Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1042
Peer-review started: February 21, 2021
First decision: May 3, 2021
Revised: May 7, 2021
Accepted: July 28, 2021
Article in press: July 28, 2021
Published online: September 27, 2021
Processing time: 213 Days and 1.4 Hours
Long-term antiviral treatment of chronic hepatitis B patients has been proven to be beneficial in reducing liver-related complications. However, lengthy periods of daily administration of medication have some inevitable drawbacks, including decreased medication adherence, increased cost of treatment, and possible long-term side effects. Currently, discontinuation of antiviral agent has become the strategy of interest to many hepatologists, as it might alleviate the aforementioned drawbacks and increase the probability of achieving functional cure. This review focuses on the current evidence of the outcomes following stopping antiviral treatment and the factors associated with subsequent hepatitis B virus relapse, hepatitis B surface antigen clearance, and unmet needs.
Core Tip: Stop strategy is one of the options to get closer to functional cure with a finite duration of treatment in chronic hepatitis B patients. Virological relapse and clinical re
- Citation: Kaewdech A, Sripongpun P. Challenges in the discontinuation of chronic hepatitis B antiviral agents. World J Hepatol 2021; 13(9): 1042-1057
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/1042.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.1042
Hepatitis B virus (HBV) infection is a major health problem globally; approximately 292 million people are affected by this virus[1]. Patients with chronic hepatitis B (CHB) infection are at risk of developing long-term liver-related complications, e.g., cirrhosis, decompensation, and malignant liver tumors[2]. Although the prevalence of CHB infection has declined as a result of immunization programs, the majority of Southeast Asian countries are still categorized as intermediately to highly endemic areas[3]. HBV replication occurs through the formation of covalently closed circular DNA (cccDNA), and the persistence of intrahepatic cccDNA is the major reason for disease chronicity and a major obstacle for the eradication of HBV[4]. However, the measurement of intrahepatic cccDNA is not practical in clinical practice as it can only be done through liver biopsy.
Long-term nucleos(t)ide analogs (NA) inhibit the reverse transcriptase activity of viral polymerase and effectively inhibit HBV replication, reverse liver fibrosis, and reduce the risk of hepatocellular carcinoma (HCC)[5,6]. However, NA have no direct effect on intrahepatic cccDNA or virus transcription in the liver. Therefore, because functional cure, defined as hepatitis B surface antigen (HBsAg) clearance with or without anti-HBs seroconversion, is not often achieved, and most patients need long-term or even lifelong NA therapy[7].
Currently, the best time to stop NA therapy before HBsAg clearance is still uncertain because of the high rates of nontreatment recurrence. For instance, the pooled analysis of a systematic review showed a virological relapse (VR) rate of about 50% to 60% within 12 to 36 mo after drug withdrawal[8]. Although recent clinical guidelines suggest that some patients may stop taking NA before achieving HBsAg serum clearance[9-11], sensitive and reliable biomarkers for identifying patients with low recurrence risk have not yet been established[12,13]. This review focuses on both benefits and risks of discontinuing antiviral agents, as well as the current recommendations, factors, and novel biomarkers for predicting outcomes following NA cessation, and unfulfilled demands.
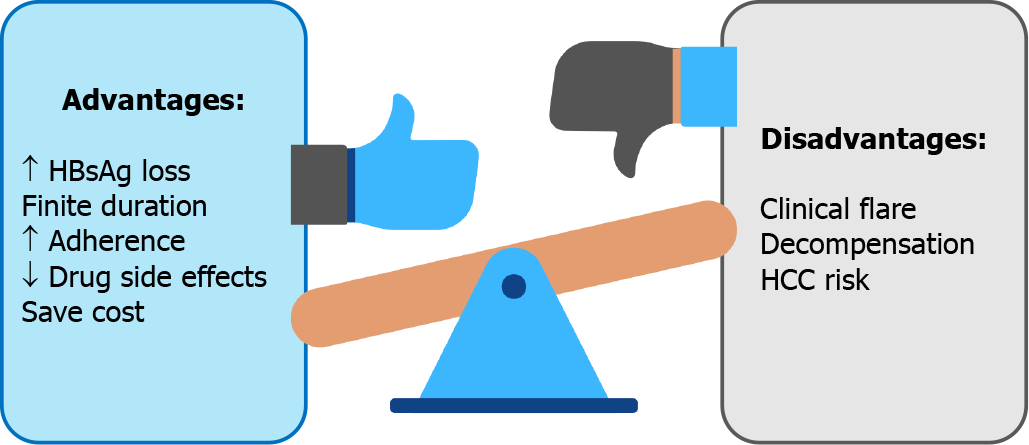
Benefit and risk concerns of CHB antiviral cessation are summarized in Figure 1.
Increased HBsAg loss: The ultimate goal of CHB treatment is clearance of intrahepatic cccDNA. Nonetheless, this endpoint seems to be unrealistic with the current treatment options[9-11]. A more pragmatic endpoint is HBsAg loss with undetectable HBV DNA or a so called “functional cure,” yet HBsAg loss is rarely achieved with long-term NA therapy. In a French study of 18 CHB patients with NA treatment, the annual decrease of HBsAg levels was only 0.084 log10 IU/mL[14], with a study-derived model pre
On the other hand, cessation of NA therapy may increase HBsAg clearance. An initial study by Hadziyannis et al[15] showed a high rate of HBsAg loss of 39.4% at 6 years after stopped adefovir (ADV) in hepatitis B e-antigen (HBeAg) negative CHB patients. That study was followed by a peak of interest in NA discontinuation[15]. A recent systematic review including 1085 patients reported a rate of HBsAg loss of approximately 8%[8]. In contrast, a subsequent study reported HBsAg loss in a minority of patients on continuous NA therapy, approximately 2.1% after 10 years of follow-up[16].
Finite duration: Generally, long-term treatment with NA is required, in contrast to the definable duration of interferon-based therapy, 12 mo in HBeAg-negative, and 6-12 mo in HBeAg-positive patients[17]. Even though the side effects after several years of medication are very few, they can be problematic in real-life practice. An attempt to define a limited duration of NA therapy was first proposed in the Asian Pacific Association for the Study of the Liver (APASL) 2008 guidelines[18]. Finite duration may increase drug adherence, lower the chances of developing side effects from the drug, and reduce costs[19].
Increased adherence: Longer use of NA treatment is associated with lower medication compliance. Drug adherence is of concern in real-life practice[20]. Poor antiviral agent compliance is associated with emerging resistance, particularly in agents with a low genetic barrier[21]. A large retrospective study that included 11,100 CHB patients in the United States found a rate of adherence of 87%[20]. Moreover, a systematic review and meta-analysis included of 30 studies reported that the long-term adherence rate was only 74.7% after a median follow-up of 16 mo[22]. Notably, it was suboptimal compared with a good adherence rate of 95% defined in previous studies[20,23-25]. Compliance to antiviral agent use may improve with finite duration of treatment.
Decreased side effects: A recent systematic review indicated adverse events asso
Cost savings: As mentioned above, hepatitis B treatment with NA might be a long-term therapy. According to a survey in Singapore, fewer than half the patients preferred lifelong treatment[31]. One of the most concerns of lifelong therapy is the cost of treatment. Moreover, only about a quarter of the patients were willing to pay for lifelong therapy, with an acceptable daily cost of 8 United States dollars.
Clinical flare and decompensation following off-therapy: The concerning issue after NA discontinuation is HBV flare, especially clinical relapse (CR). Most studies defined CR as an off-therapy HBV DNA > 2000 IU/mL plus an alanine aminotransferase (ALT) level > 2 times the upper limit of normal (ULN)[8,32]. The overall CR rate from a pooled data analysis with a follow-up ranging from 12-69 mo duration after NA discontinuation was 34.6% in which CR was higher in HBeAg-negative patients (43.7%) than in HBeAg-positive (23.8%)[8]. CR, particularly severe CR, may lead to jaundice, prolonged prothrombin time (PT), or eventually liver failure. In our study in Thai patients, two noncirrhotic HBeAg-negative patients developed jaundice (classified as severe CR) 3 mo after NA discontinuation[12]. Jaundice and hepatitis resolved in both patients after retreatment. Clinical decompensation and death following NA discontinuation has been reported in Asian studies; decompensation and fatality were observed in 0%-1.58% and 0%-0.19% in noncirrhotic patients at 1-3 years of follow-up, while there was a limited number of studies in cirrhotic patients[33-35]. The annual incidence of liver decompensation and death were recently re
HCC risk: There are several well-known benefits of NA treatment in CHB patients[9-11]. Antiviral therapy with NA results in viral suppression, fibrosis improvement, and lower risk of HCC development[37]. Whether patients who stop NA will experience an increased occurrence of HCC in the future than those with continuous treatment is not clear. Nevertheless, to date, HCC development in patients who discontinued NA is not significantly higher than in those who continued NA treatment[33].
Currently, international practice guidelines for CHB management suggest that patients who had consecutive findings of undetectable HBV DNA for a certain duration can stop NA[9-11]. The expert consensus from the APASL first mentioned treatment discontinuation in 2008, advocating that NA therapy can be stopped in selected patients because of drug resistance concerns in long-term NA treatment[18]. The latest recommendations from international hepatology societies for considering stopping NA therapy are shown in Table 1.
| Guidelines | HBeAg-positive CHB | HBeAg-negative CHB |
| APASL 2015[11] | HBeAg seroconversion: + undetectable HBV DNA + normal ALT for ≥ 12 mo (or preferably 3 yr). Cirrhotic patients may be stopped with careful monitoring | Undetectable HBV DNA at least 2 yr with documented on three separate occasions, 6 mo apart: Or HBsAg clearance either at least for 1 yr; Or until anti-HBs seroconversion. Cirrhotic patients may be stopped with careful monitoring |
| AASLD 2018[10] | HBeAg seroconversion + undetectable DNA + normal ALT for ≥ 12 mo. Not recommended in cirrhosis | HBsAg clearance. Not recommended in cirrhosis |
| EASL 2017[9] | HBeAg seroconversion + undetectable DNA for ≥ 12 mo. Not recommended in cirrhosis | HBsAg clearance. Or selected noncirrhotic with undetectable HBV DNA ≥ 3 yr. Not recommended in cirrhosis |
In HBeAg-positive CHB patients, all guidelines allow NA discontinuation in patients who develop HBeAg seroconversion with persistent normal ALT levels and undetectable HBV DNA following consolidation therapy after e-seroconversion for at least 12 mo[38] or preferably 3 years in the APASL guidelines[11]. For patients who are HBeAg-negative, the APASL guideline states that NA can be withdrawn in noncirrhotic patients after treatment for at least 2 years, with an undetectable HBV DNA documented on three consecutive visits, 6 mo apart, or until HBsAg loss with or without development of anti-HBs[11]. Likewise, the European Association for the Study of the Liver (EASL) allows stopping NA in highly selected patients with 3 years of continuously suppression of HBV DNA in noncirrhotic patients[9]. On the contrary, the American Association for the Study of Liver Diseases (AASLD) recommend continuing NA treatment indefinitely unless HBsAg loss is achieved[10]. In patients with liver cirrhosis, the APASL recommends that the discontinuation of NA might be considered, but only with close monitoring[11].
HBV relapse is a common event after NA discontinuation and can be simply ca
| Ref. | Country | n (%) | HBeAg-negative, n (%) | Follow-up time (mo) | Virological relapse rate (%) | Clinical relapse rate (%) | HBsAg loss, n (%) |
| Fung et al[67], 2004 | Canada | 27 | 27 | 18 | 44.4 | 25.9 | NR1 |
| Enomoto et al[68], 2008 | Japan | 22 | 22 | 48 | 68.7 | 68.7 | NR |
| Yeh et al[69], 2009 | Taiwan | 71 | 0 | 15 | 26.8 | 26.8 | 0 |
| Fung et al[70], 2009 | Hongkong | 22 | 0 | 20 | 63.6 | 31.8 | NR |
| Wang et al[71], 2010 | China | 125 | 125 | 24 | 30.4 | NR | NR |
| Kuo et al[72], 2010 | Taiwan | 124 | 0 | > 12 | 66.1 | 66.1 | NR |
| Cai et al[73], 2010 | China | 11 | 0 | 22 | 42.8 | 0 | NR |
| Liu et al[74], 2011 | China | 61 | 61 | 15 | 50.8 | 45.9 | 8/61 |
| Jung et al[75], 2011 | South Korea | 19 | 9 | 12 | 31.6 | 21 | 0 |
| Chan et al[76], 2011 | Hongkong | 53 | 53 | 47 | 69.8 | NR | 9/53 |
| Liang et al[77], 2011 | Hongkong | 84 | 43 | 44 | 14.3 | NR | |
| Chaung et al[78], 2012 | United States | 39 | 0 | 14 | 89.7 | 38.5 | 0 |
| Hadziyannis et al[15], 2012 | Greece | 33 | 33 | 69 | 45.4 | 45.4 | 13/33 |
| Ha et al[79], 2012 | China | 145 | 145 | 16 | 65.5 | 64.1 | NR |
| Song et al[80], 2012 | South Korea | 48 | 0 | 18 | 41.6 | NR | NR |
| He et al[81], 2013 | China | 66 | 66 | 17 | 28.8 | NR | 2/66 |
| Kim et al[82], 2013 | Korea | 45 | 45 | 26 | 73.3 | 53.3 | NR |
| Jeng et al[83], 2013 | Taiwan | 95 | 95 | > 12 | 57.9 | 45.3 | 0/95 |
| Kwon et al[84], 2013 | South Korea | 16 | NR | 32 | 25 | 25 | 2/16 |
| Ridruejo et al[85], 2014 | Argentina | 35 | 0 | 15 | 25.7 | NR | 18/35 |
| Sohn et al[86], 2014 | South Korea | 95 | 54 | 22 | 83.1 | NR | 0/95 |
| Patwardhan et al[87], 2014 | United States | 33 | 33 | 36 | 63.6 | 48.5 | 0/33 |
| He et al[88], 2014 | China | 97 | 0 | 32 | 8.2 | 1 | 11/97 |
| Chen et al[40], 2014 | Taiwan | 188 | 105 | 49 | 66.5 | NR | 33/185 |
| Jiang et al[89], 2015 | China | 72 | 39 | 13 | 65.3 | 41.7 | NR |
| Seto et al[90], 2015 | Hongkong | 184 | 184 | 12 | 91.8 | 22.8 | 0 |
| Peng et al[91], 2015 | China | 65 | 21 | 12 | 43.1 | 27.7 | 1/65 |
| Jeng et al[92], 2016 | Taiwan | 85 | 85 | 155 | 69 | 52 | 2/85 |
| Qiu et al[93], 2016 | China | 112 | 0 | 52 | 48.2 | NR | 1/112 |
| Yao et al[94], 2017 | Taiwan | 119 | 119 | 6 yr | 25.2 | 12.7 | 44/1192 |
| Cao et al[95], 2017 | China | 82 | 22 | 91 | 70.7 | 34.1 | 5/82 |
| Chen et al[96], 2018 | Taiwan | 143 | 104 | 104 | 67.1 | 48.9 | 7/143 |
| Hung et al[97], 2017 | Taiwan | 73 | 73 | 6 yr | 54.8 | 6.8 | 20/73 |
| Berg et al[42], 2017 | German | 21 | 21 | 144 | 52 | 23 | 4/21 |
| Jeng et al[33], 2018 | Taiwan | 691 | 691 | 6 yr | 79.2 | 60.6 | 42/691 |
| Liem et al[39], 2019 | Canada | 45 | 27 | 72 | 71 | 13 | 1/45 |
| Kaewdech et al[12], 2020 | Thailand | 92 | 70 | 48 | 63 | 33.7 | 2/92 |
Various baseline and on-treatment factors are associated with VR off-therapy patients. At pretreatment, the baseline characteristics of increasing age and male sex have been associated with an increased relapse rate[40]. During treatment, extension of consolidation treatment duration by more than 1 to 3 years reduces the risk of VR in both HBeAg-positive and HBeAg-negative patients[38]. For that reason, the international guidelines recommend at least 1 year of consolidation therapy, and preferably 3 years in the APASL guidelines[11], after HBeAg seroconversion before considering NA discontinuation in HBeAg-positive patients. Moreover, the end of treatment (EOT) HBsAg level is highly predictive of HBV relapse, a higher level is correlated with a higher HBV relapse rate[40,41].
From our point of view, the CR is more clinically important than VR, as it may be followed by liver-related complications. A study in a Thai cohort demonstrated that EOT hepatitis B core-related antigen (HBcrAg) and HBV RNA level were independent risk factors for the subsequent development of CR[12]. A recent meta-analysis in
| Baseline at pretreatment | On-treatment | End of treatment |
| Virological relapse | ||
| High age[40,44] | Short consolidation duration[38] | High HBsAg level[40,41] |
| Male sex[40] | High HBcrAg level[12] | |
| High HBsAg level[44] | High HBV RNA level[12] | |
| Clinical relapse | ||
| High HBsAg level[44] | Short consolidation duration[44] | High HBsAg level[13,40,41] |
| High HBcrAg level[12,13,52] | ||
| High HBV RNA level[12,52] | ||
HBsAg clearance is the desired goal of hepatitis B treatment. Nonetheless, as men
A large retrospective Taiwanese study that included 691 patients, demonstrated a shorter time to undetectable HBV DNA (especially if assayed less than 12 wk after NA initiation), on-treatment reduction of HBsAg level of > 1 log10 IU/mL, and an EOT HBsAg level of < 100 IU/mL were independently associated with an increase in the likelihood of off-therapy HBsAg loss[33]. Furthermore, lower pretreatment ALT and HBV DNA levels, lower EOT HBsAg level, and longer treatment duration predicted HBsAg loss in another study[40]. The predictive factors for HBsAg loss in off-therapy patients are summarized in Table 4.
Quantitative serum HBsAg (qHBsAg) has been around in the management of CHB for a while. In untreated patients, serum HBsAg quantification can help to define disease stage, predict spontaneous HBsAg clearance, and predict long-term liver-related complications[43]. As qHBsAg has been used in the clinical practice nowadays, commercial assay kits are widely available. There is increasing evidence of qHBsAg as a marker to aid physicians in deciding whether to discontinue NA. A Taiwanese study by Chen et al[40] found that a cutoff level of < 120 IU/mL predicted HBsAg clearance in HBeAg-negative patients and < 300 IU/mL in HBeAg-positive, respectively[40]. A systematic review by Liu et al[41] indicated that an EOT HBsAg level < 100 IU/mL was the optimal cutoff[41] to predict low rates of HBV relapse and a high chance of HBsAg loss. A meta-analysis involving 1573 patients found that the same EOT HBsAg level (> 100 IU/mL) was associated with an increased risk of VR and CR, however, it is not predictive of CR in a subgroup of Asian patients[44]. The finding is consistent with our study in Thai patients in which the HBsAg level was not associated with the development of CR. A recent multicenter study by Sonneveld et al[13] found that a cutoff level of < 50 IU/mL was the best for predicting a sustained response and HBsAg loss[13]. In conclusion, HBsAg level is a good predictor of HBsAg loss after NA cessation, but its use as a biomarker to predict CR, especially in Asian patients, is still not clear.
Serum HBcrAg has emerged as a novel biomarker in CHB patients. Serum HBcrAg measurement is the combined assay of hepatitis B core antigen, HBeAg, and p22 protein, and it has been shown to be a potential surrogate marker of intrahepatic cccDNA[45,46]. In previous Japanese reports, an increased HBcrAg level was asso
| Ref. | n (%) | End of treatment HBcrAg level (log10 U/mL) | Clinical application |
| Shinkai et al[98], 2006 | 22 | < 3.4 | Predictive factor for absence of the off-therapy relapse |
| Matsumoto et al[47], 2007 | 34 | < 3.2 | Predictive factor for absence of the off-therapy relapse |
| Jung et al[99], 2016 | 113 | ≤ 3.7 | Virological relapse within 1 yr of NA cessation |
| Hsu et al[48], 2019 | 135 | NR | Predictive factors of HBsAg loss and lower clinical relapse |
| Kaewdech et al[12], 2020 | 92 | < 3 | Low risk of off-therapy relapse |
| Papatheodoridi et al[54], 2020 | 57 | < 2 | Predictive factor of HBsAg loss, not required retreatment |
| Sonneveld et al[13], 2020 | 572 | < 2 | Higher risk of sustained response and HBsAg loss |
Serum HBV RNA is closely associated with the transcriptional activity of intrahepatic cccDNA and can be quantified by polymerase chain reaction-based techniques[31]. Moreover, this novel marker is potentially valuable in monitoring for relapse after NA discontinuation[49]. A study by Wang et al[49] reported that viral rebound occurred in 100% of patients who had detectable HBV RNA at EOT[49]. A recent study in HBeAg-positive patients found that positive serum HBV RNA at EOT was associated with the development of off-therapy CR[50].
Together, the data suggest that serum qHBsAg, HBcrAg, and HBV RNA, especially at EOT, are predictive of the outcomes following NA cessation. A few studies have explored the usefulness of combining the biomarkers to select the best candidates for stopping NA[12,48,51,52]. A post-hoc analysis from China included 130 CHB patients who discontinued NA and serial followed-up HBV DNA, qHBsAg and HBV RNA[50] found that the combination of negative HBV DNA and HBV RNA at EOT correlated with lower a CR rate and had an excellent 92% negative predictive value (NPV). Another study, combining qHBsAg, and HBcrAg reported that lower qHBsAg, and HBcrAg levels were associated with lower CR and increased HBsAg clearance[48]. Furthermore, a combination of the two biomarkers before stopping NA showed that no patients with negative HBV RNA, and HBcrAg < 4 log10 U/mL at EOT developed CR[52]. The result is consistent with that observed in our study of the combination of the three biomarkers, i.e. qHBsAg, HBcrAg, and HBV RNA in the prediction of CR after cessation of NA. We found that HBcrAg of < 3 log10 U/mL and HBV RNA of < 2 log10 U/mL had 100% NPV for CR[12]. Nonetheless, when combining all three bio
Apart from using only biomarkers, previous studies illustrated that other clinical and laboratory parameters were significantly associated with post off-treatment outcomes. Therefore, the development of scoring systems utilizing various variables to predict HBV relapse and HBsAg clearance is foreseeable. The first score to predict CR after NA discontinuation is the Japan society of hepatology (JSH) score that consisted of the HBsAg level and HBcrAg level at the time of cessation. The JSH scores are divided into low, moderate, and high-risk groups for HBV relapse after NA cessation[53]. How
The SCALE-B scoring system was developed using data from 135 Taiwanese CHB patients[48]. The score is comprised of the HBsAg level (S), HBcrAg (C), age (A), ALT (L), and tenofovir (E) for HBV (B) and is calculated as HBsAg (log10 IU/mL) + 20 × HBcrAg (log10 U/mL) + 2 × age (yr) + ALT (U/L) + 40 for the use of tenofovir. The scores are divided into three strata, low (< 260 points), intermediate (260-320 points), and high (> 320 points) risk of CR. A score of < 260 points was associated with a subsequent HBsAg loss in 27.1% of the patients at 3 years[48]. The SCALE-B score has been validated in a Caucasian population in which it predicted HBsAg clearance, but not relapse[54]. Recently, the CREATE study, which included a large number of Asian as well as Caucasian patients reported that the SCALE-B score predicted CR and HBsAg loss regardless of HBeAg status or ethnicity[13].
T cells contribute to the control of HBV infection by killing infected hepatocytes[55]. However, chronic HBV infection can exhaust immune activity, particularly T cell function[55], as the longer time of HBV infection is associated with the length of exposure to high antigenicity[56]. With NA therapy, T cell function decreases over time. With discontinuation of NA, T cell function may recover with the increase in the number of active T cells and less exhausted phenotypes[57,58].
After the cessation of NA treatment, the HBV DNA usually becomes detectable and often triggers ALT flares that reflect the immune response. Increased numbers of HBV-specific T cells were observed in patients in virological remission after NA discontinuation[59]. A study by Rinker et al[58] that high function of HBV-specific T cells was observed after NA cessation in patients with subsequent HBsAg loss, especially HBV-specific CD4* T cells[58]. In addition, T cell function increased after programmed death-ligand 1 blockage. More recently, a study by a Spanish group[60] reported that an HBsAg level of ≤ 1000 IU/mL, lower cccDNA transcriptional activity, and a higher HBV-specific T cell response were associated with the development of HBsAg loss.
A new concept of the immune response after NA cessation, beneficial flare vs bad flare is of interest, and was introduced by a Taiwanese group[61]. HBsAg kinetics may be useful in predicting whether patients will require retreatment after CR. Initiation of retreatment is considered in patients who have an increase in HBsAg level before or during ALT flare, which reflects an ineffective immune response. On the other hand, patients in whom a reduction on the HBsAg level was observed before or during ALT flare may not need retreatment, and spontaneous HBsAg clearance may eventually occur[62].
At present, there is no consensus on how to monitor and when to restart NA therapy. Previous studies reported that most HBV relapses occurred within 1 year after the discontinuation of antiviral agents. Most studies recommend careful monitoring, with physical examinations, liver function tests, and serum HBV DNA assays every 1-2 mo for the first 3 mo, every 3 mo for 1 year, and every 6 mo thereafter[12-14,63]. If the patient experiences ALT flare, then close follow-up every week with liver function tests and PT are mandatory for deciding whether prompt retreatment is needed.
Currently, retreatment criteria differ among the studies summarized in Table 6[12,13,39,63]. Most suggested that retreatment should be initiated in patients with an ALT level > 10 times above the ULN regardless of bilirubin level, with an ALT level > 5 times above ULN plus a bilirubin > 1.5-2 mg/dL, persistent of ALT level > 5 times the ULN for 4 wk, or an ALT elevation with either a prolonged PT > 2 sec or a bilirubin level >1.5-2 mg/dL. The retreatment strategy is challenging as CR may reflect the immune restoration and reintroduction of NA might alleviate the effect. However, delayed initiation of retreatment can cause severe ALT flare, and eventually liver decompensation. The biomarkers or tools to aid justification of the optimal timing of retreatment are unmet needs.
| Ref. | Follow-up interval | Criteria of retreatment |
| Berg et al[42], 2017 | Every 2 wk in the first 3 mo, every 4 wk until week 48, and every 12 wk thereafter until week 144 | Two consecutive total bilirubin > 1.5 mg/dL plus ALT > ULN |
| Two consecutive PT ≥ 2.0 seconds (INR ≥ 0.5) prolonged from baseline with adequate vitamin K therapy plus ALT > ULN | ||
| Two consecutive ALT > 10 × ULN | ||
| ALT > 2 × but ≤ 5 × ULN persisting for ≥ 12 wk plus HBV DNA > 20000 copies/mL | ||
| ALT 5 × but ≤ 10 × ULN persisting for ≥ 4 wk | ||
| Papatheodoridi et al[63], 2018 | Every mo in the first 3 mo then at least every 3 mo until month 12 | Greece cohort: (1) ALT > 10 × ULN; (2) ALT > 5 × ULN plus total bilirubin > 2 mg/dL; (3) ALT > 3 × ULN plus HBV DNA > 100000 IU/mL; and (4) ALT > ULN plus HBV DNA > 2000 IU/mL on three sequential occasions |
| Taiwanese cohort: (1) ALT > 2 × ULN twice 3 mo apart plus HBV DNA > 2000 IU/mL; (2) Total bilirubin > 2 mg/dL; and (3) PT ≥ 3 seconds of control range | ||
| Liem et al[39], 2019 | Wk 4, 6, 12, 18, 24, 36, 48, 60, and 72 | HBeAg seroreversion |
| HBV DNA > 2000 IU/mL plus ALT > 600 IU/mL | ||
| HBV DNA > 2000 IU/mL plus ALT > 5 × ULN (40 IU/mL) on two consecutive visits | ||
| HBV DNA > 2000 IU/mL plus ALT > 200 IU/mL but < 600 IU/mL for > 6–8 wk | ||
| HBV DNA > 20000 IU/mL on two consecutive visits at least 4 wk apart | ||
| García-López et al[60], 2020 | Monthly in the first 6 mo then every 3-4 mo until 24 mo | Two consecutive ALT > 10 × ULN regardless of HBV DNA level |
| ALT > 5-10 × ULN and HBV DNA > 2000 IU/mL persisting for ≥ 4 wk | ||
| ALT > 2-5 × ULN and HBV DNA > 2000 IU/mL persisting for ≥ 6 mo | ||
| Need for immunosuppressive treatment |
In Asian and Caucasian populations, there are differences in rates of HBsAg clearance and HBV relapse. Caucasians have a higher probability of achieving a functional cure after NA cessation[13]. HBsAg clearance has been observed in 19%-29% of Caucasians at 2 years[42,64] whereas it had been found in only 1.78%/year in Asians. This phenomenon might be explained by the difference of HBV genotypes between Asians and Caucasians, and the duration of infectivity. In Asians, the most common ge
Another discrepancy between East and West is the consideration of stopping NA in cirrhotic patients. The APASL recommends that in highly selected cirrhotic patients, NA discontinuation may be considered according to the stopping criteria and safety results of previous Asian studies[11,33]. On the contrary, the AASLD and EASL do not recommend NA cessation in cirrhotic patients because safety concerns[9,10].
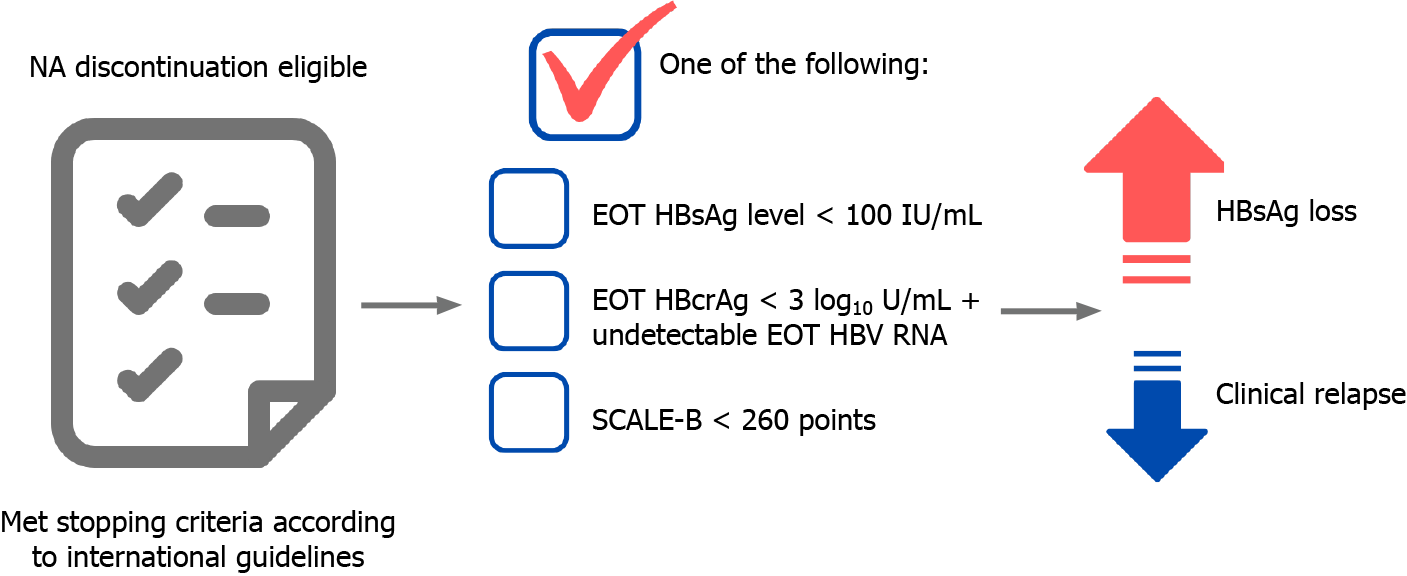
From our perspective, the stop strategy is optimal in highly selected noncirrhotic CHB patients. At present, we propose the ideal candidates for NA discontinuation in CHB patients as shown in Figure 2. The major benefit of this strategy is it enhances the chance of achieving a functional cure faster than continuous long-term NA therapy. However, there are some caveats, including severe CR, liver decompensation, or HCC development to be considered. The current unmet needs for NA discontinuation strategy in CHB patients are the better prediction of the patients who are good can
The authors thank the Faculty of Medicine, Prince of Songkla University, Hat Yai, Thailand and Thai Association for the Study of the Liver (THASL) for the inspiration and their support for this review. The work is that of the authors only.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: The Gastroenterological Association of Thailand; American Association for the Study of Liver Diseases; Thai Association for the Study of the Liver.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bottaro M S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1216] [Article Influence: 173.7] [Reference Citation Analysis (2)] |
| 2. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1700] [Cited by in RCA: 1712] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 3. | Udompap P, Tanwandee T, Gani R. Affordability of Antiviral Therapy in Asia-Pacific Countries and Its Impact on Public Health Outcomes. Clin Liver Dis (Hoboken). 2020;16:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kumar R, Pérez-Del-Pulgar S, Testoni B, Lebossé F, Zoulim F. Clinical relevance of the study of hepatitis B virus covalently closed circular DNA. Liver Int. 2016;36 Suppl 1:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 696] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 6. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1369] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 7. | Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 8. | Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, Petersen J. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology. 2016;63:1481-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 10. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2845] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 11. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 12. | Kaewdech A, Tangkijvanich P, Sripongpun P, Witeerungrot T, Jandee S, Tanaka Y, Piratvisuth T. Hepatitis B surface antigen, core-related antigen and HBV RNA: Predicting clinical relapse after NA therapy discontinuation. Liver Int. 2020;40:2961-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Sonneveld MJ, Park JY, Kaewdech A, Seto WK, Tanaka Y, Carey I, Papatheodoridi M, van Bömmel F, Berg T, Zoulim F, Ahn SH, Dalekos GN, Erler NS, Höner Zu Siederdissen C, Wedemeyer H, Cornberg M, Yuen MF, Agarwal K, Boonstra A, Buti M, Piratvisuth T, Papatheodoridis G, Maasoumy B; CREATE Study Group. Prediction of Sustained Response After Nucleo(s)tide Analogue Cessation Using HBsAg and HBcrAg Levels: A Multicenter Study (CREATE). Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143:629-636.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 16. | Lok AS, Zoulim F, Dusheiko G, Chan HLY, Buti M, Ghany MG, Gaggar A, Yang JC, Wu G, Flaherty JF, Subramanian GM, Locarnini S, Marcellin P. Durability of Hepatitis B Surface Antigen Loss With Nucleotide Analogue and Peginterferon Therapy in Patients With Chronic Hepatitis B. Hepatol Commun. 2020;4:8-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Brunetto MR, Bonino F. Interferon therapy of chronic hepatitis B. Intervirology. 2014;57:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 18. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S; Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 19. | Liaw YF. Finite nucleos(t)ide analog therapy in HBeAg-negative chronic hepatitis B: an emerging paradigm shift. Hepatol Int. 2019;13:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Thompson AJ, Ayres A, Yuen L, Bartholomeusz A, Bowden DS, Iser DM, Chen RY, Demediuk B, Shaw G, Bell SJ, Watson KJ, Locarnini SA, Desmond PV. Lamivudine resistance in patients with chronic hepatitis B: role of clinical and virological factors. J Gastroenterol Hepatol. 2007;22:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Ford N, Scourse R, Lemoine M, Hutin Y, Bulterys M, Shubber Z, Donchuk D, Wandeler G. Adherence to Nucleos(t)ide Analogue Therapies for Chronic Hepatitis B Infection: A Systematic Review and Meta-Analysis. Hepatol Commun. 2018;2:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Allard N, Dev A, Dwyer J, Srivatsa G, Thompson A, Cowie B. Factors associated with poor adherence to antiviral treatment for hepatitis B. J Viral Hepat. 2017;24:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Lieveld FI, van Vlerken LG, Siersema PD, van Erpecum KJ. Patient adherence to antiviral treatment for chronic hepatitis B and C: a systematic review. Ann Hepatol. 2013;12:380-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D, Sorbel J, Borroto-Esoda K, Oldach D, Rousseau F. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | de Fraga RS, Van Vaisberg V, Mendes LCA, Carrilho FJ, Ono SK. Adverse events of nucleos(t)ide analogues for chronic hepatitis B: a systematic review. J Gastroenterol. 2020;55:496-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Udompap P, Kim D, Ahmed A, Kim WR. Longitudinal trends in renal function in chronic hepatitis B patients receiving oral antiviral treatment. Aliment Pharmacol Ther. 2018;48:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL, Acharya SK, Flaherty JF, Massetto B, Cathcart AL, Kim K, Gaggar A, Subramanian GM, McHutchison JG, Pan CQ, Brunetto M, Izumi N, Marcellin P; GS-US-320-0108 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 29. | Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, Hui AJ, Janssen HL, Chowdhury A, Tsang TY, Mehta R, Gane E, Flaherty JF, Massetto B, Gaggar A, Kitrinos KM, Lin L, Subramanian GM, McHutchison JG, Lim YS, Acharya SK, Agarwal K; GS-US-320-0110 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 30. | Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, Sharma M, Janssen HLA, Pan CQ, Çelen MK, Furusyo N, Shalimar D, Yoon KT, Trinh H, Flaherty JF, Gaggar A, Lau AH, Cathcart AL, Lin L, Bhardwaj N, Suri V, Mani Subramanian G, Gane EJ, Buti M, Chan HLY; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 31. | Lim SG, Aung MO, Chung SW, Soon CS, Mak BH, Lee KH. Patient preferences for hepatitis B therapy. Antivir Ther. 2013;18:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment Pharmacol Ther. 2015;42:243-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 33. | Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 34. | Kuo MT, Hu TH, Hung CH, Wang JH, Lu SN, Tsai KL, Chen CH. Hepatitis B virus relapse rates in chronic hepatitis B patients who discontinue either entecavir or tenofovir. Aliment Pharmacol Ther. 2019;49:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Ma TL, Hu TH, Hung CH, Wang JH, Lu SN, Chen CH. Incidence and predictors of retreatment in chronic hepatitis B patients after discontinuation of entecavir or tenofovir treatment. PLoS One. 2019;14:e0222221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Ahn J, Lee HM, Lim JK, Pan CQ, Nguyen MH, Ray Kim W, Mannalithara A, Trinh H, Chu D, Tran T, Min A, Do S, Te H, Reddy KR, Lok AS. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir Is Associated With Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients With Chronic HBV Infection in China. Gastroenterology. 2020;158:215-225.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 38. | Chi H, Hansen BE, Yim C, Arends P, Abu-Amara M, van der Eijk AA, Feld JJ, de Knegt RJ, Wong DK, Janssen HL. Reduced risk of relapse after long-term nucleos(t)ide analogue consolidation therapy for chronic hepatitis B. Aliment Pharmacol Ther. 2015;41:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 39. | Liem KS, Fung S, Wong DK, Yim C, Noureldin S, Chen J, Feld JJ, Hansen BE, Janssen HLA. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut. 2019;68:2206-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 40. | Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 41. | Liu J, Li T, Zhang L, Xu A. The Role of Hepatitis B Surface Antigen in Nucleos(t)ide Analogues Cessation Among Asian Patients With Chronic Hepatitis B: A Systematic Review. Hepatology. 2019;70:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 42. | Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, Eisenbach C, Welzel TM, Zachoval R, Felten G, Schulze-Zur-Wiesch J, Cornberg M, Op den Brouw ML, Jump B, Reiser H, Gallo L, Warger T, Petersen J; FINITE CHB study investigators [First investigation in stopping TDF treatment after long-term virological suppression in HBeAg-negative chronic hepatitis B]. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 43. | Mak LY, Seto WK, Fung J, Yuen MF. Use of HBsAg quantification in the natural history and treatment of chronic hepatitis B. Hepatol Int. 2020;14:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Liu Y, Jia M, Wu S, Jiang W, Feng Y. Predictors of relapse after cessation of nucleos(t)ide analog treatment in HBeAg-negative chronic hepatitis B patients: A meta-analysis. Int J Infect Dis. 2019;86:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2018;47:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 46. | Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, Loglio A, Facchetti F, Lampertico P, Levrero M, Zoulim F. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 47. | Matsumoto A, Tanaka E, Minami M, Okanoue T, Yatsuhashi H, Nagaoka S, Suzuki F, Kobayashi M, Chayama K, Imamura M, Yotsuyanagi H, Nakaoka S, Maki N, Kawata S, Kumada H, Iino S, Kiyosawa K. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol Res. 2007;37:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Hsu YC, Nguyen MH, Mo LR, Wu MS, Yang TH, Chen CC, Tseng CH, Tai CM, Wu CY, Lin JT, Tanaka Y, Chang CY. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment Pharmacol Ther. 2019;49:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 49. | Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, Zhang R, Chen R, Li T, Zhang T, Yuan Q, Li PC, Huang Q, Colonno R, Jia J, Hou J, McCrae MA, Gao Z, Ren H, Xia N, Zhuang H, Lu F. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 50. | Fan R, Zhou B, Xu M, Tan D, Niu J, Wang H, Ren H, Chen X, Wang M, Ning Q, Shi G, Sheng J, Tang H, Bai X, Liu S, Lu F, Peng J, Sun J, Xie Q, Hou J; Chronic Hepatitis B Study Consortium. Association Between Negative Results From Tests for HBV DNA and RNA and Durability of Response After Discontinuation of Nucles(t)ide Analogue Therapy. Clin Gastroenterol Hepatol. 2020;18:719-727.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 51. | Carey I, Gersch J, Wang B, Moigboi C, Kuhns M, Cloherty G, Dusheiko G, Agarwal K. Pregenomic HBV RNA and Hepatitis B Core-Related Antigen Predict Outcomes in Hepatitis B e Antigen-Negative Chronic Hepatitis B Patients Suppressed on Nucleos(T)ide Analogue Therapy. Hepatology. 2020;72:42-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 52. | Fan R, Peng J, Xie Q, Tan D, Xu M, Niu J, Wang H, Ren H, Chen X, Wang M, Sheng J, Tang H, Bai X, Wu Y, Zhou B, Sun J, Hou J; Chronic Hepatitis B Study Consortium. Combining Hepatitis B Virus RNA and Hepatitis B Core-Related Antigen: Guidance for Safely Stopping Nucleos(t)ide Analogues in Hepatitis B e Antigen-Positive Patients With Chronic Hepatitis B. J Infect Dis. 2020;222:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 53. | Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis B Virus Infection. Hepatol Res. 2014;44 Suppl S1:1-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 54. | Papatheodoridi M, Hadziyannis E, Berby F, Zachou K, Testoni B, Rigopoulou E, Gatselis NK, Lyberopoulou A, Vlachogiannakos I, Manolakopoulos S, Dalekos GN, Zoulim F, Papatheodoridis GV. Predictors of hepatitis B surface antigen loss, relapse and retreatment after discontinuation of effective oral antiviral therapy in noncirrhotic HBeAg-negative chronic hepatitis B. J Viral Hepat. 2020;27:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 55. | Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G, Colombo M, Missale G, Ferrari C. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963-73.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 56. | Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215-4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 738] [Article Influence: 41.0] [Reference Citation Analysis (1)] |
| 57. | Höner Zu Siederdissen C, Rinker F, Maasoumy B, Wiegand SB, Filmann N, Falk CS, Deterding K, Port K, Mix C, Manns MP, Herrmann E, Wedemeyer H, Kraft AR, Cornberg M. Viral and Host Responses After Stopping Long-term Nucleos(t)ide Analogue Therapy in HBeAg-Negative Chronic Hepatitis B. J Infect Dis. 2016;214:1492-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 58. | Rinker F, Zimmer CL, Höner Zu Siederdissen C, Manns MP, Kraft ARM, Wedemeyer H, Björkström NK, Cornberg M. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 59. | Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, Becht E, Hansi NK, Foster GR, Su TH, Tseng TC, Lim SG, Kao JH, Newell EW, Kennedy PT, Bertoletti A. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 60. | García-López M, Lens S, Pallett LJ, Testoni B, Rodríguez-Tajes S, Mariño Z, Bartres C, García-Pras E, Leonel T, Perpiñán E, Lozano JJ, Rodríguez-Frías F, Koutsoudakis G, Zoulim F, Maini MK, Forns X, Pérez-Del-Pulgar S. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol. 2021;74:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 61. | Liaw YF, Jeng WJ, Chang ML. HBsAg Kinetics in Retreatment Decision for Off-Therapy Hepatitis B Flare in HBeAg-Negative Patients. Gastroenterology. 2018;154:2280-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Jeng WJ, Chang ML, Liaw YF. Off-therapy precipitous HBsAg decline predicts HBsAg loss after finite entecavir therapy in HBeAg-negative patients. J Viral Hepat. 2019;26:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Papatheodoridis GV, Manolakopoulos S, Su TH, Siakavellas S, Liu CJ, Kourikou A, Yang HC, Kao JH. Significance of definitions of relapse after discontinuation of oral antivirals in HBeAg-negative chronic hepatitis B. Hepatology. 2018;68:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Papatheodoridis GV, Rigopoulou EI, Papatheodoridi M, Zachou K, Xourafas V, Gatselis N, Hadziyannis E, Vlachogiannakos J, Manolakopoulos S, Dalekos GN. DARING-B: discontinuation of effective entecavir or tenofovir disoproxil fumarate long-term therapy before HBsAg loss in non-cirrhotic HBeAg-negative chronic hepatitis B. Antivir Ther. 2018;23:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Tangkijvanich P, Mahachai V, Komolmit P, Fongsarun J, Theamboonlers A, Poovorawan Y. Hepatitis B virus genotypes and hepatocellular carcinoma in Thailand. World J Gastroenterol. 2005;11:2238-2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Chamroonkul N, Piratvisuth T. Hepatitis B During Pregnancy in Endemic Areas: Screening, Treatment, and Prevention of Mother-to-Child Transmission. Paediatr Drugs. 2017;19:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Fung SK, Wong F, Hussain M, Lok AS. Sustained response after a 2-year course of lamivudine treatment of hepatitis B e antigen-negative chronic hepatitis B. J Viral Hepat. 2004;11:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 68. | Enomoto M, Tamori A, Kohmoto MT, Hayashi T, Morikawa H, Jomura H, Sakaguchi H, Habu D, Kawada N, Shiomi S, Nishiguchi S. Optimal duration of additional therapy after biochemical and virological responses to lamivudine in patients with HBeAg-negative chronic hepatitis B: a randomized trial. Hepatol Res. 2008;38:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Yeh CT, Hsu CW, Chen YC, Liaw YF. Withdrawal of lamivudine in HBeAg-positive chronic hepatitis B patients after achieving effective maintained virological suppression. J Clin Virol. 2009;45:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Fung J, Lai CL, Tanaka Y, Mizokami M, Yuen J, Wong DK, Yuen MF. The duration of lamivudine therapy for chronic hepatitis B: cessation vs. continuation of treatment after HBeAg seroconversion. Am J Gastroenterol. 2009;104:1940-1946; quiz 1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Wang L, Liu F, Liu YD, Li XY, Wang JB, Zhang ZH, Wang YZ. Stringent cessation criterion results in better durability of lamivudine treatment: a prospective clinical study in hepatitis B e antigen-positive chronic hepatitis B patients. J Viral Hepat. 2010;17:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Kuo YH, Chen CH, Wang JH, Hung CH, Tseng PL, Lu SN, Changchien CS, Lee CM. Extended lamivudine consolidation therapy in hepatitis B e antigen-positive chronic hepatitis B patients improves sustained hepatitis B e antigen seroconversion. Scand J Gastroenterol. 2010;45:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Cai W, Xie Q, An B, Wang H, Zhou X, Zhao G, Guo Q, Gu R, Bao S. On-treatment serum HBsAg level is predictive of sustained off-treatment virologic response to telbivudine in HBeAg-positive chronic hepatitis B patients. J Clin Virol. 2010;48:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Liu F, Wang L, Li XY, Liu YD, Wang JB, Zhang ZH, Wang YZ. Poor durability of lamivudine effectiveness despite stringent cessation criteria: a prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients. J Gastroenterol Hepatol. 2011;26:456-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Jung YK, Yeon JE, Lee KG, Jung ES, Kim JH, Seo YS, Yim HJ, Um SH, Ryu HS, Byun KS. Virologic response is not durable after adefovir discontinuation in lamivudine-resistant chronic hepatitis B patients. Korean J Hepatol. 2011;17:261-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Chan HL, Wong GL, Chim AM, Chan HY, Chu SH, Wong VW. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther. 2011;16:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 77. | Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, Xie R, Ge S, Hu J, Jiang Z, Zhu M, Wong VW, Chan HL. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment Pharmacol Ther. 2011;34:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Chaung KT, Ha NB, Trinh HN, Garcia RT, Nguyen HA, Nguyen KK, Garcia G, Ahmed A, Keeffe EB, Nguyen MH. High frequency of recurrent viremia after hepatitis B e antigen seroconversion and consolidation therapy. J Clin Gastroenterol. 2012;46:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Ha M, Zhang G, Diao S, Lin M, Sun L, She H, Kuan C, Shen L, Huang C, Shen W, Huang Z. A prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients with stringent cessation criteria for adefovir. Arch Virol. 2012;157:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Song MJ, Song DS, Kim HY, Yoo SH, Bae SH, Choi JY, Yoon SK, Paik YH, Lee JS, Lee HW, Kim HJ. Durability of viral response after off-treatment in HBeAg positive chronic hepatitis B. World J Gastroenterol. 2012;18:6277-6283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | He D, Guo S, Chen W, Chen X, Yan G, Wang J, Li M, Zhu P, Huang H, Wang Y. Long-term outcomes after nucleos(t)ide analogues discontinuation in chronic hepatitis B patients with HBeAg-negative. BMC Infect Dis. 2013;13:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Kim YJ, Kim K, Hwang SH, Kim SS, Lee D, Cheong JY, Cho SW. Durability after discontinuation of nucleos(t)ide therapy in chronic HBeAg negative hepatitis patients. Clin Mol Hepatol. 2013;19:300-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, Liaw YF. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology. 2013;58:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 84. | Kwon JH, Jang JW, Choi JY, Park CH, Yoo SH, Bae SH, Yoon SK. Should lamivudine monotherapy be stopped or continued in patients infected with hepatitis B with favorable responses after more than 5 years of treatment? J Med Virol. 2013;85:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Ridruejo E, Marciano S, Galdame O, Reggiardo MV, Muñoz AE, Adrover R, Cocozzella D, Fernandez N, Estepo C, Mendizábal M, Romero GA, Levi D, Schroder T, Paz S, Fainboim H, Mandó OG, Gadano AC, Silva MO. Relapse rates in chronic hepatitis B naïve patients after discontinuation of antiviral therapy with entecavir. J Viral Hepat. 2014;21:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Sohn HR, Min BY, Song JC, Seong MH, Lee SS, Jang ES, Shin CM, Park YS, Hwang JH, Jeong SH, Kim N, Lee DH, Kim JW. Off-treatment virologic relapse and outcomes of re-treatment in chronic hepatitis B patients who achieved complete viral suppression with oral nucleos(t)ide analogs. BMC Infect Dis. 2014;14:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Patwardhan VR, Sengupta N, Bonder A, Lau D, Afdhal NH. Treatment cessation in noncirrhotic, e-antigen negative chronic hepatitis B is safe and effective following prolonged anti-viral suppression with nucleosides/nucleotides. Aliment Pharmacol Ther. 2014;40:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | He D, Guo S, Zhu P, Tao S, Li M, Huang H, Wang J, Wang Y, Ding M. Long-term outcomes after nucleos(t)ide analogue discontinuation in HBeAg-positive chronic hepatitis B patients. Clin Microbiol Infect. 2014;20:O687-O693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Jiang JN, Huang ZL, He LX, Huang YH, Su MH, Xie R, Liang YX, Fu WD, Huang XH, Guo WW, Zhong SH, Liu ZH, Li SH, Zhu TF, Gao ZL. Residual amount of HBV DNA in serum is related to relapse in chronic hepatitis B patients after cessation of nucleos(t)ide analogs. J Clin Gastroenterol. 2015;49:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, Yuen MF, Chan HL. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut. 2015;64:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 91. | Peng J, Cao J, Yu T, Cai S, Li Z, Zhang X, Sun J. Predictors of sustained virologic response after discontinuation of nucleos(t)ide analog treatment for chronic hepatitis B. Saudi J Gastroenterol. 2015;21:245-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Jeng WJ, Chen YC, Sheen IS, Lin CL, Hu TH, Chien RN, Liaw YF. Clinical Relapse After Cessation of Tenofovir Therapy in Hepatitis B e Antigen-Negative Patients. Clin Gastroenterol Hepatol. 2016;14:1813-1820.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 93. | Qiu YW, Huang LH, Yang WL, Wang Z, Zhang B, Li YG, Su TT, Zhou HY, Xu W, Wang XD, Dai YP, Gan JH. Hepatitis B surface antigen quantification at hepatitis B e antigen seroconversion predicts virological relapse after the cessation of entecavir treatment in hepatitis B e antigen-positive patients. Int J Infect Dis. 2016;43:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Yao CC, Hung CH, Hu TH, Lu SN, Wang JH, Lee CM, Chen CH. Incidence and predictors of HBV relapse after cessation of nucleoside analogues in HBeAg-negative patients with HBsAg ≤ 200 IU/mL. Sci Rep. 2017;7:1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Cao J, Chi H, Yu T, Li Z, Hansen BE, Zhang X, Zhong C, Sun J, Hou J, Janssen HLA, Peng J. Off-Treatment Hepatitis B Virus (HBV) DNA Levels and the Prediction of Relapse After Discontinuation of Nucleos(t)ide Analogue Therapy in Patients With Chronic Hepatitis B: A Prospective Stop Study. J Infect Dis. 2017;215:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 96. | Chen CH, Hsu YC, Lu SN, Hung CH, Wang JH, Lee CM, Hu TH. The incidence and predictors of HBV relapse after cessation of tenofovir therapy in chronic hepatitis B patients. J Viral Hepat. 2018;25:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 97. | Hung CH, Wang JH, Lu SN, Hu TH, Lee CM, Chen CH. Hepatitis B surface antigen loss and clinical outcomes between HBeAg-negative cirrhosis patients who discontinued or continued nucleoside analogue therapy. J Viral Hepat. 2017;24:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 98. | Shinkai N, Tanaka Y, Orito E, Ito K, Ohno T, Hirashima N, Hasegawa I, Sugauchi F, Ueda R, Mizokami M. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol Res. 2006;36:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 99. | Jung KS, Park JY, Chon YE, Kim HS, Kang W, Kim BK, Kim SU, Kim do Y, Han KH, Ahn SH. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J Gastroenterol. 2016;51:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |