Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1480
Peer-review started: February 25, 2022
First decision: April 8, 2022
Revised: April 20, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: July 27, 2022
Processing time: 152 Days and 7.8 Hours
The Mac-2 binding protein glycosylation isomer (M2BPGi), a fibrosis marker in various liver diseases, is reportedly a prognostic marker in patients with hepatocellular carcinoma (HCC) who underwent hepatectomy.
To evaluate whether the M2BPGi value, M2BP, and pre-sarcopenia before radiofrequency ablation (RFA) could be useful recurrence and prognostic markers in patients with early-stage HCC.
In total, 160 patients with early-stage primary HCC treated with RFA were separately analyzed as hepatitis C virus (HCV)-positive and HCV-negative. Factors contributing to recurrence and liver-related death, including M2BP, M2BPGi, and skeletal muscle mass index, were statistically analyzed. Eighty-three patients were HCV-positive and 77 were HCV-negative.
In HCV-positive patients, only des-γ-carboxy-prothrombin ≥ 23 mAU/mL was a significant poor prognostic factor affecting survival after RFA. In HCV-negative patients, M2BPGi ≥ 1.86 cutoff index was significantly associated with tumor recurrence, while M2BP was not. M2BPGi ≥ 1.86 cutoff index (hazard ratio, 4.89; 95% confidence interval: 1.97-12.18; P < 0.001) and pre-sarcopenia (hazard ratio, 3.34, 95% confidence interval: 1.19-9.37; P = 0.022) were independent significant poor prognostic factors in HCV-negative patients.
In HCV-negative patients with primary HCC treated with RFA, lower M2BPGi contributed to a lower tumor recurrence rate and longer survival period. Pre-sarcopenia contributed to the poor prognosis independently in HCV-negative patients. These factors might be useful recurrence and prognostic markers for early-stage primary HCC.
Core Tip: Hepatocellular carcinoma (HCC) is prone to recurrence, even if cured at an early stage. Pre-sarcopenia is a poor prognostic factor in the elderly population. The usefulness of the Mac-2 binding protein glycosylation isomer (M2BPGi) to treat HCC has recently attracted attention. In this study, we investigated the recurrence and prognostic factors in patients who underwent radiofrequency ablation for early-stage HCC. Based on our data, pre-sarcopenia and higher M2BPGi, but not M2BP, were useful predictors of the recurrence and poor prognosis of early-stage primary HCC in hepatitis C virus-negative patients.
- Citation: Nakai M, Morikawa K, Hosoda S, Yoshida S, Kubo A, Tokuchi Y, Kitagataya T, Yamada R, Ohara M, Sho T, Suda G, Ogawa K, Sakamoto N. Pre-sarcopenia and Mac-2 binding protein glycosylation isomer as predictors of recurrence and prognosis of early-stage hepatocellular carcinoma. World J Hepatol 2022; 14(7): 1480-1494
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1480.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1480
Hepatocellular carcinoma (HCC) is an important health problem affecting approximately 900000 new cancer cases worldwide. In 2020, > 800000 people died from HCC, accounting for approximately 8.3% of cancer deaths[1]. HCC often results from cirrhosis or chronic liver injury caused by background diseases, such as hepatitis C virus (HCV), hepatitis B virus (HBV), primary biliary cholangitis (PBC), autoimmune hepatitis (AIH), alcoholic liver disease, and nonalcoholic steatohepatitis (NASH). In the last 25 years, treatment for viral hepatitis has made great strides. Notably, HCV can be eliminated in almost all cases using direct-acting antivirals (DAAs). Although HBV is still an important risk factor that accounts for approximately 50% of the causes of HCC, the proportion of non-viral liver diseases, especially steatohepatitis, as the causative disease of HCC is increasing[2].
HCC is prone to recurrence, even if cured at an early stage. In the Barcelona Clinic Liver Cancer (BCLC) staging system[3-5], which is widely used in the treatment of HCC, early-stage HCC is classified as stage 0 or A. BCLC stage 0 is defined as very early stage, for single nodule ≤ 2 cm, Child-Pugh A, Eastern Cooperative Oncology Group Performance status (PS) 0. BCLC stage A is defined as the early stage and is the case of maximum tumor diameter ≤ 3 cm, number of tumors ≤ 3, Child-Pugh A-B, and PS 0. Liver transplantation is considered in some unresectable cases of stage A disease, but resection and ablation are often recommended as curative treatments. In recent years, a median overall survival > 6 years has been expected for early-stage liver cancer patients undergoing BCLC-0 of A liver resection and ablation[6]. However, even in the case of liver resection for early-stage HCC, the prognosis is poor in cases of portal hypertension[7,8].
Radiofrequency ablation (RFA) is the most widely used local therapy for HCC treatment. It has been reported that the 4-year local recurrence rate after RFA in the early stage is approximately 5%-10% and the 5-year survival rate is approximately 70%[9-13]. However, it has been reported that cases with impaired liver function and/or bad tumor conditions (large tumor diameter and large number of tumors) have a poor prognosis[13].
In recent years, many studies have demonstrated that sarcopenia is a poor prognostic factor in patients with chronic liver disease and HCC, because it is related to frailty, loss of function, and low quality of life. Sarcopenia is diagnosed using both muscle power loss and muscle volume loss according to the Japan Society of Hepatology (JSH) diagnostic guidelines or European diagnostic guidelines[14-16]. Pre-sarcopenia is defined as muscle volume loss without muscle power loss, and has been reported to be a poor prognostic factor in the elderly population[17].
In addition, the usefulness of the Mac-2 binding protein glycosylation isomer (M2BPGi), or Wisteria floribunda agglutinin (WFA)-positive M2BP, which was first reported as a fibrosis marker in HCV patients, to treat HCC has recently attracted attention[18]. M2BPGi is a serum marker predicting fibrosis in HCV and other liver diseases, such as HBV, AIH, PBC, and NASH[19-22]. It is also a useful predictor of HCC in various liver diseases[23-27].
In this study, we investigated the usefulness of pre-sarcopenia, M2BPGi, and M2BP as recurrence and prognostic factors in patients who underwent RFA for early-stage HCC.
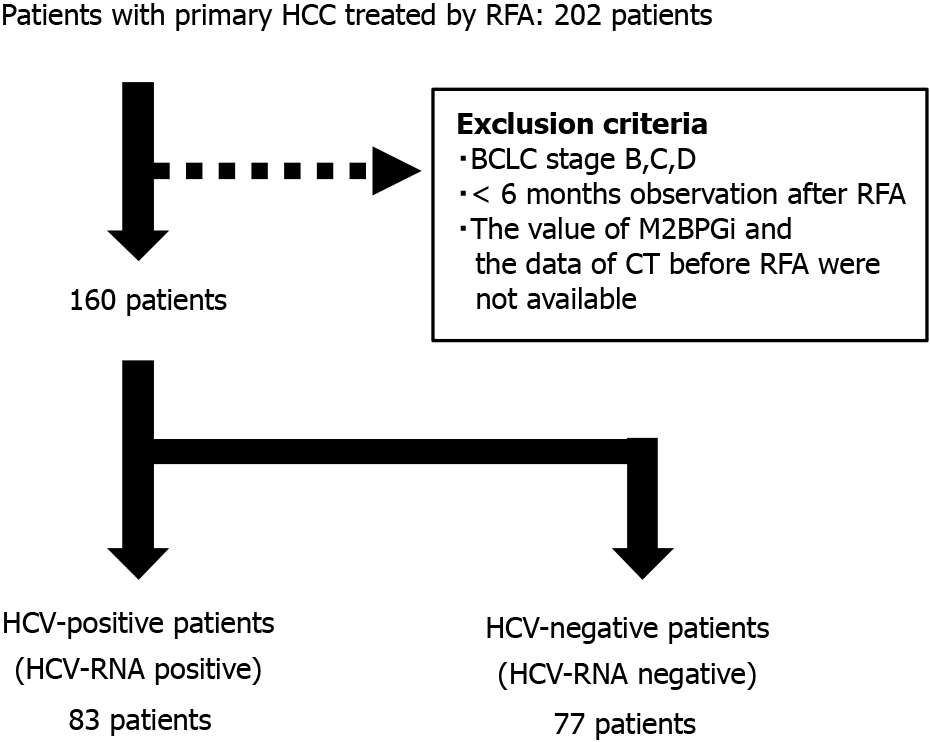
A total of 202 patients underwent RFA for primary HCC between 2001 and 2017 at Hokkaido University Hospital, 160 of whom were diagnosed with BCLC stage 0 or A and followed up > 6 mo after RFA. Patients with HCV-RNA positive were classified to “HCV-positive” group and HCV-RNA negative were classified to “HCV-negative” group. Blood chemistry data, tumor factors (tumor number, size, and form), and clinical symptoms including ascites, pleural effusion, and hepatic encephalopathy were obtained before RFA.
Percutaneous RFA was performed using a cooled-tip electrode (Cool-Tip; Ablation Systems, Covidien, Boulder, Colombia, CO) after ultrasonography (US) planning. RFA was performed by experienced operators under real-time ultrasound guidance. In some cases, we used a contrast-enhanced US technique or a real-time visual support system to detect the tumor more clearly. Moreover, in some cases, artificial ascites or pleural fluid can prevent thermal injury to extrahepatic organs or avoid the lungs in the tracking line. The ablation time, including three occurrences of roll-off, was 3-12 min. The ablated lesion and ablative margin were assessed using dynamic computed tomography (CT) or magnetic resonance imaging (MRI) 1-4 d after RFA.
Because of the early detection of local and distant recurrence, the first imaging test (dynamic CT or MRI) after RFA was performed 4-8 wk after RFA. After the initial evaluation, follow-up by imaging (dynamic CT or MRI) and serum tumor markers such as alpha-fetoprotein (AFP), lens culinaris agglutinin-A reactive AFP isoform (AFP-L3), and des-γ-carboxy-prothrombin (DCP) were performed every 3-4 mo. Chest CT was regularly performed to detect distant metastases.
For HCC recurrence, appropriate treatment was performed according to liver cancer treatment guidelines[28-31]. Deaths due to liver cancer, liver failure (including acute or chronic liver failure), hemorrhage due to gastroesophageal varices, and infections associated with spontaneous bacterial peritonitis were defined as liver-related deaths. Deaths other than liver diseases, such as other organ cancers, ischemic heart disease, and pneumonia, were analyzed as survival sensors.
Pre-sarcopenia was assessed according to the sarcopenia assessment criteria of the JSH guidelines for sarcopenia in liver disease[14]. Skeletal muscle mass index (SMI) calculated using simple methods[14,16]. In particular, the left-right sum of the long axis times the short axis of the iliopsoas muscles at the level of the third lumbar vertebra (L3) divided by height squared. This method has been reported to correlate well with SMI calculated using a muscle mass measurement software.
M2BPGi levels were measured in the conserved serum before RFA and at 1 mo after RFA. M2BPGi detection was based on a lectin-antibody sandwich immunoassay (Sysmex Co., Kobe, Japan) and expressed as a cutoff index (COI), with a range of 0.1-20 COI as previously reported[18].
M2BP was measured in conserved serum using enzyme-linked immunosorbent assay methods (Human Mac-2 binding protein (Mac-2bp) Assay Kit, Immuno-Biological Laboratories Co., Ltd., Fujioka, Japan).
Statistical analyses were performed using EZR software[32]. The Mann-Whitney U test was used to analyze continuous variables. Fisher’s exact test was used for univariate analysis of ordered variables. The Kaplan-Meier method was used to determine recurrence and survival rates, and the log-rank test was used to analyze differences. The median value was used as the cutoff. For the multivariate analysis of factors related to recurrence and survival, Cox proportional hazards models with stepwise methods using P value were used.
The study protocol was approved by the Institutional Ethics Committee of Hokkaido University (IRB-No. 015-1412) and conformed to the ethical guidelines of the Declaration of Helsinki.
As shown in Figure 1, 202 patients underwent RFA for primary HCCs. Of these, 160 cases were classified as BCLC stage 0 or A, and the data were analyzed. Eighty-three patients were classified into the HCV-positive group, and 77 patients were classified into the HCV-negative group. The ratio of older age and Child-Pugh Grade B was higher in the HCV-positive group than in the HCV-negative group. Serum transaminase and fibrosis-4 (FIB-4) index were higher in the HCV-positive group than in the HCV-negative group. In addition, the serum AFP and AFP-L3 levels were higher in the HCV-positive group. The median tumor diameter and number were not significantly different; however, they tended to be larger in the HCV-positive group than in the HCV-negative group. In contrast, the SMI of the HCV-positive group was significantly lower than that of the HCV-negative group (Table 1).
| HCV-positive (n = 83) | HCV-negative (n = 77) | P value | |
| Sex (male/female) | 45/38 | 50/27 | 0.20 |
| Age (years)1 | 70 (44-90) | 64 (41-88) | < 0.01 |
| Tumor factors | |||
| Tumor number (solitary/multiple) | 63/20 | 68/9 | 0.07 |
| Tumor size (mm)1 | 17 (8-30) | 15 (6-30) | 0.05 |
| Tumor form (only boundary/others) | 67/16 | 68/9 | 0.20 |
| Stage (LCSG) (I/II/III) | 39/38/6 | 45/27/5 | 0.35 |
| Liver function | |||
| Child-Pugh Score (5-6/7-9) | 66/17 | 66/11 | < 0.01 |
| ALBI grade (1/2-3) | 27/56 | 43/34 | 0.41 |
| Blood data | |||
| Platelet (×104/µL)1 | 10.2 (2.7-43.7) | 11.8 (3.7-36.8) | 0.04 |
| AST (U/L)1 | 56 (18-139) | 39 (16-100) | < 0.01 |
| ALT (U/L)1 | 49 (12-155) | 30 (9-87) | < 0.01 |
| FIB-4 index1 | 5.90 (0.96-37.86) | 3.61 (0.88-14.16) | < 0.01 |
| APRI1 | 2.00 (0.15-15.06) | 1.08 (0.28-4.32) | < 0.01 |
| PT (%)1 | 84.6 (48.6-125.0) | 81.3 (51.8-117.1) | 0.51 |
| Total bilirubin (mg/dL)1 | 0.9 (0.2-2.8) | 0.9 (0.4-2.7) | 0.56 |
| Albumin (g/dL)1 | 3.7 (2.2-4.7) | 4.0 (2.4-5.0) | < 0.01 |
| AFP (ng/mL)1 | 17.4 (3.0-621.6) | 6.4 (1.3-1962.9) | < 0.01 |
| DCP (mAU/mL)1 | 23 (4-1086) | 22 (7-6308) | 0.66 |
| AFP-L3 (%)1 | 5.1 (< 0.5-69.1) | < 0.5 (< 0.5-85.6) | 0.03 |
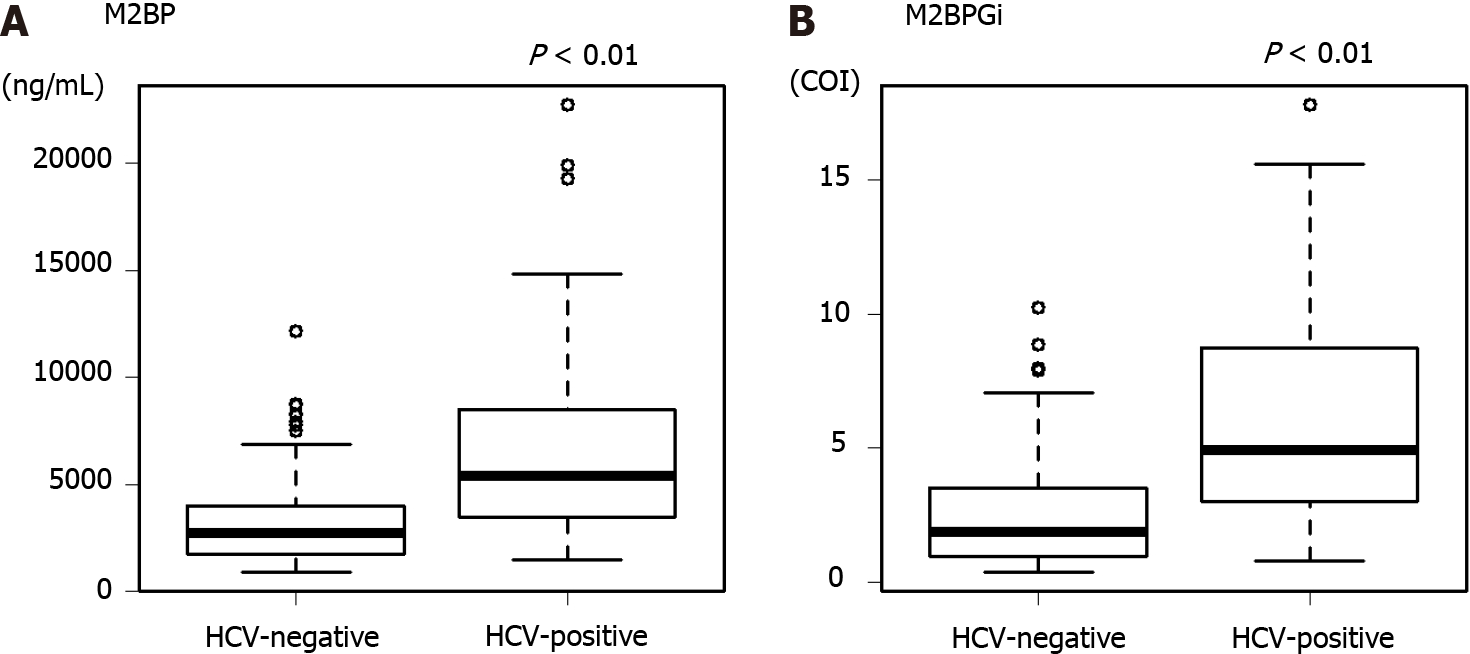
| M2BPGi (COI)1 | 4.94 (0.78-17.81) | 1.86 (0.36-10.23) | < 0.01 |
| M2BP (ng/mL)1 | 5385 (1460-22770) | 2745 (865-12150) | < 0.01 |
| SMI (cm2/m2)1 | 5.28 (2.62-11.75) | 6.51 (2.58-10.89) | < 0.01 |
| Pre-sarcopenia, n (%) | 21 (25.3) | 14 (18.2) | 0.34 |
| Observation period (mo)1 | 46 (6-157) | 56 (6-185) | 0.19 |
In the HCV-positive group, the median M2BP was 5385 ng/mL and that of M2BPGi was 4.94 COI. On the other hand, in the HCV-negative group, the median M2BP was 2745 ng/mL and that of M2BPGi was 1.86 COI. M2BP and M2BPGi levels were significantly higher in the HCV-positive group than in the negative group (Figure 2). Therefore, we used the median as the cutoff value in the following analysis for each group.
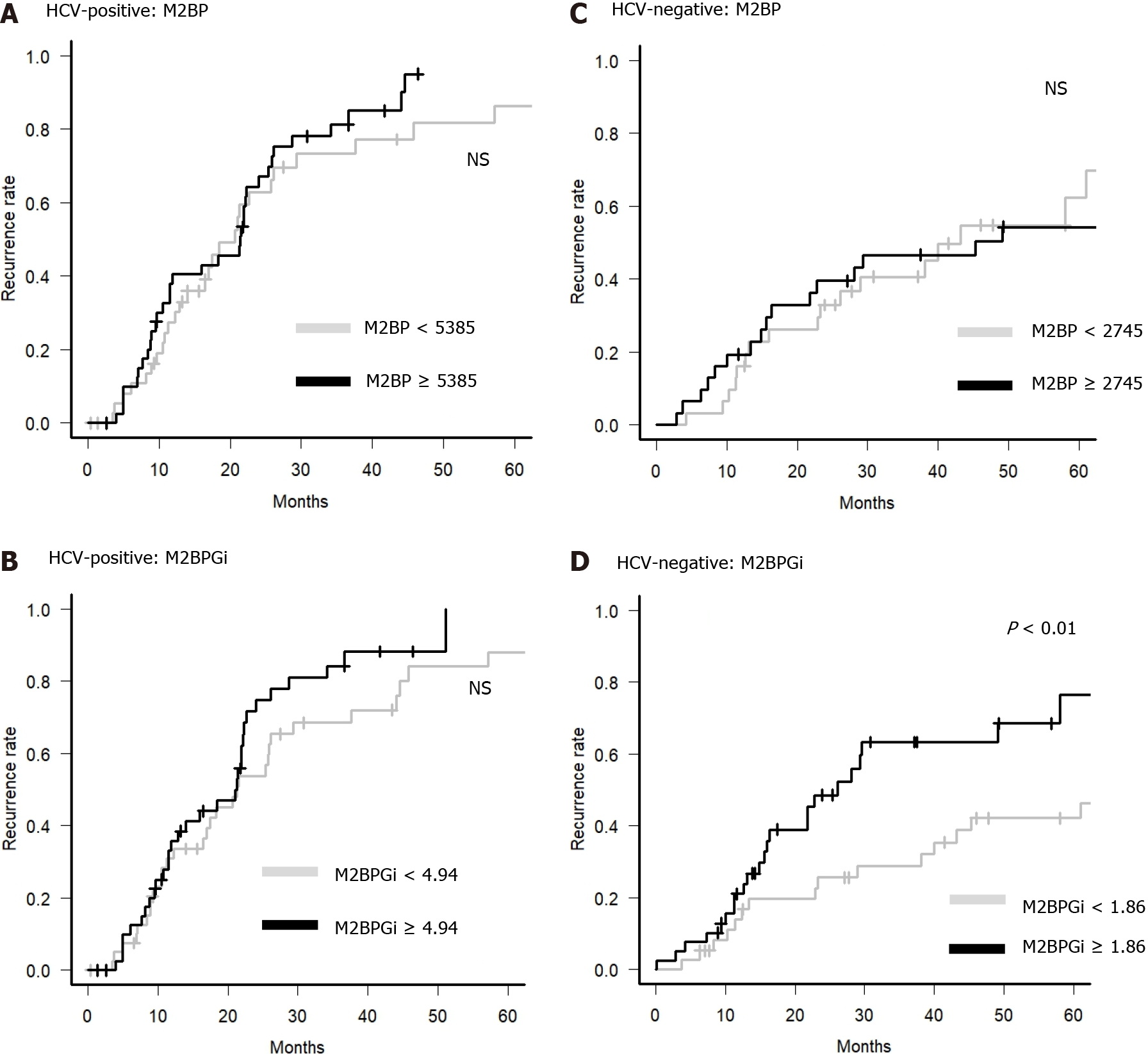
Next, we examined whether M2BP and M2BPGi could be predictive factors for HCC recurrence in primary HCC patients with BCLC stage 0 or A. M2BP could not be a predictive factor for HCC recurrence in each group, but M2BPGi could be a clinical predictor for HCC recurrence only in the HCV-negative group (Figure 3). Therefore, it is suggested that M2BPGi, but not M2BP, is a predictive factor for HCC recurrence in patients without current HCV infection.
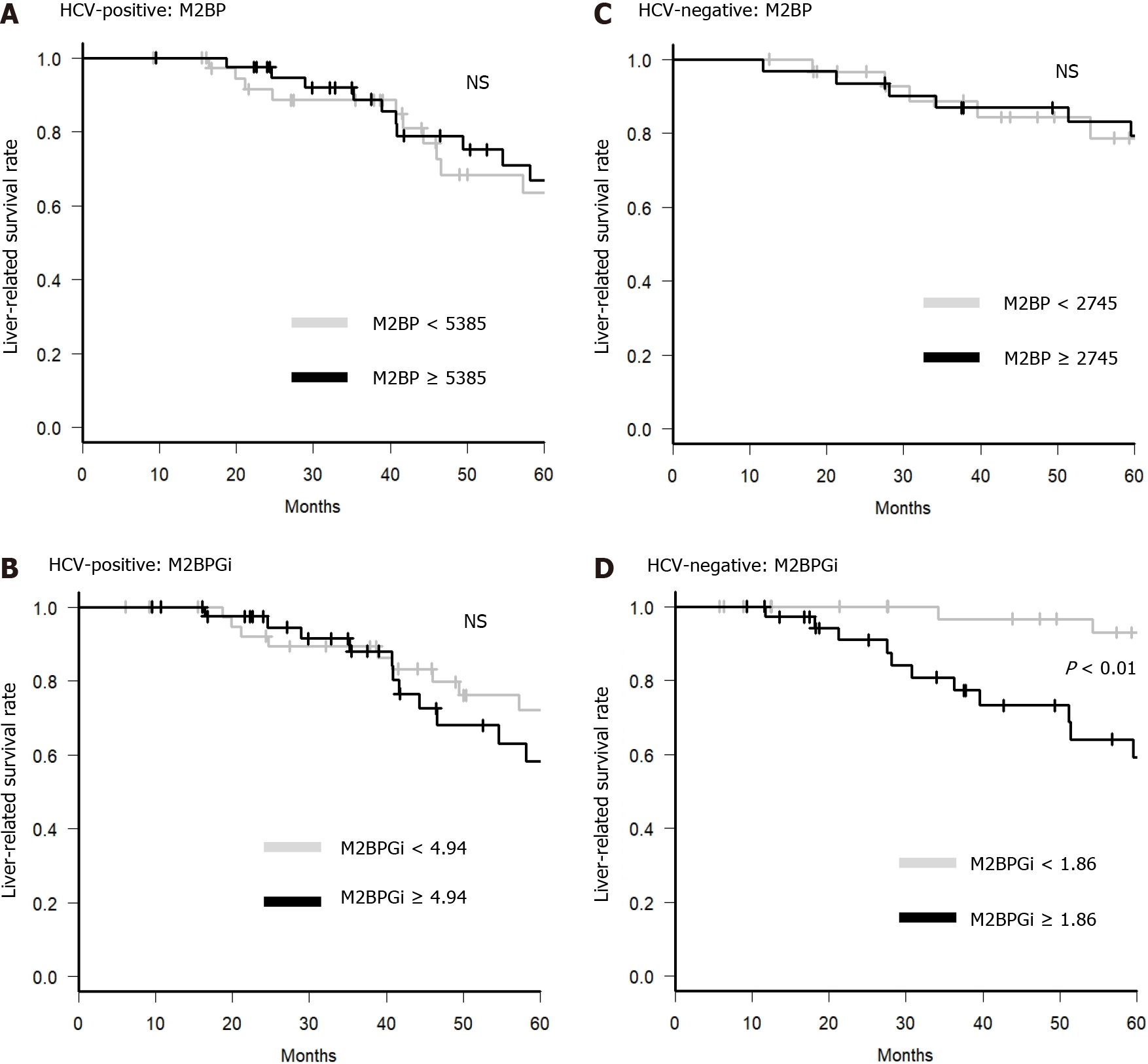
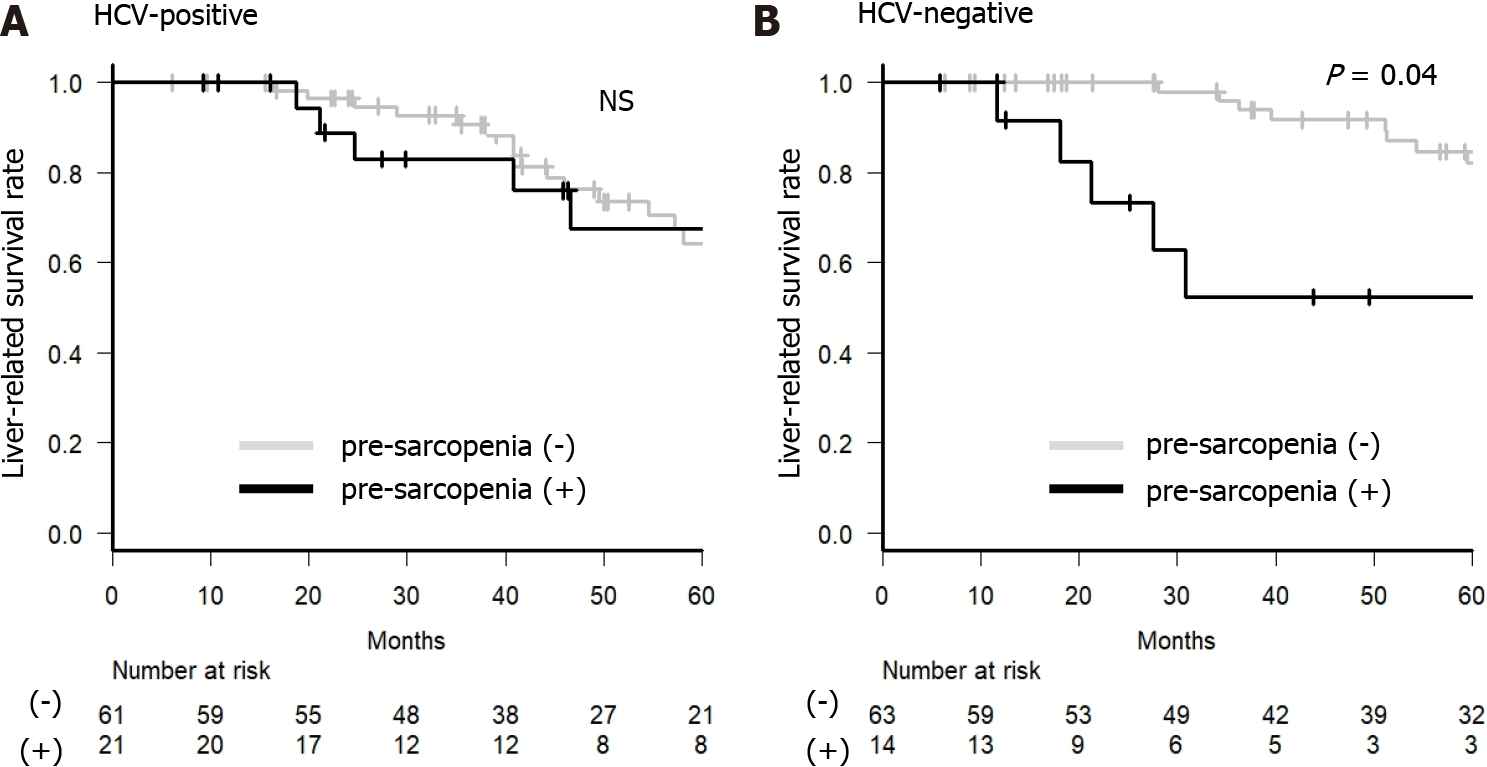
For further analysis, we examined whether M2BP and M2BPGi could be predictive factors of liver-related death in BCLC stage 0 or A. In the HCV-positive group, older age (≥ 70 years), albumin-bilirubin (ALBI) grade 2 or 3, DCP ≥ 23 mAU/L, and AFP-L3 ≥ 10% were factors contributing to a negative effect on survival on univariate analysis. Only DCP ≥ 23 mAU/L was a factor contributing to a negative effect on survival on multivariate analysis, and higher M2BP and M2BPGi were not significant factors for a negative effect on survival in the HCV-positive group (Table 2). In contrast, in the HCV-negative group, M2BPGi ≥ 1.86 COI and pre-sarcopenia were significant factors contributing to a negative effect on survival (Table 3). In the HCV-negative patient group, M2BPGi, but not M2BP, was a poor prognostic factor (Figure 4). Similarly, pre-sarcopenia was a poor prognostic factor only in the HCV-negative group (Figure 5). Therefore, higher M2BPGi and pre-sarcopenia were poor prognostic factors in patients without active HCV infection.
| Subject | Univariate | Multivariate | ||
| P value | HR | 95%CI | P value | |
| Age (< 70/≥ 70 years) | 0.08 | 1.92 | 0.94-3.94 | 0.074 |
| Sex (Female/Male) | 0.13 | |||
| ALBI grade (1/2,3) | 0.06 | 1.81 | 0.84-3.90 | 0.129 |
| Child-Pugh Score (5-6/7-15) | 0.15 | |||
| Stage (LCSG) (I/II+III) | 0.47 | |||
| Tumor number (solitary/multiple) | 0.97 | |||
| Tumor form (only boundary/others) | 0.43 | |||
| Tumor size (< 20 mm/≥ 20 mm) | 0.54 | |||
| AFP (< 17.2/≥ 17.2 ng/mL) | 0.12 | |||
| DCP (< 23/≥ 23 mAU/mL) | < 0.01 | 2.54 | 1.23-5.23 | 0.012 |
| AFP-L3 (< 10/≥ 10%) | 0.02 | 1.72 | 0.80-3.71 | 0.167 |
| M2BPGi (< 4.94/≥ 4.94 COI) | 0.26 | |||
| M2BP (< 5385/≥ 5385 ng/mL) | 0.24 | |||
| APRI (< 2.0/≥ 2.0) | 0.58 | |||
| FIB-4 index (< 4.5/≥ 4.5) | 0.31 | |||
| Pre-sarcopenia (No/Yes) | 0.28 | |||
| Subject | Univariate | Multivariate | ||
| P value | HR | 95%CI | P value | |
| Age (< 65/≥ 65) | 0.03 | - | ||
| Sex (Female/Male) | 0.88 | |||
| ALBI grade (1/2,3) | < 0.01 | 2.41 | 0.81-7.12 | 0.115 |
| Child-Pugh Score (5-6/7-15) | < 0.01 | - | ||
| Stage (LCSG) (I/II+III) | 0.91 | |||
| Tumor number (solitary/multiple) | 0.54 | |||
| Tumor form (boundary/others) | 0.11 | |||
| Tumor size (< 20 mm/≥ 20 mm) | 0.74 | |||
| AFP (< 6.4/≥ 6.4 ng/mL) | 0.64 | |||
| DCP (< 22/≥ 22 mAU/mL) | 0.23 | |||
| AFP-L3 (< 10/≥ 10%) | 0.29 | |||
| M2BPGi (< 1.86/≥ 1.86 COI) | < 0.01 | 4.89 | 1.97-12.18 | < 0.001 |
| M2BP (< 2745/≥ 2745 ng/mL) | 0.92 | |||
| APRI (< 1.5/≥ 1.5) | 0.04 | - | ||
| FIB-4 index (< 3.6/≥ 3.6) | < 0.01 | 1.86 | 0.63-5.44 | 0.257 |
| Pre-sarcopenia (no/yes) | 0.04 | 3.34 | 1.19-9.37 | 0.022 |
In this study, we retrospectively analyzed the prognostic factors of early-stage HCC (BCLC stage 0-A) after RFA treatment. Here, we investigated the usefulness of M2BGi and M2BP as predictors of HCC recurrence and prognosis. As a result, M2BPGi and pre-sarcopenia were useful in HCC recurrence and as prognostic factors in patients without current HCV infection.
Many randomized controlled trials[11,33-40] have compared the treatment outcomes of hepatectomy and RFA for early-stage HCC, but there are few reports with high quality evidence[11,39,40]. In recent years, Ng et al[11] reported no statistically significant difference in recurrence-free survival between hepatectomy and RFA in 109 cases. In the SURF trial, hepatectomy and RFA for HCC with a Child-Pugh score ≤ 7, tumor diameter ≤ 3 cm, and tumor number ≤ 3 had equivalent recurrence-free survival[39]. Based on the above, RFA has almost the same therapeutic results as hepatectomy for BCLC stage 0/A HCC. Considering that RFA is less invasive than hepatectomy, it is expected to become a standard treatment.
However, it has been reported that local recurrence is observed in approximately 10% of cases in which a sufficient ablation area is obtained by RFA[41,42]. The risk factors for recurrence have also been reported. Shiina et al[13] reported that, in a large number of cases, a higher DCP was associated with local recurrence. Ectopic recurrence is associated with HCV positivity, Child-Pugh grade B or C, platelet counts ≤ 100000, higher AFP, higher DCP, large tumor diameter, and a large number of tumors. Thus, regarding the recurrence of HCC after RFA, not only tumor factors but also factors related to liver function are largely involved. Contrarily, factors related to survival after RFA including younger age, lack of portosystemic shunt, Child-Pugh grade A, lower bilirubin, lower ALBI score, higher albumin, higher prothrombin time, lower AFP, HBV positivity, lower neutrophil to lymphocyte ratio, small tumor diameter, and low tumor number have been reported in a meta-analysis[43]. Therefore, liver function and pretreatment tumor factors are considered important factors not only for recurrence but also for survival.
In this study, we focused on M2BPGi and muscle mass, which are not direct tumor factors and liver function. M2BP is a secreted glycoprotein of approximately 90 kDa that was originally reported as a ligand for galectin[44]. The serum concentration of M2BP has been reported to increase in various cancers, such as breast and lung cancers[45]. Furthermore, Kamada et al[46] reported its usefulness as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. M2BPGi has a sugar chain with an affinity for WFA and distinguishes the glycan structure of WFA-detectable M2BP. The usefulness of M2BPGi as a marker of liver fibrosis in patients with HCV infection was reported in 2013[18]. M2BPGi has also been reported to be useful as a fibrosis marker in various liver diseases[19-22]. However, the M2BPGi value differs depending on the background liver disease, and it has been reported that the predicted cutoff value of METAVIR scoring system in the F4 stage is 5.2 COI for HCV, 3.1 COI for HBV, and 0.91 COI for NASH[47,48]. M2BPGi is an interferon (IFN)-simulated protein, and the amount of M2BPGi decreases after HCV eradication[49]. Therefore, it is suggested that M2BPGi is high in patients currently infected with HCV, even with the same degree of liver fibrosis. In this study, the median values differed significantly between the HCV-positive and HCV-negative patients. The M2BPGi levels were significantly higher in HCV-positive patients than in HCV-negative patients (Figure 2). Therefore, we analyzed the M2BPGi values separately in HCV-positive and HCV-negative patients.
M2BPGi has also been reported as a useful marker for predicting the occurrence of HCC. Specifically, it has been reported as a marker for predicting HCC in HCV, HBV and post-HCV eradication cases[19,23,25,27,49-57]. In this study, M2BPGi significantly predicted recurrence in HCV-negative cases. In contrast, M2BP level was not be a predictor of recurrence. Progression of liver fibrosis is a risk factor for HCC. As M2BPGi reflects liver fibrosis, M2BPGi may be indirectly associated with the development of HCC. M2BPGi may show higher levels in HCV cases than in others, even at similar levels of liver fibrosis. This is because the inflammation caused by the current HCV infection might affect the M2BPGi value in the HCV-positive group. Therefore, predicting HCC recurrence may be difficult using the value of M2BPGi only in HCV-positive cases. Based on the results of this study, prediction of cases at a high risk for recurrence after RFA was possible in early-stage HCC by focusing on the value of M2BPGi in HCV-negative patients.
Furthermore, M2BPGi has been reported to be a useful marker for predicting the prognosis of patients after HCV eradication, hepatectomy, and transcatheter arterial chemoembolization[25,58,59]. In this study, we analyzed prognostic factors after RFA for early-stage HCC, focusing on M2BP, M2BPGi, and pre-sarcopenia. In HCV-positive cases, DCP that is one of the serum tumor markers of HCC was a significant poor prognosis factor. In contrast, in HCV-negative cases, M2BPGi and pre-sarcopenia were significant poor prognostic factors, but tumor factors (tumor number, size, form, and serum markers) were not. In addition, M2BP was not a prognostic predictor in either group. M2BPGi levels are affected by various factors, including acute liver failure, and are associated with liver inflammation, damage, and hepatocyte degeneration[60]. Furthermore, M2BPGi was reported to correlate with inflammatory cytokines and was reduced by steroid treatment in patients with autoimmune hepatitis[61]. In HCV-negative cases, high M2BPGi levels may indicate advanced fibrosis or coexistence of inflammation because these cases are not affected by HCV. Therefore, M2BPGi may be a predictor of liver-related death. Notably, M2BPGi was a more sensitive prognostic marker than other liver function or fibrosis markers such as ALBI and FIB-4 in HCV-negative patients. Thus, M2BPGi may be a marker that can predict poor prognosis, including the effects of other factors, such as inflammation and liver fibrosis.
Patients with chronic liver disease and sarcopenia have a significantly poorer prognosis[62]. Furthermore, it has been reported that in the elderly, pre-sarcopenia cases have a poorer prognosis than non-sarcopenia cases[17]. In this study, pre-sarcopenia was a significant poor prognostic factor in HCV-negative cases but was not a significant prognostic factor in HCV-positive cases. The reason for this might be related to the fact that HCV-positive patients had significantly less SMI than the HCV RNA-negative patient group (Table 1). Because muscle volume increases after IFN-free treatment in HCV-positive patients and HCV elimination suppresses pre-sarcopenia, the current HCV infection itself may contribute to pre-sarcopenia. In this study, the high proportion of cases of pre-sarcopenia and the elderly may have affected the observation that pre-sarcopenia was not a significant prognostic factor in HCV-positive cases[63,64].
This study has several limitations. First, it was a retrospective observational study involving a single hospital and a small number of patients. Second, SMI was evaluated using only the simple CT method. Further studies with larger patient numbers and multicenter evaluations are needed.
In the near future, almost all HCVs will be eradicated by DAA treatment. Henceforth, almost no HCC cases were derived from the current HCV infection. In this study, we investigated the predictive factors of survival after RFA for HCC in BCLC stage 0 or A patients divided into two groups: HCV-RNA positive and negative. Pre-sarcopenia and M2BPGi, but not M2BP, might be useful tools for the prediction of survival in early-stage HCC in the era of HCV eradication.
Hepatocellular carcinoma (HCC) is prone to recurrence, even if cured at an early stage. In recent years, many studies have demonstrated that sarcopenia is a poor prognostic factor in patients with chronic liver disease and HCC, because it is related to frailty, loss of function, and low quality of life. Pre-sarcopenia is defined as muscle volume loss without muscle power loss and is a poor prognostic factor in the elderly population. In addition, the usefulness of the Mac-2 binding protein glycosylation isomer (M2BPGi), or Wisteria floribunda agglutinin-positive M2BP, which was first reported as a fibrosis marker in hepatitis C virus (HCV) patients, to treat HCC has recently attracted attention.
The M2BPGi, a fibrosis marker in various liver diseases, is reportedly a prognostic marker in patients with HCC who underwent hepatectomy. In recent years, many studies have demonstrated that sarcopenia is a poor prognostic factor in patients with chronic liver disease and HCC, because it is related to frailty, loss of function, and low quality of life. Sarcopenia is diagnosed using both muscle power loss and muscle volume loss. Pre-sarcopenia is defined as muscle volume loss without muscle power loss and is a poor prognostic factor in the elderly population.
To investigate the usefulness of pre-sarcopenia, M2BPGi, and M2BP as recurrence and prognostic factors in patients who underwent RFA for early-stage HCC.
In this study, 202 patients underwent radiofrequency ablation (RFA) for primary HCCs. Of these, 160 cases were classified as BCLC stage 0 or A, and the data were analyzed. Eighty-three patients were classified into the HCV-positive group, and 77 patients were classified into the HCV-negative group.
In HCV-positive patients, only des-γ-carboxy-prothrombin (DCP) ≥ 23 mAU/mL was a significant poor prognostic factor affecting survival after RFA. In HCV-negative patients, M2BPGi ≥ 1.86 cutoff index was significantly associated with tumor recurrence, but M2BP was not. M2BPGi ≥ 1.86 cutoff index (hazard ratio, 4.89; 95% confidence interval: 1.97-12.18; P < 0.001) and pre-sarcopenia (hazard ratio, 3.34, 95% confidence interval: 1.19-9.37; P = 0.022) were independent significant poor prognostic factors in HCV-negative patients.
In HCV-negative patients with primary HCC treated with RFA, lower M2BPGi contributed to a lower tumor recurrence rate and longer survival period. Pre-sarcopenia contributed to the poor prognosis independently in HCV-negative patients.
In the near future, almost all HCVs will be eradicated by DAA treatment. Almost no HCC cases were derived from the current HCV infection. Pre-sarcopenia and M2BPGi, but not M2BP, might be useful tools to predict survival in early-stage HCC in the era of HCV eradication.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mohamed GA, Egypt; Yuan H, China S-Editor: Wang LL L-Editor: A P-Editor: Yuan YY
| 1. | International Agency for Reserach on Cancer. Cancer fact sheets, 2020. [cited 20 April 2022]. Available from: https://gco.iarc.fr/today/fact-sheets-cancers. |
| 2. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1501] [Article Influence: 187.6] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, Lencioni R, Greten TF, Kudo M, Mandrekar SJ, Zhu AX, Finn RS, Roberts LR; AASLD Panel of Experts on Trial Design in HCC. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology. 2021;73 Suppl 1:158-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6065] [Article Influence: 866.4] [Reference Citation Analysis (3)] |
| 5. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 6. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 7. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 584] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection vs transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1272] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 9. | Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020;6:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 10. | Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation vs Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 11. | Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, Yuen J, Poon RTP, Fan ST, Lo CM. Randomized clinical trial of hepatic resection vs radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 12. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 13. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-577; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 14. | Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 479] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 15. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7810] [Article Influence: 1301.7] [Reference Citation Analysis (1)] |
| 16. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8473] [Article Influence: 564.9] [Reference Citation Analysis (0)] |
| 17. | Lera L, Angel B, Marquez C, Saguez R, Albala C. Besides Sarcopenia, Pre-Sarcopenia Also Predicts All-Cause Mortality in Older Chileans. Clin Interv Aging. 2021;16:611-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, Narimatsu H. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 19. | Ishii A, Nishikawa H, Enomoto H, Iwata Y, Kishino K, Shimono Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Iijima H, Nishiguchi S. Clinical implications of serum Wisteria floribunda agglutinin-positive Mac-2-binding protein in treatment-naïve chronic hepatitis B. Hepatol Res. 2017;47:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Iijima H, Nishiguchi S. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol Res. 2016;46:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Umemura T, Joshita S, Sekiguchi T, Usami Y, Shibata S, Kimura T, Komatsu M, Matsumoto A, Ota M, Tanaka E. Serum Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein Level Predicts Liver Fibrosis and Prognosis in Primary Biliary Cirrhosis. Am J Gastroenterol. 2015;110:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, Hara Y, Hige S, Sakamoto M, Yamada G, Kage M, Korenaga M, Hiasa Y, Mizokami M, Narimatsu H. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015;50:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, Hashimoto S, Sasaki R, Bekki S, Kugiyama Y, Miyazoe Y, Kuno A, Korenaga M, Togayachi A, Ocho M, Mizokami M, Narimatsu H, Yatsuhashi H. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 24. | Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Korenaga M, Mizokami M, Narimatsu H. Serum WFA+ -M2BP levels as a prognostic factor in patients with early hepatocellular carcinoma undergoing curative resection. Liver Int. 2016;36:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, Murakawa M, Nitta S, Itsui Y, Azuma S, Kakinuma S, Nouchi T, Sakai H, Tomita M, Watanabe M; Ochanomizu Liver Conference Study Group. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 26. | Kawanaka M, Tomiyama Y, Hyogo H, Koda M, Shima T, Tobita H, Hiramatsu A, Nishino K, Okamoto T, Sato S, Hara Y, Nishina S, Kawamoto H, Chayama K, Okanoue T, Hino K. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts the development of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Hepatol Res. 2018;48:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Tseng TC, Peng CY, Hsu YC, Su TH, Wang CC, Liu CJ, Yang HC, Yang WT, Lin CH, Yu ML, Lai HC, Tanaka Y, Nguyen MH, Liu CH, Chen PJ, Chen DS, Kao JH. Baseline Mac-2 Binding Protein Glycosylation Isomer Level Stratifies Risks of Hepatocellular Carcinoma in Chronic Hepatitis B Patients with Oral Antiviral Therapy. Liver Cancer. 2020;9:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J Gastroenterol. 2006;12:828-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society of Hepatology 2009 update. Hepatol Res. 2010;40 Suppl 1:2-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, Kudo M, Kubo S, Takayama T, Tateishi R, Fukuda T, Matsui O, Matsuyama Y, Murakami T, Arii S, Okazaki M, Makuuchi M. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 31. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 398] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 32. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13331] [Article Influence: 1110.9] [Reference Citation Analysis (0)] |
| 33. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1104] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 34. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 35. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 598] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 36. | Fang Y, Chen W, Liang X, Li D, Lou H, Chen R, Wang K, Pan H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Liu H, Wang ZG, Fu SY, Li AJ, Pan ZY, Zhou WP, Lau WY, Wu MC. Randomized clinical trial of chemoembolization plus radiofrequency ablation vs partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg. 2016;103:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Lee HW, Lee JM, Yoon JH, Kim YJ, Park JW, Park SJ, Kim SH, Yi NJ, Suh KS. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018;94:74-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Izumi N, Hasegawa K, Nishioka Y, Takayama T, Yamanaka N, Kudo M, Shimada M, Inomata M, Kaneko S, Baba H, Koike K, Omata M, Makuuchi M, Matsuyama Y, Kokudo N. A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). Journal of Clinical Oncology. 2019;37:4002-4002. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Kudo M, Hasegawa K, Kawaguchi Y, Takayama T, Izumi N, Yamanaka N, Shimada M, Inomata M, Kaneko S, Baba H, Koike K, Omata M, Makuuchi M, Matsuyama Y, Kokudo N. A multicenter randomized controlled trial to evaluate the efficacy of surgery vs radiofrequency ablation for small hepatocellular carcinoma (SURF trial): Analysis of overall survival. Journal of Clinical Oncology. 2021;39:4093-4093. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 42. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 43. | Lee MW, Kang D, Lim HK, Cho J, Sinn DH, Kang TW, Song KD, Rhim H, Cha DI, Lu DSK. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30:2391-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 44. | Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactoside lectin. J Biol Chem. 1991;266:18731-18736. [PubMed] |
| 45. | Grassadonia A, Tinari N, Iurisci I, Piccolo E, Cumashi A, Innominato P, D'Egidio M, Natoli C, Piantelli M, Iacobelli S. 90K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj J. 2002;19:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Kamada Y, Ono M, Hyogo H, Fujii H, Sumida Y, Yamada M, Mori K, Tanaka S, Maekawa T, Ebisutani Y, Yamamoto A, Takamatsu S, Yoneda M, Kawada N, Chayama K, Saibara T, Takehara T, Miyoshi E; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG‐NAFLD). Use of Mac-2 binding protein as a biomarker for nonalcoholic fatty liver disease diagnosis. Hepatol Commun. 2017;1:780-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 48. | Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, Narimatsu H, Mizokami M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. 2018;53:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 49. | Sasaki R, Yamasaki K, Abiru S, Komori A, Nagaoka S, Saeki A, Hashimoto S, Bekki S, Kugiyama Y, Kuno A, Korenaga M, Togayachi A, Ocho M, Mizokami M, Narimatsu H, Ichikawa T, Nakao K, Yatsuhashi H. Serum Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein Values Predict the Development of Hepatocellular Carcinoma among Patients with Chronic Hepatitis C after Sustained Virological Response. PLoS One. 2015;10:e0129053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Tamaki N, Kurosaki M, Kuno A, Korenaga M, Togayachi A, Gotoh M, Nakakuki N, Takada H, Matsuda S, Hattori N, Yasui Y, Suzuki S, Hosokawa T, Tsuchiya K, Nakanishi H, Itakura J, Takahashi Y, Mizokami M, Narimatsu H, Izumi N. Wisteria floribunda agglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol Res. 2015;45:E82-E88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Kawaguchi K, Honda M, Ohta H, Terashima T, Shimakami T, Arai K, Yamashita T, Sakai Y, Mizukoshi E, Komura T, Unoura M, Kaneko S. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts hepatocellular carcinoma incidence and recurrence in nucleos(t)ide analogue therapy for chronic hepatitis B. J Gastroenterol. 2018;53:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Liu J, Hu HH, Lee MH, Korenaga M, Jen CL, Batrla-Utermann R, Lu SN, Wang LY, Mizokami M, Chen CJ, Yang HI. Serum Levels of M2BPGi as Short-Term Predictors of Hepatocellular Carcinoma in Untreated Chronic Hepatitis B Patients. Sci Rep. 2017;7:14352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Ichikawa Y, Joshita S, Umemura T, Shobugawa Y, Usami Y, Shibata S, Yamazaki T, Fujimori N, Komatsu M, Matsumoto A, Tanaka E. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol Res. 2017;47:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Cheung KS, Seto WK, Wong DK, Mak LY, Lai CL, Yuen MF. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts liver cancer development in chronic hepatitis B patients under antiviral treatment. Oncotarget. 2017;8:47507-47517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Heo JY, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Park YN, Ahn SS, Han KH, Kim HS. Use of Wisteria Floribunda Agglutinin-Positive Human Mac-2 Binding Protein in Assessing Risk of Hepatocellular Carcinoma Due to Hepatitis B Virus. Medicine (Baltimore). 2016;95:e3328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Yasui Y, Kurosaki M, Komiyama Y, Takada H, Tamaki N, Watakabe K, Okada M, Wang W, Shimizu T, Kubota Y, Higuchi M, Takaura K, Tsuchiya K, Nakanishi H, Takahashi Y, Itakura J, Enomoto N, Izumi N. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts early occurrence of hepatocellular carcinoma after sustained virologic response by direct-acting antivirals for hepatitis C virus. Hepatol Res. 2018;48:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Sato S, Genda T, Ichida T, Amano N, Sato S, Murata A, Tsuzura H, Narita Y, Kanemitsu Y, Hirano K, Shimada Y, Iijima K, Wada R, Nagahara A, Watanabe S. Prediction of Hepatocellular Carcinoma Development after Hepatitis C Virus Eradication Using Serum Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Fujiyoshi M, Kuno A, Gotoh M, Fukai M, Yokoo H, Kamachi H, Kamiyama T, Korenaga M, Mizokami M, Narimatsu H, Taketomi A; Hepatitis Glyco-biomarker Study Group. Clinicopathological characteristics and diagnostic performance of Wisteria floribunda agglutinin positive Mac-2-binding protein as a preoperative serum marker of liver fibrosis in hepatocellular carcinoma. J Gastroenterol. 2015;50:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Tak KY, Jang B, Lee SK, Nam HC, Sung PS, Bae SH, Choi JY, Yoon SK, Jang JW. Use of M2BPGi in HCC patients with TACE. J Gastroenterol Hepatol. 2021;36:2917-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Morio K, Imamura M, Daijo K, Teraoka Y, Honda F, Nakamura Y, Kobayashi T, Nakahara T, Nagaoki Y, Kawaoka T, Tsuge M, Hiramatsu A, Kawakami Y, Aikata H, Nelson Hayes C, Tsugawa K, Yokozaki M, Chayama K. Wisteria floribunda agglutinin positive Mac-2-binding protein level increases in patients with acute liver injury. J Gastroenterol. 2017;52:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Migita K, Horai Y, Kozuru H, Koga T, Abiru S, Yamasaki K, Komori A, Fujita Y, Asano T, Sato S, Suzuki E, Matsuoka N, Kobayashi H, Watanabe H, Naganuma A, Naeshiro N, Yoshizawa K, Ohta H, Sakai H, Shimada M, Nishimura H, Tomizawa M, Ario K, Yamashita H, Kamitsukasa H, Kohno H, Nakamura M, Furukawa H, Takahashi A, Kawakami A, Ohira H, Yastuhashi H. Serum cytokine profiles and Mac-2 binding protein glycosylation isomer (M2BPGi) level in patients with autoimmune hepatitis. Medicine (Baltimore). 2018;97:e13450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 63. | Sugimoto R, Iwasa M, Hara N, Tamai Y, Yoshikawa K, Ogura S, Tanaka H, Eguchi A, Yamamoto N, Kobayashi Y, Hasegawa H, Takei Y. Changes in liver function and body composition by direct-acting antiviral therapy for hepatitis C virus infection. Hepatol Res. 2018;48:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Endo K, Sato T, Suzuki A, Yoshida Y, Kakisaka K, Miyasaka A, Takikawa Y. Sustained virologic response by direct-acting antivirals suppresses skeletal muscle loss in hepatitis C virus infection. J Gastroenterol Hepatol. 2020;35:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |