Published online Jan 27, 2021. doi: 10.4254/wjh.v13.i1.151
Peer-review started: September 23, 2020
First decision: October 21, 2020
Revised: November 12, 2020
Accepted: November 28, 2020
Article in press: November 28, 2020
Published online: January 27, 2021
Processing time: 124 Days and 18.7 Hours
Budd-Chiari syndrome (BCS) is a challenging indication for liver transplantation (LT) due to a combination of massive liver, increased bleeding, retroperitoneal fibrosis and frequently presents with stenosis of the inferior vena cava (IVC). Occasionally, it may be totally thrombosed, increasing the complexity of the procedure, as it should also be resected. The challenge is even greater when performing living-donor LT as the graft does not contain the retrohepatic IVC; thus, it may be necessary to reconstruct it.
A 35-year-old male patient with liver cirrhosis due to BCS and hepatocellular carcinoma beyond the Milan criteria underwent living-donor LT with IVC reconstruction. It was necessary to remove the IVC as its retrohepatic portion was completely thrombosed, up to almost the right atrium. A right-lobe graft was retrieved from his sister, with outflow reconstruction including the right hepatic vein and the branches of segment V and VIII to the middle hepatic vein. Owing to massive subcutaneous collaterals in the abdominal wall, venovenous bypass was implemented before incising the skin. The right atrium was reached via a transdiaphragramatic approach. Hepatectomy was performed en bloc with the retrohepatic vena cava. It was reconstructed with an infra-hepatic vena cava graft obtained from a deceased donor. The patient remains well on outpatient clinic follow-up 25 mo after the procedure, under an anticoagulation protocol with warfarin.
Living-donor LT in BCS with IVC thrombosis is feasible using a meticulous surgical technique and tailored strategies.
Core Tip: A right-lobe living-donor liver transplantation (LT) with inferior vena cava (IVC) resection and reconstruction was performed in a patient with liver cirrhosis due to Budd-Chiari syndrome and hepatocellular carcinoma beyond the Milan criteria. It was necessary to remove the IVC because its retrohepatic portion was completely thrombosed, up to almost the right atrium. It was reconstructed with an infra-hepatic vena cava graft obtained from a deceased donor. The patient remains well 25 mo after the procedure. This case highlights the meticulous surgical technique and tailored strategies required for dealing with these challenging procedures in living-donor LT.
- Citation: Rocha-Santos V, Waisberg DR, Pinheiro RS, Nacif LS, Arantes RM, Ducatti L, Martino RB, Haddad LB, Galvao FH, Andraus W, Carneiro-D'Alburquerque LA. Living-donor liver transplantation in Budd-Chiari syndrome with inferior vena cava complete thrombosis: A case report and review of the literature. World J Hepatol 2021; 13(1): 151-161
- URL: https://www.wjgnet.com/1948-5182/full/v13/i1/151.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i1.151
Budd-Chiari Syndrome (BCS) is characterized by the obstruction of hepatic venous drainage that leads to progressive hepatic congestion and, ultimately, portal hypertension and liver cirrhosis[1]. This blockage may be present in the hepatic venules, main hepatic veins, inferior vena cava (IVC) or right atrium[2]. Several nonsurgical therapeutics have been described, such as anticoagulation therapy, percutaneous transluminal angioplasty and interventional radiologic placement of a transjugular intrahepatic portosystemic shunt (TIPS) or direct intrahepatic portocaval shunt[1-3]. Liver transplantation (LT) is indicated in acute cases of fulminant hepatic failure or chronic cases with cirrhosis, which commonly evolve with gastrointestinal bleeding, untreatable ascites, sarcopenia, encephalopathy and hepatocellular carcinoma (HCC)[4]. In such scenarios, TIPS is often unfeasible due to extensive venous thrombosis or advanced liver disease[5].
Venous thrombosis can affect not only the hepatic veins but also a prolonged segment of the retrohepatic IVC, occasionally very close to the right atrium. The association between the severity of the disease, the extension of the venous thrombosis and the massive liver that is frequently present in BCS makes LT a particularly difficult procedure in these cases[1]. The hypercoagulative nature of the syndrome further increases the challenge, owing to vascular complications[6].
The challenge is even greater when considering living donor liver transplantation (LDLT) since the graft does not contain the retrohepatic IVC, as in deceased-donor liver transplantation (DDLT). Therefore, hepatic venous reconstruction is more complex, especially if the IVC is also obliterated[7]. That is the reason why only approximately 70 patients with BCS underwent LDLT worldwide between 1989 and 2015[1,8]. When LDLT is performed and HCC is also present, DDLT may not be possible in case of postoperative complications if the patient is beyond the Milan criteria[9], depending on local legislation in some countries, such as Brazil. Thus, performing LDLT for BSC in such a scenario is even more risky.
We report a case of a complex retrohepatic IVC thrombosis due to BCS in a patient with HCC beyond the Milan criteria. As the patient had a good response to transarterial chemoembolization (TACE) and his alfa fetoprotein levels decreased, we decided to perform LDLT.
A 35-year-old cirrhotic male patient was referred for LT evaluation due to BCS and HCC.
The patient had been diagnosed with cirrhosis and BCS four years previously, after presenting with ascites and hematemesis due to esophageal varices. Abdominal computed tomography (CT) scan on this occasion showed hepatic veins thrombosis and signs of chronic hepatopathy with paraumbilical vein recanalization and extensive collateral circulation in the splenic hilum, around the stomach, and in the anterior and lateral abdominal walls. The liver also showed multiple hepatic nodules of up to 1.5 cm in diameter, some them hypervascularized, which in the context of BCS, were compatible with regenerative hepatic nodules. Hepatic biopsy revealed chronic hepatic outflow obstruction. Laboratory testing for autoimmune hepatitis was negative, as were serological markers for hepatitis C and B viruses. The patient also denied previous alcohol abuse. No thrombophilia was diagnosed, despite extensive hematological investigation. The patient was then maintained on oral anticoagulation with warfarin.
The patient had no previous medical history.
The patient was a smoker (10 cigarettes/day for 20 years). There was no relevant family history concerning this case.
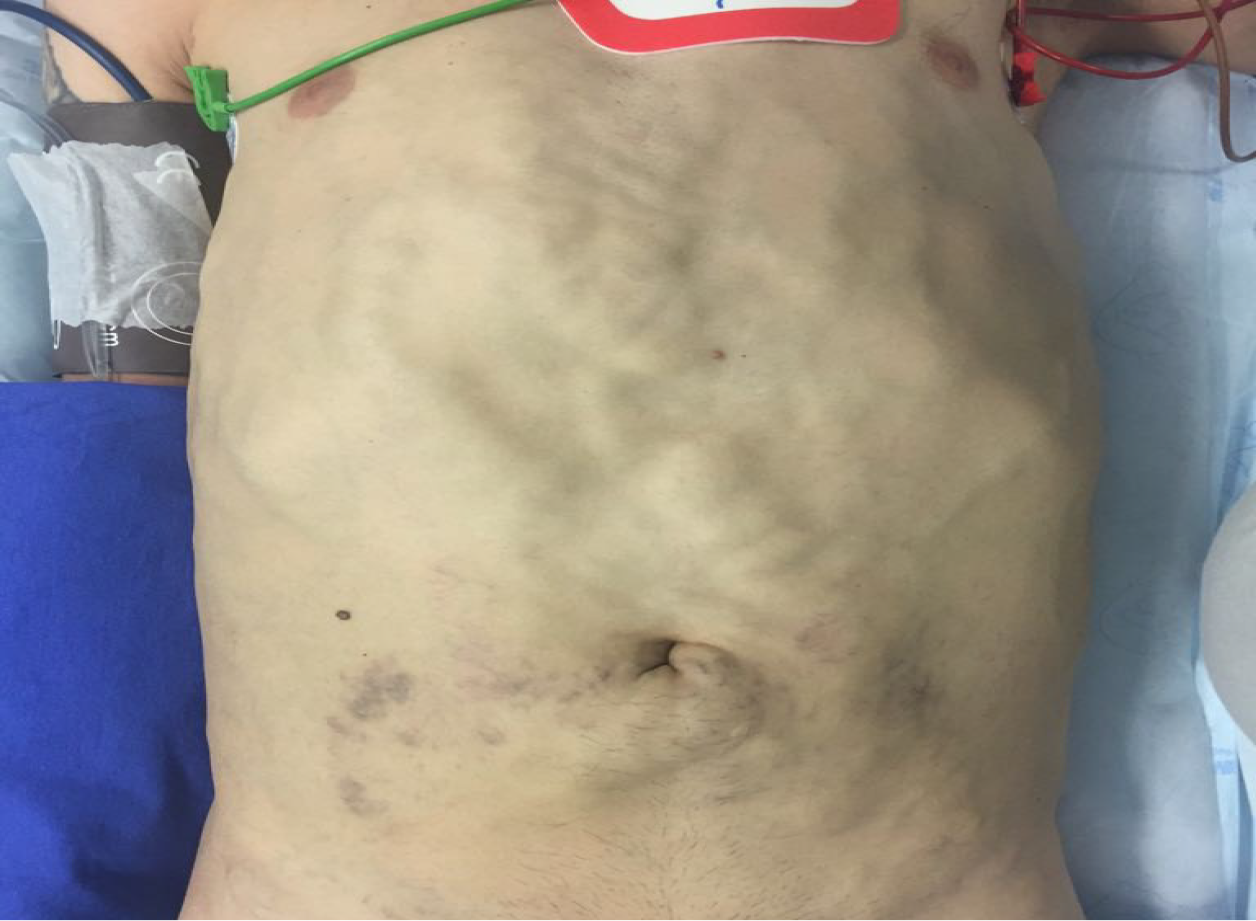
The patient exhibited mild jaundice and extensive subcutaneous collateral veins in the anterior abdominal wall (Figure 1). Further physical examination was unremarkable.
Blood analysis revealed normal hemoglobin, mild leukopenia and mild thrombocytopenia with mildly elevated total bilirubin, direct bilirubin and gamma-glutamyl-transferase (Table 1). Kidney function and electrolytes were normal as well as serum albumin, alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase. The patient’s prothrombin time was elevated even without warfarin (Table 1). Considering that the patient did not present encephalopathy or ascites, his Child-Pugh score was A6, and his Model of End-Stage Liver Disease (MELD) score was 15. His alpha-fetoprotein level was 58.7 ng/mL (normal range < 10 ng/mL), although 6 mo earlier, it was 9.4 ng/mL.
| Laboratory test | Result | Normal range |
| Hemoglobin | 12.6 g/dL | 12.5-17.5 g/dL |
| Leukocytes | 3.5 × 109/L | 4-11 × 109/L |
| Platelets | 80 × 103/mm3 | 150-400 × 103/mm3 |
| Total bilirubin | 1.73 mg/dL | 0.2-1 mg/dL |
| Direct bilirubin | 0.85 mg/dL | < 0.3 mg/dL |
| Alanine aminotransferase | 20 U/L | < 41 U/L |
| Aspartate aminotransferase | 35 U/L | < 37 U/L |
| Alkaline phosphatase | 78 U/L | 40-129 U/L |
| Gamma-glutamyl-transferase | 115 U/L | 8-91 U/L |
| Creatinine | 0.79 mg/dL | 0.7-1.2 mg/dL |
| Blood urea nitrogen | 31 mg/dL | 10-50 mg/dL |
| Sodium | 143 mEq/L | 135-145 mEq/L |
| Potassium | 3.9 mEq/L | 3.5-4.5 mEq/L |
| Albumin | 4.4 g/dL | 3.4-4.8 g/dL |
| Prothrombin time | 21.8 s | 9.4-12.5 s |
| International normalized ratio | 1.75 | 0.95-1.2 |
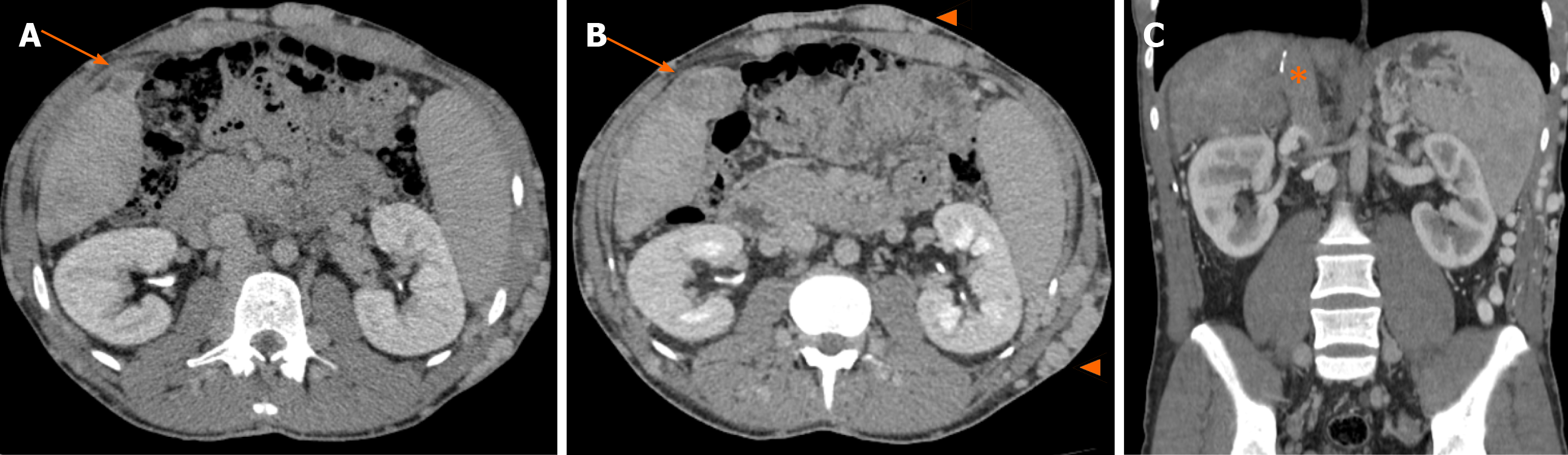
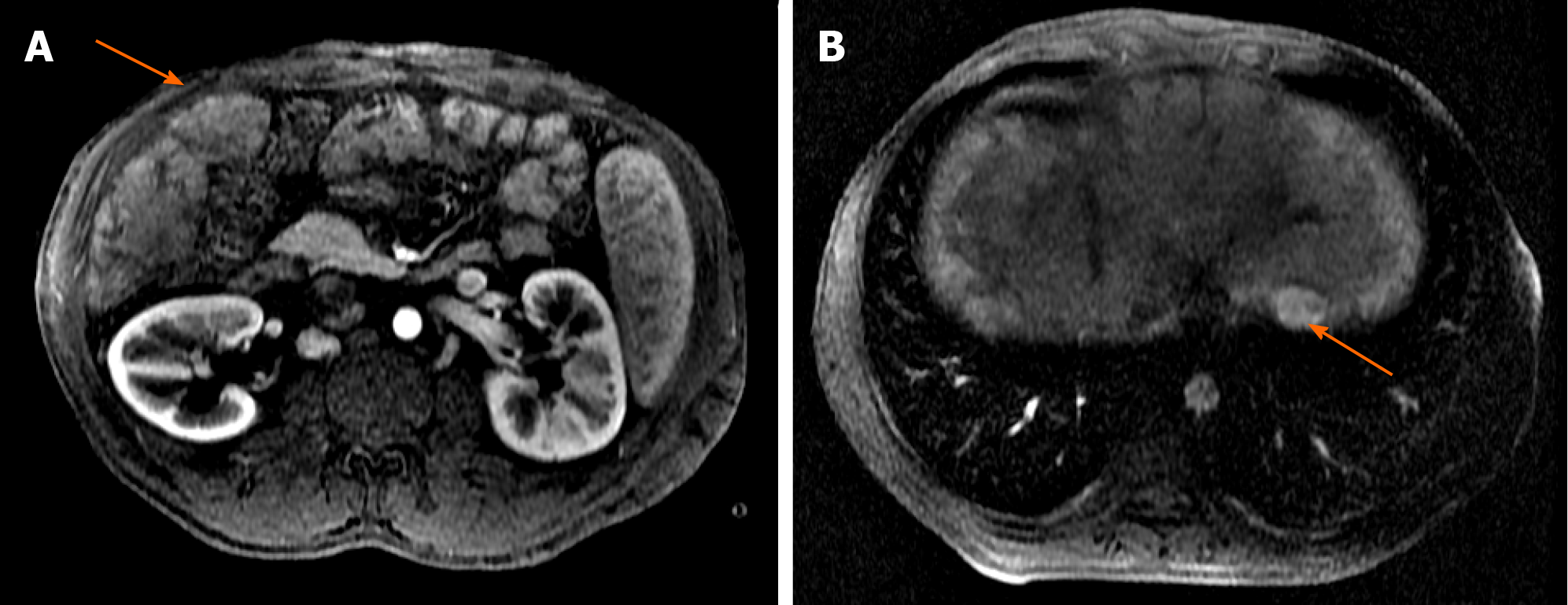
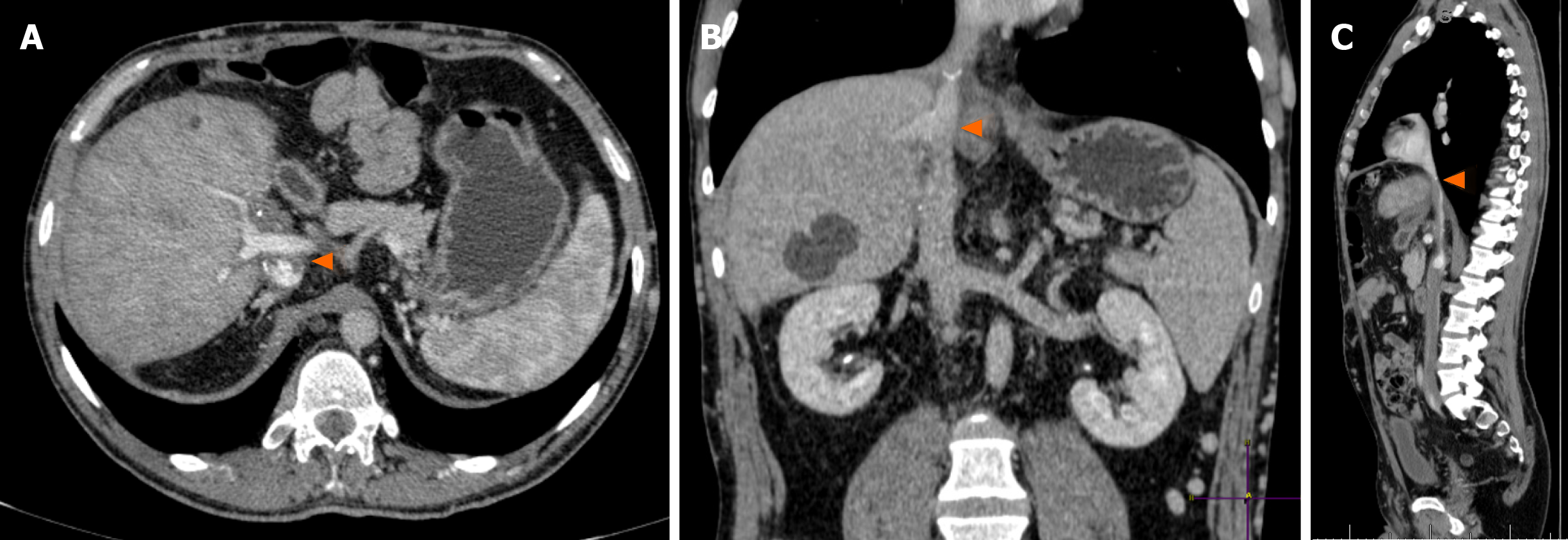
During outpatient follow-up, an abdominal CT scan showed a heterogeneously vascularized nodule in segment V, which increased from 2 cm to 4 cm in three years (Figure 2A and B). He also showed complete thrombosis of the retrohepatic IVC, up to almost the right atrium, with large subcutaneous veins in his abdominal wall (Figure 2C). Further evaluation with abdominal liver magnetic resonance imaging with hepatobiliary contrast showed two hypervascularized nodules with hypocaptation in the biliary phase in segments V and II, 4 and 2.3 cm in size, respectively (Figure 3). Considering the previous CT scans with multiple regenerative nodules, these 2 specific nodules were classified as indeterminate lesions. Given their growth, the atypical pattern of contrast uptake and the rise in alpha-fetoprotein serum levels, further investigation with biopsy of these nodules was indicated due to the suspicion of HCC.
Percutaneous ultrasound-guided biopsy of the largest nodule confirmed a moderately differentiated HCC (grade 3 Edmondson-Steiner grading system). Therefore, the patient presented liver cirrhosis due to BCS with retrohepatic vena cava thrombosis and multicentric HCC beyond the Milan criteria.
According to Brazilian legislation, the patient could not be listed for DDLT due to being beyond the Milan criteria. He underwent 2 TACE procedures in order to downstage the lesions to within the Milan criteria so that he could be listed. Even though the serum alfa-fetoprotein level decreased from 58.7 to 18 ng/mL, the nodules did not decrease in size and the patient remained beyond the Milan criteria. His sister then volunteered for liver donation and the patient was selected for LDLT. She was a healthy 51-year-old female with a body mass index of 22.6 kg/m2. Liver volumetry revealed a right lobe of 724 cm3 (66% of the entire organ), and usual biliary tree anatomy was found on magnetic resonance cholangiopancreatography. Liver parenchyma also showed simple cysts.
The patient weighed 71 kg, resulting in a predicted graft-to-recipient weight ratio (GRWR) of 0.81%. Donor operation consisted of a right hepatectomy with middle hepatic vein preservation. The procedure was uneventful, resulting in a 560 g right lobe graft with usual anatomy (GRWR of 0.79%). In the backtable operation, the right hepatic vein and the V5 and V8 branches of the middle hepatic vein were reconstructed to avoid outflow blockage.
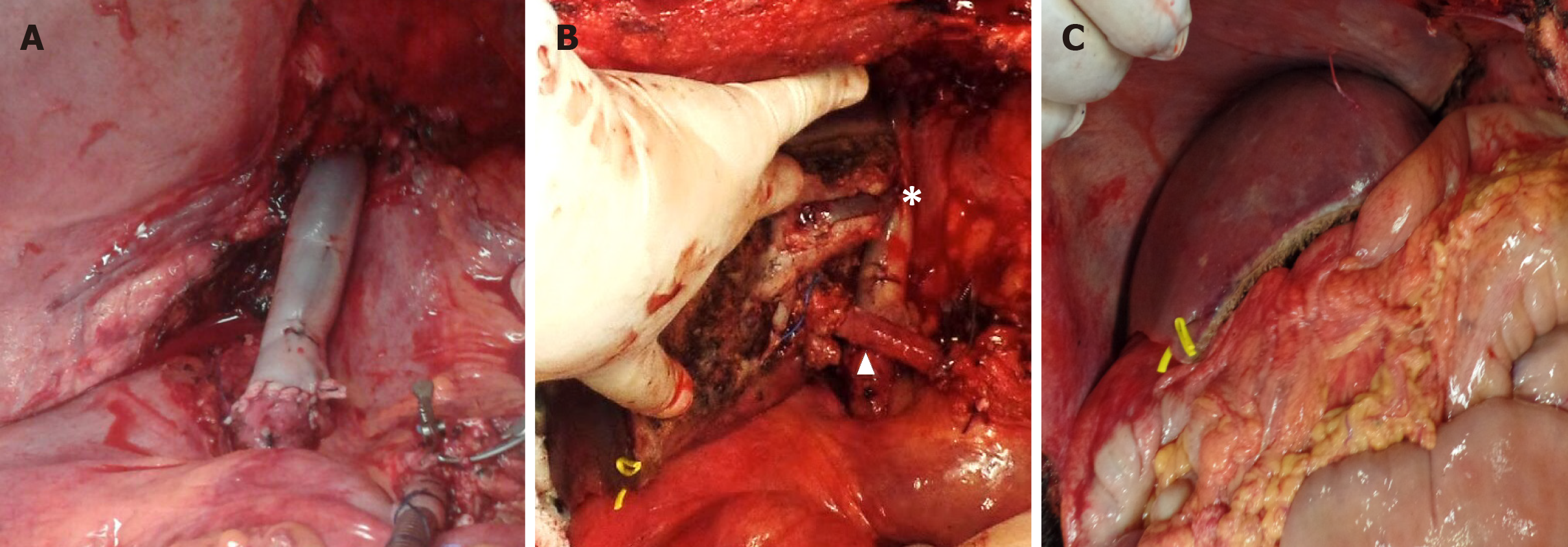
For the recipient, the surgical strategy included the use of a venovenous bypass prior to incising the abdomen due to very large subcutaneous collaterals in the abdominal and thoracic walls. The left femoral and left axillary veins were used to implement the venovenous bypass. Hepatectomy was performed with the retrohepatic vena cava, close to the right atrium. The explanted liver weighed 1880 g. The portal vein was then cannulated and added to the venovenous bypass. As the right lobe graft did not include the retrohepatic vena cava, it was reconstructed using an infra-hepatic IVC from a deceased donor (Figure 4A). The graft was then implanted using this newly formed IVC to be anastomosed with the graft venous conduit for the outflow reconstruction. The right portal vein, right hepatic artery and right hepatic duct of the graft were then respectively anastomosed to their counterparts in the recipient (Figure 4B and C). Total and warm ischemia times were 370 and 30 min, respectively.
The donor’s postoperative course was uneventful, and she was discharged home on postoperative day (POD) 5. The recipient was extubated on POD 2, and anticoagulation with enoxaparin was restarted, as well as low-dose aspirin. Liver Doppler ultrasound on POD 1 and 15 showed preserved graft vascularization. Renal function remained preserved, and the patient’s condition progressively improved. The patient’s immunosuppression regimen included intraoperative corticoid bolus and tapering associated with tacrolimus. The patient was discharged home on POD 19. Everolimus was added to the tacrolimus regimen 3 mo after the transplantation. Low-dose corticoid was maintained for 6 mo.
On histopathological analysis, the explanted liver confirmed hepatic cirrhosis related to chronic BCS and two moderately differentiated HCCs in segment V (4.5 cm) and segment II (2.5 cm).
Routine abdominal CT scan performed 23 mo after transplant showed a patent retrohepatic vena cava and adequate right lobe vascularization (Figure 5). The patient remains well on outpatient clinic follow-up 25 mo after the procedure, under an anticoagulation protocol with warfarin and without signs of HCC recurrence (alpha-fetoprotein 6.5 ng/mL).
Despite the numerous treatment modalities available for BCS, LT is performed in 10% to 20% of patients[1,2]. Nevertheless, it is a rare cause for LT, accounting for approximately 1%[10,11]. This a challenging indication for LT due to a combination of massive liver and increased bleeding, caudate lobe enlargement, retroperitoneal diffuse fibrosis, firm retrohepatic IVC adhesions and frequently presents with stenosis and/or thrombosis of the IVC[3]. Especially in LDLT, in which the donor’s IVC cannot be used, the retrohepatic IVC dissection performed during the piggyback technique and the venous outflow reconstruction are particularly problematic. Novel alternative techniques, aimed at eliminating stenosis or obstruction in the recipient IVC, are thus needed for LDLT in the context of BCS[6]. Some of them include cross-clamping the supra- and infrahepatic IVC and excising its thickened wall to create a wide orifice for graft implantation[7] or the V-Y plasty technique[12].
Nevertheless, when the IVC is completely occluded, which is known as obliterative hepatocavopathy (OHC), it is advisable to remove the IVC en bloc with the native liver[13], as the piggyback dissection becomes technically unfeasible due to dense inflammatory adhesions, enlarged collaterals and hypertrophied caudate lobe. If an LDLT is performed in this situation, it may be necessary to reconstruct the retrohepatic IVC. In 2006, Yan et al[14] reported the first LDLT for BCS with IVC reconstruction using an interposed cryopreserved cadaveric IVC graft[14]. Since then, many other studies have addressed IVC reconstruction with interposing autologous veins[15], cadaveric venous allografts[3,7,16-18], cadaveric aortic allografts[7,17-20], synthetic material[12,13,18] or a combination of synthetic material and autologous vein[21,22] or venous allografts[18,23]. Table 2 provides a review of all cases found in the literature of LDLT for BCS with IVC resection.
| Ref. | Number of cases | Technique | Venovenous bypass use | Outcomes |
| Yan et al[14], 2006 | n = 1 | IVC replacement with cadaveric IVC allograft | Yes | Alive after 3 mo |
| Yamada et al[2], 2006 | n = 1 | IVC resection without replacement | No | Alive after 10 mo |
| Shimoda et al[15], 2007 | n = 1 | IVC replacement with autologous internal jugular vein, external iliac vein and suprarenal IVC | No | Alive after 17 mo |
| Sasaki et al[16], 2009 | n = 1 | IVC replacement with cadaveric IVC allograft | No | N/A |
| Kazimi et al[32], 2009 | n = 1 | IVC resection without replacement | No | Alive after 3 mo |
| Choi et al[3], 2010 | n = 2 | IVC replacement with cadaveric IVC allograft (n = 1) and RHV-atrial shunt using preexisting mesoatrial shunt (n = 1) | No | Both alive after a median follow-up of 18 mo |
| Ogura et al[21], 2011 | n = 1 | IVC replacement with an inverted composite graft (Gore-Tex stretch vascular graft and transposed IVC) | Yes | Alive after 24 mo |
| Sakçak et al[19], 2012 | n = 1 | IVC replacement with cadaveric aortic allografts | No | Alive after 4 mo |
| Fukuda et al[24], 2013 | n = 1 | IVC resection without replacement | No | Alive after 60 mo |
| Yagci et al[17], 2015 | n = 4 | IVC replacement with cadaveric IVC (n = 1), iliac vein (n = 1) and aorta allografts (n = 2) | No | 2 patients died due to biliary complications after 5 mo of follow-up |
| Cetinkunar et al[20], 2015 | n = 1 | IVC replacement by cadaveric aortic allograft | No | Alive after 4 mo |
| Ara et al[7], 2016 | n = 7 | IVC replacement with cadaveric IVC (n = 4) and cadaveric aorta allografts (n = 2). No replacement in one case | No | 2 patients died due to recent HAT after LT, and 2 patients died of sepsis during follow-up |
| Pahari et al[12], 2016 | n = 2 | IVC replacement with e-PTFE graft | No | Both alive after a median follow-up of 18 mo |
| Karaca et al[6], 2017 | n = 3 | IVC resection without replacement | No | N/A |
| Sabra et al[25], 2018 | n = 1 | IVC resection without replacement | No | Alive after 3 mo |
| Yagi et al[22], 2018 | n = 1 | IVC replacement with an inverted composite graft (e-PTFE graft and transposed IVC) | Yes | Alive after 36 mo |
| Ionescu et al[23], 2018 | n = 2 | IVC replacement with caval-dacron composite graft | No | Both alive (follow-up not available) |
| Yoon et al[13], 2019 | n = 5 | IVC replacement with synthetic material (ringed polyester) | Yes (n=3) | All alive after a median follow-up of 10.5 years |
| Gonultas et al[18], 2020 | n = 12 | IVC replacement with cadaveric IVC allograft (n = 6), cadaveric aorta allograft (n = 1), synthetic material (n = 3, Dacron) and caval-dacron composite graft (n = 2) | No | All alive after median follow-up of 15 mo |
| Present study | n = 1 | IVC replacement with cadaveric IVC allograft | Yes | Alive after 25 mo |
In the present report, we faced three ordeals in the preoperative period. First, the massive liver was associated with extensive IVC thrombosis starting close to the renal veins and progressing up to the transition between the IVC and the right atrium. Second, it was necessary to use a living donor right lobe with the potential risk of postoperative small-for-size syndrome, given the association of extensive thrombosis, portal hypertension and partial graft[12]. Finally, the LDLT was performed in a patient with HCC beyond the Milan criteria, which, according to Brazilian law, prevented the use of a deceased-donor graft in case of postoperative graft dysfunction.
Most authors describe a transdiaphragmatic access to the supradiaphragmatic IVC or even the right atrium, although a rarely performed lower median sternotomy may be helpful in some cases[13,24]. In the present report, through a standard Makuuchi incision, the recipient’s liver was removed en bloc with the retrohepatic vena cava, from just above the renal veins to the beginning of the right atrium. This surgical approach, without thoracic access, was very useful as the patient had no major bleeding or hemodynamic instability. The interposition of a conduit replacing the retrohepatic IVC was necessary because we could observe considerable venous flow from the suprarenal vena cava. There is no consensus in the literature regarding the best material for IVC reconstruction[18]. The use of synthetic material raises concerns regarding the long-term patency of the anastomosis between the hepatic vein from the liver graft and the prosthesis, due to the possibility of thrombosis, deformity of the synthetic orifice and anastomosis kinking consequent to growth of the liver graft[25]. Infection of prosthetic material is also an issue[26]. Many centers, including ours, therefore prefer autologous or allogeneic grafts, which present less thrombosis and infection risk[18,27]. Even cadaveric IVC recovered 25 h after the donor´s circulatory death has been successfully used[28]. As a high-volume center of DDLT, there is great availability of allografts in our institution biobank. Storage of such grafts is feasible and inexpensive, only requiring sterile Ringer Lactate solution and a laboratory freezer[29]. However, in countries with scarce deceased donor organ donation and in centers with a high volume of LDLT, access to these grafts may be difficult[18].
Given the complexity of such procedures, it is paramount to obtain a suitable amount of liver parenchyma[30]. Therefore, we used the right lobe, as in most reported cases; however, some authors have also used the right posterior segment[15], the left lateral segment (pediatric recipients)[7,17,19], the left lobe[2,22,24,25] and dual grafts[13]. Another concern is the possibly elevated portal inflow to the graft[31]. That is the reason why we routinely measure the portal venous pressure by a catheter inserted via a jejunal branch. As the portal pressure was below 14 mmHg in this case after graft implantation, we did not implement further strategies to decrease the portal inflow.
In most cases reported, venovenous bypass was not used (Table 2). Due to the chronicity of IVC obstruction, venous return is expected to occur via collaterals involving the azygos, hemiazygos, accessory hemiazygos and thoracolumbar veins[24]. In a large series addressing LDLT with IVC resection for various reasons in 29 patients by Gonultas et al[18], venovenous bypass was not used in any case, as there was no hemodynamic instability during IVC clamping. In our case, the patient presented a well-developed collateral circulation; however, we observed that it was mainly composed of a massive subcutaneous plexus in the abdominal and thoracic wall (Figures 1 and 2). Thus, we decided to use the extracorporeal venovenous bypass before the abdominal skin was incised. We feared that an abdominal incision could lead to hemodynamic instability, since it was necessary to ligate the collaterals forming this enormous subcutaneous plexus. Therefore, when we accessed the abdominal cavity and clamped the IVC, the patient was already on venovenous bypass.
Retrohepatic IVC resection without replacement in LDLT for BCS has also been reported[2,6,7,24,25,32], in which the liver graft is anastomosed directly to the right atrium[6,32], to the intrapericardical IVC[24,25] or to the rarely preserved supra-hepatic IVC[2,6,7]. In one patient, the graft was directly anastomosed to a previous mesoatrial shunt[3]. This raises the question of whether or not it necessary to reconstruct the IVC. As addressed by Gonultas et al[18], the venous continuity should be maintained in patients without a venous collateral circulation system or in those with insufficient venous drainage. For patients that have a well-developed venous collateral, on the other hand, the liver graft may be, in theory, anastomosed directly to the suprahepatic IVC without the need for reconstruction. In our case, as the collaterals forming the subcutaneous plexus were ligated during the skin incision, the IVC reconstruction was required. We also observed a significant blood flow in the infra-hepatic IVC after the native liver was removed, suggesting the necessity of venous continuity restoration with an IVC interposition graft.
Despite the complexity of cases, most studies describe successful outcomes after LDLT (Table 2). The literature review identified 2 deaths due to early hepatic arterial thrombosis and another 4 patients died during follow-up due to infectious and biliary complications occurring months after transplant. In the series by Gonultas et al[18], 4 patients experienced late thrombosis of the replaced IVC during follow-up that were successfully treated with percutaneous balloon dilatation and/or stenting. The early use of low-dose aspirin and low molecular weight heparin a few days after LDLT is important to prevent the recurrence of thrombosis[12,13,18,32].
We describe a novel surgical approach for LDLT in BCS with OHC and HCC beyond the Milan criteria that can be used in highly selected patients. Due to its complexity and rarity, LDLT in such situations is feasible using a meticulous surgical technique and tailored strategies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Park SB S-Editor: Fan JR L-Editor: Webster JR P-Editor: Wang LL
| 1. | Akamatsu N, Sugawara Y, Kokudo N. Budd-Chiari syndrome and liver transplantation. Intractable Rare Dis Res. 2015;4:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Yamada T, Tanaka K, Ogura Y, Ko S, Nakajima Y, Takada Y, Uemoto S. Surgical techniques and long-term outcomes of living donor liver transplantation for Budd-Chiari syndrome. Am J Transplant. 2006;6:2463-2469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Choi GS, Park JB, Jung GO, Chun JM, Kim JM, Moon JI, Kwon CH, Kim SJ, Joh JW, Lee SK. Living donor liver transplantation in Budd-Chiari syndrome: a single-center experience. Transplant Proc. 2010;42:839-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Valla DC. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2018;12:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Parekh J, Matei VM, Canas-Coto A, Friedman D, Lee WM; Acute Liver Failure Study Group. Budd-chiari syndrome causing acute liver failure: A multicenter case series. Liver Transpl. 2017;23:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Karaca C, Yilmaz C, Ferecov R, Iakobadze Z, Kilic K, Caglayan L, Aydogdu S, Kilic M. Living-Donor Liver Transplantation for Budd-Chiari Syndrome: Case Series. Transplant Proc. 2017;49:1841-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Ara C, Akbulut S, Ince V, Karakas S, Baskiran A, Yilmaz S. Living donor liver transplantation for Budd-Chiari syndrome: Overcoming a troublesome situation. Medicine (Baltimore). 2016;95:e5136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Doğrul AB, Yankol Y, Mecit N, Kanmaz T, Acarlı K, Kalayoğlu M. Orthotopic Liver Transplant for Budd-Chiari Syndrome: An Analysis of 14 Cases. Exp Clin Transplant. 2016;14:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5287] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 10. | Segev DL, Nguyen GC, Locke JE, Simpkins CE, Montgomery RA, Maley WR, Thuluvath PJ. Twenty years of liver transplantation for Budd-Chiari syndrome: a national registry analysis. Liver Transpl. 2007;13:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Mackiewicz A, Kotulski M, Zieniewicz K, Krawczyk M. Results of liver transplantation in the treatment of Budd-Chiari syndrome. Ann Transplant. 2012;17:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Pahari H, Chaudhary RJ, Thiagarajan S, Raut V, Babu R, Bhangui P, Goja S, Rastogi A, Vohra V, Soin AS. Hepatic Venous and Inferior Vena Cava Morphology No Longer a Barrier to Living Donor Liver Transplantation for Budd-Chiari Syndrome: Surgical Techniques and Outcomes. Transplant Proc. 2016;48:2732-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Yoon YI, Lee SG, Moon DB, Ahn CS, Hwang S, Kim KH, Ha TY, Song GW, Jung DH, Park GC, Kim DS, Choo SJ. Surgical Techniques and Long-term Outcomes of Living-donor Liver Transplantation With Inferior Vena Cava Replacement Using Atriocaval Synthetic Interposition Graft for Budd-Chiari Syndrome. Ann Surg. 2019;269:e43-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Yan L, Li B, Zeng Y, Wen T, Zhao J, Wang W, Xu M, Yang J, Ma Y, Chen Z, Wu H. Living donor liver transplantation for Budd-Chiari syndrome using cryopreserved vena cava graft in retrohepatic vena cava reconstruction. Liver Transpl. 2006;12:1017-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Shimoda M, Marubashi S, Dono K, Miyamoto A, Takeda Y, Nagano H, Umeshita K, Monden M. Utilization of autologous vein graft for replacement of the inferior vena cava in living-donor liver transplantation for obliterative hepatocavopathy. Transpl Int. 2007;20:804-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sasaki K, Kasahara M, Fukuda A, Shigeta T, Tanaka H, Nakagawa S, Nakagawa A, Nakayasiro M. Living donor liver transplantation with vena cava reconstruction using a cryopreserved allograft for a pediatric patient with Budd-Chiari syndrome. Transplantation. 2009;87:304-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Yagci MA, Tardu A, Karagul S, Ince V, Ertugrul I, Kirmizi S, Unal B, Aydin C, Kayaalp C, Yilmaz S. Living Donor Liver Transplantation With Vena Cava Replacement. Transplant Proc. 2015;47:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Gonultas F, Akbulut S, Barut B, Usta S, Kutluturk K, Kutlu R, Yilmaz S. Usability of Inferior Vena Cava Interposition Graft During Living Donor Liver Transplantation: Is This Approach Always Necessary? J Gastrointest Surg. 2020;24:1540-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Sakçak I, Eriş C, Ölmez A, Kayaalp C, Yılmaz S. Replacement of the vena cava with aortic graft for living donor liver transplantation in Budd-Chiari syndrome associated with hydatid cyst surgery: a case report. Transplant Proc. 2012;44:1757-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Cetinkunar S, Ince V, Ozdemir F, Ersan V, Yaylak F, Unal B, Yilmaz S. Living-Donor Liver Transplantation for Budd-Chiari Syndrome--Resection and Reconstruction of the Suprahepatic Inferior Vena Cava With the Use of Cadaveric Aortic Allograft: Case Report. Transplant Proc. 2015;47:1537-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ogura Y, Kanazawa H, Yoshizawa A, Nitta T, Ikeda T, Uemoto S. Supradiaphragmatic approach for Budd-Chiari syndrome with transjugular intrahepatic portosystemic shunt stent in combination with inferior vena cava reconstruction during living donor liver transplantation: a case report. Transplant Proc. 2011;43:2093-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Yagi T, Takagi K, Yoshida R, Umeda Y, Nobuoka D, Kuise T, Fujiwara T, Takaki A. New Left Lobe Transplantation Procedure with Caval Reconstruction Using an Inverted Composite Graft for Chronic Budd-Chiari Syndrome in Living-Donor Liver Transplantation-A Case Report. Transplant Proc. 2018;50:1192-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Ionescu MI, de Usera MA, Muiesan P, Mirza D, Isaac JR. Donation after Circulatory Death Type 2 Liver Transplantation in a Large Referral Centre in the United Kingdom: A Feasibility Study. 2018 Congress of the International Liver Transplantation Society. May 23 - 26, 2018 in Lisbon, Portugal. [DOI] [Full Text] |
| 24. | Fukuda A, Ogura Y, Kanazawa H, Mori A, Kawaguchi M, Takada Y, Uemoto S. Living donor liver transplantation for Budd-Chiari syndrome with hepatic inferior vena cava obstruction after open pericardial procedures. Surg Today. 2013;43:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Sabra TA, Okajima H, Tajima T, Fukumitsu K, Hata K, Yasuchika K, Masui T, Taura K, Kaido T, Uemoto S. Living donor liver transplantation for adult Budd Chiari syndrome - Resection without replacement of retrohepatic IVC: A case report. Int J Surg Case Rep. 2018;42:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Mancuso A, Martinelli L, De Carlis L, Rampoldi AG, Magenta G, Cannata A, Belli LS. A caval homograft for Budd-Chiari syndrome due to inferior vena cava obstruction. World J Hepatol. 2013;5:292-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Koc C, Akbulut S, Ozdemir F, Kose A, Isik B, Yologlu S, Yilmaz S. Analysis of Risk Factors Affecting the Development of Infection in Artificial Vascular Grafts Used for Reconstruction of Middle Hepatic Vein Tributaries in Living Donor Liver Transplantation. Transplantation. 2019;103:1871-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Palma AF, Oberkofler CE, Raptis DA, Eshmuminov D, de Rougemont O, Schnyder A, Dimitroulis D, Lesurtel M, Dutkowski P, Clavien PA. Novel rescue procedure for inferior vena cava reconstruction in living-donor liver transplantation using a vascular graft recovered 25 h after donors' circulatory death and systematic review. Transpl Int. 2014;27:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Aydin C, Ince V, Otan E, Akbulut S, Koc C, Kayaalp C, Yilmaz S. Storage of allogeneic vascular grafts: experience from a high-volume liver transplant institute. Int Surg. 2013;98:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 31. | Soin AS. Smoothing the path: reducing biliary complications, addressing small-for-size syndrome, and making other adaptations to decrease the risk for living donor liver transplant recipients. Liver Transpl. 2012;18 Suppl 2:S20-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Kazimi M, Karaca C, Ozsoy M, Ozdemir M, Apaydin AZ, Ulukaya S, Zeytunlu M, Kilic M. Live donor liver transplantation for Budd-Chiari syndrome: anastomosis of the right hepatic vein to the right atrium. Liver Transpl. 2009;15:1374-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |