Published online Apr 27, 2021. doi: 10.4254/wjh.v13.i4.483
Peer-review started: February 3, 2021
First decision: March 1, 2021
Revised: March 7, 2021
Accepted: March 19, 2021
Article in press: March 19, 2021
Published online: April 27, 2021
Processing time: 71 Days and 23.7 Hours
Although arterial hemorrhage after pancreaticoduodenectomy (PD) is not frequent, it is fatal. Arterial hemorrhage is caused by pseudoaneurysm rupture, and the gastroduodenal artery stump and hepatic artery (HA) are frequent culprit vessels. Diagnostic procedures and imaging modalities are associated with certain difficulties. Simultaneous accomplishment of complete hemostasis and HA flow preservation is difficult after PD. Although complete hemostasis may be obtained by endovascular treatment (EVT) or surgery, liver infarction caused by hepatic ischemia and/or liver abscesses caused by biliary ischemia may occur. We herein discuss therapeutic options for fatal arterial hemorrhage after PD.
To present our data here along with a discussion of therapeutic strategies for fatal arterial hemorrhage after PD.
We retrospectively investigated 16 patients who developed arterial hemorrhage after PD. The patients’ clinical characteristics, diagnostic procedures, actual treatments [transcatheter arterial embolization (TAE), stent-graft placement, or surgery], clinical courses, and outcomes were evaluated.
The frequency of arterial hemorrhage after PD was 5.5%. Pancreatic leakage was observed in 12 patients. The onset of hemorrhage occurred at a median of 18 d after PD. Sentinel bleeding was observed in five patients. The initial EVT procedures were stent-graft placement in seven patients, TAE in six patients, and combined therapy in two patients. The rate of technical success of the initial EVT was 75.0%, and additional EVTs were performed in four patients. Surgical approaches including arterioportal shunting were performed in eight patients. Liver infarction was observed in two patients after TAE. Two patients showed a poor outcome even after successful EVT. These four patients with poor clinical courses and outcomes had a poor clinical condition before EVT. Fourteen patients were successfully treated.
Transcatheter placement of a covered stent may be useful for simultaneous accomplishment of complete hemostasis and HA flow preservation.
Core Tip: Arterial hemorrhage after pancreaticoduodenectomy is fatal. This hemorrhage is caused by pseudoaneurysm rupture, and the gastroduodenal artery stump and hepatic artery are frequent culprit vessels. Simultaneous accomplishment of complete hemostasis and hepatic artery flow preservation is difficult after pancreaticoduodenectomy. Although complete hemostasis may be obtained by transcatheter arterial embolization or surgery, liver infarction and/or abscesses may occur. We here evaluate our experience including actual treatments (transcatheter arterial embolization, stent-graft placement, or surgery), and discuss therapeutic strategies. Transcatheter placement of a covered stent is useful for simultaneous accomplishment of complete hemostasis and hepatic arterial flow preservation.
- Citation: Kamada Y, Hori T, Yamamoto H, Harada H, Yamamoto M, Yamada M, Yazawa T, Sasaki B, Tani M, Sato A, Katsura H, Tani R, Aoyama R, Sasaki Y, Okada M, Zaima M. Fatal arterial hemorrhage after pancreaticoduodenectomy: How do we simultaneously accomplish complete hemostasis and hepatic arterial flow? World J Hepatol 2021; 13(4): 483-503
- URL: https://www.wjgnet.com/1948-5182/full/v13/i4/483.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i4.483
The mortality rate after pancreaticoduodenectomy (PD) is currently < 5%[1-8] because surgical procedures and perioperative management techniques have been well established[1,9-11]. However, postoperative complications still remain a matter of concern[1,2,4,6,8,11-13]. Although arterial hemorrhage after PD is not frequent, it is fatal. Its mortality rate reportedly ranges from 10% to 60%[1,2,4,7,12,14-23], and it easily results in shock and coagulopathy[1,18,23]. Arterial hemorrhage is mainly caused by pseudoaneurysm rupture of a splanchnic artery[18,24], and the gastroduodenal artery (GDA) stump, common hepatic artery (CHA), and proper hepatic artery (PHA) are the most frequent culprit vessels[1-3,6,18,25-28]. Diagnostic and treatment strategies should be decided on a case-by-case basis[18,28,29].
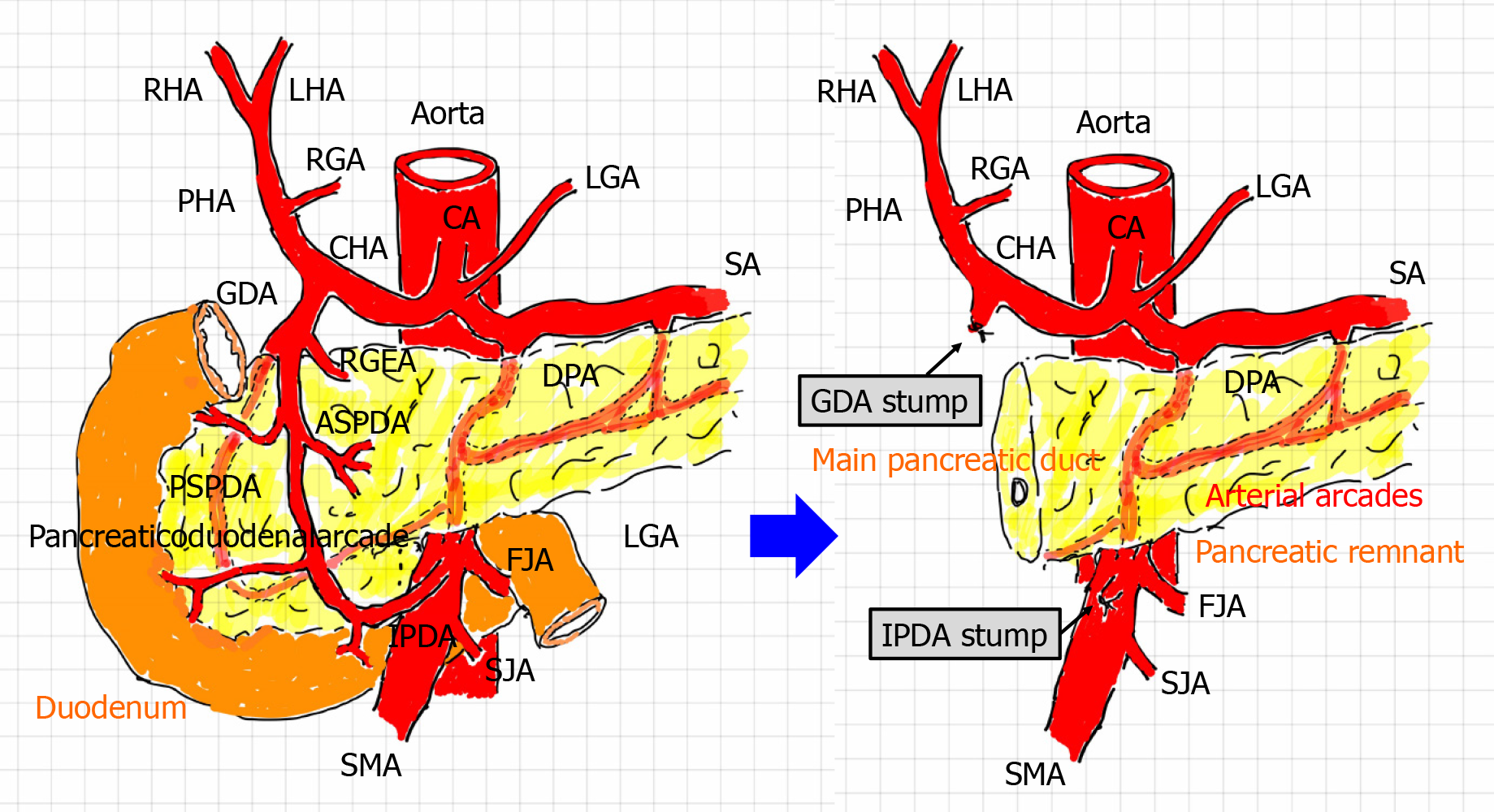
Arterial flow, especially in the liver, is modified after PD (Figure 1). Briefly, the hepatopetal flow of the hepatic artery (HA) depends on the blood supply from the celiac artery (e.g., the CHA and PHA), not from the superior mesenteric artery (SMA) [e.g., the inferior pancreaticoduodenal artery (IPDA) and retrograde-flowing GDA] and collateral circulation (e.g., hepatopetal collaterals via the inferior phrenic artery)[1,28,30,31]. This leads to a simple question: How do we simultaneously accomplish complete hemostasis and HA flow preservation? Endovascular treatment (EVT) [e.g., transcatheter arterial embolization (TAE) and stent-graft placement] are currently available[1,6,13,16,18,19,32-40], and surgical arterioportal shunting has therapeutic potential for the arterial blood supply[41,42].
TAE provides complete hemostasis[1,2,13,19,20,23,39,43], although this approach increases the risk of severe complications associated with liver infarction caused by hepatic ischemia[1,13,18,19,23,30,32,33,39] and/or liver abscesses caused by biliary ischemia[6,34,35]. In contrast, transcatheter placement of a stent-graft (bare or covered stent) preserves HA flow[1,13,16,18,36-38,40], although technical failure of hemostasis may rarely occur[1]. From the viewpoint of cost-effectiveness, EVT is more advantageous than conventional surgery[29].
Complete hemostasis of fatal hemorrhage and preservation of HA flow should be simultaneously obtained; however, this may be difficult after PD because the hepatopetal arterial supply has been modified (Figure 1). In the present study, we retrospectively investigated our treatments for fatal arterial hemorrhage after PD and evaluated our own results. We also herein discuss the safety and feasibility of transcatheter stent-graft placement and especially validate the therapeutic potential of using a covered (not bare) stent for simultaneous accomplishment of complete hemostasis and HA flow preservation.
This study focused on the postoperative state after PD (Figure 1); therefore, patients who underwent other surgeries (e.g., distal pancreatectomy or gastrectomy) were excluded from further analysis. During a 14-year period (from January 2007 to December 2020), 291 PDs were performed in our institution. Fatal arterial hemorrhage occurred in 16 patients who underwent PD, and these patients were enrolled in this study. The patients’ mean age at the time of PD was 73.4 ± 7.7 years, and the patients comprised 11 men and 5 women. The types of PD and postoperative complications are summarized in Table 1. The median follow-up duration after PD was 1.34 years [range, 14 d (death) to 9.55 years].
| Case number | Primary disease | Type of PD | Lymphadenectomy (categorization1) | Nerve dissection | Associated pancreatitis | Pancreatic leakage | Postoperative complications | Hemorrhage oncet2 | Sentinel bleeding | Symptoms | Sepsis | Shock | Liver ischemia |
| 1 | Insulinoma | SSpPD | No | No | No | Yes | - | 7 | No | Active bleeding from intraperitoneal drain | Yes | Yes | No |
| 2 | Gastric cancer | PD | Yes (D2) | No | No | Yes | - | 20 | No | Bleeding from wound | Yes | Yes | No |
| 3 | Gallbladder cancer | HPD | Yes (regional) | Yes | No | Yes | - | 58 | No | Hematemesis | No | No | No |
| 4 | Neuroendoicrine tumor | Lasparoscopic PD | No | No | No | Yes | - | 18 | Yes | Active bleeding from intraperitoneal drain | No | Yes | No |
| 5 | Bile duct cancer | PD | Yes (regional) | No | Yes | No | Digestive anastomotic failure | 11 | No | Active bleeding from intraperitoneal drain | No | No | No |
| 6 | Pancreatic cancer | SSpPD | Yes (D2) | Yes | Yes | Yes | - | 22 | No | Active bleeding from intraperitoneal drain | No | No | No |
| 7 | Bile duct cancer | SSpPD | Yes (regional) | Yes | No | Yes | - | 14 | No | Active bleeding from intraperitoneal drain | Yes | No | No |
| 8 | Gastric cancer | PD | Yes (D2+) | No | No | No | Ruptured suture (staple line) | 32 | Yes | Active bleeding fromintraperitoneal drain | Yes | Yes | No |
| 9 | Pancreatic cancer | SSpPD | Yes (D2) | Yes | Yes | No | - | 6 | Yes | Active bleeding from intraperitoneal drain | Yes | Yes | No |
| 10 | Pancreatic cancer | SSpPD | Yes (D2) | Yes | Yes | Yes | - | 16 | No | Melena | No | No | Yes |
| 11 | Pancreatic metastasis from renal cancer | SSpPD | No | No | No | Yes | - | 30 | Yes | Active bleeding from intraperitoneal drain | No | Yes | No |
| 12 | Ampullary cancer | SSpPD | Yes (D1) | No | No | Yes | - | 6 | Yes | Active bleeding from intraperitoneal drain | No | Yes | No |
| 13 | Pancreatic cancer | PD | Yes (D2) | Yes | Yes | Yes | - | 14 | No | Active bleeding from intraperitoneal drain | Yes | Yes | No |
| 14 | Intraductal papillary mucinous neoplasm | PpPD | No | No | No | Yes | - | 22 | No | Active bleeding from intraperitoneal drain | Yes | Yes | No |
| 15 | Pancreatic cancer | SSpPD | Yes (D1) | No | Yes | No | Biliary necrosis | 12 | No | Active bleeding from intraperitoneal drain | Yes | Yes | No |
| Ruptured cholangiojejunostomy | |||||||||||||
| 16 | Pancreatic cancer | SSpPD | Yes (D2) | Yes | Yes | Yes | - | 28 | No | Abdominal pain | Yes | Yes | Yes |
The clinical features, management strategy, and outcome of arterial hemorrhage were evaluated.
The surgical procedures of PD have been described in detail elsewhere[9,44]. Lymphadenectomy and nerve dissection were performed in patients with malignancies in accordance with the Japanese guideline[45]. Briefly, the GDA from the celiac artery and IPDA from the SMA were cut after double ligation using a locking loop knot. Inherent reconstructions during subtotal stomach-preserving PD were performed by the modified Child’s method with Braun’s anastomosis. During pancreaticojejunostomy, an intraductal lost stent (pancreatic duct tube, 5 Fr, burled, MD41515; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) was placed, and duct-to-jejunal anastomosis was performed with interrupted polydioxanone sutures (4-0 PDS II, violet, RB-1, Z712D; Ethicon, Inc., Cincinnati, OH, United States). Adequate approximation of the pancreatic stump and jejunal wall was ensured with interrupted polyvinylidene fluoride sutures (4-0 ASSP504-0IIN, ASFLEX, 75 cm; Kono Seisakusho Co., Ltd., Ichikawa, Chiba, Japan). choledochojejunostomy was performed with interrupted polydioxanone sutures. A linear stapler was employed for gastrojejunostomy, and the entry hole was closed by hand suturing in a layer-to-layer fashion. Braun’s anastomosis was also performed by hand suturing in a layer-to-layer fashion.
Liver infarction was mainly diagnosed by imaging findings. A sudden increase in the serum aspartate aminotransferase concentration or a gradual increase in the total bilirubin concentration was used as supporting data[1].
Pancreatic leakage was diagnosed according to the criteria established by the International Study Group of Pancreatic Surgery[46].
TAE was performed as the EVT procedure in this study. We intend to arrest fatal hemorrhage by placement of microcoils (Deltaplush; Codman & Shurtleff, Inc., Raynham, MA, United States) in the pseudoaneurysm and/or culprit artery.
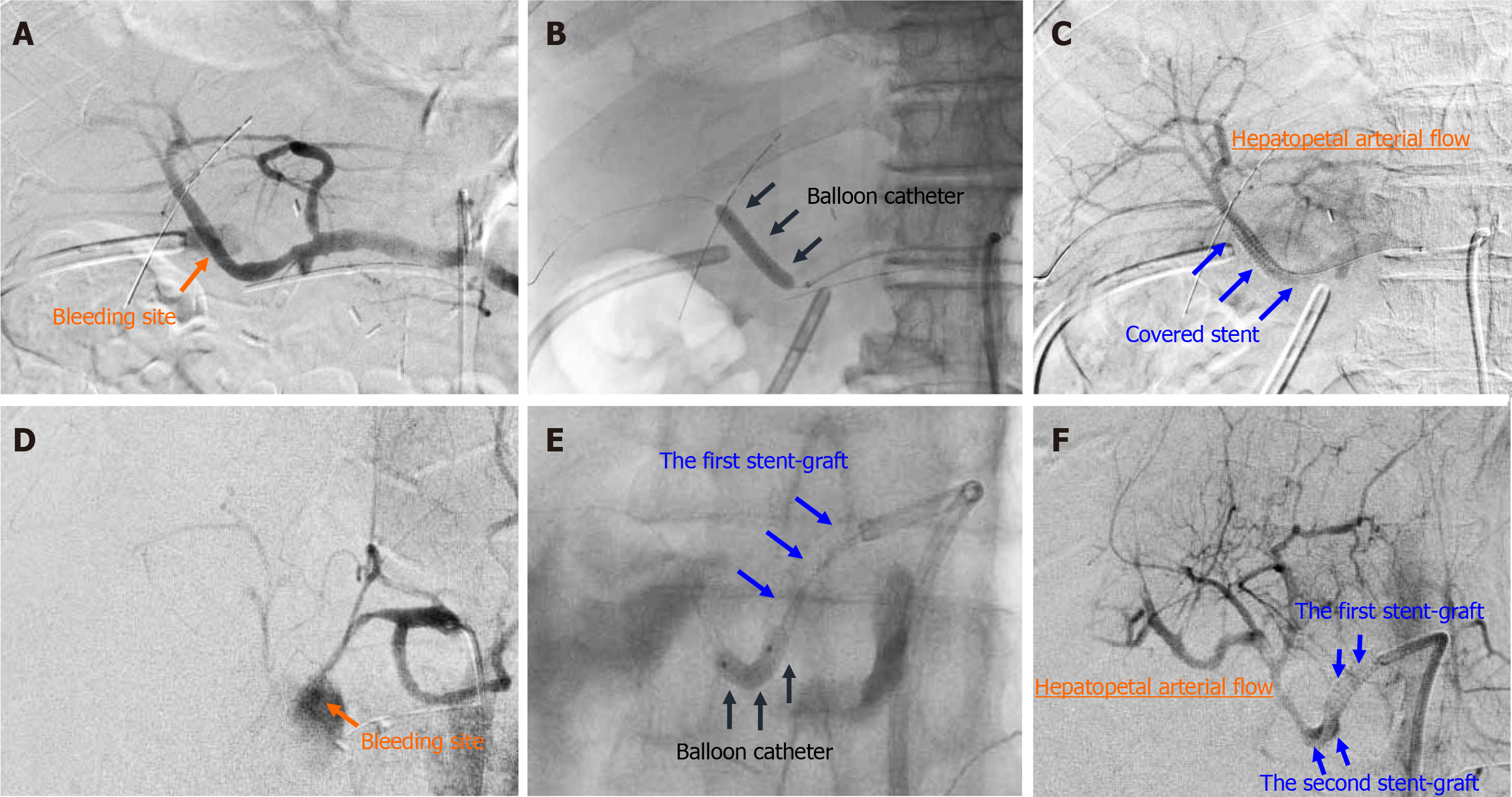
The EVT procedures involved transcatheter placement of a stent-graft. In general, procedures of stent-graft placement were performed under local anesthesia. The target artery was dilated by a balloon catheter (Graftmaster; Abbott Laboratories, Chicago, IL, United States). Balloon catheter pressures was increased in manner of 2 atm per 5 s, and the maximum of intracatheter pressure was 15 atm (1520 kPa). A covered stent (Graftmaster; Abbott Laboratories), not a bare stent, was placed at the culprit artery. The size and length of covered stent was carefully decided on a case-by-case basis, based on angiographic findings after balloon dilation. We aimed to simultaneously obtain complete hemostasis of fatal hemorrhage and preservation of HA flow. The second overlapping stent-graft was implanted in an overlapping fashion, if needed. The actual procedure is shown in Figure 2.
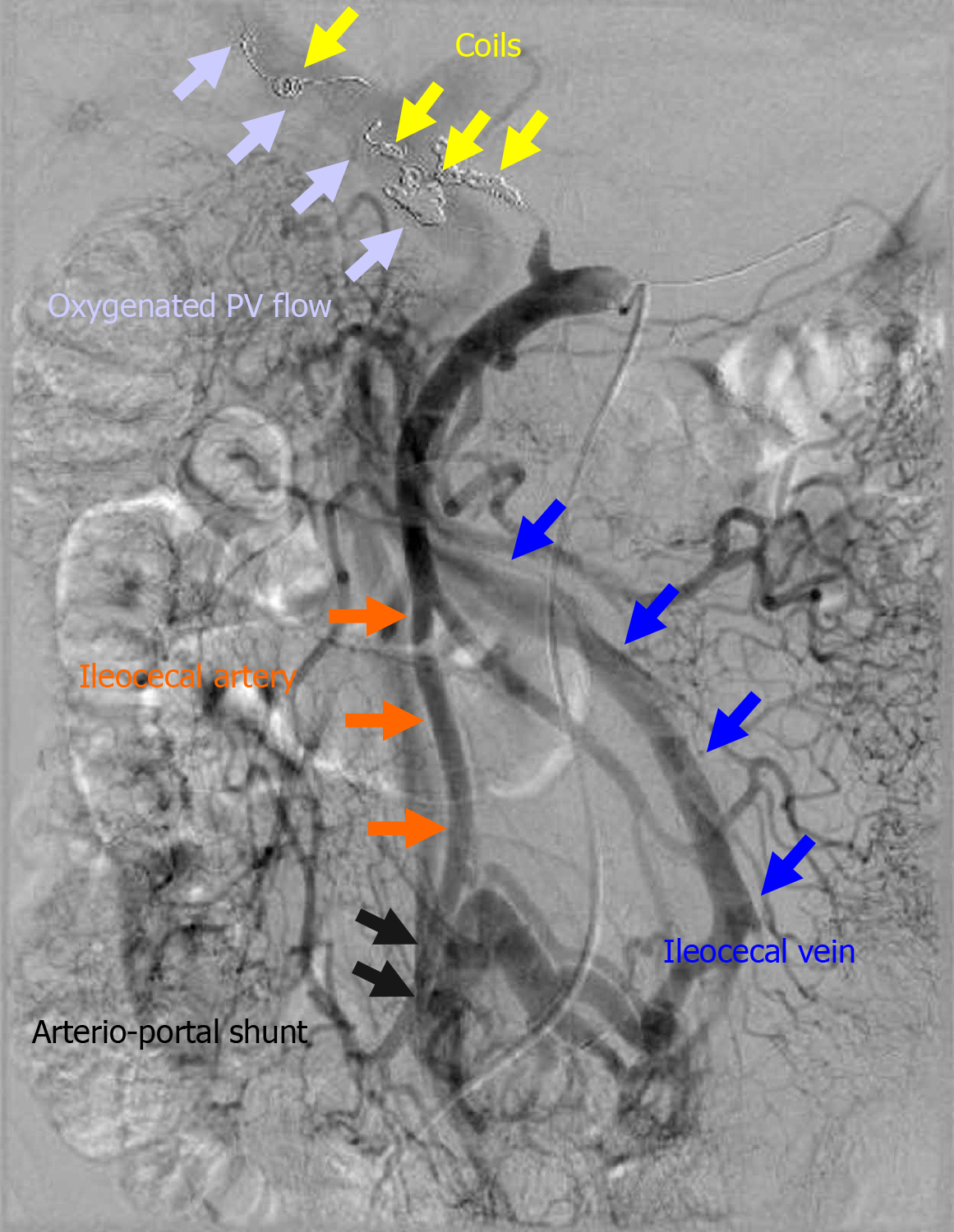
An arterioportal shunt was surgically created (Figure 3). The ileocecal vein and artery were anastomosed in a side-to-side fashion using polypropylene suture. Thereafter, the hepatopetal flow of the portal vein (PV) was well oxygenated.
This retrospective study was approved by the ethics review committee for clinical studies of our institution. The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki. All patients involved in this study provided written informed consent authorizing the use and disclosure of their protected health information.
All results are shown as mean ± SD or median (range). Survival rates were calculated using the Kaplan-Meier method. All calculations were performed using statistical software (SPSS Inc., Chicago, IL, United States).
The overall frequency of arterial hemorrhage after PD was 5.5% (16 of 291 patients who underwent PD in our institution).
The primary diseases and surgical procedures are summarized in Table 1. Thirteen (81.3%) patients had malignancies. Lymphadenectomy and/or nerve dissection was performed in 12 (75.0%) patients.
Associated pancreatitis occurred in seven patients, and nine (56.3%) patients had a soft pancreatic remnant (i.e., pancreatic remnant without associated pancreatitis) (Table 1). Pancreatic leakage was observed in 12 (75.0%) patients (Table 1).
The clinical characteristics at hemorrhage onset are summarized in Table 1. Hemorrhage onset occurred at a median of 18 d (range, 6-58 d) after PD. Sentinel bleeding was observed in 5 (31.3%) patients. Arterial hemorrhage was externalized through the intraperitoneal drain or wound in 13 (81.3%) patients and through the digestive tract in 2 (16.7%) patients. Sepsis, shock (including an unstable hemodynamic state), and liver infarction were observed in 9 (56.3%), 11 (68.8%), and 2 (16.7%) patients, respectively. Notably, four patients with poor clinical courses after EVT (Patients 13-16) had a poor clinical condition before EVT (Table 1).
The most common and second most common sites of bleeding were the GDA stump (7 patients, 43.8%) and HA (4 patients, 25.0%), respectively (Table 2). Computed tomography (CT) angiography was the diagnostic modality in 13 (81.3%) patients. The imaging findings of CT angiography and angiography are summarized in Table 2. The median time from hemorrhage onset to definitive diagnosis and the median time from hemorrhage onset to EVT were 0 d (range, 0-1 d) and 0 d (range, 0-14 d), respectively (Table 2).
| Case number | Bleeding site | Diagnostic modality | CT angiographic findings | Angiographic findings | Time from hemorrhage onset to definitive diagnosis (d) | Time from hemorrhage onset to EVT (d) |
| 1 | RGA | CT angiography | Extravasation | Extravasation | 0 | 0 |
| 2 | SA | CT angiography | Extravasation | Extravasation | 0 | 141 |
| 3 | RHA | CT angiography | Enlargement of pseudoaneurysm | Pseudoaneurysm; Extravasation | 1 | 0 |
| 4 | Cholangiojejunostomy | Clinical findings2 | None | None | 0 | 0 |
| 5 | DPA | CT angiography | Extravasation | Extravasation | 0 | 0 |
| 6 | GDA stump | CT angiography | Extravasation | Extravasation | 0 | 0 |
| 7 | RHA | CT angiography | Extravasation | Extravasation | 0 | 0 |
| 8 | GDA stump | CT angiography | Pseudoaneurysm; Extravasation | Pseudoaneurysm | 0 | 0 |
| 9 | DPA | CT angiography | Extravasation | Extravasation; Pseudoaneurysm | 0 | 0 |
| 10 | PHA | CT angiography | Pseudoaneurysm | Obstruction of CHA; Pseudoaneurysm | 0 | 1 |
| 11 | RHA | CT angiography | Pseudoaneurysm | Pseudoaneurysm | 0 | 0 |
| 12 | GDA stump | Laparotomy3 | None (hematoma only) | None (stenosis of CHA) | 0 | 0 |
| 13 | GDA stump | Angiography | Extravasation | Extravasation | 0 | 0 |
| 14 | GDA stump | CT angiography | Extravasation | Extravasation; Pseudoaneurysm | 0 | 0 |
| 15 | GDA stump | CT angiography | Minor extravasation | Extravasation; Pseudoaneurysm | 0 | 1 |
| 16 | GDA stump | CT angiography | Pseudoaneurysm; Extravasation | Pseudoaneurysm; Extravasation | 0 | 0 |
The treated arteries and ranges are summarized in Table 3. The initial EVT procedures were stent-graft placement in 7 (43.8%) patients, TAE in 6 (37.5%) patients, and combined therapy involving stent-graft placement and TAE in 2 (16.7%) patients (Table 3).
| Case number | Treated artery (target and range) | TAE | Stent-graft placement | Technical success during EVT | Reasons for failed or incomplete EVT | Additional surgical approaches (day number2) | Additional EVT (day number2) | Antiplatelet and/or anticoagulation agents (number) | Long-term results of EVT | ||||
| TAE | Stent-graft placement | ||||||||||||
| Stent type (number1) | Size (mm) | Length (mm) | Collateral circulation (yr)3 | Recanalization (yr)3 | Patency (y)3 | ||||||||
| 1 | RGA | Coiling | - | Yes | - | No | No | No | No (4.39) | No (4.39) | - | ||
| 2 | CA; SA | -; Coiling | Covered stent (1); - | 3.5; - | 19; - | No | Stenosis | Hemostasis (-7 and -6) | Stent regrafting (+ 1); Coiling (+ 1) | No | - | - | - |
| 3 | PHA-LHA | - | Covered stent (1) | 3.5 | 19 | Yes | - | No | No | Yes (1) | - | - | Patent (0.72) |
| 4 | SMA branch; RHA | Coiling; - | -; Covered stent (1) | -; 3.5 | -; 19 | Yes | - | Lavage and cholangio-jejunal anastomosis (+ 7) | Stent regrafting (+ 28) | No | No (6.14); - | No (6.14); - | -; Patent (6.14) |
| 5 | SA branch | Coiling | - | Yes | - | No | No | Yes (1) | No (0.46) | No (0.46) | - | ||
| 6 | CHA-PHA | - | Covered stent (1) | 3.5 | 19 | Yes | - | No | No | No | - | - | Patent (0.93) |
| 7 | RHA | - | Covered stent (1) | 3.5 | 19 | Yes | - | Lavage and cholangio-jejunal anastomosis (+ 3) | No | No | - | - | Patent (1.27) |
| 8 | GDA | Coiling | - | - | - | No | Subtle bleeding4 | No | No | No | No (0.24) | No (0.24) | - |
| 9 | DPA | Coiling | - | - | - | Yes | - | No | No | No | No (0.44) | No (0.44) | - |
| 10 | - | - | - | - | - | No | Stenosis | Hemostasis and ligation of CHA (± 0) | No | Yes (2) | - | - | Patent (1.95) |
| 11 | RHA | - | Covered stent (2) | 3.0 | 19 | Yes | - | Removal of hematoma (- 18) | Stent regrafting (+33) | Yes (2) | - | - | Patent (1.53) |
| 12 | CHA-PHA | - | Covered stent (1) | 3.5 | 19 | Yes | - | Removal of hematoma (± 0) | No | No | - | - | Patent (0.98) |
| 13 | CHA-PHA | Coiling | - | - | - | Yes | - | No | No | No | Yes (1.53) | No (1.53) | - |
| 14 | GDA | Coiling | - | - | - | No | Difficulty in packing | Arterio-portal shunting5 (+ 4) | CHA coiling (+ 4) | Yes (1) | Yes (7.72) | No (7.72) | - |
| 15 | GDA | - | Covered stent (1) | 3.5 | 19 | Yes | - | No | No | No | - | - | Patent (0.00) |
| 16 | GDA | - | Covered stent (2) | 2.6 | 19 | Yes | - | Removal of hematoma (± 0) | No | No | - | - | Patent (0.01) |
The initial EVT failed and/or was incomplete in 4 (25.0%) patients, and the rate of technical success of the initial EVT was 75.0% (Table 3). The reasons for failed and/or incomplete EVT were stenosis in 2 patients, and subtle bleeding in one patient, and difficulty in packing in 1 patient (Table 3). Additional EVTs were performed in 4 (25.0%) patients (Table 3). Antiplatelet and/or anticoagulation agents were administered to 5 (31.3%) patients (Table 3), and these 5 patients continuously received medications even after discharge from our hospital.
Recanalization did not occur (0.0%) throughout the long-term follow-up after TAE (Table 3). Collateral circulation was observed in 2 (25.0%) of eight patients who underwent TAE (Table 3). Additionally, all implanted stent-grafts (100.0%) maintained their patency throughout the long-term follow-up after stent-graft placement (Table 3).
Surgical approaches were utilized in eight patients and are summarized in Table 3. In one patient who underwent failed EVT (patient 10), hemostasis and ligation of the CHA were surgically performed under laparotomy. In one patient in whom the initial EVT failed (patient 14), hemostasis was completed by additional TAE, and liver infarction subsequently occurred. Therefore, an arterioportal shunt was surgically created to oxygenate the PV flow (Figure 3). In this case, arterioportal shunting minimized the patient’s progression to fatal liver infarction due to hepatic ischemia and a refractory liver abscess caused by biliary ischemia.
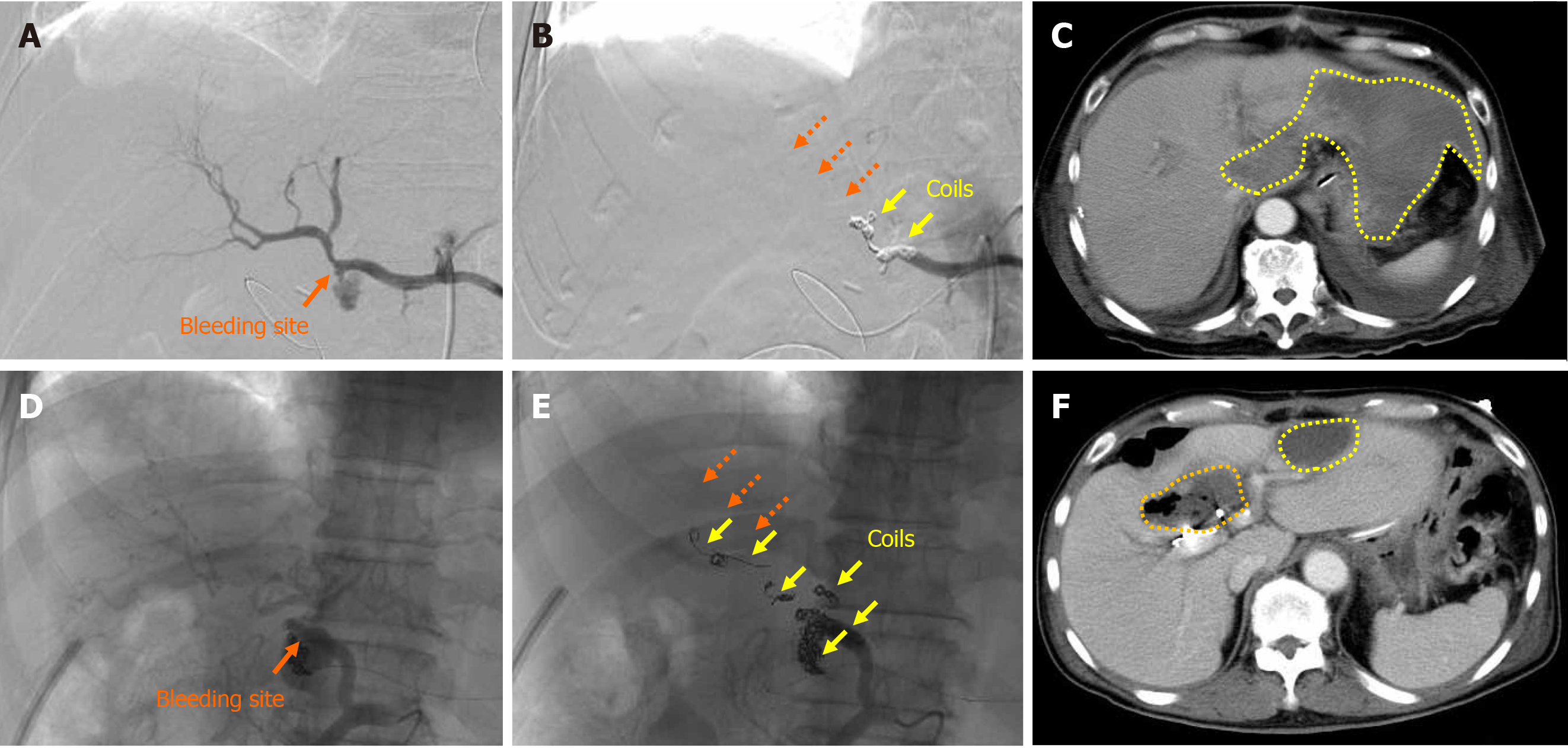
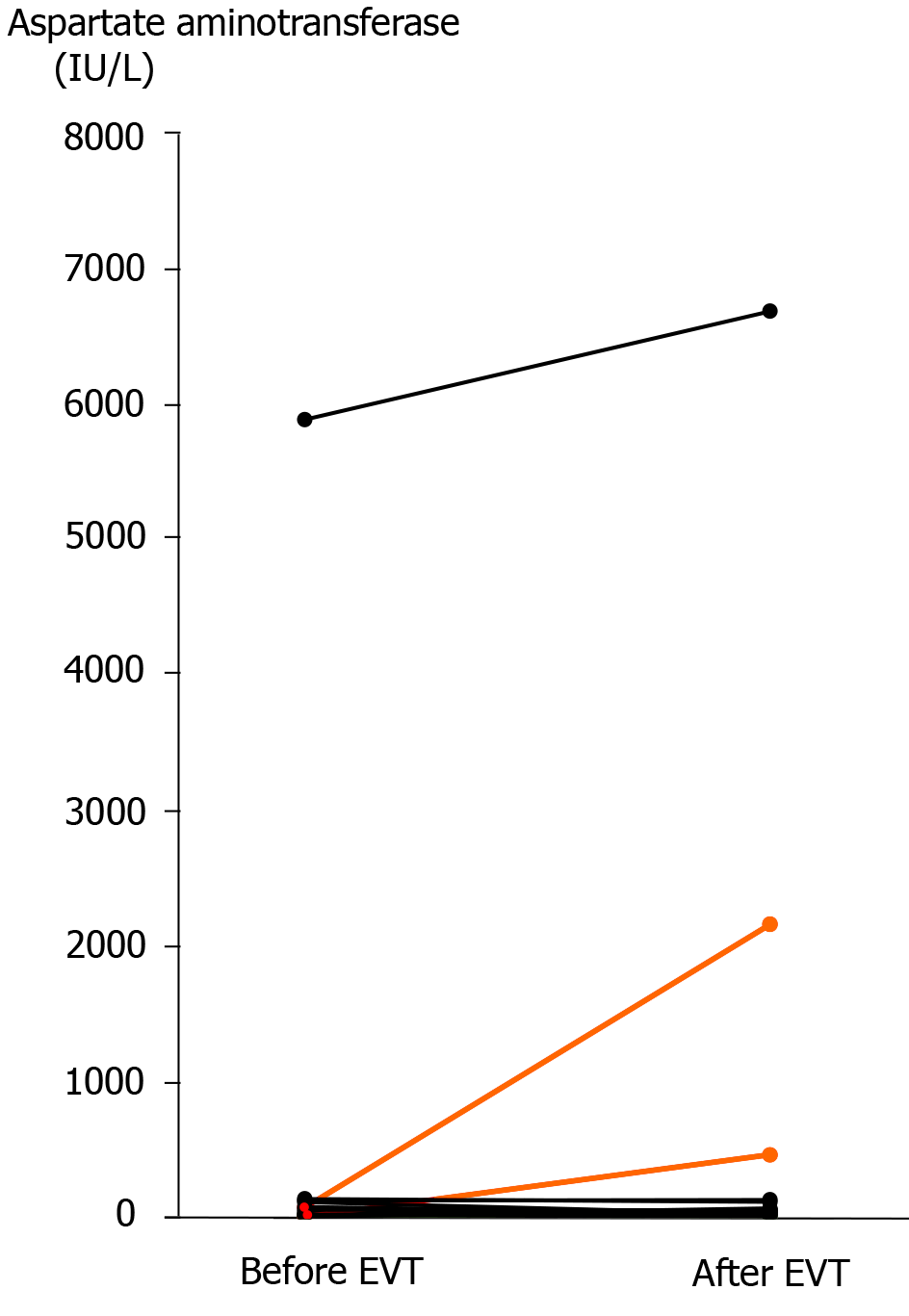
Liver infarction after EVT was observed in 2 (12.5%) patients (patients 13 and 14), and these patients underwent TAE (Tables 3 and 4). Complete hemostasis was obtained by TAE, but hepatopetal arterial flow was completely lost (Figure 4). Liver infarction due to hepatic ischemia subsequently occurred (Figure 4). In these patients, the serum aspartate aminotransferase concentration clearly increased after EVT (Figure 5). Fortunately, both patients successfully recovered from arterial hemorrhage after PD and liver infarction after TAE (Table 4).
| Case number | Complication after EVT | Liver infarction after EVT | Hospital death (day number1 and POD) | Clinical success2 | Follow-up term (yr) | Cause of death | Prognosis (dead or alive) |
| 1 | - | No | No | Yes | 5.57 | Cancer-related death | Dead |
| 2 | - | No | Yes (+ 61 and 94) | Yes | 0.26 | Cancer-related death | Dead |
| 3 | - | No | No | Yes | 0.72 | Cancer-related death | Dead |
| 4 | - | No | No | Yes | 8.36 | - | Alive |
| 5 | - | No | No | Yes | 0.56 | Cancer-related death | Dead |
| 6 | Bleeding | No | No | Yes | 1.04 | Cancer-related death | Dead |
| 7 | - | No | No | Yes | 1.34 | Cancer-related death | Dead |
| 8 | - | No | No | Yes | 0.52 | Cancer-related death | Dead |
| 9 | - | No | No | Yes | 0.47 | Cancer-related death | Dead |
| 10 | - | No | No | Yes | 1.95 | - | Alive |
| 11 | - | No | No | Yes | 1.69 | - | Alive |
| 12 | - | No | No | Yes | 1.46 | - | Alive |
| 13 | - | Yes | No | Yes | 1.74 | Cancer-related death | Dead |
| 14 | Bleeding; Liver abscess | Yes | No | Yes | 9.55 | - | Alive |
| 15 | - | No | Yes (+ 1 and 14) | No | 0.04 | Bleeding, sepsis and DIC | Dead |
| 16 | - | No | Yes (+ 3 and 31) | No | 0.08 | Liver failure | Dead |
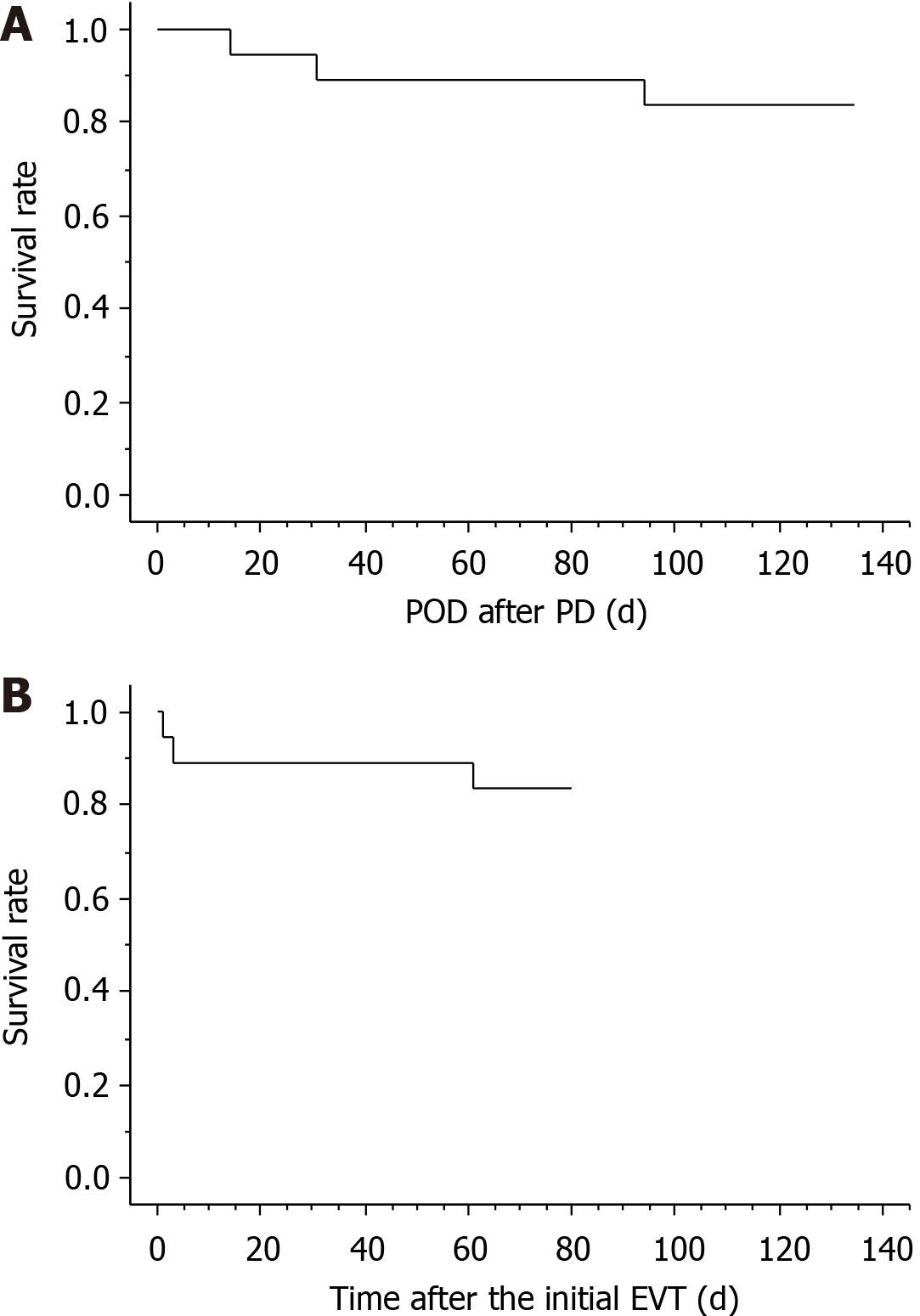
The patients’ clinical courses and outcomes after EVT are summarized in Table 4. Three patients (patients 2, 15, and 16) died during hospitalization, and the actual survival curves after PD and EVT are shown in Figure 6. The mean hospital stay after PD was 66.8 ± 27.7 d among 13 patients who achieved hospital discharge. Fourteen (87.5%) patients were successfully treated because the cause of death in 1 patient (patient 2) was unrelated to arterial hemorrhage (cancer-related death).
Two patients (patients 15 and 16) had a poor outcome even after successful EVT. These 2 patients had a poor clinical condition before EVT (Table 1). One patient (patient 15) had sepsis, shock, and disseminated intravascular coagulation before EVT and died of these conditions even after successful stent-graft placement (Tables 1 and 4). The other patient (patient 16) had sepsis, shock, and liver infarction before EVT (Table 1 and Figure 5) and finally died of liver failure even after successful stent-graft placement (Table 4).
In general, visceral artery pseudoaneurysms are rare but fatal[1,13,18,19,21,23,29,30]. The HA (i.e., the CHA, PHA, and lobular branches) is the second most frequent site of visceral pseudoaneurysms, and the splenic artery is generally the most common[47]. Pseudoaneurysms of the HA are usually iatrogenic[2,16,30,44] but may also be associated with localized infection or trauma[30]. Possible causes of intraoperative pseudoaneurysms include direct vascular injury during dissection or retraction, clamp injury to the vessel, or thermal injury via electrocautery[17,30]. Lymphadenectomy and/or nerve dissection for malignancy renders visceral arteries more vulnerable to further wall injuries[2,16,17,30,39,44,48]. Complications following PD commonly consist of localized infection, anastomotic failure, delayed gastric emptying, and gastrointestinal bleeding[2,6,8,9,29,30]. Although arterial hemorrhage after PD is not frequent, it is fatal[1,2,4,7,12,14-23]. The GDA stump is the most common site of arterial hemorrhage, and the CHA and PHA are the next most common sites[16,18,19,21,22,27]. Arterial hemorrhage of the SMA after PD has also been reported[49,50].
Pancreatic leakage compromises the arterial wall[2,6,16,17,19,22,24,29,30,39,44,51]. Pancreatic juice or localized infection gradually causes arterial wall erosions, resulting in pseudo-aneurysms[2,6,16,17,22,24,29,39,44,51]. Pseudoaneurysm rupture causes sudden-onset, massive, and active hemorrhage[18]. Studies have shown a trend toward a higher prevalence of a soft pancreatic remnant in patients with arterial hemorrhage[2,6,44]. Leaving approximately 1 cm of the GDA stump, spreading an omental flap, and winding the HA by the round ligament of the liver have been suggested to minimize direct contact of pancreatic juice with adjacent vessels[18,22,51-53].
Diagnostic procedures and imaging modalities are associated with certain difficulties[6,7,17,18,28,54]. Even based on laparotomy findings, definitive diagnosis may be difficult[54]. Bleeding from the digestive tract or intraperitoneal drain should be considered a warning because it is an important prelude to massive and active hemorrhage. The term “sentinel bleeding” was first coined in 1989 by Shankar and Russell[55] and was further discussed by Brodsky and Turnbull in 1991[34]. The incidence of sentinel bleeding is approximately 30% to 80%[2,18]. Pseudoaneurysms can be detected by CT in patients with sentinel bleeding[6,7]. Sentinel bleeding should be regarded very seriously[2,4,6,18], even in asymptomatic patients with conservatively treated pancreatic leakage[6,56,57]. An accurate definitive diagnosis should be made immediately, before the patient’s unstable hemodynamic state deteriorates[6,18,29]. Diagnostic digestive endoscopy delays adequate treatment in hemodynamically unstable patients because of pseudoaneurysm rupture[7,58]. The diagnostic potential of CT angiography[6,16,19,28,43] and diagnostic angiography[6,7,17,18,29,39,54] have been established. Diagnostic angiographic findings include extravasation of contrast medium, pseudoaneurysm formation, non-smooth arterial intima, local vascular spasm, stenosis, and distal arterial branch expansion[20,54]. Diagnostic angiography should be considered even in patients with suspected hemorrhage[6,7,17,18,29,39,54], and subsequent EVT should be adequately performed if necessary[1,7,16-18,30,54].
EVT represents the first-line treatment for arterial hemorrhage after PD[2,7,17,40,59-61]. Arterial hemorrhage easily results in unstable hemodynamic state[1,6,16,18,23,29,62], sepsis[2,6,7,13], and hepatic ischemia[6,28,39,63]. Prolonged hemorrhage leads to shock and coagulopathy[1,16,18,23], and further hemorrhage results in disseminated intravascular coagulation[1,16,18,23]. Complicated homeostasis is associated with a poor prognosis even after successful EVT[1,16,18], and the patient’s condition before EVT is strongly associated with complications after EVT[1,16,29]. In fact, our two patients who had a poor clinical condition before EVT (e.g., sepsis, shock, and liver infarction) finally died even after successful EVT (Tables 1 and 4). If a patient shows any signs of a suspected hemorrhage, EVT should be performed as soon as possible before the development of complicated homeostasis[1,2,16,18,23,30]. Concern exists regarding the placement of foreign bodies (i.e., coils and stent-grafts) in the setting of infection or inflammation[40]. Intravascular stent infection can be a devastating complication, but it is very rare[40,64-66]. In fact, stent-grafts have been used to repair infected pseudoaneurysms[67,68]. Though pancreatic juice-related localized infection may associate with pseudoaneurysm and arterial wall erosion[2,6,16,17,22,24,29,39,44,51], we consider that stent-grafts can be placed even in suspicious infectious site.
Arterial hemorrhage after PD usually occurs after at least 1 d[24], and delayed hemorrhage generally occurs after 1 wk[6,58]. In one study, one-third of arterial hemorrhages occurred 1 mo after PD[21]. Hence, delayed hemorrhage is common after PD[2,6,7-19,21,22,28,58,62], and the median or mean time point of hemorrhage onset ranges from 18 d to 21 d after PD[2,7,21,28]. Pancreatic leakage is a possible cause of delayed arterial hemorrhage[2,6,44], and delayed hemorrhage after PD carries a significantly higher mortality rate[2,6,7,21,28].
Because the GDA and IPDA were ligated and the pancreaticoduodenal arcade was resected during PD in the present study, hepatopetal blood supply via these arteries could no longer be expected (Figure 1). EVT may lead to severe complications (e.g., hepatic ischemia, liver abscess formation, and PV stenosis)[1]. The EVT technique should be decided on a case-by-case basis[18,28,29]. Notably, the EVT procedure is strongly associated with complications after EVT[1,16]. Although TAE is technically easier than stent-graft placement[19], liver infarction secondary to hepatic ischemia frequently occurs[6,34,35]. Even a subtle ischemic change in the biliary tree results in intractable liver abscesses[6,34,35]. We also experienced a case of a refractory liver abscess due to biliary ischemia (patient 14) (Figure 4F). PV stenosis easily disturbs the hepatic parenchymal perfusion, resulting in liver infarction with a poor prognosis[23,69]. The rates of mortality and serious hepatic complications after EVT are approximately 20% to 50% and 20% to 80%, respectively[1,18,19,21,23,31,35,70-72].
TAE is advantageous for ensuring complete hemostasis[2,13,20,23,43,54,62,73-77], and the hemostatic rate is reportedly > 90%[17,20,23,54,71]. TAE is technically user-friendly at the most frequent site (i.e., the GDA stump)[19,43], although both the proximal and distal sides of the GDA should be completely embolized[19]. To prevent recanalization and rebleeding, all arterial flows to the pseudoaneurysm should be completely interrupted[19,44,47,48]. Although the pancreaticoduodenal arcade is removed during PD, arterial arcades remain in the pancreatic remnant (Figure 1)[29,47]. Recanalization via the collateral circulation has been reported after TAE[29]; however, transcatheter techniques (e.g., isolation, packing, and embolization) are available for various forms of pseudoaneurysms[29]. Notably, TAE is occasionally associated with serious hepatic complications caused by hepatic ischemia[1,16,23,30,32,33,69,70]. The liver has many potential collateral pathways that communicate with the adjacent arterial system[16,19,23,29,78,79], and a sudden complete block of HA flow immediately after surgery may induce an ischemic insult to the liver parenchyma[16,29,78,79]. Whether extrahepatic arteries (e.g., the inferior phrenic artery and left gastric artery) provide sufficient hepatopetal collateral circulation to avoid fatal hepatic ischemia after TAE remains unclear[1,19,23,32]. Additionally, the liver can tolerate considerable TAE without significant liver infarction because it has a dual blood supply from the HA and PV[19,20,23,29]. TAE may cause liver infarction in patients with poor collateral circulation because of their postoperative status[29]. Approximately 30% to 80% of patients develop hepatic ischemia after TAE[69,71], and approximately 20% to 40% of patients progress to liver infarction[23,70,71]. The reported mortality rate ranges from 30% to 50%[19,31,35,72].
Simultaneous accomplishment of complete hemostasis and HA flow preservation is difficult[1,7,13,16,18,28,59]. Transcatheter placement of a covered stent may be of value in maintaining the patency of adjacent arteries, and stent-graft placement is an ideal technique to preserve HA flow[1,6,13,16,17,21,27-31,40,54,61,63,80-82]. If necessary, a second overlapping stent-graft can be implanted[40,83]. Actually, we placed a second stent in two patients (Table 3). Some researchers have described patients who underwent this EVT technique[30,31,63,80-82,84], and others have documented such cases in published case series[13,36,38,85]. The success rate of stent-graft placement reportedly ranges from 75% to 80% because the target arteries require a specialized stent size and/or exhibit narrowness and tortuosity[16,37,38,59]. The overall mortality and clinical outcomes are affected by the patients’ conditions before stent-graft placement[16,29]. The use of antiplatelet agents or heparinization after stent-graft placement in the HA is still controversial[13,16,36,37,40,85]. Some clinicians do not use such agents in patients with an unstable hemodynamic state after arterial hemorrhage[37]. However, stent-graft placement in a patient with arterial hemorrhage after surgery carries a high risk of thrombosis because the damaged wall of the HA is surrounded by localized infection and/or massive hematoma, and the HA diameter is very small[16,40]. Hence, some clinicians use these agents after stent-graft placement[13,16,36,40,85].
Stent grafting is a technically difficult procedure and requires adaptation to vessels of various sizes[18,40]. However, this EVT technique is considered the most appropriate treatment method in patients with a favorable vascular anatomy. Stent-graft placement may fail for anatomical reasons (e.g., tortuosity or variation)[13,17,40,59,84,86] or because of catheter-induced vasospasm or spontaneous thrombosis within the aneurysmal wall[17,87-89]. Actual reasons for failed or incomplete EVT in our institution were summarized in Table 3. Since stent-graft placement may technically failed due to tortuosity, variation, stenosis, vasospasm or thrombosis at the culprit artery[13,17,40,59,84,86-89], preliminary dilatation by balloon catheter is indispensable even for self-expandable covered stent. The interventional radiologist who performs the procedure must have adequate experience and skill[59,62].
If EVT fails or is incomplete, the next management option for arterial hemorrhage is still a surgical approach[2,7,62]. Surgical exploration and complete hemostasis are difficult and hazardous because of postoperative adhesions and the patient’s critical condition[17,18,48]. Surgical treatment is usually associated with a high mortality rate (29%-58%)[2,17-19,48]. Hepatopetal flow of the PV can be well oxygenated by creation of an arterioportal shunt, and some reports have described such cases[41,42]. The impact of a surgical approach involving arterioportal shunting on the prevention of liver infarction has been documented[90,91]. Although the clinical decision and optimal timing for the surgical approach of arterioportal shunting are still controversial, we consider that the presence of subtle clinical signs of progressive liver infarction after EVT is a clear indication for arterioportal shunting.
To our knowledge, most reports to date are limited to case reports or small series[13,30,31,36,38,63,80-82,84,85]. We acknowledge that this study has several limitations. The main limitation is that this was a retrospective study with a small number of patients from a single center. Of course, we have demonstrated our individual-tailored approach. Potential limitations due to bias and a small sample size are inherent to this type of study. This represents our experience in a single institution and our views may be affected by various biases. Hence, we understand that our conclusions must be drawn with extreme caution. However, we believe that transcatheter placement of a covered stent has therapeutic advantages for arterial hemorrhage after PD, with simultaneous accomplishment of complete hemostasis and HA flow preservation.
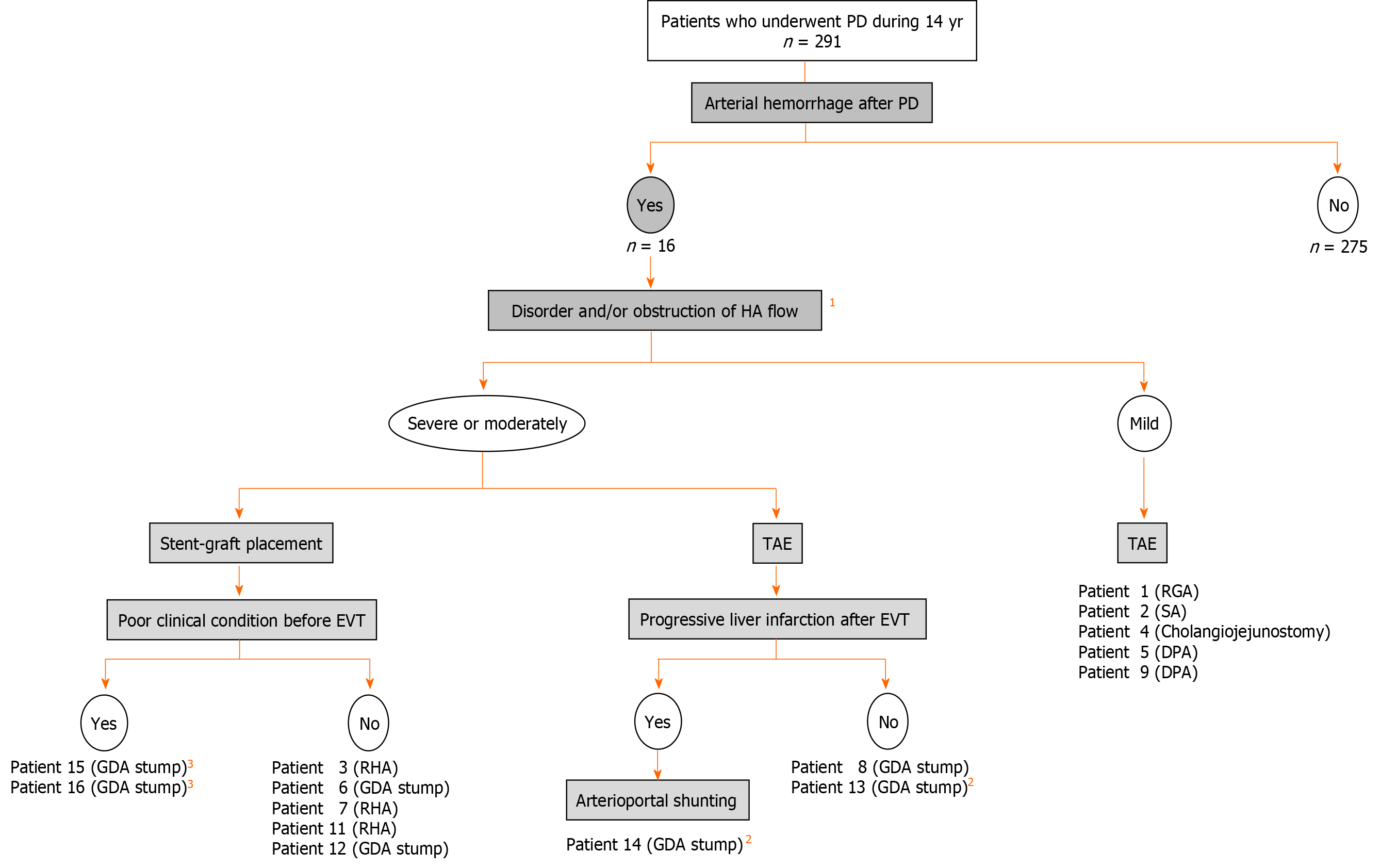
Actual therapeutic strategies for our patients who caused arterial hemorrhage after PD were summarized in Figure 7. We currently have an institutional therapeutic strategy for arterial hemorrhage after PD based on our own experiences: (1) CT angiography is performed if general condition is stable; (2) Diagnostic angiography is immediately performed even in a suspicious patient; and (3) Covered stent is subsequently placed at the culprit artery as the first line treatment.
In conclusion, transcatheter placement of a covered stent may be a powerful tool for simultaneous accomplishment of complete hemostasis and HA flow preservation, although arterial hemorrhage after PD is generally fatal.
Arterial hemorrhage after pancreaticoduodenectomy (PD) is fatal.
This hemorrhage is caused by pseudoaneurysm rupture, and the gastroduodenal artery stump and hepatic artery are frequent culprit vessels.
Simultaneous accomplishment of complete hemostasis and hepatic artery flow preservation is difficult after PD. Although complete hemostasis may be obtained by transcatheter arterial embolization or surgery, liver infarction and/or abscesses may occur.
Arterial hemorrhage after PD is fatal. This hemorrhage is caused by pseudoaneurysm.
We here evaluate our experience including actual treatments (transcatheter arterial embolization, stent-graft placement, or surgery), and discuss therapeutic strategies.
Transcatheter placement of a covered stent is useful for simultaneous accomplishment of complete hemostasis and hepatic arterial flow preservation.
Therapeutic options for fatal arterial hemorrhage after PD is shown.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Japanese Board of Cancer Therapy, No. 12101244; The Japanese Society for Transplantation, No. 20120644; The Japanese Breast Cancer Society, No. 2266; The Japan Society of Hepatology, No. 4330; The Japanese Society of Gastroenterological Surgery, No. 3006223; Japan Gastroenterological Endoscopy Society, No. 20040703; The Japanese Society of Gastroenterology, No. 27782; Japan Surgical Society, No. 16437; The American Association for the Study of Liver Diseases, No. 121820; American Society of Transplantation, No. 511350; Japanese Society of Hepato-Biliary-Pancreatic Surgery, No. 5227717554; Japan Society for Endoscopic Surgery, No. 15GS015; and American College of Surgeons, No. 03258534.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mezzetto L, Suc B S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Hasegawa T, Ota H, Matsuura T, Seiji K, Mugikura S, Motoi F, Unno M, Takase K. Endovascular Treatment of Hepatic Artery Pseudoaneurysm after Pancreaticoduodenectomy: Risk Factors Associated with Mortality and Complications. J Vasc Interv Radiol 2017; 28: 50-59. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Tien YW, Lee PH, Yang CY, Ho MC, Chiu YF. Risk factors of massive bleeding related to pancreatic leak after pancreaticoduodenectomy. J Am Coll Surg. 2005;201:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Schäfer M, Heinrich S, Pfammatter T, Clavien PA. Management of delayed major visceral arterial bleeding after pancreatic surgery. HPB (Oxford). 2011;13:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, Kaifi J, Schurr PG, Bubenheim M, Nolte-Ernsting C, Adam G, Izbicki JR. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Hackert T, Büchler MW, Werner J. Surgical options in the management of pancreatic cancer. Minerva Chir. 2009;64:465-476. [PubMed] |
| 6. | Blanc T, Cortes A, Goere D, Sibert A, Pessaux P, Belghiti J, Sauvanet A. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, Obertop H, Gouma DJ. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Kapoor VK. Complications of pancreato-duodenectomy. Rozhl Chir. 2016;95:53-59. [PubMed] |
| 9. | Hori T, Yamamoto H, Harada H, Yamamoto M, Yamada M, Yazawa T, Tani M, Kamada Y, Tani R, Aoyama R, Sasaki Y, Zaima M. Inferior Pancreaticoduodenal Artery Aneurysm Related with Groove Pancreatitis Persistently Repeated Hemosuccus Pancreaticus Even After Coil Embolization. Am J Case Rep. 2019;20:567-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Kimura Y, Yasukawa D, Aisu Y, Hori T. Imanaga's First Method for Reconstruction with Preservation of Mesojejunal Autonomic Nerves During Pylorus-Preserving Pancreatoduodenectomy. Am J Case Rep. 2018;19:608-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Sinha S, Karthikesalingam A, Holt PJ. Importance of outcomes research in surgery. ANZ J Surg. 2012;82:861-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Sanjay P, Fawzi A, Fulke JL, Kulli C, Tait IS, Zealley IA, Polignano FM. Late post pancreatectomy haemorrhage. Risk factors and modern management. JOP. 2010;11:220-225. [PubMed] |
| 13. | Wang MQ, Liu FY, Duan F, Wang ZJ, Song P, Fan QS. Stent-grafts placement for treatment of massive hemorrhage from ruptured hepatic artery after pancreaticoduodenectomy. World J Gastroenterol. 2010;16:3716-3722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Ellison EC. Evidence-based management of hemorrhage after pancreaticoduodenectomy. Am J Surg. 2007;194:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Santoro R, Carlini M, Carboni F, Nicolas C, Santoro E. Delayed massive arterial hemorrhage after pancreaticoduodenectomy for cancer. Management of a life-threatening complication. Hepatogastroenterology. 2003;50:2199-2204. [PubMed] |
| 16. | Lim SJ, Park KB, Hyun DH, Do YS, Park HS, Shin SW, Cho SK, Choi DW. Stent graft placement for postsurgical hemorrhage from the hepatic artery: clinical outcome and CT findings. J Vasc Interv Radiol. 2014;25:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Ding X, Zhu J, Zhu M, Li C, Jian W, Jiang J, Wang Z, Hu S, Jiang X. Therapeutic management of hemorrhage from visceral artery pseudoaneurysms after pancreatic surgery. J Gastrointest Surg. 2011;15:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Lee HG, Heo JS, Choi SH, Choi DW. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy. World J Gastroenterol. 2010;16:1239-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Hur S, Yoon CJ, Kang SG, Dixon R, Han HS, Yoon YS, Cho JY. Transcatheter arterial embolization of gastroduodenal artery stump pseudoaneurysms after pancreaticoduodenectomy: safety and efficacy of two embolization techniques. J Vasc Interv Radiol. 2011;22:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Xu HF, Zhu X, Chen H, Wang XD, Cao G, Liu P, Gao S, Guo JH. [Angiographic findings and interventional therapy for post-pancreaticoduodenectomy hemorrhage]. Zhonghua Yi Xue Za Zhi. 2013;93:55-57. [PubMed] |
| 21. | Lee JH, Hwang DW, Lee SY, Hwang JW, Song DK, Gwon DI, Shin JH, Ko GY, Park KM, Lee YJ. Clinical features and management of pseudoaneurysmal bleeding after pancreatoduodenectomy. Am Surg. 2012;78:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Neri G, Moro E, Zamprogno R, Sandri R. [Anomalous chordae tendineae of the left ventricle. Echocardiographic study]. G Ital Cardiol. 1984;14:939-940. [PubMed] |
| 23. | Mine T, Murata S, Ueda T, Takeda M, Onozawa S, Yamaguchi H, Kawano Y, Kumita S. Contribution of extrahepatic collaterals to liver parenchymal circulation after proper hepatic artery embolization. J Gastroenterol Hepatol. 2014;29:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Rumstadt B, Schwab M, Korth P, Samman M, Trede M. Hemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, Tanaka M. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg. 1998;133:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Okuno A, Miyazaki M, Ito H, Ambiru S, Yoshidome H, Shimizu H, Nakagawa K, Shimizu Y, Nukui Y, Nakajima N. Nonsurgical management of ruptured pseudoaneurysm in patients with hepatobiliary pancreatic diseases. Am J Gastroenterol. 2001;96:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Desai GS, Pande PM. Gastroduodenal artery: single key for many locks. J Hepatobiliary Pancreat Sci. 2019;26:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Adam G, Tas S, Cinar C, Bozkaya H, Adam F, Uysal F, Resorlu M, Unalp OV, Parildar M, Koçak E, Ozdemir H. Endovascular treatment of delayed hemorrhage developing after the pancreaticoduodenectomy procedure. Wien Klin Wochenschr. 2014;126:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Chatani S, Inoue A, Ohta S, Takaki K, Sato S, Iwai T, Murakami Y, Watanabe S, Sonoda A, Nitta N, Maehira H, Tani M, Murata K. Transcatheter Arterial Embolization for Postoperative Bleeding Following Abdominal Surgery. Cardiovasc Intervent Radiol. 2018;41:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Harvey J, Dardik H, Impeduglia T, Woo D, DeBernardis F. Endovascular management of hepatic artery pseudoaneurysm hemorrhage complicating pancreaticoduodenectomy. J Vasc Surg. 2006;43:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 31. | Kaw LL Jr, Saeed M, Brunson M, Delaria GA, Dilley RB. Use of a stent graft for bleeding hepatic artery pseudoaneurysm following pancreaticoduodenectomy. Asian J Surg. 2006;29:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Sato A, Yamada T, Takase K, Matsuhashi T, Higano S, Kaneda T, Egawa S, Takeda K, Ishibashi T, Takahashi S. The fatal risk in hepatic artery embolization for hemostasis after pancreatic and hepatic surgery: importance of collateral arterial pathways. J Vasc Interv Radiol. 2011;22:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Fujii Y, Shimada H, Endo I, Yoshida K, Matsuo K, Takeda K, Ueda M, Morioka D, Tanaka K, Togo S. Management of massive arterial hemorrhage after pancreatobiliary surgery: does embolotherapy contribute to successful outcome? J Gastrointest Surg. 2007;11:432-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Brodsky JT, Turnbull AD. Arterial hemorrhage after pancreatoduodenectomy. The 'sentinel bleed'. Arch Surg. 1991;126:1037-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Otah E, Cushin BJ, Rozenblit GN, Neff R, Otah KE, Cooperman AM. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg. 2002;137:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Stoupis C, Ludwig K, Inderbitzin D, Do DD, Triller J. Stent grafting of acute hepatic artery bleeding following pancreatic head resection. Eur Radiol. 2007;17:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Boufi M, Belmir H, Hartung O, Ramis O, Beyer L, Alimi YS. Emergency stent graft implantation for ruptured visceral artery pseudoaneurysm. J Vasc Surg. 2011;53:1625-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Stampfl U, Sommer CM, Bellemann N, Weitz J, Böckler D, Richter GM, Kauczor HU, Radeleff B. The use of balloon-expandable stent grafts for the management of acute arterial bleeding. J Vasc Interv Radiol. 2012;23:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Zhou CG, Shi HB, Liu S, Yang ZQ, Zhao LB, Xia JG, Zhou WZ, Li LS. Transarterial embolization for massive gastrointestinal hemorrhage following abdominal surgery. World J Gastroenterol. 2013;19:6869-6875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Pedersoli F, Isfort P, Keil S, Goerg F, Zimmermann M, Liebl M, Schulze-Hagen M, Schmeding M, Kuhl CK, Bruners P. Stentgraft Implantation for the Treatment of Postoperative Hepatic Artery Pseudoaneurysm. Cardiovasc Intervent Radiol. 2016;39:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Reese T, von Rittberg Y, Oldhafer KJ. Portal vein arterialization for iatrogenic embolization of the hepatic artery. An old but still useful technique? Int J Surg Case Rep. 2020;71:91-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Shi Y, Su Y, Li C, Shi H, Liang Y. Revascularization of iatrogenic intraoperative injury to a major artery during hepatobiliary-pancreatic surgery: a single-center experience in China. Minerva Chir. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Robinson K, Rajebi MR, Zimmerman N, Zeinati C. Post-pancreaticoduodenectomy hemorrhage of unusual origin: treatment with endovascular embolization and the value of preoperative CT angiography. J Radiol Case Rep. 2013;7:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Hori T, Ogawa K, Yamamoto H, Harada H, Matsumura K, Yamamoto M, Yamada M, Yazawa T, Kuriyama K, Tani M, Yasukawa D, Kamada Y, Aisu Y, Tani R, Aoyama R, Nakayama S, Sasaki Y, Nishimoto K, Zaima M. Impact of continuous local lavage on pancreatic juice-related postoperative complications: Three case reports. World J Clin Cases. 2019;7:2526-2535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Society JP. General rules for the study of pancreatic cancer. 7th ed. Tokyo: Kanehara Shuppan, 2016. |
| 46. | Pulvirenti A, Ramera M, Bassi C. Modifications in the International Study Group for Pancreatic Surgery (ISGPS) definition of postoperative pancreatic fistula. Transl Gastroenterol Hepatol. 2017;2:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 47. | Tani R, Hori T, Yamamoto H, Harada H, Yamamoto M, Yamada M, Yazawa T, Tani M, Kamada Y, Aoyama R, Sasaki Y, Zaima M. Severely Calcified True Aneurysm: A Thought-Provoking Case of Solitary Origin and Postoperative Management. Am J Case Rep. 2019;20:620-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Yang J, Zhang XH, Huang YH, Chen B, Xu JB, Chen CQ, Cai SR, Zhan WH, He YL, Ma JP. Diagnosis and Treatment of Abdominal Arterial Bleeding After Radical Gastrectomy: a Retrospective Analysis of 1875 Consecutive Resections for Gastric Cancer. J Gastrointest Surg. 2016;20:510-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Davtian OIa, Saakian AB, Akopian AA, Simonian SIu, Agavelian AM. [The content of middle molecules in the blood plasma of patients with proctologic diseases]. Vrach Delo. 1989: 69-71. [PubMed] |
| 50. | Mizuno S, Imai H, Takaki H, Tanemura A, Kuriyama N, Sakurai H, Yamakado K, Sakuma H, Isaji S. Spontaneous Rupture of an Intrasplenic Aneurysm After Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma. J Emerg Med. 2015;48:729-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Nakatsuka H, Sawatsubashi T, Morioka N, Shimizu T, Kanda T. [Use of the round ligament of the liver to prevent post-pancreatectomy hemorrhage]. Gan To Kagaku Ryoho. 2013;40:1903-1905. [PubMed] |
| 52. | Turrini O, Moutardier V, Guiramand J, Lelong B, Bories E, Sannini A, Magnin V, Viret F, Blache JL, Giovannini M, Delpero JR. Hemorrhage after duodenopancreatectomy: impact of neoadjuvant radiochemotherapy and experience with sentinel bleeding. World J Surg. 2005;29:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Koukoutsis I, Bellagamba R, Morris-Stiff G, Wickremesekera S, Coldham C, Wigmore SJ, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Haemorrhage following pancreaticoduodenectomy: risk factors and the importance of sentinel bleed. Dig Surg. 2006;23:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Zhou T, Sun J, Zhang Y, Nie C, Zhou G, Zhu T, Wang W, Zheng S. [Diagnosis and treatment of hemorrhage after pancreatoduodenectomy via digital subtraction angiography and transcatheter arterial embolization]. Zhonghua Yi Xue Za Zhi. 2015;95:368-370. [PubMed] |
| 55. | Shankar S, Russell RC. Haemorrhage in pancreatic disease. Br J Surg. 1989;76:863-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Munoz-Bongrand N, Sauvanet A, Denys A, Sibert A, Vilgrain V, Belghiti J. Conservative management of pancreatic fistula after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2004;199:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Yeo CJ. Management of complications following pancreaticoduodenectomy. Surg Clin North Am. 1995;75:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Belli AM, Markose G, Morgan R. The role of interventional radiology in the management of abdominal visceral artery aneurysms. Cardiovasc Intervent Radiol. 2012;35:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Sachdev-Ost U. Visceral artery aneurysms: review of current management options. Mt Sinai J Med. 2010;77:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Eyheremendy EP, Méndez P, McCormack L, Primo JC, Montaño Y, Sierre S. [Endovascular treatment of an hepatic artery pseudoaneurysm following pancreaticoduodenectomy]. Acta Gastroenterol Latinoam. 2015;45:80-84. [PubMed] |
| 62. | Tasu JP, Vesselle G, Herpe G, Ferrie JC, Chan P, Boucebci S, Velasco S. Postoperative abdominal bleeding. Diagn Interv Imaging. 2015;96:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Miyazawa R, Kamo M, Nishiyama T, Ohigashi S, Yagihashi K. Covered Stent Placement Using "Pull-Through" Technique for a Gastroduodenal Artery Stump Pseudoaneurysm after Pancreaticoduodenectomy. J Vasc Interv Radiol. 2016;27:1743-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Clarke MG, Thomas HG, Chester JF. MRSA-infected external iliac artery pseudoaneurysm treated with endovascular stenting. Cardiovasc Intervent Radiol. 2005;28:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Gandini R, Pipitone V, Konda D, Pendenza G, Spinelli A, Stefanini M, Simonetti G. Endovascular treatment of a giant superior mesenteric artery pseudoaneurysm using a nitinol stent-graft. Cardiovasc Intervent Radiol. 2005;28:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Kinney EV, Kaebnick HW, Mitchell RA, Jung MT. Repair of mycotic paravisceral aneurysm with a fenestrated stent-graft. J Endovasc Ther. 2000;7:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Qu L, Jing Z, Feng R. Endoaortic stent grafting of a giant infected hepatic-celiac pseudoaneurysm. J Vasc Surg. 2005;42:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Sanada J, Matsui O, Arakawa F, Tawara M, Endo T, Ito H, Ushijima S, Endo M, Ikeda M, Miyazu K. Endovascular stent-grafting for infected iliac artery pseudoaneurysms. Cardiovasc Intervent Radiol. 2005;28:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Cho SK, Kim SS, Do YS, Park KB, Shin SW, Park HS, Choo SW, Choo IW. Ischemic liver injuries after hepatic artery embolization in patients with delayed postoperative hemorrhage following hepatobiliary pancreatic surgery. Acta Radiol. 2011;52:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Miura F, Asano T, Amano H, Yoshida M, Toyota N, Wada K, Kato K, Yamazaki E, Kadowaki S, Shibuya M, Maeno S, Furui S, Takeshita K, Kotake Y, Takada T. Management of postoperative arterial hemorrhage after pancreato-biliary surgery according to the site of bleeding: re-laparotomy or interventional radiology. J Hepatobiliary Pancreat Surg. 2009;16:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Gwon DI, Ko GY, Sung KB, Shin JH, Kim JH, Yoon HK. Endovascular management of extrahepatic artery hemorrhage after pancreatobiliary surgery: clinical features and outcomes of transcatheter arterial embolization and stent-graft placement. AJR Am J Roentgenol. 2011;196:W627-W634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Yoon YS, Kim SW, Her KH, Park YC, Ahn YJ, Jang JY, Park SJ, Suh KS, Han JK, Lee KU, Park YH. Management of postoperative hemorrhage after pancreatoduodenectomy. Hepatogastroenterology. 2003;50:2208-2212. [PubMed] |
| 73. | Tessier DJ, Fowl RJ, Stone WM, McKusick MA, Abbas MA, Sarr MG, Nagorney DM, Cherry KJ, Gloviczki P. Iatrogenic hepatic artery pseudoaneurysms: an uncommon complication after hepatic, biliary, and pancreatic procedures. Ann Vasc Surg. 2003;17:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Bassi C, Falconi M, Salvia R, Mascetta G, Molinari E, Pederzoli P. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453-7; discussion 458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Tsirlis T, Vasiliades G, Koliopanos A, Kopanakis N, Katseli A, Tsipras H, Margaris H. Pancreatic leak related hemorrhage following pancreaticoduodenectomy. A case series. JOP. 2009;10:492-495. [PubMed] |
| 76. | Khorsandi SE, Limongelli P, Jackson JE, Tait P, Williamson RC, Habib NA, Jiao LR. Management of delayed arterial hemorrhage after pancreaticoduodenectomy. A case series. JOP. 2008;9:172-178. [PubMed] |
| 77. | Tipaldi MA, Orgera G, Krokidis M, Rebonato A, Maiettini D, Vagnarelli S, Ambrogi C, Rossi M. Trans Arterial Embolization of Non-variceal Upper Gastrointestinal Bleeding: Is the Use of Ethylene-Vinyl Alcohol Copolymer as Safe as Coils? Cardiovasc Intervent Radiol. 2018;41:1340-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Charnsangavej C, Chuang VP, Wallace S, Soo CS, Bowers T. Angiographic classification of hepatic arterial collaterals. Radiology. 1982;144:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Tajima Y, Kuroki T, Tsutsumi R, Sakamoto I, Uetani M, Kanematsu T. Extrahepatic collaterals and liver damage in embolotherapy for ruptured hepatic artery pseudoaneurysm following hepatobiliary pancreatic surgery. World J Gastroenterol. 2007;13:408-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Onizawa S, Hamano M, Tsuchiya A, Araida T, Toda J, Yamamoto M. Successful treatment of pseudoaneurysm rupture after pylorus preserving pancreaticoduodenectomy by covered stent placement. Surg Technol Int. 2012;22:77-82. [PubMed] |
| 81. | Nakai M, Sato H, Sato M, Ikoma A, Sanda H, Nakata K, Minamiguchi H, Kawai N, Sonomura T, Nishimura Y, Okamura Y. Endovascular stenting and stent-graft repair of a hemorrhagic superior mesenteric artery pseudoaneurysm and dissection associated with pancreaticoduodenectomy. J Vasc Interv Radiol. 2012;23:1381-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Asai K, Watanabe M, Kusachi S, Matsukiyo H, Saito T, Kodama H, Enomoto T, Nakamura Y, Okamoto Y, Saida Y, Iijima R, Nagao J. Successful treatment of a common hepatic artery pseudoaneurysm using a coronary covered stent following pancreatoduodenectomy: report of a case. Surg Today. 2014;44:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Singh CS, Giri K, Gupta R, Aladdin M, Sawhney H. Successful management of hepatic artery pseudoaneurysm complicating chronic pancreatitis by stenting. World J Gastroenterol. 2006;12:5733-5734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Suzuki K, Mori Y, Komada T, Matsushima M, Ota T, Naganawa S. Stent-graft treatment for bleeding superior mesenteric artery pseudoaneurysm after pancreaticoduodenectomy. Cardiovasc Intervent Radiol. 2009;32:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Lü PH, Zhang XC, Wang LF, Chen ZL, Shi HB. Stent graft in the treatment of pseudoaneurysms of the hepatic arteries. Vasc Endovascular Surg. 2013;47:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Herzog T, Suelberg D, Belyaev O, Uhl W, Seemann M, Seelig MH. Treatment of acute delayed visceral hemorrhage after pancreatic surgery from hepatic arteries with covered stents. J Gastrointest Surg. 2011;15:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Cynamon J, Atar E, Steiner A, Hoppenfeld BM, Jagust MB, Rosado M, Sprayregen S. Catheter-induced vasospasm in the treatment of acute lower gastrointestinal bleeding. J Vasc Interv Radiol. 2003;14:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Inoue Y, Ikegawa H, Ukai I, Yoshiya K, Sumi Y, Ogura H, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. Spontaneous occlusion of splenic and renal pseudoaneurysm after blunt abdominal trauma: a case report and literature review. J Emerg Med. 2010;38:e17-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Dror S, Dani BZ, Ur M, Yoram K. Spontaneous thrombosis of a splenic pseudoaneurysm after blunt abdominal trauma. J Trauma. 2002;53:383-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Bhangui P, Salloum C, Lim C, Andreani P, Ariche A, Adam R, Castaing D, Kerba T, Azoulay D. Portal vein arterialization: a salvage procedure for a totally de-arterialized liver. The Paul Brousse Hospital experience. HPB (Oxford). 2014;16:723-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Noji T, Tsuchikawa T, Okamura K, Nakamura T, Tamoto E, Shichinohe T, Hirano S. Resection and reconstruction of the hepatic artery for advanced perihilar cholangiocarcinoma: result of arterioportal shunting. J Gastrointest Surg. 2015;19:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |