Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1190
Peer-review started: January 12, 2022
First decision: March 16, 2022
Revised: March 18, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: June 27, 2022
Processing time: 162 Days and 7.2 Hours
Hepatocellular carcinoma (HCC) in hepatitis C virus (HCV)-infected patients has a high risk of recurrence. Although eradication of HCV is expected to reduce this risk, the risk in patients with a history of HCC may be high after treatment with direct-acting antivirals (DAAs).
To determine the risk factors for HCC recurrence in patients with HCV and a history of HCC.
The risk of HCC recurrence in patients with a history of HCC and/or of HCC occurrence in patients without a history of HCC after DAA therapy was retrospectively analyzed in 311 HCV patients treated at our institution and several neighboring hospitals. The frequency and predictors of HCC recurrence/ occu
HCV patients with a history of HCC were older and had greater progression of liver fibrosis and diabetes than patients without a history of HCC. Median recurrence-free survival (RFS) was 1092 d in patients with a history of HCC, and post-DAA HCC recurrence/occurrence was observed in 29 patients (53.7%) with and 5 (1.9%) without a history of HCC over 6 years (P < 0.001). RFS in patients with a history of HCC did not differ significantly before and after DAA treatment. The frequency of HCC recurrence/occurrence in patients with a history of HCC was lower after than before DAA treatment. Multivariate analysis showed that the incidence rate of HCC recurrence/occurrence before DAA treatment was the only independent predictor of HCC recurrence/occurrence after DAA treatment. Liver function was well preserved and clinical course was good in patients with HCC recurrence/occurrence after DAA therapy.
DAA therapy in patients infected with HCV is also effective in patients with a history of HCC. Curative treatment for HCC is desirable before DAA therapy. The frequency of HCC recurrence/occurrence before DAA therapy was associated with a significantly increased risk of HCC recurrence after DAA therapy. Careful observation after DAA therapy is required in patients with a history of HCC.
Core Tip: To estimate the therapeutic value of direct-acting antivirals (DAAs) in hepatitis C virus (HCV)-infected patients with a history of hepatocellular carcinoma (HCC), the clinical course of HCV patients with or without a history of HCC after DAA therapy was retrospectively analyzed. DAA treatment did not increase the incidence rate of HCC recurrence/occurrence or enhance malignant transformation of HCC in patients with a history of HCC. The risk of HCC recurrence after DAA therapy was significantly associated with the frequency of HCC recurrence/occurrence before DAA therapy.
- Citation: Tajiri K, Ito H, Kawai K, Kashii Y, Hayashi Y, Murayama A, Minemura M, Takahara T, Shimizu Y, Yasuda I. Direct-acting antivirals for hepatitis C virus-infected patients with hepatocellular carcinoma. World J Hepatol 2022; 14(6): 1190-1199
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1190.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1190
Hepatocellular carcinoma (HCC) is one of the most frequent malignancies and a major cause of cancer-related deaths worldwide. Although HCC detected at an early stage can often be cured by surgical resection or local ablative therapy, HCC is often diagnosed at an advanced stage, precluding curative treatment and resulting in a high mortality rate[1]. Viral hepatitis is associated with the development of HCC, with hepatitis C virus (HCV) and hepatitis B virus (HBV) infections being major causes of HCC, along with nonviral etiologies such as alcoholic liver disease and nonalcoholic fatty liver disease[2]. HCV-related HCC often recurs after curative therapies for HCC, such as surgical resection or ablative therapies, with 5-year recurrence rates ranging from 60%-80%[3].
Interferon-based HCV eradication reduces the incidence rates of HCC[4]. The anti-HCV and anti-carcinogenic effects of interferon reduce liver inflammation, contributing to reductions in the rate of HCC recurrence/occurrence. It is unclear, however, whether HCV eradication with direct-acting antivirals (DAAs) increase the risk of HCC, as DAA treatment disrupts immune surveillance during rapid elimination of HCV[5]. Large-scale studies, however, have shown that DAA eradication of HCV increases the risk of HCC, whereas basal liver fibrosis is associated with the risk of HCC[6-8]. Because other studies have reported that DAA eradication results in malignant transformation, suggesting that DAA had adverse carcinogenic effects[5,9], these carcinogenic risks should be especially considered in patients with a history of HCC. The effects of DAA therapy have therefore been assessed in patients with a history of HCC. Studies have suggested that factors associated with pre-existing malignant potential, such as advanced liver fibrosis, high serum alpha-fetoprotein (AFP) concentration, and the presence of precancerous nodules, might lead to HCC recurrence in patients with a history of HCC[10-14].
This study retrospectively evaluated the risks of HCC recurrence/occurrence, defined as HCC recurrence in patients with a history of HCC and/or of HCC occurrence in those without a history of HCC, and the clinical course of HCC in HCV patients treated with DAA. The results of this study suggest that a history of HCC prior to DAA treatment is a major factor contributing to HCC recurrence/occurrence after DAA treatment.
This study enrolled HCV patients treated with DAA at Toyama University Hospital, Takaoka Municipal Hospital, Nanto Municipal Hospital, and Saiseikai Toyama Hospital (all in Toyama, Japan) between November 2014 and July 2020. HCV infection was confirmed by HCV-RNA quantification and the genotype of HCV was determined in all patients. The fibrosis-4 (Fib-4) index, a useful noninvasive method of assessing liver fibrosis[15], was also evaluated in all patients. Liver cirrhosis was diagnosed by hepatologists, each with over 20 years’ of experience, based on the results of imaging modalities such as ultrasonography (US), computed tomography (CT), and elastography, and the titers of fibrosis markers such as platelet count and Fib-4 index. HCC was diagnosed based on histological and/or imaging data such as contrast-enhanced CT or magnetic resonance imaging (MRI), according to the diagnostic criteria of the American Association for the Study of Liver Diseases[16]. Before DAA therapy, all patients were screened using US, CT, or MRI to rule out the presence of viable HCC. This multicenter study was performed in accordance with the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Toyama University (Approval No. R2019-131).
Before the start of DAA therapy, patients with viable HCC were treated with surgery, radiofrequency ablation (RFA), or transarterial chemoembolization (TACE). Patients who did not show viable HCC lesions on contrast-enhanced CT or MRI performed 1 to 3 mo after HCC treatment were considered eligible for DAA therapy. Treatment regimens were determined by hepatologists according to HCV treatment guidelines[17,18]. Treatment regimens included daclatasvir plus asunaprevir (DCV + ASV) in patients with HCV genotype 1b from 2014 to 2016; sofosbuvir plus ledipasvir (SOF + LDV) for patients with HCV genotypes 1b and 2a/2b from 2015 to 2020; SOF plus ribavirin (SOF + Rib) for patients with HCV genotypes 2a/2b from 2015 to 2017; and glecaprevir and pibrentasvir (GLE + PIB) for patients with any HCV genotype from 2017 to 2020. Other regimens considered included ombitasvir, paritaprevir, and ritonavir from 2016 to 2017; elbasvir plus grazoprevir in 2017; and SOF plus velpatasvir from 2019 to 2020 depending on the patient’s condition and the timing of treatment. Patients were monitored every 4 wk during DAA treatment, and every 12 wk thereafter, with HCC evaluated by imaging modalities. A sustained viral response (SVR) was defined as complete clearance of HCV-RNA clearance 12 wk after the end of DAA treatment. The flow chart of this study is shown in Supplementary Figure 1. Patients were monitored for a median 1311 d (range: 28 d to 2231 d) after the end of DAA therapy.
HCC treatment in each patient was determined by discussions among surgeons, hepatologists, and radiologists at each institution and was based on Japanese practice guidelines for HCC[19]. Treatments of patients with early-stage HCC included surgical resection or RFA. Treatments of patients with multiple HCCs included TACE or systemic chemotherapy such as sorafenib, according to liver function and tumor progression and following treatment guidelines.
Variable distributions were reported as mean ± SD. Categorical variables were compared by the Fisher’s exact test. Continuous variables were compared by the Student’s t-test or the Mann-Whitney U test. Survival was evaluated using the Kaplan-Meier method, with differences in survival curve compared by log-rank tests. The incidence rates of HCC recurrence/occurrence were reported as person-years. All statistical analyses were performed using SPSS software, version 19.0 (IBM Corp., Armonk, NY, United States), with P < 0.05 considered statistically significant.
A total of 311 patients, 143 (46.0%) men and 168 (54.0%) women, were included in this study (Table 1). Of these 311 patients, 87 (28.0%) had cirrhosis, 229 (73.6%) were infected with HCV genotype 1b, and 53 (17.0%) had a previous history of HCC. Their mean Fib-4 index was 3.87 ± 3.24 and their mean AFP concentration was 12.0 ± 35.2 ng/mL. The 53 patients with a history of HCC were significantly older (75.6 years vs 66.5 years; P < 0.01) than the 258 patients with no history of HCC. The rates of diabetes, a risk factor for HCC after DAA treatment[20] (35.8% vs 3.1%; P < 0.01) and liver cirrhosis (34.0% vs 20.2%; P < 0.01) were significantly higher, whereas the rates of HCV genotype 2 (13.2% vs 25.6%; P = 0.04) were significantly lower, in patients with than without a history of HCC. In addition, serum albumin concentrations (3.5 g/dL vs 4.0 g/dL; P < 0.01) and platelet counts (12.9 × 104/mL vs 16.7 × 104/mL; P < 0.01) were significantly lower, whereas Fib-4 index (6.27 vs 3.37; P < 0.01) and AFP concentrations (23.7 ng/mL vs 9.4 ng/mL; P = 0.047) were significantly higher in patients who had a previous history of HCC. Of the 311 patients, 56 (21.9%) had a history of habitual alcohol use, but these rates did not differ significantly in patients with and without a history of HCC. Thus patients with a history of HCC were older and had more advanced liver fibrosis progression and diabetes than patients without a history of HCC.
| Overall | With HCC | Without HCC | P value1 | |
| Case | 311 | 53 | 258 | |
| Age in yr | 68.1 ± 13.5 | 75.8 ± 6.7 | 66.5 ± 14.1 | < 0.01 |
| Male/Female | 143/168 | 27/26 | 116/142 | 0.45 |
| Diabetes, yes/no | 47/264 | 19/34 | 28/230 | < 0.01 |
| Habitual alcohol use2, yes/no | 56/255 | 12/41 | 44/214 | 0.33 |
| Liver cirrhosis, yes/no | 224/87 | 18/35 | 52/206 | < 0.01 |
| Genotype, 1b/2a, 2b/others | 229/73/10 | 45/7/1 | 183/66/9 | 0.04 |
| Alb in g/dL | 3.9 ± 0.4 | 3.5 ± 0.4 | 4.0 ± 0.4 | < 0.01 |
| ALT in U/L | 44.3 ± 45.0 | 40.3 ± 22.0 | 45.1 ± 48.3 | 0.48 |
| Plt as × 104/μL | 16.0 ± 5.9 | 12.9 ± 5.6 | 16.7 ± 5.8 | < 0.01 |
| Fib-4 index | 3.87 ± 3.24 | 6.27 ± 4.64 | 3.37 ± 2.60 | < 0.01 |
| AFP in ng/mL | 12.0 ± 35.2 | 23.7 ± 52.4 | 9.4 ± 29.6 | 0.047 |
Patients infected with HCV genotype 1b were administered DCV + ASV, SOF + LDV, GLE + PIB, or other regimens in accordance with contemporary guidelines. Similarly patients infected with HCV genotypes 2a/2b were administered SOF + Rib, SOF + LDV, GLE + PIB, or other regimens; and patients with other genotypes such as genotypes 3a/3b/4s were administered GLE + PIB. SVR was achieved by 52 (98.1%) of the 53 patients with and by 250 (96.9%) of the 258 patients without a history of HCC (P = 1.00). Several patients who did not initially achieve SVR were switched to another DAA regimen, with SVR achieved in all treated patients. Post-DAA treatment AFP levels were higher in patients with, than without, a history of HCC history, both at end of treatment and SVR, but these concentrations were lower than those before DAA therapy (Table 2).
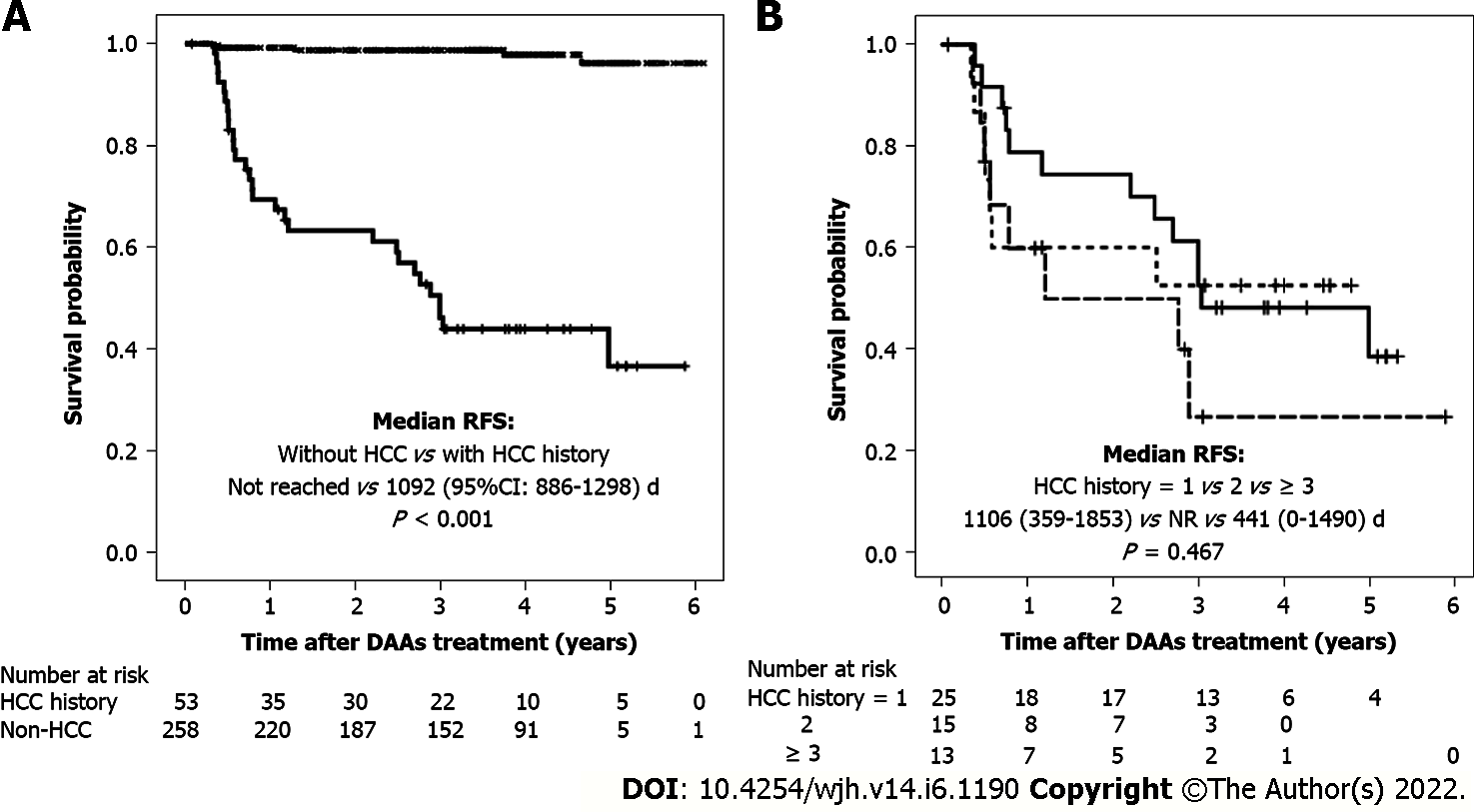
Following DAA therapy, HCC recurrence/occurrence was found in 29 patients (53.7%) with and 5 (1.9%) without a history of HCC, with 3-year incidence rates of 50.9% (27/53) and 1.2% (3/258), respectively. Median recurrence-free survival (RFS) in patients with a history of HCC was 1092 d, whereas none of those without a history of HCC died during the 6-year study period (P < 0.001; Figure 1A).
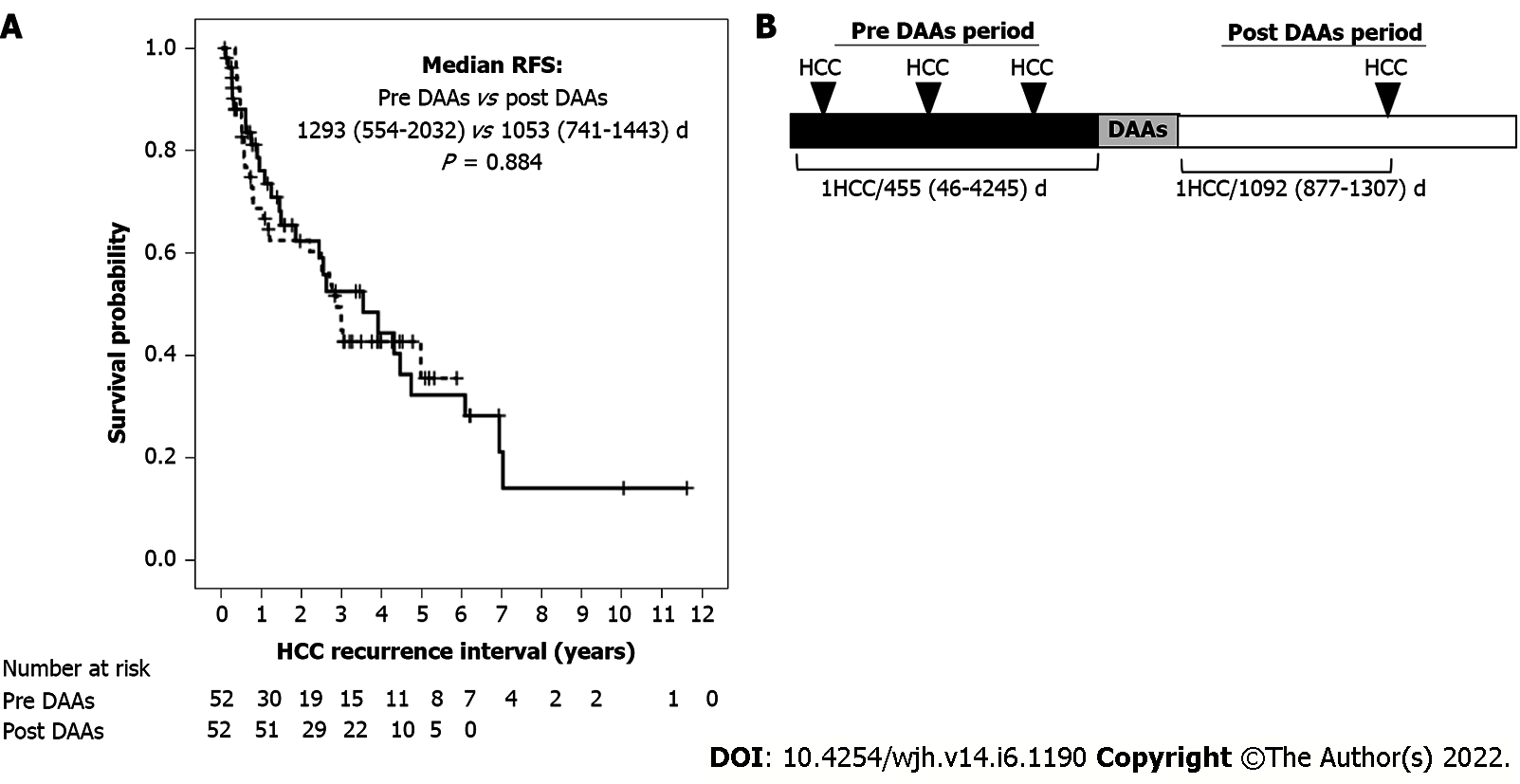
HCC recurrence and other parameters before and after DAA therapy were compared in patients with a history of HCC. Median RFS did not differ significantly in patients with HCC recurrence before and after DAA therapy [1293 d (range 554-2032 d) vs 1053 d (range 741-1443 d); P = 0.884) (Figure 2A), with incidence rates of HCC recurrence of 1/1.25 and 1/2.99 person-years, respectively (Figure 2B). HCV clearance induced by DAA treatment did not increase HCC recurrence rate. Univariate analysis showed that AFP concentration at SVR and frequency of HCC recurrence before DAA treatment were risk factors for HCC recurrence after DAA treatment, whereas multivariate analysis showed that only the frequency of HCC recurrence before DAA treatment was an independent predictor of HCC recurrence after DAA treatment (Table 3). Only a history of HCC before DAA treatment contributed to the risk of HCC recurrence after DAA treatment, whereas HCV clearance by DAA alone did not. The 1-year rates of HCC recurrence after DAA treatment in patients with 1, 2, and ≥ 3 HCC events before DAA treatment were 28%, 40% and 38.5%, respectively (Figure 1B).
| Factors | Univariate | Multivariate | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 0.98 | 0.92-1.04 | 0.52 | |||
| CH or LC | 0.50 | 0.21-1.21 | 0.12 | |||
| Diabetes | 1.12 | 0.79-1.59 | 0.53 | |||
| Habitual alcohol use | 1.08 | 0.68-1.51 | 0.51 | |||
| Fib-4 | 1.01 | 0.93-1.09 | 0.86 | |||
| AFP at baseline | 1.01 | 1.00-1.01 | 0.22 | |||
| AFP at EOT | 1.09 | 1.00-1.19 | 0.05 | 1.10 | 1.00-1.01 | 0.05 |
| AFP at SVR | 1.01 | 1.00-1.01 | 0.04 | 1.01 | 1.00-1.01 | 0.08 |
| Duration between first HCC and DAAs treatment | 1.00 | 1.00-1.00 | 0.18 | |||
| Number of HCC occurrence | 1.32 | 1.06-1.64 | 0.02 | 1.61 | 1.18-2.19 | < 0.01 |
All 29 patients with a history of HCC who experienced HCC recurrence after DAA therapy had been treated according to HCC treatment guidelines[19]. Six and seventeen of these patients underwent surgical resection and RFA, respectively. Multiple recurrences were observed in 6 patients, including one with portal invasion. These 6 patients were subsequently treated with TACE, hepatic artery infusion chemotherapy, or sorafenib. Two died due to advanced HCC, with survival times following DAA therapy completion of 49.7 and 52.6 mo, respectively.
This study found that DAA-induced eradication of HCV did not increase the risk of HCC recurrence, with multivariate analysis showing that a prior history of HCC was the only independent factor predicting the risk of HCC recurrence after DAA therapy. DAA treatment, however, did not worsen the clinical course of subsequent HCC events. Rather, liver reserve function was preserved following DAA treatment, allowing curative and continuous treatment of HCC. Although malignant transformation after DAA treatment has been reported[5,9], this study found that DAA therapy itself was not the causal agent.
In this study, SVR rates in DAA-treated patients were similar in those with (98.1%) and without (96.9%), a previous history of HCC. Systematic reviews, however, have reported lower SVR rates in patients with a history of HCC[21]. This study found that treatment with DAAs was highly effective in eradicating HCV in patients with a history of HCC, despite their being older and more likely to have liver fibrosis and diabetes mellitus than patients without a history of HCC. DAAs are also effective in patients with advanced HCC[22-24]. HCV eradication by DAAs ameliorates liver inflammation and suppresses liver fibrosis progression, preserving or improving liver function. Since the introduction of DAAs as treatment for HCV, mortality rates in patients with HCV-associated HCC have improved compared with mortality rates in patients with HBV-related and nonviral HCC[25]. These findings suggest that HCV eradication might prolong overall survival in patients with HCV-related HCC.
Although HCV eradication by DAAs has been suggested to increase the subsequent risk of HCC, most studies have found that preexisting risk factors for HCC development were present at the time of DAA initiation. The progression of liver fibrosis and the presence of cirrhosis have been shown to be associated with HCC development[6-8]. Chronic HCV infection leads to the progression of liver fibrosis, the factor that contributes most to HCC development through various epigenetic changes and the creation of a microenvironment favorable to carcinogenesis[26]. The risk of HCC recurrence/occurrence after DAA treatment was shown to be higher in patients with than without advanced liver fibrosis[27], suggesting that earlier achievement of SVR before the development of fibrosis may reduce the likelihood of HCC recurrence/occurrence.
Serum AFP concentration has also been found to predict HCC development[10,13]. Higher AFP concentration is a major biomarker for HCC occurrence after SVR[28,29], as well as being associated with liver inflammation, making AFP concentration at the end of treatment very important[30]. AFP concentrations before and after DAA treatment should therefore be measured to estimate the risk of HCC recurrence/occurrence. Another factor associated with HCC development is the presence of preexisting hepatic nodules[14]. Although all patients in the present study who were treated with DAAs were evaluated by imaging modalities, some did not undergo enhanced CT or MRI. Thus, the exact proportion of patients with dysplastic nodules was unclear. For example, a patient found to have a 1.5 cm dysplastic nodule in the liver on ethoxybenzyl-diethylenetriamine pentaacetic acid enhanced (EOB)-MRI developed HCC from the dysplastic nodule 3-years after DAA completion, akin to hypervascular transformation of 9 mm hypovascular nodules with a 3-year incidence rate of 30%[31]. Certain types of DAAs, such as SOF and DCV, were found to have greater oncogenic potential through off-target DAA effects[32]. In the present study, HCC recurrence/occurrence was not frequent in patients treated with SOF or DCV (data not shown).
Collectively, DAA treatment was effective in patients with a history of HCC, as shown by their high SVR rates. DAAs eliminated hepatic inflammation and suppressed the progression of hepatic fibrosis, leading to preserved liver function. Improvement or preservation of liver function provides benefits in the management of HCC. Further prospective studies are required to evaluate the risk of DAA-associated transformation of precancerous lesions to HCC and the effects of specific DAAs on the risks of HCC recurrence/occurrence.
Multivariate analysis of patients in the present study also found that liver fibrosis, diabetes mellitus, and serum AFP concentration before DAA treatment were unassociated with HCC recurrence/ occurrence after DAA treatment. Rather, the only factor significantly associated with HCC recurrence/ occurrence after DAA treatment was history of prior HCC events. DAA treatment has been reported effective in patients with multiple prior courses of HCC recurrence[33], suggesting the need for careful screening for HCC before DAA treatment of patients with a history of HCC, as well as diligent follow-up of these patients after DAA therapy. Estimating the risk of HCC after DAA treatment is important, with the degree of liver fibrosis predicting the risk HCC recurrence[34,35]. A previous history of HCC and stratification by the Fib-4 index can be used to construct a novel predictive model for HCC development after DAA treatment[36]. The need for careful screening and follow-up in patients with a history of HCC increases with the number of times patients have experienced HCC recurrence.
This study had several limitations. First, its retrospective design precluded accurate determination of the effects of DAA treatment on the risks of HCC recurrence/occurrence. Second, the number of patients included in the present study, especially of those with a history of multiple HCC events, was relatively small. Third, not all patients underwent EOB-MRI, preventing actual determination of their HCC or non-HCC status. Although all underwent enhanced CT or US performed by experienced hepatologists rather than EOB-MRI, further studies are required to evaluate precancerous lesions and HCC more precisely. In addition, other risk factors for HCC, including tobacco use, obesity, and metabolic diseases, were not analyzed.
DAA treatment of HCV-infected patients can also preserve liver function in patients with HCC. Curative treatment of HCC is desirable before DAA therapy. A history of multiple courses of HCC events before DAA treatment significantly increases the risk of HCC recurrence. Careful HCC screening prior to DAA treatment and thorough follow-up observation after DAA treatment is recommended in such patients.
Treatment with direct-acting antivirals (DAAs) has provided many benefits to hepatitis C virus (HCV)-infected patients. Hepatocellular carcinoma (HCC) development after treatment with DAAs remains a serious issue.
The effect of DAA treatment on the risk of HCC development is an important clinical question.
To clarify the risk of HCC development after DAA treatment in patients HCV-infected patients at high risk for HCC development.
HCC occurrence after DAA treatment was retrospectively evaluated in patients with and without a history of HCC.
The frequency of HCC recurrence/occurrence was similar before and after treatment with DAAs. The number of HCC occurrences before DAA treatment was an independent risk factor for HCC recurrence/occurrence.
HCV-infected patients with a history of multiple HCCs should be monitored carefully for HCC recurrence.
An effective screening method should be established for patients at high risk of HCC recurren- ce/occurrence.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen C, China; Ghoneim S, United States A-Editor: Yao QG, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, Wagman LD, Nissen N, Colquhoun SD, Kim J. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, Yoshiji H, Yatsuhashi H, Shimizu M, Torimura T, Moriyama M, Sakaida I, Okada H, Chiba T, Chuma M, Nakao K, Isomoto H, Sasaki Y, Kaneko S, Masaki T, Chayama K, Koike K. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011-2015 update. J Gastroenterol. 2019;54:367-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 3. | Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, Okita K, Omata M, Kudo M, Kojiro M, Nakanuma Y, Takayasu K, Monden M, Matsuyama Y, Ikai I. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H, Kawanishi M. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 362] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 6. | Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996-1005.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 682] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 7. | Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH, Berry K. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157:1264-1278.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 290] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 8. | Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Wong R, Huang A, Misra S, Schwartz M, Mitrani R, Nakka S, Noureddine W, Ho C, Konjeti VR, Dao A, Nelson K, Delarosa K, Rahim U, Mavuram M, Xie JJ, Murphy CC, Parikh ND. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156:1683-1692.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 9. | Fouad M, El Kassas M, Ahmed E, El Sheemy R. Tumor characteristics of hepatocellular carcinoma after direct-acting antiviral treatment for hepatitis C: Comparative analysis with antiviral therapy-naive patients. World J Hepatol. 2021;13:1743-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ikeda K, Kawamura Y, Kobayashi M, Kominami Y, Fujiyama S, Sezaki H, Hosaka T, Akuta N, Saitoh S, Suzuki F, Suzuki Y, Arase Y, Kumada H. Direct-Acting Antivirals Decreased Tumor Recurrence After Initial Treatment of Hepatitis C Virus-Related Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:2932-2942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, Distefano M, Larocca L, Prestileo T, Tinè F, Bertino G, Giannitrapani L, Benanti F, Licata A, Scalisi I, Mazzola G, Cartabellotta F, Alessi N, Barbàra M, Russello M, Scifo G, Squadrito G, Raimondo G, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia - HCV (RESIST-HCV). Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? Aliment Pharmacol Ther. 2017;46:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 12. | Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, Koyanagi T, Kato M, Shimoda S, Kajiwara E, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther. 2018;47:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Mashiba T, Joko K, Kurosaki M, Ochi H, Osaki Y, Kojima Y, Nakata R, Goto T, Takehiro A, Kimura H, Mitsuda A, Kawanami C, Uchida Y, Ogawa C, Kusakabe A, Narita R, Ide Y, Abe T, Tsuji K, Kitamura T, Okada K, Sohda T, Shigeno M, Satou T, Izumi N. Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? PLoS One. 2018;13:e0194704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Ooka Y, Miho K, Shuntaro O, Nakamura M, Ogasawara S, Suzuki E, Yasui S, Chiba T, Arai M, Kanda T, Maruyama H, Yokosuka O, Kato N, Mochizuki H, Omata M. Prediction of the very early occurrence of HCC right after DAA therapy for HCV infection. Hepatol Int. 2018;12:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3525] [Article Influence: 185.5] [Reference Citation Analysis (0)] |
| 16. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3207] [Article Influence: 458.1] [Reference Citation Analysis (1)] |
| 17. | Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, Lesmana CR, Sollano J, Kumar M, Jindal A, Sharma BC, Hamid SS, Dokmeci AK, Mamun-Al-Mahtab, McCaughan GW, Wasim J, Crawford DH, Kao JH, Yokosuka O, Lau GK, Sarin SK. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | Tanaka A. [JSH guidelines for the management of hepatitis C virus infection (version 3)]. Nihon Rinsho. 2015;73:221-227. [PubMed] |
| 19. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, Kudo M, Kubo S, Takayama T, Tateishi R, Fukuda T, Matsui O, Matsuyama Y, Murakami T, Arii S, Okazaki M, Makuuchi M. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 20. | Váncsa S, Németh D, Hegyi P, Szakács Z, Farkas Á, Kiss S, Hegyi PJ, Kanjo A, Sarlós P, Erőss B, Pár G. Diabetes Mellitus Increases the Risk of Hepatocellular Carcinoma After Direct-Acting Antiviral Therapy: Systematic Review and Meta-Analysis. Front Med (Lausanne). 2021;8:744512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | He S, Lockart I, Alavi M, Danta M, Hajarizadeh B, Dore GJ. Systematic review with meta-analysis: effectiveness of direct-acting antiviral treatment for hepatitis C in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51:34-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Tsai HY, Chang HP, Chen CJ, Hsu WL, Huang LY, Lee PC. Effects of direct-acting antiviral therapy for patients with advanced hepatocellular carcinoma and concomitant hepatitis C-A population-based cohort study. Eur Rev Med Pharmacol Sci. 2021;25:7543-7552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Kawaoka T, Aikata H, Teraoka Y, Inagaki Y, Honda F, Hatooka M, Morio K, Morio R, Kobayashi T, Nagaoki Y, Nakahara T, Hiramatsu A, Tsuge M, Imamura M, Kawakami Y, Chayama K. Impact of Hepatitis C Virus Eradication on the Clinical Outcome of Patients with Hepatitis C Virus-Related Advanced Hepatocellular Carcinoma Treated with Sorafenib. Oncology. 2017;92:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Yeh ML, Kuo HT, Huang CI, Huang CF, Hsieh MY, Liang PC, Lin IH, Hsieh MH, Lin ZY, Chen SC, Dai CY, Huang JF, Yu ML, Chuang WL. Eradication of hepatitis C virus preserve liver function and prolong survival in advanced hepatocellular carcinoma patients with limited life expectancy. Kaohsiung J Med Sci. 2021;37:145-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Lockart I, Hajarizadeh B, Buckley N, Davison S, Prakoso E, Levy MT, George J, Dore GJ, Danta M. All-cause hepatocellular carcinoma survival in the era of direct-acting antiviral therapy. J Gastroenterol Hepatol. 2021;36:3515-3523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Ahumada A, Rayón L, Usón C, Bañares R, Alonso Lopez S. Hepatocellular carcinoma risk after viral response in hepatitis C virus-advanced fibrosis: Who to screen and for how long? World J Gastroenterol. 2021;27:6737-6749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Sanduzzi-Zamparelli M, Mariño Z, Lens S, Sapena V, Iserte G, Pla A, Granel N, Bartres C, Llarch N, Vilana R, Nuñez I, Darnell A, Belmonte E, García-Criado A, Díaz A, Muñoz-Martinez S, Ayuso C, Bianchi L, Fuster-Anglada C, Rimola J, Forner A, Torres F, Bruix J, Forns X, Reig M. Liver cancer risk after HCV cure in patients with advanced liver disease without non-characterized nodules. J Hepatol. 2022;76:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Oze T, Hiramatsu N, Yakushijin T, Miyazaki M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H, Inui Y, Hijioka T, Inada M, Katayama K, Tamura S, Yoshihara H, Inoue A, Imai Y, Hayashi E, Kato M, Miyagi T, Yoshida Y, Tatsumi T, Kasahara A, Hamasaki T, Hayashi N, Takehara T; Osaka Liver Forum. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Huang CM, Hu TH, Chang KC, Tseng PL, Lu SN, Chen CH, Wang JH, Lee CM, Tsai MC, Lin MT, Yen YH, Hung CH, Cho CL, Wu CK. Dynamic noninvasive markers predict hepatocellular carcinoma in chronic hepatitis C patients without sustained virological response after interferon-based therapy: Prioritize who needs urgent direct-acting antiviral agents. Medicine (Baltimore). 2017;96:e8696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Kuwano A, Yada M, Nagasawa S, Tanaka K, Morita Y, Masumoto A, Motomura K. Serum α-fetoprotein level at treatment completion is a useful predictor of hepatocellular carcinoma occurrence more than one year after hepatitis C virus eradication by direct-acting antiviral treatment. J Viral Hepat. 2022;29:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Suh CH, Kim KW, Pyo J, Lee J, Kim SY, Park SH. Hypervascular Transformation of Hypovascular Hypointense Nodules in the Hepatobiliary Phase of Gadoxetic Acid-Enhanced MRI: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2017;209:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Giovannini C, Fornari F, Indio V, Trerè D, Renzulli M, Vasuri F, Cescon M, Ravaioli M, Perrucci A, Astolfi A, Piscaglia F, Gramantieri L. Direct Antiviral Treatments for Hepatitis C Virus Have Off-Target Effects of Oncologic Relevance in Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Ohki T, Sato K, Kondo M, Goto E, Sato T, Kondo Y, Akamatsu M, Sato S, Yoshida H, Koike Y, Obi S. Effectiveness of direct acting antiviral agents for hepatitis C virus related recurrent hepatocellular carcinoma patients who had multiple courses of recurrence. J Viral Hepat. 2021;28:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Degasperi E, D'Ambrosio R, Iavarone M, Sangiovanni A, Aghemo A, Soffredini R, Borghi M, Lunghi G, Colombo M, Lampertico P. Factors Associated With Increased Risk of De Novo or Recurrent Hepatocellular Carcinoma in Patients With Cirrhosis Treated With Direct-Acting Antivirals for HCV Infection. Clin Gastroenterol Hepatol. 2019;17:1183-1191.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Ogasawara N, Saitoh S, Akuta N, Sezaki H, Suzuki F, Fujiyama S, Kawamura Y, Hosaka T, Kobayashi M, Suzuki Y, Arase Y, Ikeda K, Kumada H. Advantage of liver stiffness measurement before and after direct-acting antiviral therapy to predict hepatocellular carcinoma and exacerbation of esophageal varices in chronic hepatitis C. Hepatol Res. 2020;50:426-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Miyasaka A, Yoshida Y, Suzuki A, Sawara K, Takikawa Y. A Novel Standard for Hepatocellular Carcinoma Screening Intensity After Hepatitis C Elimination. Int J Gen Med. 2021;14:8935-8943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |