Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1357
Peer-review started: March 11, 2022
First decision: April 8, 2022
Revised: April 12, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: July 27, 2022
Processing time: 137 Days and 13.3 Hours
Ultrasonic devices are widely used in many surgical fields, including hepatectomy; however, the negative effects of tissue pad degradation of ultrasonic devices, including those in liver surgery, remain unknown. The Harmonic® 1100 (H-1100) scalpel has advanced heat control technology than previous models, such as the Harmonic® HD1000i (H-HD1000i). We hypothesized that, because of its advanced temperature-control technology, the H-1100 scalpel would show less tissue pad degradation, resulting in superior sealing performance, compared to that with the H-HD1000i scalpel.
To elucidate ultrasonic device tissue pad degradation effects on instrument temperature and sealing performance using ex vivo porcine liver/vessel models.
Two different harmonic scalpels were used and compared: A newer model, the H-1100 scalpel, and an older model, the H-HD1000i scalpel. Using ex vivo porcine livers, each instrument was activated until the liver parenchyma was dissected. The device temperature (passive jaw temperature) was measured after every 10 consecutive activations, until 300 transections of the porcine liver were performed. Tissue pad degradation was evaluated after 300 activations. Sealing performance was evaluated using excised porcine carotid vessels; vessel sealing speed and frequency of vessel burst pressure below 700 mmHg were determined after 300 transections of porcine liver parenchyma.
The temperature of the H-HD1000i scalpel was approximately 10°C higher than that of the H-1100 scalpel, and gradually increased as the number of activations increased. The median passive jaw temperature of the H-HD1000i scalpel was significantly higher than that of the H-1100 scalpel (73.4°C vs 65.1°C; P < 0.001). After 300 transections of porcine liver parenchyma, less tissue pad degradation was observed with the H-1100 scalpel than with the H-HD1000i scalpel (0.08 mm vs 0.51 mm). The H-1100 scalpel demonstrated faster vessel-sealing speed (4.9 sec. vs 5.1 sec.) and less frequent vessel burst pressure < 700 mmHg (0% vs 40%) after 300 activations than the H-HD1000i scalpel; however, the difference did not reach statistical significance (P = 0.21 and P = 0.09, respectively).
In an ex vivo porcine hepatectomy model, the H-1100 scalpel shows lower passive jaw temperature and maintains its sealing performance by avoiding tissue pad degradation compared to that with the H-HD1000i.
Core Tip: The present study showed that the tissue pad of the Harmonic® 1100 (H-1100) ultrasonic scalpel, with improved heat control technology, was preserved even in a hepatectomy model, which is a severe condition for ultrasonic devices. It can be inferred that by using the H-1100 scalpel, even surgeons-in-training inexperienced in handling ultrasonic devices do not need to worry about device issues related to tissue pad degradation. Furthermore, use of the H-1100 scalpel may eventually reduce hospital costs.
- Citation: Kajiwara M, Fujikawa T, Hasegawa S. Tissue pad degradation of ultrasonic device may enhance thermal injury and impair its sealing performance in liver surgery. World J Hepatol 2022; 14(7): 1357-1364
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1357.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1357
Advanced-energy devices have become indispensable in modern surgery for tissue dissection and vascular control. In hepatectomy, these devices are known to shorten the liver transection time[1] and reduce blood loss during liver transection[2]. Ultrasonic devices, such as the harmonic scalpel (Ethicon, Cincinnati, Ohio, United States), are representative of the various energy devices that provide precise tissue dissection and secure hemostasis during meticulous procedures and improve surgical outcomes[3-5]. However, the tissue pad on the passive jaw of ultrasonic devices is reported to degrade with repeated long activations, or with activations without any tissue intervention[6].
To maintain a satisfactory burst pressure, a higher compression force is important[7,8]. Tissue pad degradation in an ultrasonic device generates a substantial gap between the active blade and the tissue pad, leading to decreased compression pressure of the targeted tissue and increased sealing time. With longer sealing time, the device temperature becomes higher[6,8]. Consequently, tissue pad degradation can result in undesirable thermal damage to the adjacent tissues.
The Harmonic® 1100 (H-1100) scalpel is a state-of-the-art ultrasonic device equipped with an improved heat control algorithm compared to that of the Harmonic® HD1000i (H-HD1000i), a previous model. The H-1100 scalpel actively lowers the blade heat as soon as the targeted tissue is dissected. This advanced H-1100 technology prevents overheating of the blade, which may protect the surrounding tissue from thermal injury and enhance tissue pad life throughout the procedure[9,10]. However, the advantage of the H-1100 scalpel in the field of liver surgery remains understudied.
As the liver is a solid organ, liver parenchymal dissection may burden ultrasonic devices with more mechanical stress compared to that with digestive tract surgery (in which, membrane and fat are the main dissection targets) and demand the enhanced tissue pad life. We hypothesized that, because of its advanced temperature-control technology, the H-1100 scalpel would show less tissue pad degradation, resulting in superior sealing performance, compared to that with the H-HD1000i scalpel. Therefore, we compared tissue pad degradation between H-1100 and H-HD1000i devices and examined the negative effects of tissue pad degradation on the device temperature and sealing capability (vessel sealing speed and burst pressure) using ex vivo porcine liver and vessel models, respectively.
Two different models of harmonic scalpels were used: a newer Harmonic® 1100 (H-1100) model and an older Harmonic® HD1000i (H-HD1000i) model. Both devices were powered by a GEN11 generator (Ethicon, Cincinnati, Ohio, United States), and the power level was set to five for all experiments. The H-1100 scalpel employs a new version of intelligent heat control technology (adaptive tissue technology), which prevents inadvertent activation when tissue is not on the device by cooling down the blade temperature immediately after tissue dissection[9,10]. With the exception of this difference in thermal control technology, the H-1100 and H-HD1000i models are considered as mechanically identical products[10].
Each experiment was performed twice (n = 2), and a new set of harmonic devices was used in each experiment. All experiments were conducted by a single attending surgeon familiar with hepatectomy using ultrasonic devices.
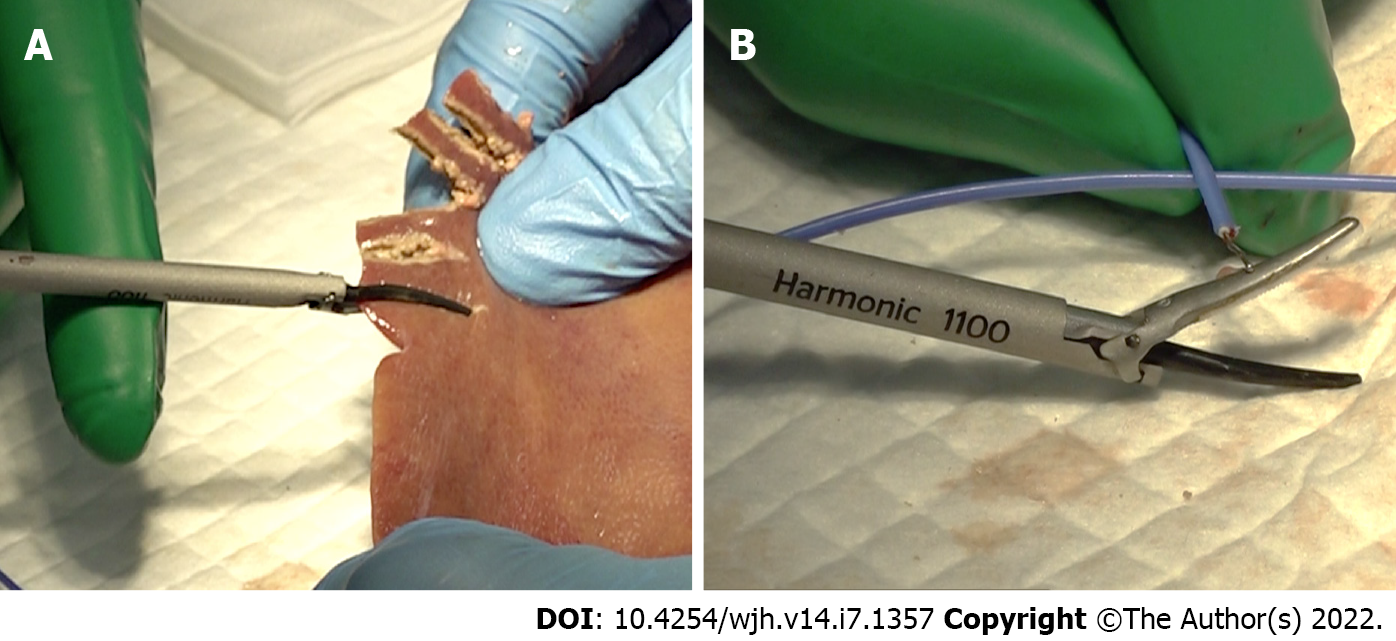
We measured the temperature of the back of the passive jaw as the device temperature. Ex vivo porcine liver (Tokyo-Shibaura-Zouki Co., Ltd., Tokyo, Japan) was used for this experiment. Commercially available porcine liver was harvested from living bodies at six days before the experiment, maintained and transported at 4°C, and then returned to room temperature on the day of the experiment. The harmonic scalpel was applied to the edge of the porcine liver with an almost full tissue bite and gradually clamped with continuous activation (Figure 1A). Activation was stopped when the liver tissue was completely dissected. A thermocouple temperature probe (Multichannel Recorder, MCR-4TC; T&D Corporation, Nagano, Japan) was attached to the back side of the passive jaw as soon as activation was completed (Figure 1B). The device temperature was measured repeatedly after every 10 consecutive activations, until 300 porcine liver transections were performed. A sufficient instrument cooling time was set after each device temperature measurement.
The extent of tissue pad degradation of the harmonic scalpel was measured using a digital indicator with a resolution of 0.01 mm (ID-S1012X, Mitutoyo Corporation, Kanagawa, Japan), after 300 activations.
Commercially available excised porcine carotid arteries (5-7 mm diameter; Tokyo-Shibaura-Zouki Co., Ltd., Tokyo, Japan) were used to evaluate the vessel sealing speed and burst pressure. The evaluated vessel size has been reported as safely sealed and cut using an ultrasonic device[11,12]. A catheter, securely ligated to the carotid vessel, was connected to a syringe and a manometer (Artfreak, Tokyo, Japan). Vessel sealing time was defined as the duration from harmonic scalpel activation to complete transection of the vessels. After sealing the vessel, saline was gradually infused into the vessel lumen at a constant rate by manually pushing a syringe. The burst pressure was identified as the pressure at the moment that the sealed vessel ruptured. Five replicates were performed for each harmonic device after 300 activations of the porcine liver parenchyma. We counted the frequency of burst pressure below 700 mmHg, which is considered to be a reliable measurement threshold for the manometer, although the scale of the manometer was up to 760 mmHg.
Continuous variables, such as device temperature and vessel sealing speed, are expressed as medians with ranges, and were non-parametrically compared between groups using the Mann-Whitney U test. The frequency of vessel burst pressure below 700 mmHg was compared between devices using the Fisher’s exact test.
All P values were two-sided, and P values < 0.05 were considered statistically significant. All statistical analyses were performed in EZR (Saitama Medical Centre, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria)[13].
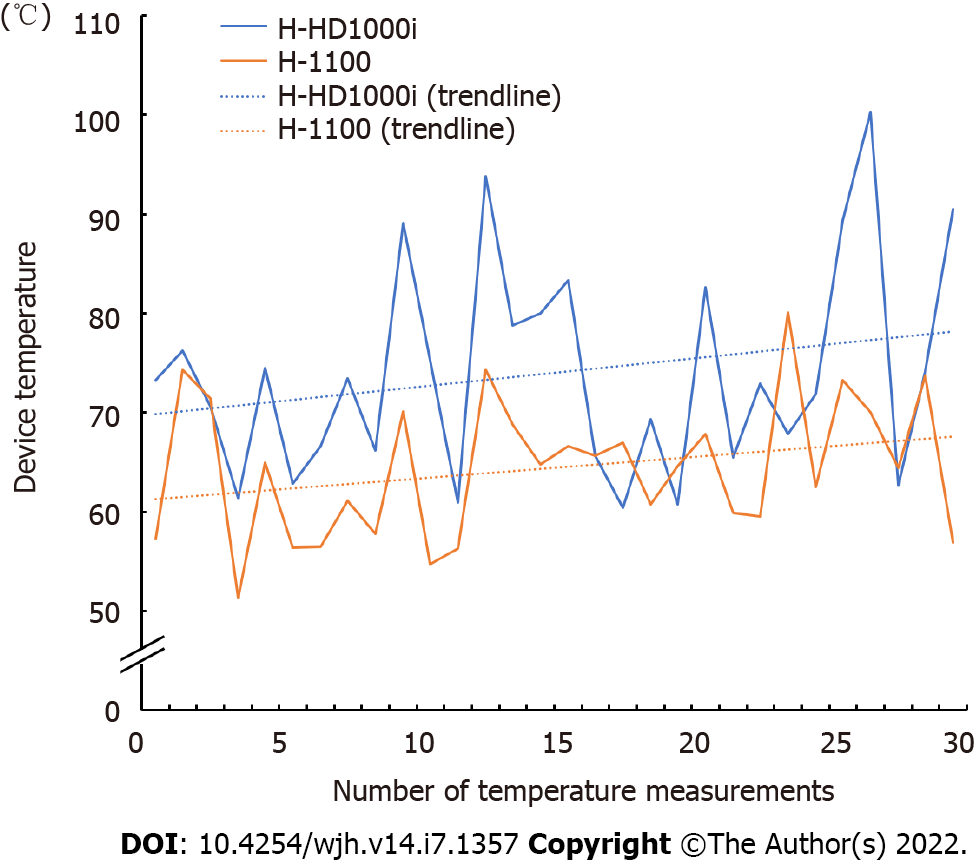
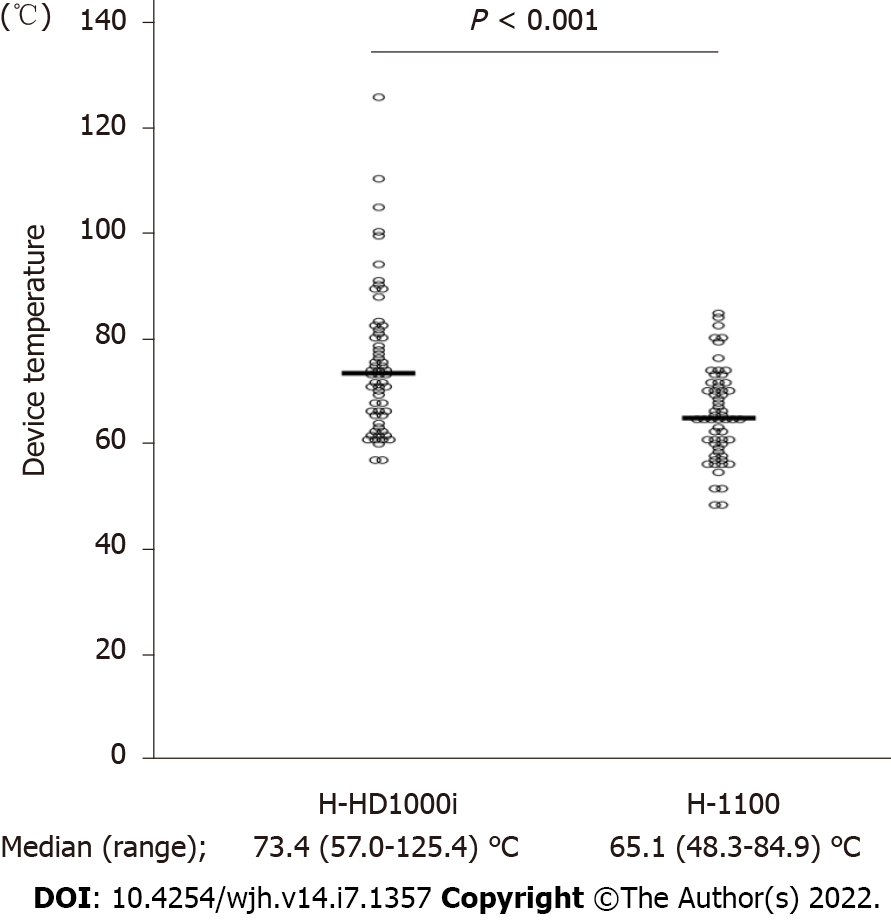
Figure 2 shows the representative trend in device temperature, which was measured after every 10 consecutive activations, until 300 porcine liver dissections were performed. The temperature of the H-HD1000i scalpel was approximately 10°C higher than that of the H-1100 scalpel, and gradually increased as the number of activations increased. All device temperatures measured in the two independent experiments are shown in Figure 3. The median device temperature of the HD-1000i scalpel was significantly higher than that of the H-1100 scalpel (73.4°C [range, 57.0°C-125.4°C] vs 65.1°C [range, 48.3°C-84.9°C]; P < 0.001).
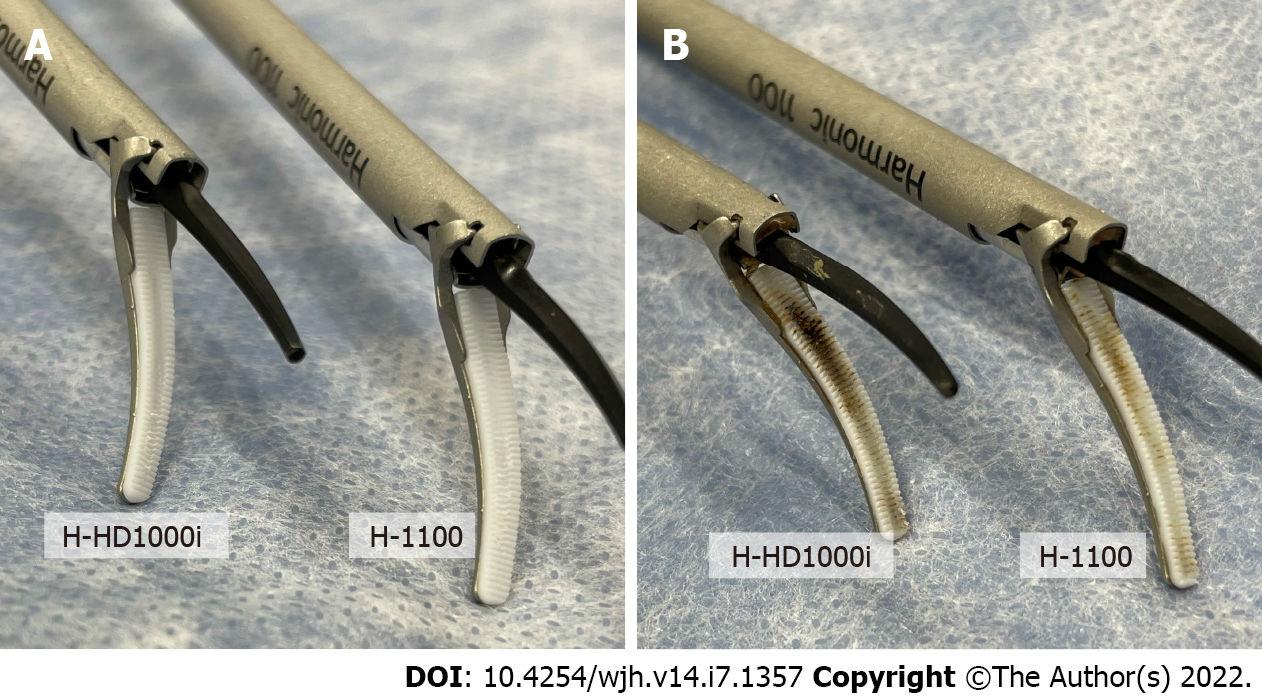
Figure 4 shows the tissue pads of both devices, before use and after 300 repeated activations. More prominent tissue pad degradation was observed with the H-HD1000i scalpel than with the H-1100 scalpel in both experiments. The mean depth of tissue pad degradation was 0.51 mm and 0.08 mm for the H-HD1000i and H-1100 scalpels, respectively (Table 1).
| H-HD1000i | H-1100 | |
| 1st experiment (mm) | 0.59 | 0.06 |
| 2nd experiment (mm) | 0.42 | 0.09 |
| Mean (mm) | 0.51 | 0.08 |
The vessel sealing speeds of the H-1100 and H-HD1000i scalpels after 300 transections of porcine liver were 4.9 sec. and 5.1 sec., respectively. To evaluate the vessel burst pressure, we counted the frequency of vessel burst pressure below 700 mmHg after 300 activations (Table 2). Four of 10 (40%) vessel burst trials (five replicates per independent experiment) showed a burst pressure below 700 mmHg with the H-HD1000i scalpel (650, 600, 600, and 500 mmHg), whereas zero of 10 (0%) vessel burst trials with the H-1100 device exhibited a vessel sealing pressure less than 700 mmHg. Although the H-1100 device demonstrated superior vessel sealing performance in both experiments, the difference did not reach statistical significance (P = 0.21 and P = 0.09, respectively).
| H-HD1000i | H-1100 | P value | |
| Vessel sealing spead (sec.) | 5.1 (3.9-5.9) | 4.9 (3.2-5.2) | 0.21 |
| Vessel burst pressure < 700 mmHg | 4 (40) | 0 (0) | 0.09 |
The tissue pad on the passive jaw of ultrasonic devices is made of polytetrafluoroethylene, also known as Teflon, which is durable, resistant to heat, and chemically stable, but begins to degrade when it reaches approximately 260°C[6]. The tissue pad degrades and melts with repeated long or inadvertent activation without any tissue intervention[6]. Possible reasons for tissue pad degradation are considered to comprise both thermal and mechanical stresses generated by the frictional motion of ultrasonic energy.
To the best of our knowledge, no study has focused on tissue pad degradation in ultrasonic devices. The results of the present study demonstrated that the H-1100 scalpel consistently maintained a lower passive jaw temperature and sustained its sealing capability by minimizing tissue pad degradation compared to that with the H-HD1000i scalpel in an ex vivo hepatectomy model. When using the H-HD1000i scalpel, which uses an older version of heat control technology, tissue pad degradation must be prevented manually by stopping the activation as soon as the target tissue is dissected. All experiments in the present study were performed by a single experienced surgeon who was familiar with hepatectomy using ultrasonic devices. Thus, the present results suggest that even a well-trained surgeon was not able to completely eliminate “air-activation” (inadvertent activation without tissue between the jaws) when using a harmonic scalpel, and thermal and mechanical stresses derived from an accumulation of minute air-activations following every single transection led to degradation of the tissue pad on the passive jaw.
The device temperature after 10 consecutive activations of the H-HD1000i scalpel gradually increased as the number of liver dissections increased. This finding likely indicates that the H-HD1000i tissue pad steadily degraded in accordance with the increase in the number of activations. The H-HD1000i scalpel was approximately 10°C higher than that of the H-1100 scalpel, on average, and became well above 60°C, which has been reported as the critical temperature for causing irreversible tissue denaturation (e.g. recurrent laryngeal nerve injury during esophagectomy)[14,15]. Interestingly, sporadic extremely high temperatures above 90°C were only observed with the H-HD1000i scalpel, which was more affected by prominent tissue pad degradation (Figures 3 and 4). To be more precise, the temperature accounting for lateral thermal damage should be measured a few millimeters from the point of instrument application, not at the device itself. Nevertheless, physicians should be aware of the potential risk of thermal injury when using ultrasonic devices and avoid it by ensuring an adequate cooling time for the device[6,16].
Regarding vessel sealing performance, the H-1100 scalpel showed sufficient sealing performance even after repeated activations of the porcine liver parenchyma. By preventing significant tissue pad degradation, the H-1100 scalpel can maintain the optimal clamping force. However, it must be noted that the burst pressure obtained by the H-HD1000i device after dissecting the liver parenchyma as many as 300 times was also well above the physiological level of blood pressure and, thus, is adequately within the safety limit for clinical purposes.
It has been reported that the temperature of liver tissue at 1 mm away from the harmonic scalpel is significantly higher than that of thyroid tissue or muscle tissue in a live porcine model, which could be a reflection of the tissue consistency, size, and relatively higher volume of blood flow through the liver[17]. Accordingly, the liver may constitute a heavy load in ultrasonic device dissection. In cases of major anatomical liver resection, many parenchymal dissections are required. The present study showed that the H-1100 tissue pad was preserved even in a hepatectomy model, which is a severe condition for ultrasonic devices. Accordingly, it can be inferred that by using the H-1100 scalpel, even surgeons-in-training inexperienced in handling ultrasonic devices do not need to worry about device issues related to tissue pad degradation. Furthermore, use of the H-1100 scalpel may eventually reduce hospital costs.
The present study has several limitations. First, the data were obtained from benchtop experiments using porcine tissues at room temperature, which may not reflect actual human liver surgery. Second, the real targets to be sealed with energy devices during hepatectomy are the intrahepatic Glissonian triads and hepatic veins. In the present study, vessel sealing was investigated using only on porcine carotid arteries without blood flow. Therefore, care should be taken when applying our results to the clinical setting of human hepatectomy. Finally, the data were obtained from duplicate experiments. The reason for the lack of a statistically significant difference in vessel sealing performance (even though the H-1100 scalpel exhibited superior sealing performance after repeated activations compared to that with the H-HD1000i scalpel) may be attributed to the small number of experiments.
In conclusion, the cutting-edge H-1100 scalpel, with improved heat control technology, maintains a lower passive jaw temperature than the previous model (H-HD1000i), and may reduce undesirable collateral thermal damage. Moreover, the H-1100 scalpel maintains its sealing performance by avoiding tissue pad degradation better than the previous model.
Ultrasonic devices are widely used in many surgical fields including hepatectomy in the modern era, while the negative effects of tissue pad degradation of ultrasonic devices in liver surgery still remain unknown.
As the liver is a solid organ, liver parenchymal dissection may burden ultrasonic devices with more mechanical stresses compared to the digestive tract surgery (in which, membrane and fat are the main dissection targets) and demand the enhanced tissue pad life. Therefore, we chose liver surgery for evaluating the effect of the tissue pad degradation.
To elucidate ultrasonic device tissue pad degradation effects on instrument temperature and sealing performance using ex vivo porcine liver/vessel models.
Two different harmonic scalpels were used and compared: Harmonic® 1100 (a new model; H-1100) and Harmonic® HD1000i (a previous model; H-HD1000i). The device temperature (passive jaw temperature), tissue pad degradation after 300 repeated activations, vessel sealing speed and burst pressure were measured.
H-1100 scalpel consistently maintained a lower passive jaw temperature and sustained its superior sealing performance by avoiding tissue pad degradation compared to that with the H-HD1000i scalpel.
In an ex vivo porcine hepatectomy model, the cutting-edge H-1100 scalpel maintains excellent performance throughout the procedure with the enhanced tissue pad life.
This study provides a new insight into understanding the negative influence of tissue pad degradation of ultrasonic devices on device temperature and sealing performance. H-1100 scalpel solves issues related to tissue pad degradation. Furthermore, use of the H-1100 scalpel may eventually reduce hospital costs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cassese G, Italy; Shen F, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Gotohda N, Yamanaka T, Saiura A, Uesaka K, Hashimoto M, Konishi M, Shimada K. Impact of energy devices during liver parenchymal transection: a multicenter randomized controlled trial. World J Surg. 2015;39:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Ichida A, Hasegawa K, Takayama T, Kudo H, Sakamoto Y, Yamazaki S, Midorikawa Y, Higaki T, Matsuyama Y, Kokudo N. Randomized clinical trial comparing two vessel-sealing devices with crush clamping during liver transection. Br J Surg. 2016;103:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Devassy R, Hanif S, Krentel H, Verhoeven HC, la Roche LAT, De Wilde RL. Laparoscopic ultrasonic dissectors: technology update by a review of literature. Med Devices (Auckl). 2019;12:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Cheng H, Clymer JW, Sadeghirad B, Ferko NC, Cameron CG, Amaral JF. Performance of Harmonic devices in surgical oncology: an umbrella review of the evidence. World J Surg Oncol. 2018;16:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | McCarus SD, Parnell LKS. The Origin and Evolution of the HARMONIC® Scalpel. Surg Technol Int. 2019;35:201-213. [PubMed] |
| 6. | Shibao K, Joden F, Adachi Y, Kohi S, Kudou Y, Kikuchi Y, Matayoshi N, Sato N, Murayama R, Hirata K. Repeated partial tissue bite with inadequate cooling time for an energy device may cause thermal injury. Surg Endosc. 2021;35:3189-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Kirschbaum A, Rüdell F, Pehl A, Bartsch DK. More compression improves sealing effect on larger pulmonary arteries. J Surg Res. 2016;201:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Reyes DA, Brown SI, Cochrane L, Motta LS, Cuschieri A. Thermal fusion: effects and interactions of temperature, compression, and duration variables. Surg Endosc. 2012;26:3626-3633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 9. | Harmonic® 1100 Shears Fact Sheet. Available from: https://www.jnjmedicaldevices.com/sites/default/files/user_uploaded_assets/pdf_assets/2020-11/HARMONIC-1100-Shears-Fact-Sheet-148803-201013.pdf. |
| 10. | Singleton D, Juncosa-Melvin N, Scoggins P, Paulin-Curlee G, Cummings J, Ricketts C. Intelligent Ultrasonic Energy: New Adaptive Tissue Technology in Harmonic Shears. Journal of Surgery. 2020; 8: 178-183.. [DOI] [Full Text] |
| 11. | Goudie E, Oliveira RL, Thiffault V, Jouquan A, Lafontaine E, Ferraro P, Liberman M. Phase 1 Trial Evaluating Safety of Pulmonary Artery Sealing With Ultrasonic Energy in VATS Lobectomy. Ann Thorac Surg. 2018;105:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Timm RW, Asher RM, Tellio KR, Welling AL, Clymer JW, Amaral JF. Sealing vessels up to 7 mm in diameter solely with ultrasonic technology. Med Devices (Auckl). 2014;7:263-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13298] [Article Influence: 1108.2] [Reference Citation Analysis (0)] |
| 14. | Sankaranarayanan G, Resapu RR, Jones DB, Schwaitzberg S, De S. Common uses and cited complications of energy in surgery. Surg Endosc. 2013;27:3056-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Lin YC, Dionigi G, Randolph GW, Lu IC, Chang PY, Tsai SY, Kim HY, Lee HY, Tufano RP, Sun H, Liu X, Chiang FY, Wu CW. Electrophysiologic monitoring correlates of recurrent laryngeal nerve heat thermal injury in a porcine model. Laryngoscope. 2015;125:E283-E290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Pogorelić Z, Perko Z, Druzijanić N, Tomić S, Mrklić I. How to prevent lateral thermal damage to tissue using the harmonic scalpel: experimental study on pig small intestine and abdominal wall. Eur Surg Res. 2009;43:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Applewhite MK, White MG, James BC, Abdulrasool L, Kaplan EL, Angelos P, Grogan RH. Ultrasonic, bipolar, and integrated energy devices: comparing heat spread in collateral tissues. J Surg Res. 2017;207:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |