Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.969
Peer-review started: May 28, 2021
First decision: July 6, 2021
Revised: July 15, 2021
Accepted: August 11, 2021
Article in press: August 11, 2021
Published online: September 27, 2021
Processing time: 116 Days and 11.1 Hours
The coronavirus disease 2019 (COVID-19) pandemic may present with a broad range of clinical manifestations, from no or mild symptoms to severe disease. Patients with specific pre-existing comorbidities, such as obesity and type 2 diabetes, are at high risk of coming out with a critical form of COVID-19. Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, and, because of its frequent association with metabolic alterations including obesity and type 2 diabetes, it has recently been re-named as metabolic-associated fatty liver disease (MAFLD). Several studies and systematic reviews pointed out the increased risk of severe COVID-19 in NAFLD/MAFLD patients. Even though dedicated mechanistic studies are missing, this higher probability may be justified by systemic low-grade chronic inflammation associated with immune dysregulation in NAFLD/MAFLD, which could trigger cytokine storm and hypercoagulable state after severe acute respiratory syndrome coronavirus 2 infection. This review focuses on the predisposing role of NAFLD/MAFLD in favoring severe COVID-19, discussing the available information on specific risk factors, clinical features, outcomes, and pathogenetic mechanisms.
Core Tip: Non-alcoholic fatty liver disease is the most widespread hepatic disorder. Recently re-named as metabolic-associated fatty liver disease, it has been lately pointed out as a predisposing factor for severe coronavirus disease 2019 (COVID-19). We herein discuss the epidemiology and possible underlying pathways predisposing severe COVID-19 in non-alcoholic fatty liver disease/metabolic-associated fatty liver disease patients.
- Citation: Bellanti F, Vendemiale G. Coronavirus disease 2019 and non-alcoholic fatty liver disease. World J Hepatol 2021; 13(9): 969-978
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/969.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.969
The coronavirus disease 2019 (COVID-19) was declared as a global pandemic by the World Health Organization (WHO) on March 11, 2020[1]. Indeed, after the first diagnosis of COVID-19 case in Wuhan (China) in December 2019, the virus spread quickly, affecting 220 countries and territories[2]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative virus of COVID-19, whose most likely origin is natural selection in an animal host followed by zoonotic transfer[3]. Features of SARS-CoV-2 infectivity and transmissibility, as well as multiple clinical presentations of COVID-19, represent burning research topics, especially with the alarming rise of new variants. Severe COVID-19 most frequently presents with acute respiratory failure, even though several non-respiratory manifestations may characterize both the acute phase of the disease and the post-COVID syndrome (or long COVID)[4].
COVID-19 patients may show hepatic injury – largely characterized by a mild increase in serum aminotransferase levels – or may experience worsening of a pre-existing liver disease[5]. Most patients presenting with moderate-severe COVID-19 are old and/or affected by metabolic comorbidities, such as diabetes mellitus and obesity[6]. These conditions are also strongly associated with unrecognized underlying liver disease, mostly non-alcoholic fatty liver disease (NAFLD)[7,8]. Affecting almost 1 billion people, NAFLD is considered as the most common chronic liver disease all over the world, and its prevalence is estimated to become higher together with the epidemics of type 2 diabetes and obesity[9]. Recent international consensus panel proposed to rename NAFLD to metabolic-associated fatty liver disease (MAFLD), giving importance to the underlying systemic metabolic dysfunction rather than alcohol abstinence[10]. Of interest, NAFLD/MAFLD patients are more likely to develop liver damage when infected by SARS-CoV-2[11].
To date, the available reviews on this topic focused on the impact of COVID-19 infection on NAFLD/MAFLD worsening and progression. The present review aims to consider the ongoing relationship between COVID-19 and NAFLD/MAFLD, targeting the predisposing role of NAFLD/MAFLD in favoring severe COVID-19. The available information since the beginning of pandemic, specific risk factors, clinical features, outcomes, and pathogenetic mechanisms will be analyzed and discussed.
NAFLD/MAFLD is characterized by steatosis in > 5% of liver parenchyma, in association with metabolic alterations (mostly type 2 diabetes and obesity), without any chronic liver disease, and with ethanol intake not exceeding 30 g/d for men and 20 g/d for women[12]. In the histological spectrum of NAFLD/MAFLD, steatosis may be accompanied by mild inflammation (non-alcoholic fatty liver) or necro-inflammation with hepatocyte ballooning (non-alcoholic steatohepatitis, NASH)[13].
Being the most widespread chronic liver disease worldwide, NAFLD/MAFLD prevalence ranges from 13.5% in Africa to 31.8% in the Middle East, consistent with differences in genetic predisposition, caloric intake, physical activity, body fat distribution, and socio-economic status[14]. In the general population, NAFLD/MAFLD prevalence increases with age, and it is higher in men than women (particularly in the pre-menopausal period)[15,16]. NAFLD/MAFLD is diagnosed in 47.3%-63.7% of type 2 diabetes patients and up to 80% of obese people[17,18]. Type 2 diabetes is rising worldwide, affecting more than 400 million people and representing the ninth main cause of death[19]. Even though type 2 diabetes is closely related to obesity, its significance in NAFLD is two-fold. Indeed, other than a high prevalence of NAFLD in these patients, type 2 diabetes accelerates NAFLD progression and is a predictor of advanced fibrosis and mortality[20]. Similar to type 2 diabetes, obesity prevalence has doubled in the last 40 years, so that approximately a third of the population can be classified as overweight or obese[21]. Even though its prevalence is higher in older people, obesity rates increased in all ages and both sexes, regardless of country, ethnicity, or socioeconomic status[21].
COVID-19 has been declared as a global pandemic by the WHO in March 2020, since cases are reported in all continents[1]. To date, there have been 168509636 confirmed cases of COVID-19, including 3505534 deaths, reported to WHO[22]. Nevertheless, the reported case counts undervalue the global burden of COVID-19, since only a small percentage of acute infections is diagnosed[23]. COVID-19 severity is related with increasing age, male sex, and pre-existing medical diseases[24,25]. Severe COVID-19, defined as intensive care unit or hospital admission, mechanical ventilation, or death, is associated with underlying conditions as diabetes mellitus and obesity[26,27]. Indeed, prevalence studies are not conclusive on increased risk of SARS-CoV-2 infection in patients affected by diabetes mellitus, but this condition may worsen the outcome of COVID-19[28]. Similarly, investigations do not show that obesity increases the risk of contracting COVID-19, but that it may exacerbate the disease severity[27].
The diagnosis of NAFLD/MAFLD requires: (1) the presence of hepatic steatosis detected by liver imaging or histology; and (2) exclusion of significant alcohol intake, other causes of steatosis, or chronic liver disease[29]. Even though liver histology is the gold standard for the diagnosis of NAFLD/MAFLD, to differentiate NASH from simple steatosis and to assess fibrosis, liver biopsy is limited to selected patients due to its invasiveness and costs[29]. Thus, available data on NAFLD/MAFLD prevalence in COVID-19 patients are limited to non-invasive diagnosis.
The frequency of hepatic steatosis fortuitously detected by chest computed tomography in COVID-19 patients was 4.7 times higher than that in age- and sex-matched non-infected patients (31.9% vs 7.1%)[30]. This result is confirmed by further studies in which NAFLD/MAFLD was diagnosed by the hepatic steatosis index in 30.7%-37.6% COVID-19 patients from China, even though (differently from the previous investigation) associated with higher risk of disease progression[11,31]. Other studies from China demonstrated that the presence of NAFLD/MAFLD is independently associated with severe COVID-19[32,33]. These latter observations suggest that a huge percentage of patients is at risk of developing the severe form of COVID-19 due to the increasing worldwide occurrence of NAFLD/MAFLD. Nevertheless, results from a study performed in Qatar could not demonstrate that NAFLD/MAFLD was an independent predictor of mortality or COVID-19 severity[34]. A further study conducted at the Imperial College Healthcare NHS Trust in London assessed that NAFLD/MAFLD per se was not associated with adverse outcomes in COVID-19 patients[35]. Two systematic reviews with meta-analysis considered several studies to conclude that NAFLD/MAFLD was associated with increased risk of severe COVID-19[36,37].
To answer the question whether NAFLD/MAFLD could increase the risk of contracting COVID-19, the impact of genetic risk score was analyzed in hospitalized participants of the UK Biobank cohort, resulting in no evident association between genetic predisposition of NAFLD/MAFLD and severe COVID-19[38]. A review on data from a huge commercial database including electronic records from 26 national healthcare systems demonstrated that the diagnosis of NASH increases 4.93 times the risk of COVID-19[6].
Several studies tried to point out if there are any risk factors predictive of severe COVID-19 in NAFLD/MAFLD patients (summarized in Table 1). According to the results of a pooled analysis, the risk of severe disease in COVID-19 patients affected by NAFLD/MAFLD seems independent of obesity[39]. Nevertheless, a systematic review showed that obesity, together with hepatic fibrosis and younger age, are associated with increased risk of severe COVID-19[40]. A subsequent study performed in a tertiary care center from Mexico showed that the presence of liver fibrosis in NAFLD/MAFLD patients is associated with severe COVID-19[41]. A further study from three Chinese hospitals suggested that high serum interleukin-6 (IL-6) levels at admission represents an independent risk factor for severe COVID-19 in NAFLD/M
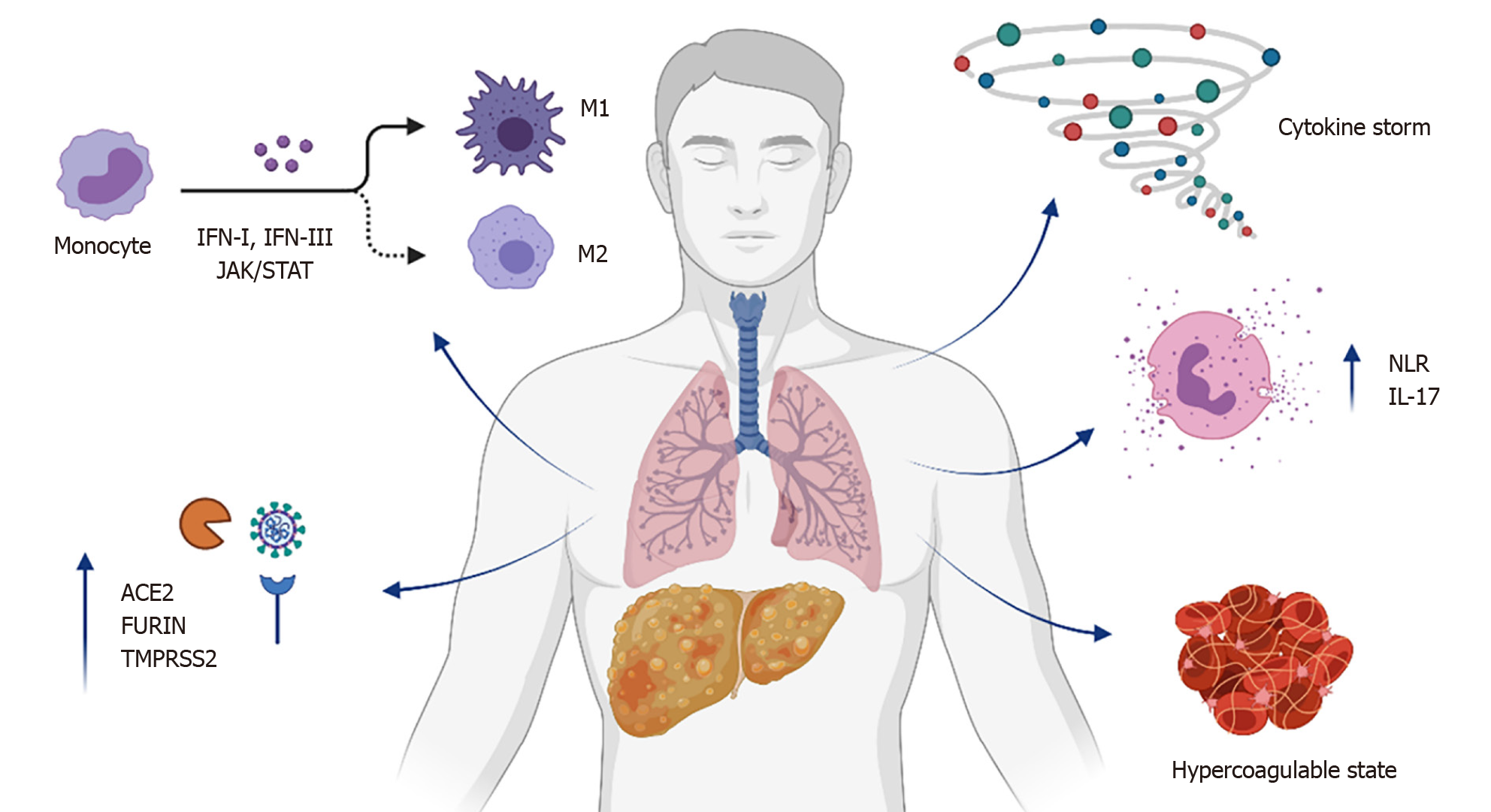
As the risk of severe COVID-19 increases in patients affected by NAFLD/MAFLD, it is conceivable that specific joint pathogenic mechanisms could be involved (Figure 1).
During the initial phase of COVID-19 infection, pathogenesis of the disease relies on binding of spike SARS-CoV-2 protein to angiotensin I converting enzyme 2 (ACE2) receptors, through which the virus enters target cells[44-46]. Even though ACE2 receptors are mainly expressed in epithelial cells of the upper respiratory tract, in type 2 alveolar epithelial cells, and in ciliated cells, they can also be found on the brush border of enterocytes and in cholangiocytes[45]. Following the binding with ACE2 receptor, the SARS-CoV-2 spike protein undergoes a cleavage by the host's FURIN serine protease, a critical process in promoting spike-mediated entry of the virus[47]. Likewise, cleavage of SARS-CoV-2 spike protein by the serine protease two key host factors of SARS-CoV-2 (transmembrane serine protease 2, TMPRSS2) is determinant for its fusogenic activity[46]. Of great interest, it has been evidenced that patients with NAFLD/MAFLD present with an increased expression of ACE2, FURIN, and TMPRSS2 genes[48]. The enhanced expression of receptors that mediate SARS-CoV-2 cellular entry can explain the increased susceptibility of NAFLD/MAFLD to COVID-19. Moreover, increased levels of FURIN and TMPRSS2 may boost the processing of SARS-CoV-2 spike, further improving its cellular entry. It is worth to note that analysis of data from rodent models and NAFLD/MAFLD patients could not show any increased hepatic expression of ACE2, FURIN, and TMPRSS2 genes[49]. On the contrary, the upregulation of these genes in multiple tissues probably represents an additional mechanism of increased susceptibility to severe COVID-19 in NAFLD/M
Several authors suggested that individuals with NAFLD/MAFLD may present with a dysregulation of both innate and adaptive immune response, which could predispose to worse outcomes in COVID-19. Innate immune response is particularly mediated by Kupffer cells in the liver, which represent the major number of resident macrophages in a single organ[51,52]. Kupffer cells are located within the hepatic sinusoids as part of the reticuloendothelial system, constituting the first line of defense against micro-organisms, and regulating immune homeostasis in the liver with the involvement of other immune cells such as neutrophils[53]. In NAFLD/MAFLD, macrophages are polarized towards a pro-inflammatory (M1, or classically activated) rather than anti-inflammatory (M2, or alternatively activated) phenotype[54]. Activation and hyperplasia of Kupffer cells was documented in patients with COVID-19 by several histopathological findings[55,56]. Nevertheless, the impact of COVID-19 on Kupffer cell polarization has not been fully characterized. Of note, ACE2 receptor is detected on the surface of Kupffer cells, leading to hypothesize that hepatic macrophages could be infected by SARS-CoV-2, triggering the primary defense response to the host[57]. This response is mostly mediated by type-I and type-III interferons, leading to the activation of janus kinase (JAK)-signal transducer and activator of transcription (STAT)-driven transcription of cytokines[58,59]. The expression of both JAK1 and STAT1, as well as interferon-encoding genes, are increased in NAFLD/MAFLD patients[48]. Of interest, a significant relationship between ACE2 and JAK-STAT signaling was described, suggesting that this pathway may be involved in the downstream action of ACE2 overexpression[60].
The progression from a mild to a severe form of COVID-19 is associated with a cytokine storm, characterized by elevated IL-6, IL-8, and tumor necrosis factor (TNF) levels[61]. Several cytokines are involved in NAFLD/MAFLD, determining a low-grade systemic inflammation that favors disease progression and comorbidities[62]. Circulating IL-6 is high in several chronic conditions, including metabolic syndrome, cardiovascular diseases, and chronic inflammatory airways diseases[63]. Furthermore, fatty liver is independently associated with elevated IL-6 levels[64]. Serum IL-6 is strongly and independently associated with COVID-19 severity, and treatment with a monoclonal antibody directed against IL-6 receptor (tocilizumab) improves clinical outcomes in patients affected by serious disease[65]. Indeed, while in physiological conditions the hepatic production of cytokines is nonexistent or mild, lipid accumulation leads to the release of pro-inflammatory molecules as TNF and IL-6 by hepatocytes, Kupffer cells, and adipose tissue, with reduced levels of the anti-inflammatory cytokine IL-10[66]. It is worth to note that adipose tissue is mainly characterized by dysfunctional and inflammatory immune response in patients affected by morbid obesity. In particular, both adipose and mesenchymal stem cells from obese patients are characterized by increased secretion of pro-inflammatory cytokines, including IL-6, IL-8, and TNF[67]. This may contribute to explain the increased probability of severe SARS-CoV-2 infections in NAFLD/MAFLD patients, but further studies are required to improve knowledge about the pathogenetic link between the altered innate liver immunity and COVID-19.
The neutrophil-to-lymphocyte ratio (NLR) is a biomarker of cellular immune imbalance in NAFLD/MAFLD[68]. A high NLR is associated with severity of disease, worse outcomes, and mortality in NAFLD/MAFLD patients[69,70]. Of interest, the presence of NAFLD/MAFLD and a NLR > 2.8 is associated with higher risk of severe COVID-19 with respect to patients not affected by NAFLD/MAFLD and normal NLR[33]. It is worth to note that NLR is also an easy-to-use prognostic biomarker in the early stage of SARS-CoV-2 infection[71]. Neutrophils are a crucial source of IL-17, especially in the liver but also in the airway[72,73]. The pro-inflammatory IL-17 axis may drive the progression of NAFLD/MAFLD, and also COVID-19 severity[74,75]. Activation of the IL-17 axis in NAFLD/MAFLD, other than complemented with the increase of additional pro-inflammatory cytokines as IL-6 and TNF, occurs with the imbalance of T helper lymphocyte subsets[76]. Hospitalized COVID-19 patients show a dysregulation in the balance of T lymphocytes, characterized by a reduced proportion of Treg cells as compared to non-hospitalized individuals[77]. Taken together, these observations suggest that the cellular immune imbalances described in NAFLD/MAFLD could predispose to severe COVID-19, even though further research is needed to clarify this aspect.
Cytokine release by pro-inflammatory cells may lead to enhanced production of pro-coagulant molecules such as the tissue factor and the von Willebrand factor, with consequent hypercoagulable state and resulting widespread micro-/macrovascular thrombosis[78,79]. NAFLD/MAFLD patients exhibit coagulation disorders, including elevated circulating levels of both tissue factor and von Willebrand factor, as well as increased platelet activation and plasmatic concentration of plasminogen activator inhibitor type 1[80-82]. COVID-19 patients affected by NAFLD/MAFLD present with higher level of circulating D-dimer with respect to those without NAFLD/MAFLD, suggesting that the NAFLD/MAFLD-associated pro-coagulant state may contribute to COVID-19 severity[83]. Results from a retrospective study on a cohort of COVID-19 patients revealed that the prevalence of NAFLD/MAFLD was higher in individuals presenting with Doppler ultrasound documented deep vein thrombosis[84]. Furthermore, mean admission and peak serum D-dimer concentration was more elevated in COVID-19 patients with NAFLD/MAFLD with respect to those without NAFLD/MAFLD[84]. It is conceivable that COVID-19 may further increase production of pro-inflammatory cytokines in NAFLD/MAFLD subjects, with consequent activation of the coagulation cascade and thrombosis. Indeed, histologic study of pulmonary vessels described widespread thrombosis with microangiopathy in COVID-19 patients, who also presented with hepatic steatosis involving 50%-60% of liver parenchyma[85]. To confirm this report, an Italian post-mortem analysis found hepatic steatosis and pulmonary thrombi in 55% and 73% COVID-19 patients, respectively[86]. These observations strongly suggest that these diseases are interlinked; the proinflammatory hypercoagulable state representing a mutual pathogenetic pathway to severe COVID-19, contributing to thrombosis and disease progression.
Since COVID-19 may present with severe disease and high mortality rate, several studies addressed predisposing factors and underlying pathways to identify patients at high risk. The severe form of SARS-CoV-2 infection occurs in individuals preliminary affected by metabolic diseases, including NAFLD/MAFLD. Chronic low-grade inflammation is suggested as the main leading process to trigger immune dysregulation, cytokine storm, and hypercoagulability in NAFLD/MAFLD patients with COVID-19. Other than being considered for specific therapeutic approaches against COVID-19, subjects affected by NAFLD/MAFLD should be acknowledged among groups with high-risk medical conditions in SARS-CoV-2 vaccination programs. Even though several concerns were raised about SARS-CoV-2 vaccine responses, vaccination with the alum-adjuvanted inactivated COVID-19 vaccine (Beijing Institute) resulted as effective and safe in NAFLD/MAFLD patients[87]. Nevertheless, further investigations are necessary to clarify whether NAFLD/MAFLD patients should be prioritized for SARS-CoV-2 vaccination.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pham TTT S-Editor: Yan JP L-Editor: Filipodia P-Editor:Guo X
| 1. | World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. 11 March 2020. [cited 10 May 2021]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. |
| 2. | Worldometer. COVID-19 coronavirus pandemic. [cited 7 May 2021]. Available from: https://www.worldometers.info/coronavirus/. |
| 3. | Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3581] [Cited by in RCA: 2864] [Article Influence: 572.8] [Reference Citation Analysis (0)] |
| 4. | Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2398] [Cited by in RCA: 2806] [Article Influence: 561.2] [Reference Citation Analysis (0)] |
| 5. | Amin M. COVID-19 and the liver: overview. Eur J Gastroenterol Hepatol. 2021;33:309-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Ghoneim S, Butt MU, Hamid O, Shah A, Asaad I. The incidence of COVID-19 in patients with metabolic syndrome and non-alcoholic steatohepatitis: A population-based study. Metabol Open. 2020;8:100057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 937] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 8. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 711] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 9. | Makri E, Goulas A, Polyzos SA. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch Med Res. 2021;52:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2201] [Article Influence: 440.2] [Reference Citation Analysis (1)] |
| 11. | Huang R, Zhu L, Wang J, Xue L, Liu L, Yan X, Huang S, Li Y, Zhang B, Xu T, Li C, Ji F, Ming F, Zhao Y, Cheng J, Wang Y, Zhao H, Hong S, Chen K, Zhao XA, Zou L, Sang D, Shao H, Guan X, Chen X, Chen Y, Wei J, Zhu C, Wu C. Clinical features of COVID-19 patients with non-alcoholic fatty liver disease. Hepatol Commun. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3176] [Article Influence: 352.9] [Reference Citation Analysis (4)] |
| 13. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 988] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 14. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7528] [Article Influence: 836.4] [Reference Citation Analysis (0)] |
| 15. | Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 16. | Salvoza NC, Giraudi PJ, Tiribelli C, Rosso N. Sex differences in non-alcoholic fatty liver disease: hints for future management of disease. Explor Med. 2020;1:51-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 806] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 18. | Younossi ZM, Henry L. The Impact of Obesity and Type 2 Diabetes on Chronic Liver Disease. Am J Gastroenterol. 2019;114:1714-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3386] [Article Influence: 483.7] [Reference Citation Analysis (0)] |
| 20. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1453] [Article Influence: 242.2] [Reference Citation Analysis (1)] |
| 21. | Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1735] [Article Influence: 289.2] [Reference Citation Analysis (0)] |
| 22. | World Health Organization. WHO coronavirus disease (COVID-19) dashboard. [cited 28 May 2021]. Available from: https://covid19.who.int/?gclid=CjwKCAjwx9_4BRAHEiwApAt0zv9_o-gc4Y31g9Mmx4jJ56WBZ8jwC1NhTcUar5dVc58mih0NGT3VRoC_XEQAvD_BwE. |
| 23. | Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ, Fry AM, Cannon DL, Chiang CF, Gibbons A, Krapiunaya I, Morales-Betoulle M, Roguski K, Rasheed MAU, Freeman B, Lester S, Mills L, Carroll DS, Owen SM, Johnson JA, Semenova V, Blackmore C, Blog D, Chai SJ, Dunn A, Hand J, Jain S, Lindquist S, Lynfield R, Pritchard S, Sokol T, Sosa L, Turabelidze G, Watkins SM, Wiesman J, Williams RW, Yendell S, Schiffer J, Thornburg NJ. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 466] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 24. | Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & amp; mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1186] [Cited by in RCA: 1464] [Article Influence: 292.8] [Reference Citation Analysis (0)] |
| 25. | Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, Stamatelopoulos K, Dimopoulos MA, Caforio ALP, Georgiopoulos G. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur J Clin Invest. 2020;50:e13362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 26. | Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 27. | Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. 2021;93:257-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 28. | Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, Bonora BM, Selmin E, Arcidiacono G, Pinelli S, Farnia F, Falaguasta D, Russo L, Voltan G, Mazzocut S, Costantini G, Ghirardini F, Tresso S, Cattelan AM, Vianello A, Avogaro A, Vettor R. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168:108374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 29. | Ando Y, Jou JH. Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin Liver Dis (Hoboken). 2021;17:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 30. | Medeiros AK, Barbisan CC, Cruz IR, de Araújo EM, Libânio BB, Albuquerque KS, Torres US. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom Radiol (NY). 2020;45:2748-2754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 32. | Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 33. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 34. | Mushtaq K, Khan MU, Iqbal F, Alsoub DH, Chaudhry HS, Ata F, Iqbal P, Elfert K, Balaraju G, Almaslamani M, Al-Ejji K, AlKaabi S, Kamel YM. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues. J Hepatol. 2021;74:482-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, Judge R, Soubieres A, Middleton P, Daunt A, Perez-Guzman P, Selvapatt N, Lemoine M, Dhar A, Thursz MR, Nayagam S, Manousou P. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15:e0240400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 36. | Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, Erőss B, Szakács Z, Hegyi P, Pár G. Metabolic Associated Fatty Liver Disease Is Associated With an Increased Risk of Severe COVID-19: A Systematic Review With Meta-Analysis. Front Med (Lausanne). 2021;8:626425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Singh A, Hussain S, Antony B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Valenti L, Jamialahmadi O, Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID-19. J Hepatol. 2020;73:709-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020;1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Sy-Janairo ML, Y Cua IH. Association of metabolic-associated fatty liver disease and risk of severe coronavirus disease 2019 illness. JGH Open. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Campos-Murguía A, Román-Calleja BM, Toledo-Coronado IV, González-Regueiro JA, Solís-Ortega AA, Kúsulas-Delint D, Cruz-Contreras M, Cruz-Yedra N, Cubero FJ, Nevzorova YA, Martínez-Cabrera CF, Moreno-Guillén P, Lozano-Cruz OA, Chapa-Ibargüengoitia M, Gulías-Herrero A, Aguilar-Salinas CA, Ruiz-Margáin A, Macías-Rodríguez RU. Liver fibrosis in patients with metabolic associated fatty liver disease is a risk factor for adverse outcomes in COVID-19. Dig Liver Dis. 2021;53:525-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Gao F, Zheng KI, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J, Zheng MH. Association and Interaction Between Serum Interleukin-6 Levels and Metabolic Dysfunction-Associated Fatty Liver Disease in Patients With Severe Coronavirus Disease 2019. Front Endocrinol (Lausanne). 2021;12:604100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Bramante C, Tignanelli CJ, Dutta N, Jones E, Tamariz L, Clark JM, Usher M, Metlon-Meaux G, Ikramuddin S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1528] [Article Influence: 305.6] [Reference Citation Analysis (0)] |
| 45. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 741] [Article Influence: 148.2] [Reference Citation Analysis (0)] |
| 46. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14257] [Article Influence: 2851.4] [Reference Citation Analysis (0)] |
| 47. | Braun E, Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunology. 2019;8:e1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 48. | Singh MK, Mobeen A, Chandra A, Joshi S, Ramachandran S. A meta-analysis of comorbidities in COVID-19: Which diseases increase the susceptibility of SARS-CoV-2 infection? Comput Biol Med. 2021;130:104219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 49. | Biquard L, Valla D, Rautou PE. No evidence for an increased liver uptake of SARS-CoV-2 in metabolic-associated fatty liver disease. J Hepatol. 2020;73:717-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Meijnikman AS, Bruin S, Groen AK, Nieuwdorp M, Herrema H. Increased expression of key SARS-CoV-2 entry points in multiple tissues in individuals with NAFLD. J Hepatol. 2021;74:748-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Blériot C, Ginhoux F. Understanding the Heterogeneity of Resident Liver Macrophages. Front Immunol. 2019;10:2694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 52. | Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 433] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 53. | Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 1001] [Article Influence: 125.1] [Reference Citation Analysis (0)] |
| 54. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 55. | Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693-7706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 56. | Fassan M, Mescoli C, Sbaraglia M, Guzzardo V, Russo FP, Fabris R, Trevenzoli M, Pelizzaro F, Cattelan AM, Basso C, Navalesi P, Farinati F, Vettor R, Dei Tos AP. Liver histopathology in COVID-19 patients: A mono-Institutional series of liver biopsies and autopsy specimens. Pathol Res Pract. 2021;221:153451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Song X, Hu W, Yu H, Zhao L, Zhao Y, Zhao X, Xue HH. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry A. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 58. | Majoros A, Platanitis E, Kernbauer-Hölzl E, Rosebrock F, Müller M, Decker T. Canonical and Non-Canonical Aspects of JAK-STAT Signaling: Lessons from Interferons for Cytokine Responses. Front Immunol. 2017;8:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 59. | Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, Wang C, Wang Y, Li L, Ren L, Guo F, Zhao Z, Zhou Z, Xiang Z, Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11:3810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 757] [Article Influence: 151.4] [Reference Citation Analysis (0)] |
| 60. | Luo J, Lu S, Yu M, Zhu L, Zhu C, Li C, Fang J, Zhu X, Wang X. The potential involvement of JAK-STAT signaling pathway in the COVID-19 infection assisted by ACE2. Gene. 2021;768:145325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1218] [Cited by in RCA: 1189] [Article Influence: 237.8] [Reference Citation Analysis (0)] |
| 62. | Lamadrid P, Alonso-Peña M, San Segundo D, Arias-Loste M, Crespo J, Lopez-Hoyos M. Innate and Adaptive Immunity Alterations in Metabolic Associated Fatty Liver Disease and Its Implication in COVID-19 Severity. Front Immunol. 2021;12:651728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Wannamethee SG, Whincup PH, Rumley A, Lowe GD. Inter-relationships of interleukin-6, cardiovascular risk factors and the metabolic syndrome among older men. J Thromb Haemost. 2007;5:1637-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Wang PW, Hsieh CJ, Psang LC, Cheng YF, Liou CW, Weng SW, Chen JF, Chen IY, Li RH, Eng HL. Fatty liver and chronic inflammation in Chinese adults. Diabetes Res Clin Pract. 2008;81:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur Cytokine Netw. 2020;31:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 66. | Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:727-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 282] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 67. | Ritter A, Kreis NN, Louwen F, Yuan J. Obesity and COVID-19: Molecular Mechanisms Linking Both Pandemics. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 68. | Paquissi FC. Immune Imbalances in Non-Alcoholic Fatty Liver Disease: From General Biomarkers and Neutrophils to Interleukin-17 Axis Activation and New Therapeutic Targets. Front Immunol. 2016;7:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 69. | Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, Zein NN, Feldstein AE. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 70. | Leithead JA, Rajoriya N, Gunson BK, Ferguson JW. Neutrophil-to-lymphocyte ratio predicts mortality in patients listed for liver transplantation. Liver Int. 2015;35:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Ciccullo A, Borghetti A, Zileri Dal Verme L, Tosoni A, Lombardi F, Garcovich M, Biscetti F, Montalto M, Cauda R, Di Giambenedetto S; GEMELLI AGAINST COVID Group. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents. 2020;56:106017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 72. | Macek Jilkova Z, Afzal S, Marche H, Decaens T, Sturm N, Jouvin-Marche E, Huard B, Marche PN. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL-17(+) neutrophils. Liver Int. 2016;36:1116-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Taylor PR, Pearlman E. IL-17A production by neutrophils. Immunol Lett. 2016;169:104-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Giles DA, Moreno-Fernandez ME, Divanovic S. IL-17 Axis Driven Inflammation in Non-Alcoholic Fatty Liver Disease Progression. Curr Drug Targets. 2015;16:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 75. | Pacha O, Sallman MA, Evans SE. COVID-19: a case for inhibiting IL-17? Nat Rev Immunol. 2020;20:345-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 76. | Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 77. | Meckiff BJ, Ramírez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, Eschweiler S, Grifoni A, Pelosi E, Weiskopf D, Sette A, Ay F, Seumois G, Ottensmeier CH, Vijayanand P. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell. 2020;183:1340-1353.e16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 409] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 78. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4038] [Article Influence: 807.6] [Reference Citation Analysis (0)] |
| 79. | Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46-e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 878] [Article Influence: 175.6] [Reference Citation Analysis (0)] |
| 80. | Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, Peyvandi F, Bertelli C, Valenti L, Fargion S. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 81. | Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, Hubens G, Van Marck E, Staels B, Michielsen P, Van Gaal L. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 82. | Virović-Jukić L, Stojsavljević-Shapeski S, Forgač J, Kukla M, Mikolašević I. Non-alcoholic fatty liver disease - a procoagulant condition? Croat Med J. 2021;62:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Ji D, Cheng G, Lau G. Reply to: "NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues". J Hepatol. 2021;74:484-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Ji D, Zhang M, Qin E, Zhang L, Xu J, Wang Y, Cheng G, Wang F, Lau G. Letter to the Editor: Obesity, diabetes, non-alcoholic fatty liver disease and metabolic dysfunction associated fatty liver disease are proinflammatory hypercoagulable states associated with severe disease and thrombosis in Covid-19. Metabolism. 2021;115:154437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 618] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 86. | Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, Antinori A, Petrosillo N, Marchioni L, Biava G, D'Offizi G, Palmieri F, Goletti D, Zumla A, Ippolito G, Piacentini M, Del Nonno F. Postmortem Findings in Italian Patients With COVID-19: A Descriptive Full Autopsy Study of Cases With and Without Comorbidities. J Infect Dis. 2020;222:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 87. | Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, Zhang A, Zhang N, Wang F, Li X, Wang Y, Liu X, Lv C, Chen S, Liu D, Ji X, Liu C, Ren T, Sun J, Zhao Z, Wu F, Li F, Wang R, Yan Y, Zhang S, Ge G, Shao J, Yang S, Huang Y, Xu D, Ai J, He Q, Zheng MH, Zhang L, Xie Q, Rockey DC, Fallowfield JA, Zhang W, Qi X. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol. 2021;75:439-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |