuchiya K, Nakanishi H, Itakura J, Izumi N. Non-alcoholic fatty liver disease fibrosis score and FIB-4 scoring system could identify patients at risk of systemic complications. Hepatol Res. 2015;45:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Ballestri S, Mantovani A, Baldelli E, Lugari S, Maurantonio M, Nascimbeni F, Marrazzo A, Romagnoli D, Targher G, Lonardo A. Liver Fibrosis Biomarkers Accurately Exclude Advanced Fibrosis and Are Associated with Higher Cardiovascular Risk Scores in Patients with NAFLD or Viral Chronic Liver Disease. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 9. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2285] [Article Influence: 126.9] [Reference Citation Analysis (1)] |
| 10. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3566] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 11. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 841] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 12. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1849] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 13. | Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6201] [Cited by in RCA: 6281] [Article Influence: 232.6] [Reference Citation Analysis (0)] |
| 14. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4123] [Article Influence: 206.2] [Reference Citation Analysis (3)] |
| 15. | Alcocer LA, Lozada O, Fanghänel G, Sánchez-Reyes L, Campos-Franco E. Global cardiovascular risk stratification: comparison of the Framingham method with the SCORE method in the Mexican population. Cir Cir. 2011;79:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7542] [Article Influence: 838.0] [Reference Citation Analysis (0)] |
| 17. | Bernal-Reyes R, Icaza-Chávez ME, Chi-Cervera LA, Remes-Troche JM, Amieva-Balmori M, Priego-Parra BA, Martínez-Vázquez S, Méndez-Guerrero IO, Martínez-Rodríguez L, Barranca-Enríquez A, Palmeros-Exsome C, Cano-Contreras AD, Triana-Romero A. Prevalence and clinical-epidemiologic characteristics of a Mexican population with metabolic (dysfunction) associated fatty liver disease: An open population study. Rev Gastroenterol Mex (Engl Ed). 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 19. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 20. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 21. | Davyduke T, Tandon P, Al-Karaghouli M, Abraldes JG, Ma MM. Impact of Implementing a "FIB-4 First" Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol Commun. 2019;3:1322-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Sumida Y, Yoneda M, Tokushige K, Kawanaka M, Fujii H, Imajo K, Takahashi H, Eguchi Y, Ono M, Nozaki Y, Hyogo H, Koseki M, Yoshida Y, Kawaguchi T, Kamada Y, Okanoue T, Nakajima A; Japan Study Group Of Nafld Jsg-Nafld. FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1168] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 24. | Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, Benjamin EJ, Levy D, Fox CS, Long MT. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Tamaki N, Kurosaki M, Takahashi Y, Itakura Y, Inada K, Kirino S, Yamashita K, Sekiguchi S, Hayakawa Y, Osawa L, Higuchi M, Takaura K, Maeyashiki C, Kaneko S, Yasui Y, Tsuchiya K, Nakanishi H, Itakura J, Izumi N. Liver fibrosis and fatty liver as independent risk factors for cardiovascular disease. J Gastroenterol Hepatol. 2021;36:2960-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313-3327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 795] [Cited by in RCA: 980] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 27. | Santos RD, Valenti L, Romeo S. Does nonalcoholic fatty liver disease cause cardiovascular disease? Atherosclerosis. 2019;282:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Chen Q, Li Q, Li D, Chen X, Liu Z, Hu G, Wang J, Ling W. Association between liver fibrosis scores and the risk of mortality among patients with coronary artery disease. Atherosclerosis. 2020;299:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |