Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1269
Peer-review started: February 3, 2022
First decision: April 8, 2022
Revised: April 13, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: July 27, 2022
Processing time: 173 Days and 17.4 Hours
Immunological checkpoint inhibitors (ICIs) have revolutionized therapy of many different malignanices. Concomitant immune-mediated adverse effects are common and can affect many organs such as the skin, lungs, gastrointestinal and endocrine organs as well as the liver. Liver injury has been reported in 3%-8% of patients with grade III-IV hepatitis in retrospective studies. The liver injury is characterized by hepatocellular injury resembling autoimmune hepatitis biochemically but not immunologically as patients with ICI induced hepatoxicity rarely have auto-antibodies or IgG elevation. The role for liver biopsy (LB) in patients with suspected liver injury due to ICIs is controversial and it is not clear whether results of a LB will change clinical management. LB can be helpful when there is diagnostic uncertainty and pre-existing liver disease is suspected. Although there are no distinctive histological features, the finding of granulomas and endothelitis may suggest a specific type of hepatitis induced by ICIs. The natural history of hepatotoxicity of ICI therapy is not well known. Recent studies have demon
Core Tip: Liver injury associated with immunological checkpoint inhibitors (ICIs) has been reported in 3%-8% of patients with grade III-IV hepatitis in retrospective studies. Although there are no distinctive histological features, the finding of granulomas and endothelitis may suggest a specific type of hepatitis induced by ICIs. Recent studies have demonstrated that 33-50% of patients improve spontaneously with discontinuation of ICIs. The high doses of corticosteroids with 1-2 mg/kg/d of methylprednisolone recommended by the oncological societies are controversial. Patients with ICI induced hepatoxicity without jaundice and/or coagulopathy should be monitored.
- Citation: Bessone F, Bjornsson ES. Checkpoint inhibitor-induced hepatotoxicity: Role of liver biopsy and management approach. World J Hepatol 2022; 14(7): 1269-1276
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1269.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1269
Immunotherapy has revolutionized the treatment of oncological diseases. Within this large type of compounds, immunological checkpoint inhibitors (ICIs) are increasingly used due to their therapeutic efficacy. These agents exert important beneficial effects on tumor regression and patient survival[1].
ICIs are a large family of co-stimulatory immunotherapy drugs with strong effects that modulate the immune response. They regulate the signaling transduction downstream of T-cell receptors via protein-kinase-mediated cascades. Major components of these immune checkpoint molecules are cytotoxic T lymphocyte-associated antigen-4 (CTLA4), programmed cell death-ligand-1, and programmed cell death protein-1 (PD1)[2]. ICIs block these proteins and disable their inhibitory effects, thus evoking an immune response leading to both activation and proliferation of T cells, which results in the killing of tumor cells. CTLA4- and PD1-mediated T-cell inhibition is involved in immunological tolerance to self-antigens as well, and the consequent immune-mediated damage can affect virtually all organs and systems, including the liver[3].
The reported incidence of drug-induced liver injury (DILI) associated with ICIs varies between 0%-30% and depends on the severity, grade, type and drug dose[4]. The occurrence of hepatitis associated with these agents is usually high, and ranges from 3-9% and 1%-2% for anti-CTLA4 and anti-PD1 drugs, respectively. Hepatotoxicity occurs more frequently if combined ICIs schemes are used (up to 17% increased risk) compared to monotherapy[5].
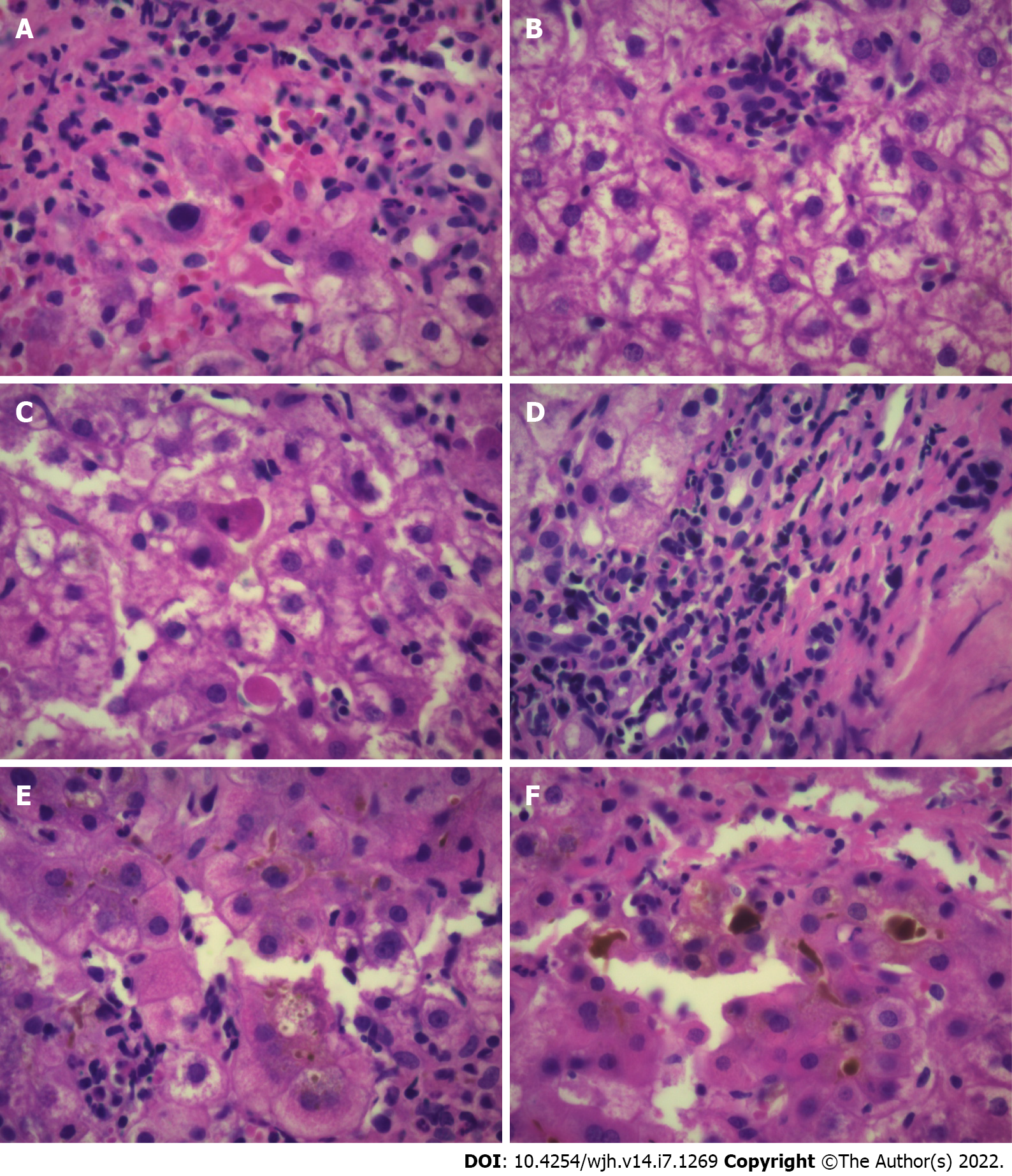
Due to the relatively short period since this type of drugs were approved, many aspects regarding the diagnosis and management of adverse effects are unknown. Besides typical clinical and analytical presentation, different histological findings have been associated with ICIs-induced liver damage (i.e. ring granuloma, endothelitis and cholangitis)[6-9]. The role of liver biopsy and the main controversies in the management of liver toxicity induced by ICIs will be discussed in this editorial.
Although liver biopsy (LB) is not always required to establish the diagnosis of DILI, it can be helpful in in patients with suspected ICIs- induced hepatotoxicity. LB is particularly indicated when there are diagnostic uncertainties despite noninvasive investigations, in patients presenting with atypical features and in those who fail to respond to conventional therapies[8]. It can also be very useful in patients with potential pre-existing liver disease that cannot be confirmed by imaging or serological tests.
Even though these compounds usually do not trigger a classical autoimmune hepatitis, there is a strong suspicion that liver injury is related to an immune-mediated mechanism[9]. In addition, there are clinicopathologic differences supporting the notion that ICIs –induced DILI is a distinct entity from autoimmune hepatitis. Hepatotoxicity due to ICIs are very rarely associated with autoantibodies and/or IgG elevations and the histological changes are different from those seen in classical autoimmune hepatis[9].
However, differentiating drug-induced autoimmune hepatitis (DIAIH) from a classical autoimmune hepatitis (AIH) is a complex issue for pathologists[10,11]. Interestingly, the histologic pattern most frequently associated with ICIs is commonly described as immune-related hepatitis to differentiate it from classical AIH. Whether liver injury caused by ICIs can be considered a DILI or a DIAIH is controversial[12].
The most conspicuous findings linked to DILI-induced by ICIs is the presence of centrilobular necrosis and acute hepatitis with lobular inflammation associated with acidophil bodies[9,12,13]. Lobular hepatitis indistinguishable from autoimmune hepatitis is one of the most reported patterns usually associated with panlobular inflammation that may be limited to zone 3[13]. Ductal damage has also been described[10] (Figure 1). Inflammatory infiltrates in ICI-induced DILI are predominantly composed of both activated CD3+ and CD8+ T lymphocytes[12]. In contrast to this pattern, autoimmune hepatitis shows higher numbers of both CD20+ and CD4+ T lymphocytes. ICIs-induced DILI may be associated with immune-mediated hepatocellular injury and do not appear to be triggered by T-helper lymphocyte activation or increased immunoglobulin production[12].
De Martin et al[9] analyzed 16 patients with liver injury associated with these drugs. A typical pattern of granulomatous hepatitis, characterized by the presence of fibrin-ring granulomas in addition to central-vein endothelitis, were found in patients treated with anti-CTLA-4 monoclonal antibodies (mAbs). Histology findings were considered useful for decision-making regarding therapy. Mild portal fibrosis was found in 50% of patients suggesting a possible trend of acute hepatitis towards chronicity. Fibrin-ring granulomas and central-vein endothelitis were also documented in patients treated with a therapeutic schema combining CTLA-4 with anti-PD-mAbs. These authors emphasized that acute hepatitis associated with immunotherapy agents for cancer treatment is not a frequent clinical event, since it is found in no more than 3.5% of treated patients[9].
Interestingly, they highlight the key role of LB stating that it provides to the clinician with valuable information about the severity of liver injury and helps them to select an appropriate treatment, sometimes avoiding the unnecessary indication of corticosteroids[9].
In a study from Barcelona, 28 cases of severe hepatitis-induced by ICIs were compared with classical AIH[14]. Histological parameters differed between the two conditions. Most patients with AIH underwent a liver biopsy in contrast to only two out of 28 cases (7%) of irH (immune-related Hepatitis) linked to a low response to immunosupression. The authors suggested that liver biopsy should be restricted to patients presenting with irH associated with poor or slow response to corticosteroids[14]. This is in line with guidelines from the European Society of Medical Oncology[15]. In addition, a consultation by a hepatologist and consideration of LB in steroid and mycophenolate-refractory cases is also recommended[15].
On the other hand, Peeraphatdit et al[8] analyzed 107 cases in a recent systematic review on management recommendations of DILI-induced by ICIs. They found 83 (78%) patients had grade 3-4 of liver injury. The authors stated that establishing causality for liver damage induced by ICIs can be challenging and a LB should be considered only in cases with at least liver injury grade 2.
Few studies have critically analyzed the predictive value of LB. A recent retrospective study analyzing 60 patients with suspected liver injury due to ICIs showed a pattern of lobular inflammation and injury, endothelitis and the presence of granulomas. The histological findings did not predict the need for corticosteroids, therapy duration, or the need for secondary immunosuppression[16]. The authors questioned the value of LB in the management of patients with typical features of ICI-induced liver injury.
Li et al[17] retrospectively analyzed a cohort of 213 patients who developed grade 3 or higher grade of hepatitis linked to ICIs therapy. The most common pattern of DILI was panlobular hepatitis. Patients who underwent a LB had a significatly longer median time to normalization of ALT vs those who did not undergo LB (42 vs 33 d respectively; P < 0.01). This study suggested that LB in patients treated with ICIs and developing grade 3 or higher liver injury presented a delay in the initiation of corticosteroid therapy and not associated with a faster resolution of liver inflammation. These authors also stated that LB can provide valuable information in patients who do not improve despite the indication of corticosteroids before another immunosuppressant is prescribed[17].
A new pattern of cholestasis induced by ICIs displaying imaging and laboratory features similar to those observed in primary eslerosing cholangitis has been recently described[18]. This type of secondary sclerosing cholangitis (SSC) has also been reported in patients with other types of DILI[19,20]. SSC induced by ICIs is characterized by diffuse dilatation and thickening of intrahepatic bile ducts[18]. The absence of biliary obstruction was demonstrated in almost 80% of the cases showing bile duct dilatation[21]. A diffuse hypertrophy in the wall of these biliary ducts was documented in most of them.
Cohen et al[16] described a predominantly cholangitic pattern in 16 patients, associated with portal-based inflammation. This histological feature was more likely to be linked to bile duct dilatation or narrowing on cholangiography. Although the biliary involvement induced by ICIs has been well documented to date, its long-term clinical consequences are unknown.
In conclusion, the use of LB is still debatable. Clinicians are faced with pros and cons considering that the final decision should be taken individually for each patient (Table 1).
| Benefits | Limitations |
| To rule out pre-existing liver diseases | Invasiveness |
| To confirm diagnosis (i.e.; granulomas) | Cost |
| Differentiate anti-PD1/PD-L1 from anti-CTLA4- induced DILI | Pathognomic histological features are lacking |
| To establish the severity of liver injury. | Unclear influence on patient management |
| To discriminate ICIs-induced DILI from typical seronegative classical AIH | Biochemical features might be sufficient |
| To assess a possible chronicity evolution |
Regarding the benefits, we should consider the usefulness of liver biopsy in different setting as follow: To rule out pre-existing diseases such as metastases or NASH. To confirm the diagnosis of liver injury especially if ring granulomas and endothelitis are observed in patients who are on anti-CTLA4. To investigate the presence of distinctive features of liver toxicity induced by anti-PD1/PD L1 and anti-CTLA4 (ring granulomas and endothelitis). To establish the severity of liver injury. To confirm diagnosis when clinical presentation is associated with features of not typical idiopathic AIH. To assess liver histology in patients with a possible trend from acute hepatitis towards chronicity.
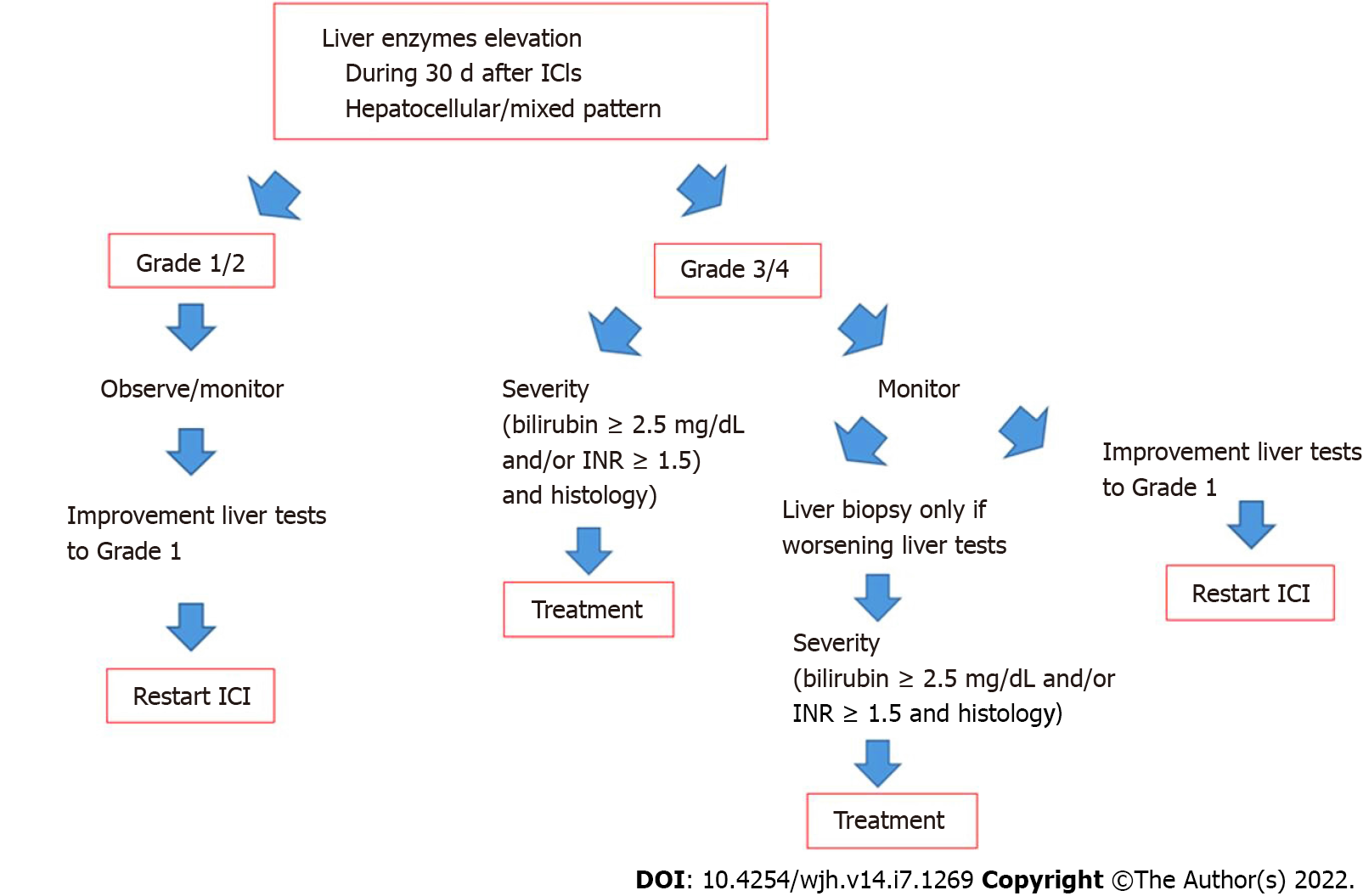
Among the limitations, the cost and invasiveness of LB should always be taken into account and whether the results will change management of the patient. Thus, frequently LB does not show pathognomonic histologic findings and, accordingly, some authors propose that is unlikely to influence patient management. Unfortunately, it is unclear if a liver biopsy is helpful in the decision if another Check point inhibitor can be tried if hepatotoxicity has occurred with the first line Check point inhibitor. A proposed algorithm on the role of liver biopsy in the management of DILI-induced by ICIs is shown in Figure 2.
Finally, a magnetic resonance cholangiopancreatography is recommended when secondary sclerosing cholangitis is suspected and to rule out biliary obstruction.
The management of liver injury considered to be due to ICIs and the role of corticosteroids as therapy for this adverse effect is not evidence based. Randomized controlled studies are lacking in this context and recommendations based on expert opinion in the guidelines of the oncological societies[15,22,23]. According to these guidelines, general advice is probably not controversial. If liver injury is mild, or < 3 × ULN in ALT, the therapy with ICIs is not interrupted and liver tests only monitored. If ALT is 3-5 × ULN, ICIs can be temporarily discontinued and if levels of elevated ALT return to baseline within a week, ICI therapy can be resumed and/or oral corticosteroids can be given. Experience from observational studies support only to monitor patients without corticosteroids in these relatively mild cases[9,24,25].
If ALT levels are > 5 × ULN, classified as grade III hepatitis by the oncological societies, which is > 5 × ULN-20 × ULN and bilirubin > 3 × ULN (15, 21-22), the patients should be monitored and patients given corticosteroids if there is no improvement in liver tests. If the levels of ALT are > 10 × ULN (grade IV hepatitis) and/or if the ALT > 5 × ULN is accompanied by rise in serum bilirubin, ICI therapy should be permanently interrupted[15,22,23]. However, in observational studies a relatively large proportion of patients of these patients have shown spontaneous improvement in liver tests without the use of corticosteroids[9,24,25]. In a study from France, 37% with > grade III hepatitis improved spontaneously and 50% in the study by Gauci et al[24] and in a recent study from Texas, 33% of patients were found not to require corticosteroids[25]. There is though no doubt that liver injury can be severe and have severe consequences. In a study from Barcelona, among 28 patients with severe hepatitis (> grade III), two patients developed acute liver failure (ALF) and one of these died from ALF[14]. Two other well characterized patients have been reported who died from hepatotoxicity[24,25]. All of these patients were treated with high dose of methylprednisolon 2mg/kg combined with mycophenolate mofetil[14,25,26]. Thus, it seems that not all cases with hepatotoxicity due to ICIs are steroid responsive. A study analyzing data from World Health Organization pharmacovigilance database (Vigilyze) also reported mortality due to hepatotoxicity[27]. Among adverse effects associated with fatality 22% were due to hepatotoxicity but this is perhaps not completely reliable data as it seems that a formal causality assessment has not been undertaken[27]. However, mortality from hepatotoxicity can occur and it is understandable that mortality from adverse effects in a patient who is in remission from the malignancy is a nightmare for the oncologist. Thus, it is understandable that they want to do everything in their power to reverse the hepatotoxicity.
High doses of corticosteroids are recommended by the oncological societies: 1 mg/kg/d for grade III hepatitis and even 2 mg/kg/d for grade IV hepatitis[15,22,23]. As pointed out earlier, these doses are not evidence based. Although high doses of corticosteroids have been used observational studies these have not always been helpful. In a study from the UK only 50% with hepatotoxicity due to ICIs responded to corticosteroids[28]. Two Japanese studies have similarly shown responsiveness between 33 and 50%[29,30]. In a recent study from France, important experience was reported on the clinical management of patients with liver injury due to ICIs[31]. In more than 300 patients with advanced melanoma, 21 had hepatotoxicity and 13/21 (62%) were treated with steroids, whereas 8 were not. Time to resolution of liver tests and survival was not statistically between the groups[31]. The authors suggested that patients with prothrombin levels > 50% and bilirubin < 50 mmol/L should be monitored and not treated with corticosteroids but ICI therapy discontinued until < 5 × ULN, whereas those with prothrombin level < 50% and bilirubin > 50, should be treated with corticosteroids 0.5-1 mg/kg/d. Riveiro-Barciela et al[14] found only 2-3 mo of corticosteroid therapy necessary. In a recent study, it was demonstrated that initial treatment with 1 mg/kg/d provided similar liver tests improvement as doses > 1.5 mg/kg/d, which was also associated with a reduced risk of steroid-induced adverse effects in comparison with higher-dose regimens[32].
In patients with worsening jaundice despite high doses of corticosteroids mycophenolate mofetil is most often used because it is probably better tolerated than tacrolimus due to potential nephrotoxicity. However, the use of secondary immunosuppression in patients is not evidence based and relies on small cases series and case reports. There is no data to guide us in patients with pre-existing liver disease.
The role for LB in the setting of DILI induced by ICIs is controversial and not yet defined. LB can be helpful when there is diagnostic uncertainty and pre-existing liver disease is suspected. Although there are no distinctive histological features, the finding of granulomas and endothelitis may suggest a specific type of hepatitis induced by ICIs. Recent data suggest that liver histology did not predict the need for corticosteroids, therapy duration, or the need for secondary immunosuppression. Patients with ICI induced hepatoxicity without jaundice and/or coagulopathy should be monitored as a large proportion of patients will recover spontaneously with discontinuation of the ICIs. Patients who develop worsening of liver tests with jaundice and/or coagulopathy despite discontinuation of ICIs should be treated with corticosteroids. Recent data suggests that 1mg/kg/d of methylprednisolon are as efficacious as higher doses but it is not clear if doses of 40-60 mg of prednisolon are less efficacious. Secondary immunosuppression mostly with mycophenolate mofetil has been reported to be helpful.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bouattour M, France; Malnick SDH, Israel; Zou Z, China A-Editor: Liu X, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Pennock GK, Chow LQ. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist. 2015;20:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Haanen JB, Robert C. Immune Checkpoint Inhibitors. Prog Tumor Res. 2015;42:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38:976-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Remash D, Prince DS, McKenzie C, Strasser SI, Kao S, Liu K. Immune checkpoint inhibitor-related hepatotoxicity: A review. World J Gastroenterol. 2021;27:5376-5391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (3)] |
| 5. | Hernandez N, Bessone F. Hepatotoxicity Induced by Biological Agents: Clinical Features and Current Controversies. J Clin Trasl Hepatol. 2022;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci. 2012;57:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Pi B, Wang J, Tong Y, Yang Q, Lv F, Yu Y. Immune-related cholangitis induced by immune checkpoint inhibitors: a systematic review of clinical features and management. Eur J Gastroenterol Hepatol. 2021;33:e858-e867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology. 2020;72:315-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 9. | De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A, Laghouati S, Robert C, Marabelle A, Guettier C, Samuel D. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 380] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 10. | Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, Lucena MI, Castiella A, Lindor K, Björnsson E. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 12. | Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 13. | Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A, Doyle LA. Ipilimumab-associated Hepatitis: Clinicopathologic Characterization in a Series of 11 Cases. Am J Surg Pathol. 2015;39:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Riveiro-Barciela M, Barreira-Díaz A, Vidal-González J, Muñoz-Couselo E, Martínez-Valle F, Viladomiu L, Mínguez B, Ortiz-Velez C, Castells L, Esteban R, Buti M. Immune-related hepatitis related to checkpoint inhibitors: Clinical and prognostic factors. Liver Int. 2020;40:1906-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119-iv142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 1504] [Article Influence: 188.0] [Reference Citation Analysis (1)] |
| 16. | Cohen JV, Dougan M, Zubiri L, Reynolds KL, Sullivan RJ, Misdraji J. Liver biopsy findings in patients on immune checkpoint inhibitors. Mod Pathol. 2021;34:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Li M, Sack JS, Bell P, Rahma OE, Srivastava A, Grover S, Zucker SD. Utility of Liver Biopsy in Diagnosis and Management of High-grade Immune Checkpoint Inhibitor Hepatitis in Patients With Cancer. JAMA Oncol. 2021;7:1711-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Hamoir C, de Vos M, Clinckart F, Nicaise G, Komuta M, Lanthier N. Hepatobiliary and Pancreatic: Nivolumab-related cholangiopathy. J Gastroenterol Hepatol. 2018;33:1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Gudnason HO, Björnsson HK, Gardarsdottir M, Thorisson HM, Olafsson S, Bergmann OM, Björnsson ES. Secondary sclerosing cholangitis in patients with drug-induced liver injury. Dig Liver Dis. 2015;47:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Ahmad J, Rossi S, Rodgers SK, Ghabril M, Fontana RJ, Stolz A, Hayashi PH, Barnhart H, Kleiner DE, Bjornsson ES. Sclerosing Cholangitis-Like Changes on Magnetic Resonance Cholangiography in Patients With Drug Induced Liver Injury. Clin Gastroenterol Hepatol. 2019;17:789-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Onoyama T, Takeda Y, Yamashita T, Hamamoto W, Sakamoto Y, Koda H, Kawata S, Matsumoto K, Isomoto H. Programmed cell death-1 inhibitor-related sclerosing cholangitis: A systematic review. World J Gastroenterol. 2020;26:353-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 22. | Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1423] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 23. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2591] [Article Influence: 370.1] [Reference Citation Analysis (0)] |
| 24. | Gauci ML, Baroudjian B, Zeboulon C, Pages C, Poté N, Roux O, Bouattour M, Lebbé C; PATIO group. Immune-related hepatitis with immunotherapy: Are corticosteroids always needed? J Hepatol. 2018;69:548-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Miller ED, Abu-Sbeih H, Styskel B, Nogueras Gonzalez GM, Blechacz B, Naing A, Chalasani N. Clinical Characteristics and Adverse Impact of Hepatotoxicity due to Immune Checkpoint Inhibitors. Am J Gastroenterol. 2020;115:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Huffman BM, Kottschade LA, Kamath PS, Markovic SN. Hepatotoxicity After Immune Checkpoint Inhibitor Therapy in Melanoma: Natural Progression and Management. Am J Clin Oncol. 2018;41:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Bhave P, Buckle A, Sandhu S, Sood S. Mortality due to immunotherapy related hepatitis. J Hepatol. 2018;69:976-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Cheung V, Gupta T, Payne M, Middleton MR, Collier JD, Simmons A, Klenerman P, Brain O, Cobbold JF. Immunotherapy-related hepatitis: real-world experience from a tertiary centre. Frontline Gastroenterol. 2019;10:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Kitagataya T, Suda G, Nagashima K, Katsurada T, Yamamoto K, Kimura M, Maehara O, Yamada R, Shigesawa T, Suzuki K, Nakamura A, Ohara M, Umemura M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Ohnishi S, Komatsu Y, Hata H, Takeuchi S, Abe T, Sakakibara-Konishi J, Teshima T, Homma A, Sakamoto N. Prevalence, clinical course, and predictive factors of immune checkpoint inhibitor monotherapy-associated hepatitis in Japan. J Gastroenterol Hepatol. 2020;35:1782-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Ito T, Ishigami M, Yamamoto T, Mizuno K, Yamamoto K, Imai N, Ishizu Y, Honda T, Kawashima H, Yasuda S, Toyoda H, Yokota K, Hase T, Nishio N, Maeda O, Kato M, Hashimoto N, Hibi H, Kodera Y, Sone M, Ando Y, Akiyama M, Shimoyama Y, Fujishiro M. Clinical course of liver injury induced by immune checkpoint inhibitors in patients with advanced malignancies. Hepatol Int. 2021;15:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Gauci ML, Baroudjian B, Bédérède U, Zeboulon C, Delyon J, Allayous C, Madelaine I, Eftekhari P, Resche-Rigon M, Poté N, Paradis V, Durand F, Lebbé C, Roux O, Bouattour M; PATIO group. Severe immune-related hepatitis induced by immune checkpoint inhibitors: Clinical features and management proposal. Clin Res Hepatol Gastroenterol. 2021;45:101491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Li M, Wong D, Vogel AS, Sack JS, Rahma OE, Hodi FS, Zucker SD, Grover S. Effect of corticosteroid dosing on outcomes in high-grade immune checkpoint inhibitor hepatitis. Hepatology. 2022;75:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |