Published online Sep 27, 2020. doi: 10.4254/wjh.v12.i9.672
Peer-review started: April 10, 2020
First decision: April 26, 2020
Revised: July 12, 2020
Accepted: July 26, 2020
Article in press: July 26, 2020
Published online: September 27, 2020
Processing time: 164 Days and 9.2 Hours
Although clinical use of sofosbuvir plus ribavirin has been approved for patients infected with genotype 2 hepatitis C virus, patients ≥ 75-years-old have not been included in previous clinical trials.
To evaluate the real-world safety and efficacy of sofosbuvir plus ribavirin for elderly patients (≥ 75-years-old) compared to nonelderly patients, we conducted a post-marketing prospective cohort study.
We treated 265 patients with genotype 2 hepatitis C virus using standard approved doses of sofosbuvir (400 mg/d) plus ribavirin adjusted by body weight, administered orally for 12 wk.
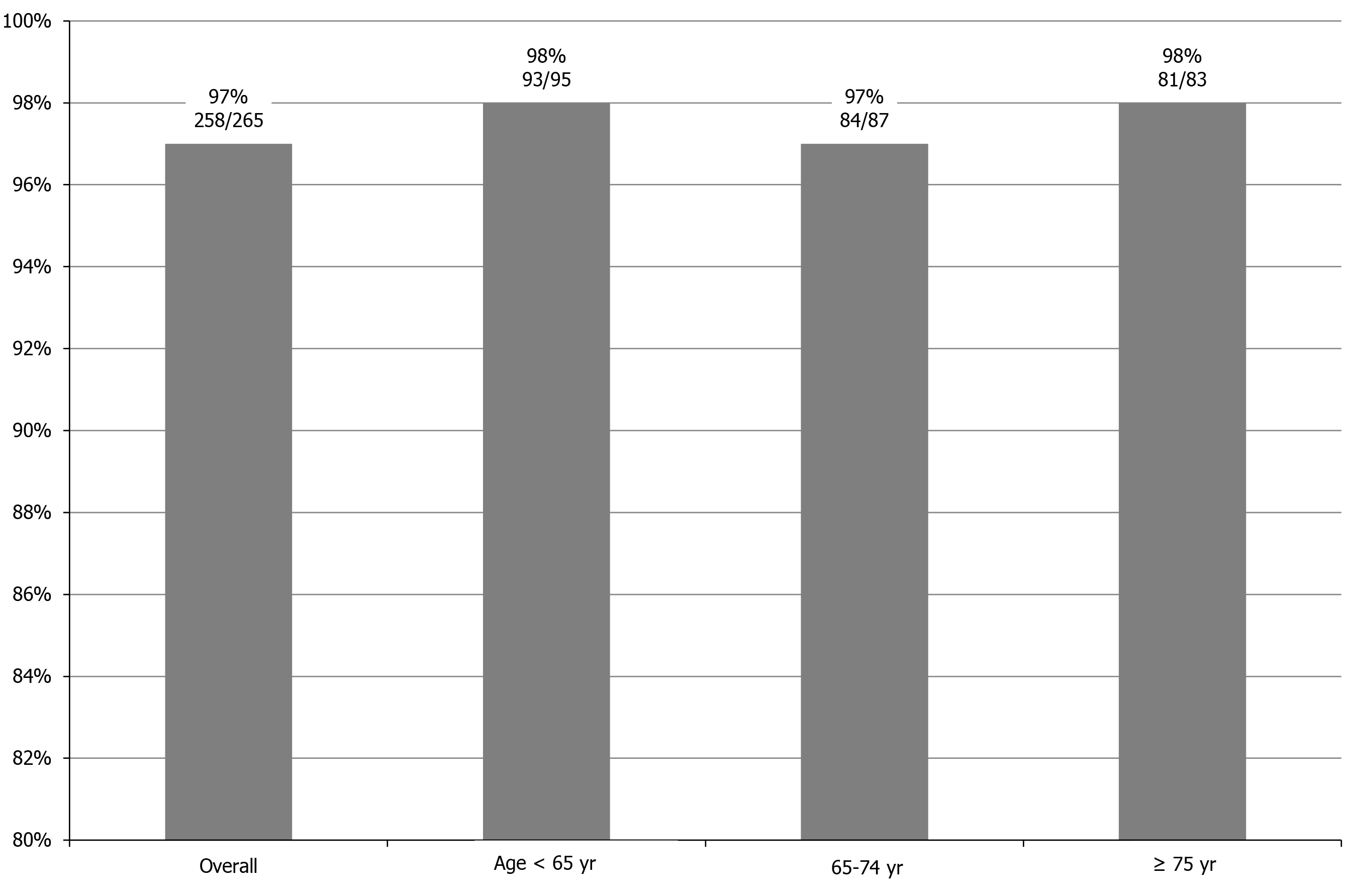
Sustained virological response rates for the overall cohort, patients < 65-years-old, ≥ 65-years-old but < 75-years-old, and ≥ 75-years-old were 97% (258/265), 98% (93/95), 97% (84/87), and 98% (81/83), respectively (P = 0.842). Logistic regression analyses identified history of hepatocellular carcinoma treatment and alpha-fetoprotein as factors significantly associated with sustained virological response. Alpha-fetoprotein was the only independent factor identified. Sustained virological response rate was significantly lower for patients with hepatocellular carcinoma treatment (91%) than for patients without history of hepatocellular carcinoma treatment (98%, P = 0.004). One patient (0.4%) discontinued treatment due to drug-induced pneumonia. Dose reduction or interruption of ribavirin was required for 12.1% (32/265) of patients because of anemia, including 7.7% (14/182) of patients < 75-years-old and 21.7% (18/83) of patients ≥ 75-years-old (P = 0.002).
Although ribavirin dose reduction or interruption was required with advanced age, sofosbuvir plus ribavirin appears tolerable and highly effective even in patients ≥ 75-years-old.
Core Tip: This was a multicenter post-marketing prospective cohort study of sofosbuvir plus ribavirin therapy for patients with genotype 2 hepatitis C virus in a real-world clinical setting. Combination therapy using sofosbuvir plus ribavirin was tolerable and highly effective even in elderly patients ≥ 75-years-old.
- Citation: Tamai H, Shingaki N, Ida Y, Shimizu R, Maeshima S, Okamura J, Kawashima A, Nakao T, Hara T, Matsutani H, Nishikawa I, Higashi K. Sofosbuvir plus ribavirin is tolerable and effective even in elderly patients 75-years-old and over. World J Hepatol 2020; 12(9): 672-684
- URL: https://www.wjgnet.com/1948-5182/full/v12/i9/672.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i9.672
Although interferon (IFN)-based therapy was standard for hepatitis C virus (HCV) infection for many years, clinical trials of IFN-free direct-acting antiviral agent (DAA) regimens using sofosbuvir and ribavirin for patients with genotype 2 HCV have been reported from Western countries since 2013[1-3]. Those clinical trials demonstrated higher viral eradication rates and lower discontinuation rates than IFN-based therapies, and treatment-related health-related quality of life impairment during treatment was reportedly mild in these clinical trials[4]. However, patients ≥ 75-years-old were not included in the sofosbuvir plus ribavirin regimen of those clinical trials. In May 2015, clinical use of combination therapy comprising sofosbuvir and ribavirin was approved as the first IFN-free therapy for patients infected with genotype 2 HCV in Japan[5]. This therapy was well tolerated and achieved a high sustained virological response (SVR) rate of 96% in a Japanese Phase III trial. However, patients ≥ 75-years-old were again not included in that trial. The safety and effectiveness of sofosbuvir plus ribavirin for elderly patients ≥ 75-years-old has thus remained unclear.
In real-world settings, elderly patients ≥ 75-years-old represent a substantial and growing population and carry a high risk of advanced liver diseases such as cirrhosis and hepatocellular carcinoma (HCC). These patients should be treated as soon as possible. However, ribavirin has various specific side effects that affect tolerability, such as hemolytic anemia, fatigue, cough, depression, and chest pain[6,7], whereas sofosbuvir is tolerable with minimal adverse effects[8]. In our previous study of low-dose pegylated IFN plus ribavirin therapy for elderly and/or cirrhotic patients infected with HCV genotype 2, drug dose reduction or interruption rates among nonelderly cirrhotic patients, elderly noncirrhotic patients, and elderly cirrhotic patients were all relatively high (65%, 63%, and 77%, respectively)[9]. A high risk of ribavirin dose reduction or interruption and lower effectiveness in elderly patients can thus be expected even with sofosbuvir plus ribavirin therapy. To evaluate the real-world safety and efficacy of sofosbuvir plus ribavirin for elderly patients ≥ 75-years-old compared to nonelderly patients, we conducted a post-marketing prospective cohort study.
This was a multicenter prospective cohort study. Between June 2015 and June 2017, all patients at Wakayama Medical University Hospital, Naga Municipal Hospital, Hidaka General Hospital, and Wakayama Rosai Hospital who were eligible were enrolled in the present study. The inclusion criterion was infection with HCV genotype 2. Exclusion criteria were any of following: (1) Infection with genotypes other than genotype 2; (2) Hemoglobin concentration < 10 g/dL; (3) Estimated glomerular filtration rate < 30 mL/min/1.73 m2; (4) Decompensated cirrhosis (Child-Pugh class B or C); and (5) Any form of cancer. However, patients who had received curative cancer treatments were not excluded from this study. Therefore, patients with HCC who had undergone surgical resection or ablation therapy were included in this study.
Liver cirrhosis was diagnosed clinically from liver biopsy or imaging studies such as ultrasonography, contrast-enhanced computed tomography, and/or magnetic resonance imaging using morphological signs of cirrhosis from portal hypertension, such as portosystemic shunt or hypersplenism. Sample size for the present study was determined by practicability but was planned to exceed the number of patients analyzed in the Japanese phase III trial[5]. All study protocols were approved by the ethics committees of the participating hospitals. Written informed consent was obtained from all patients included in this study. The present study was registered on the University Hospital Medical Information Network (trial ID: 000023269).
Standard approved doses of 400 mg sofosbuvir (Sovardi, Gilead, TKY, Japan) plus ribavirin (Copegus, Chugai Pharmaceutical, TKY, Japan or Rebetol, MSD, TKY, Japan) adjusted by body weight (1000 mg/d for patients weighing > 80 kg, 800 mg/d for patients weighing ≤ 80 but ≥ 60 kg, and 600 mg/d for patients weighing < 60 kg) were orally administered for 12 wk. If hemoglobin level fell to < 10 g/dL, ribavirin dose was reduced to 200 mg/d, and if hemoglobin level fell to < 8.5 g/dL, ribavirin was discontinued. If the attending physician judged this treatment as difficult to continue due to adverse events, both sofosbuvir and ribavirin were discontinued.
HCV-RNA load was measured using quantitative reverse transcription polymerase chain reaction (COBAS TaqMan PCR assay version 2; Roche Diagnostics, Branchburg, NJ, United States). HCV genotype was determined using the antibody serotyping method (SRL, TKY, Japan). HCV serotypes 1 and 2 correspond to genotypes 1a/1b and 2a/2b, respectively. When HCV serotype could not be determined, genotype was examined using real-time polymerase chain reaction assay (BML, TKY, Japan). HCV-RNA was checked on the day of therapy initiation and every 4 wk during treatment. Biochemical analyses including blood cell counts, C-reactive protein level, blood sugar level, and liver and renal function tests were performed every 2 wk during treatment.
Rapid virological response (RVR) was defined as serum HCV-RNA negativity in week 4 after therapy initiation. End-of-treatment response was defined as serum HCV-RNA negativity in week 12 after therapy initiation. SVR was defined as HCV-RNA negativity at 24 wk after the end of therapy. The primary end point of this study was SVR at 24 wk after the end of therapy. Treatment failure was defined as non-SVR.
Patients were assessed for the safety and tolerability of treatment by attending physicians who monitored adverse events and laboratory parameters such as blood cell counts and liver and renal function tests every 2 wk. Adverse events were assessed according to Common Terminology Criteria for Adverse Events version 4.0. The incidence of and reasons for therapy discontinuation or interruption due to adverse events were analyzed.
Therapeutic efficacy was evaluated using an intention-to-treat analysis. The Mann-Whitney U test or the t-test was used to analyze continuous variables. Fisher’s exact test or the chi-square test was used to analyze categorical variables. Logistic regression analysis for univariate comparisons was performed to investigate factors contributing to SVR. When multiple factors were significant from univariate analyses, multivariate analysis was also performed to identify independent factors. Values of P < 0.05 were considered statistically significant. SPSS for Windows version 24J statistical software (SPSS, TKY, Japan) was used for all data analyses.
A total of 265 patients met the inclusion and exclusion criteria and were enrolled in the present study. Although one patient discontinued treatment due to an adverse event, all enrolled patients completed follow-up for evaluation of safety and effectiveness. Patient characteristics are summarized in Table 1. Median age was 68-years-old (range, 17-86 years), and the cohort was comprised of 150 male and 115 female patients. Patients ≥ 75-years-old, cirrhotic patients, patients with moderate chronic kidney disease (defined as estimated glomerular filtration rate < 60 mL/min/1.73 m2), patients with a history of HCC treatment, and patients with a history of pegylated IFN plus ribavirin therapy accounted for 31%, 34%, 20%, 12%, and 15%, respectively. Median baseline hemoglobin level was 13.6 g/dL (range, 10.2-20.1 g/dL).
| n = 265 | |
| Age in yr | 68 (17-86) |
| ≥ 75 yr | 83 (31%) |
| Sex as male/female | 150/115 (57%/43%) |
| Cirrhosis | 91 (34%) |
| Moderate chronic kidney disease, eGFR < 60 mL/min/1.73 m2 | 53 (20%) |
| History of HCC treatment | 33 (12%) |
| History of pegylated IFN + ribavirin therapy | 39 (15%) |
| Height in cm | 160.2 (134.0-182.0) |
| Weight in kg | 58.0 (32.3-99.3) |
| BMI in kg/m2 | 22.6 (15.5-35.0) |
| Baseline HCV-RNA in logIU/mL | 6.0 (1.4-7.4) |
| WBC as /mm3 | 5000 (1810-13260) |
| Hb in g/dL | 13.6 (10.2-20.1) |
| Platelets as × 104/mm3 | 16.2 (4.6-38.9) |
| AST in IU/L | 43 (12-257) |
| ALT in IU/L | 39 (6-394) |
| γ-GT in IU/L | 32 (5-898) |
| eGFR | 73 (31-156) |
| AFP in ng/mL | 4.3 (1.0-88.3) |
A comparison of pre-treatment factors between patients ≥ 75-years-old and < 75-years-old is shown in Table 2. Significant differences were seen in height, weight, body mass index, cirrhosis, chronic kidney disease, history of HCC treatment, white blood cells, hemoglobin, platelets, alanine aminotransferase, γ-glutamyl transferase, alpha-fetoprotein (AFP) levels, and estimated glomerular filtration rate.
| Factors | Patients ≥ 75-yr-old, n = 83 | Patients < 75-yr-old, n = 182 | P value |
| Age in yr | 79 (75-86) | 64 (17-74) | < 0.001 |
| Sex as male/female | 42/41 (51%/49%) | 108/74 (59%/41%) | 0.183 |
| Height in cm | 156.7 (134.0-170.0) | 162.0 (140.0-182.0) | < 0.001 |
| Weight in kg | 53.5 (32.3-81.4) | 60.0 (37.6-99.3) | < 0.001 |
| BMI in kg/m2 | 22.2 (15.5-29.5) | 23.1 (16.5-35.0) | 0.009 |
| Cirrhosis | 41 (49%) | 50 (28%) | < 0.001 |
| CKD, eGFR < 60 mL/min/1.73 m2 | 28 (34%) | 25 (14%) | < 0.001 |
| History of HCC treatment | 18 (22%) | 15 (8%) | 0.002 |
| History of IFN-based therapy | 21 (25%) | 42 (23%) | 0.693 |
| HCV-RNA as logIU/mL | 6.1 (2.3-7.3) | 5.9 (1.4-7.4) | 0.894 |
| WBC in mm3 | 4540 (1810-13260) | 5200 (2200-11400) | 0.004 |
| Hb in g/dL | 12.9 (10.2-20.1) | 14.1 (10.6-17.6) | < 0.001 |
| Platelets as × 104/mm3 | 14.2 (4.9-32.8) | 17.4 (4.6-38.9) | < 0.001 |
| AST in IU/L | 40 (14-183) | 42 (12-252) | 0.589 |
| ALT in IU/L | 30 (6-139) | 44 (6-394) | < 0.001 |
| γ-GT in IU/L | 25 (7-361) | 37 (5-888) | 0.001 |
| AFP in ng/mL | 3.9 (1-32.9) | 4.8 (1.1-88.3) | 0.014 |
| eGFR | 66.0 (33.0-106.9) | 77.6 (30.9-156.0) | < 0.001 |
SVR rates overall and according to age groups are shown in Figure 1. SVR rates for the overall cohort, patients < 65-years-old, patients ≥ 65-years-old but < 75-years-old, and patients ≥ 75-years-old were 97% (258/265), 98% (93/95), 97% (84/87), and 98% (81/83), respectively. No significant differences were observed among age groups (P = 0.842).
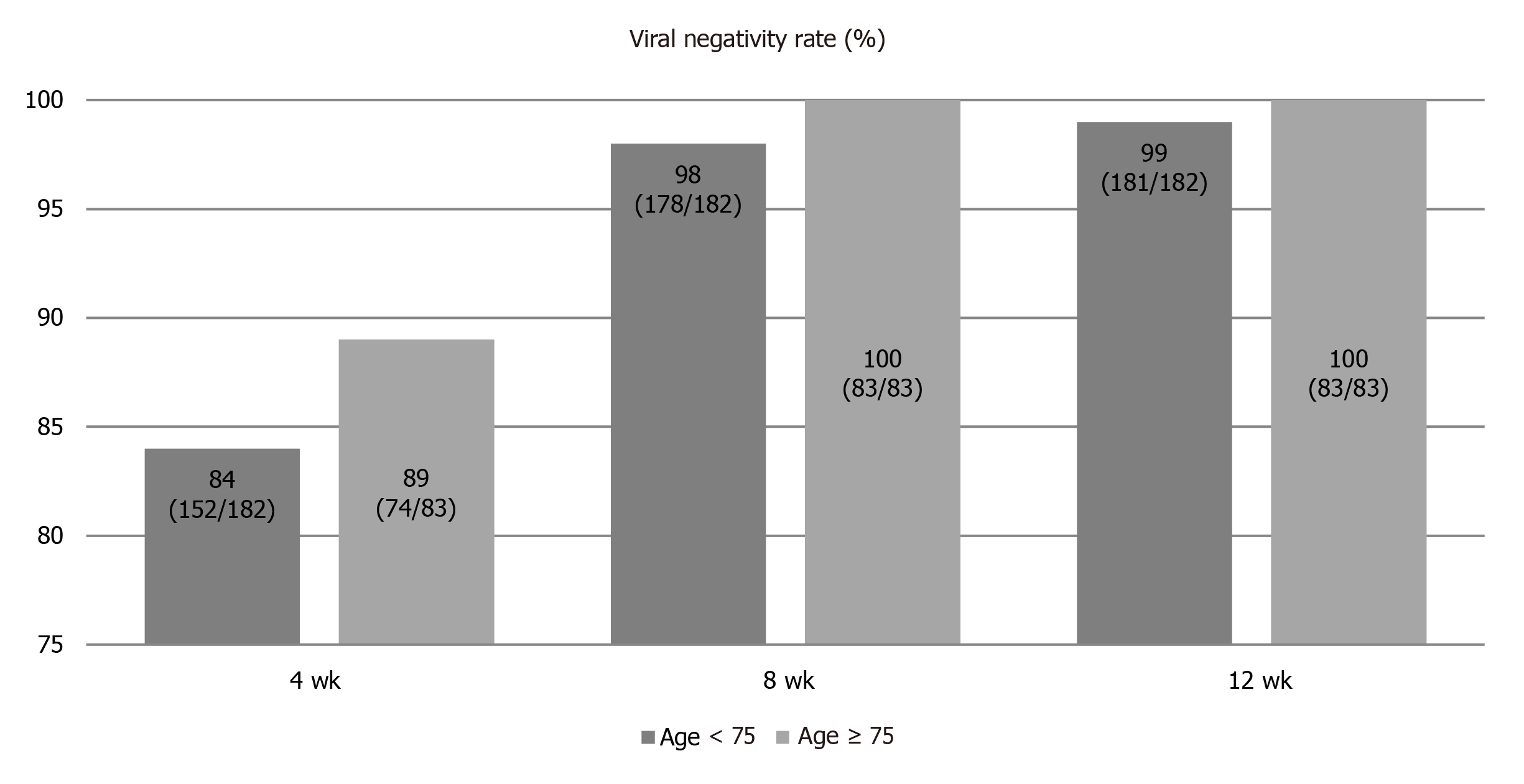
A comparison of the viral negativity rate between patients ≥ 75-years-old and < 75-years-old during treatment is shown in Figure 2. RVR rates for patients ≥ 75-years-old and < 75-years-old groups were 84% and 89%, respectively. Although RVR rate tended to be higher in patients ≥ 75-years-old than in patients < 75-years-old, the difference was not significant (P = 0.266). End-of-treatment response rates of patients ≥ 75-years-old and < 75-years-old were 99% and 100%, respectively. No significant difference was apparent between groups (P = 1.000).
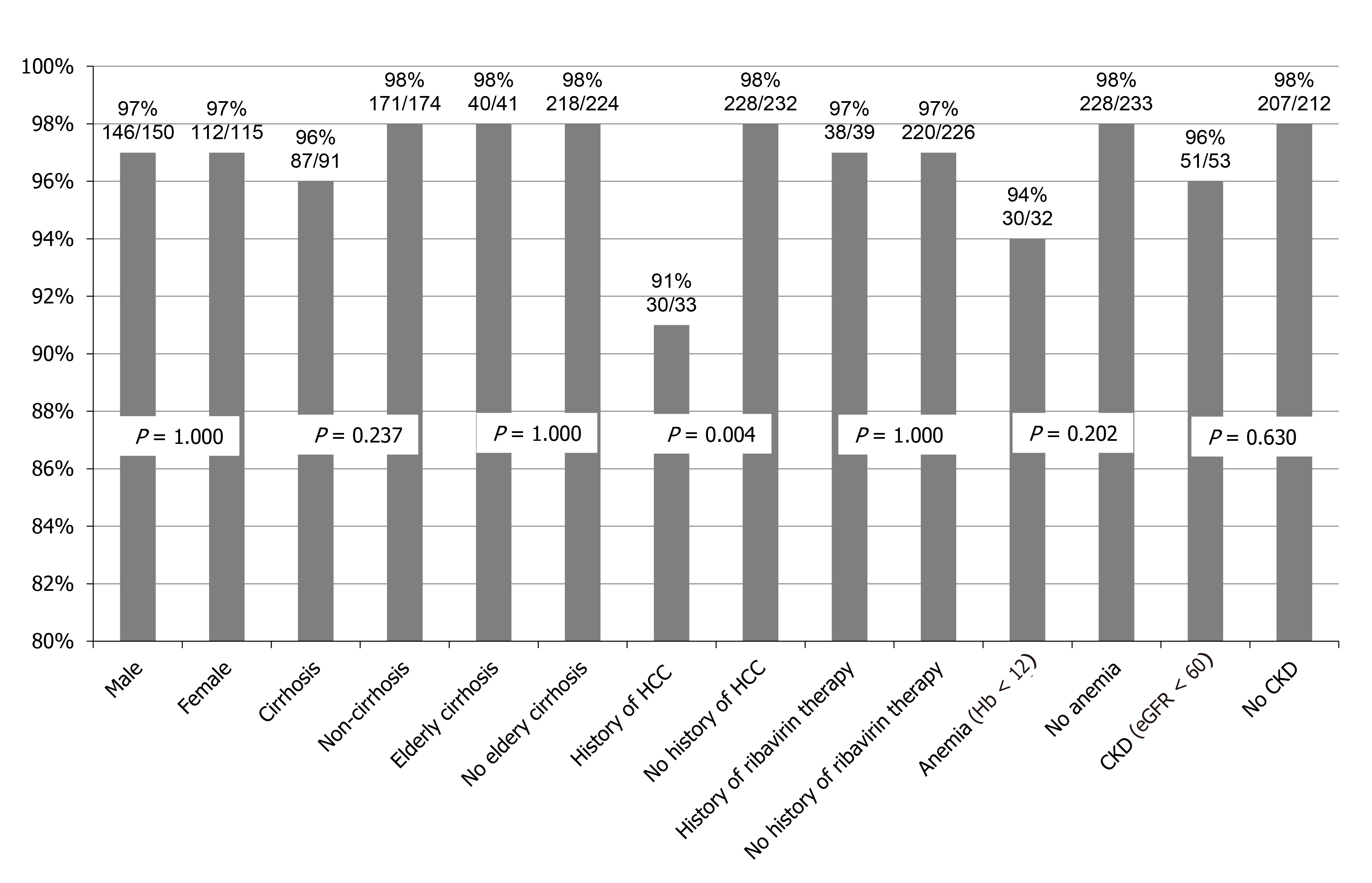
SVR rates according to background factors are summarized in Figure 3. Although no significant difference was seen in comparisons of SVR rates according to sex, cirrhosis, elderly cirrhosis, IFN plus ribavirin therapy, anemia (hemoglobin < 12 g/dL), or chronic kidney disease, a significant difference was seen between patients with and without a history of HCC treatment (P = 0.004). In patients ≥ 75-years-old with a history of IFN plus ribavirin therapy, all patients achieved SVR (100%; 14/14). In patients ≥ 75-years-old with cirrhosis, the SVR rate was 98% (40/41).
Results of logistic regression analysis to investigate factors contributing to SVR are shown in Table 3. On univariate analyses, a history of HCC treatment and AFP were factors significantly contributing to SVR. Multivariate analyses revealed AFP as the only factor independently associated with SVR.
| Univariate | Multivariate | |||||
| Factors | P value | OR | 95%CI | P value | OR | 95%CI |
| Age, per 1-yr increase | 0.782 | 0.991 | 0.931-1.055 | |||
| ≥ 75 yr | 0.874 | 1.144 | 0.217-6.022 | |||
| Sex, female | 0.977 | 1.023 | 0.224-4.663 | |||
| Height, per 1-cm increase | 0.316 | 0.957 | 0.879-1.043 | |||
| Weight, per 1-kg increase | 0.568 | 0.982 | 0.924-1.044 | |||
| BMI, per 1-kg/m2 increase | 0.929 | 0.990 | 0.792-1.238 | |||
| Cirrhosis | 0.214 | 0.382 | 0.084-1.743 | |||
| CKD, eGFR > 60 mL/min/1.73 m2 | 0.569 | 0.616 | 0.116-3.266 | |||
| History of HCC treatment | 0.027 | 0.175 | 0.037-0.822 | 0.072 | 0.225 | 0.044-1.145 |
| History of IFN-based therapy | 0.763 | 0.774 | 0.146-4.091 | |||
| HCV-RNA, per 1-logIU/mL increase | 0.644 | 0.831 | 0.380-1.821 | |||
| WBC in mm3 | 0.703 | 1.009 | 0.963-1.057 | |||
| Hb, per 1-g/dL increase | 0.196 | 1.411 | 0.837-2.380 | |||
| Platelets, per 1 × 104/mm3 increase | 0.790 | 0.984 | 0.873-1.109 | |||
| AST, per 1-IU/L increase | 0.552 | 1.007 | 0.983-1.033 | |||
| ALT, per 1-IU/L increase | 0.608 | 1.005 | 0.987-1.023 | |||
| γ-GT, per 1-IU/L increase | 0.490 | 1.007 | 0.987-1.027 | |||
| AFP, per 1-ng/mL increase | 0.004 | 0.955 | 0.926-0.985 | 0.015 | 0.959 | 0.926-0.992 |
| eGFR, per 1-mL/min/1.73 m2 increase | 0.124 | 1.036 | 0.990-1.083 | |||
Non-SVR was shown in seven patients (3%). Factors between patients with and without SVR are compared in Table 4. When drug adherence was defined as a percentage of the actual administered dose to the planned dose, RVR adherence was not identified as significantly related to non-SVR. History of HCC treatment was the only factor significantly related to non-SVR.
| Factors | SVR, n = 258 | Non-SVR, n = 7 | P value |
| Age in yr, n (range) | 68 (17-86) | 74 (39-79) | 0.682 |
| Sex as male/female | 146/112 | 4/3 | 1.000 |
| Height in cm | 160.1 (134.0-182.0) | 161.5 (155.0-177.0) | 0.350 |
| Body weight in kg | 58.0 (32.3-99.3) | 63.2 (54.0-66.2) | 0.267 |
| BMI in kg/m2 | 22.6 (15.5-38.0) | 22.6 (20.2-25.9) | 0.778 |
| Cirrhosis | 87 | 4 | 0.237 |
| CKD | 51 | 2 | 0.630 |
| History of HCC treatment | 30 | 3 | 0.044 |
| History of IFN-based therapy | 61 | 2 | 0.672 |
| HCV-RNA as logIU/mL | 6.1 (2.7-7.6) | 6.3 (3.3-7.0) | 0.713 |
| White blood cells as /mm3 | 5005 (1810-13260) | 4100 (2260-8700) | 0.581 |
| Hemoglobin in g/dL | 13.6 (10.2-20.1) | 12.5 (11.6-14.7) | 0.793 |
| Platelets as × 104/mm3 | 16.2 (4.6-38.9) | 14.9 (9.9-30.3) | 0.930 |
| AST in IU/L | 42 (12-252) | 37 (19-90) | 0.789 |
| ALT in IU/L | 39 (6-394) | 57 (19-79) | 0.843 |
| γ-GTP in IU/L | 32 (5-888) | 36 (13-461) | 0.942 |
| AFP in ng/mL | 5.2 (1.0-445.0) | 10.6 (1.3-29.7) | 0.521 |
| eGFR in mL/min/1.73 m2 | 73.1 (30-240.2) | 72.2 (50.5-105.8) | 0.166 |
| Ribavirin adherence in % | 100 (28-100) | 100 (100-100) | 0.323 |
The discontinuation rate due to adverse events was 0.4% (1/256). The reason for discontinuation was drug-induced pneumonitis with positive results for sofosbuvir on drug-induced lymphocyte stimulation testing. Adverse event profiles for the overall cohort, patients ≥ 75-years-old, and patients < 75-years-old are shown in Table 5. The most frequent adverse event other than anemia was elevated uric acid level (Grade 1). No severe liver injury or exacerbation of renal dysfunction was seen. A similar safety profile was observed between patients ≥ 75-years-old and < 75-years-old, except for ribavirin dose reduction or interruption due to anemia. Median ribavirin adherence was significantly lower for patients ≥ 75-years-old (96.8%) than for patients < 75-years-old (100%, P = 0.001). Ribavirin dose reduction or interruption was required in 12.1% (32/265) of patients because of anemia, and anemia appeared in 7.7% (14/182) of patients < 75-years-old, and 21.7% (18/83) of patients ≥ 75-years-old. A significant difference in ribavirin dose reduction or interruption rate was also seen between groups (P = 0.002).
| Total,n = 265 | Patients ≥ 75-yr-old, n = 83 | Patients < 75-yr-old, n = 182 | P value | |
| Treatment discontinuation due to adverse events1 | 1 (0.4%) | 1 (1.2%) | 0 | 0.313 |
| Dose reduction or interruption of ribavirin due to anemia2 | 32 (13.2%) | 18 (21.7%) | 14 (7.7%) | 0.002 |
| Dermatitis | 9 (3.4%) | 1 (1.2%) | 8 (4.4%) | 0.281 |
| Depression | 3 (1.1%) | 1 (1.2%) | 2 (0.5%) | 1.000 |
| Headache | 4 (1.5%) | 0 | 4 (2.2%) | 0.313 |
| Infection | 4 (1.5%) | 1 (1.2%) | 3 (1.6%) | 1.000 |
| Other adverse events | 11 (4.2%) | 5 (6.0%) | 6 (3.3%) | 0.328 |
| Elevated bilirubin level | 2 (0.8%) | 1 (1.2%) | 1 (0.5%) | 0.529 |
| Elevated transaminase level | 4 (1.5%) | 2 (2.4%) | 2 (1.1%) | 0.592 |
| Elevated serum ammonia level | 5 (1.9%) | 2 (2.4%) | 3 (1.6%) | 0.650 |
| Elevated uric acid level | 26 (9.8%) | 10 (12.0%) | 16 (8.8%) | 0.504 |
This was a multicenter post-marketing prospective cohort study of sofosbuvir plus ribavirin therapy for patients infected with genotype 2 HCV in a real-world clinical setting. In the present study, 31% of patients were ≥ 75-years-old, and 12% had a history of HCC treatment. Furthermore, 34% of enrolled patients had cirrhosis, and 20% had moderate chronic kidney disease.
Although some real-world data based on post-marketing cohort studies of sofosbuvir plus ribavirin have been reported, few reports have evaluated safety and efficacy for patients ≥ 75-years-old. Regarding safety, Ogawa et al[10] indicated that the frequency of adverse effects was higher for a ≥ 65-year-old group (18.9%) than for the < 65-year-old group (4.3%, P < 0.001). However, discontinuation of all drugs was required for only 3 of the 446 patients (0.7%)[10]. Atsukawa et al[11] indicated that the incidence of anemia increased significantly with age, and ribavirin dose reduction rate increased sharply in patients > 70-years-old[11]. Anemia during treatment occurred in 10.6% (23/218) of patients < 75-years-old, and in 48.1% (25/52) of patients ≥ 75-years-old[11]. However, none of those 270 patients discontinued use of either ribavirin or sofosbuvir[11]. In the present study, although dose reduction or interruption of ribavirin due to anemia was required in 21.7% of patients ≥ 75-years-old and 7.7% of patients < 75-years-old, treatment discontinuation was required for only one patient (0.4%). Therefore, although careful monitoring of anemia and ribavirin dose adjustment is necessary to avoid discontinuation of therapy, this treatment appears tolerable even in patients ≥ 75-years-old.
Regarding efficacy, Nishida et al[12] reported that although the difference was not significant, patients ≥ 75-years-old tended to show a lower SVR rate than patients < 75-years-old (81.3%, 13/16 for patients ≥ 75-years-old; 96.0%, 24/25 for patients < 75-years-old)[12]. Atsukawa et al[11] showed SVR rates of 98.1% (51/51) for patients ≥ 75-years-old and 96.8% (211/218) for patients < 75-years-old (P = 0.999)[11]. Ogawa et al[10] reported that although SVR12 was achieved by 95% (69/73) of patients > 75-years-old, the SVR12 rate was significantly lower in cirrhotic patients > 75-years-old with a history of IFN treatment (73.3%, 11/15) than in noncirrhotic patients > 75-years-old (100%, 17/17; P < 0.01)[10]. In the present study, the SVR rate of patients ≥ 75-years-old was 98% (81/83). Among patients ≥ 75-years-old with a history of IFN plus ribavirin therapy, the SVR rate was 100% (14/14). Furthermore, the SVR rate of cirrhotic patients ≥ 75-years-old was also extremely high (98%, 40/41). From these results, a high SVR rate (> 95%) would be expected even in patients ≥ 75-years-old, irrespective of cirrhosis or history of IFN treatment.
Discontinuation of pharmacotherapy[13], history of HCC[10,13], cirrhosis (advanced fibrosis)[10,14,15], renal dysfunction[16], history of IFN-based treatment[10,15], lower serum albumin, and ribavirin dose at baseline[14] have all been reported as factors associated with non-SVR of sofosbuvir plus ribavirin. Hirosawa et al[13] indicated that the risk factor most strongly associated with non-SVR was a history of HCC treatment (odds ratio: 9.29)[13]. In the present study, a history of HCC treatment and AFP were factors significantly associated with SVR on univariate analysis, and AFP was the only independent factor on multivariate analyses. High serum AFP levels in patients without HCC have been associated with advanced liver fibrosis and a risk of HCC occurrence[17,18]. Sofosbuvir plus ribavirin might thus be less effective in cases showing advanced liver fibrosis. Patients with a history of HCC treatment or high AFP level should probably be treated using some other ribavirin-free DAA therapy.
In recent HCV treatment guidelines from Western countries, sofosbuvir plus ribavirin therapy is no longer recommended because of the adverse effects of ribavirin and the relatively lower SVR rate compared to other DAA therapies[14,19,20]. In fact, the real-life SVR rate from nationwide German data was lower compared to SVR rates of clinical trials (83% in intention-to-treat analysis)[21]. Recently, ribavirin-free DAA therapies such as glecaprevir plus pibrentasvir, and sofosbuvir plus ledipasvir have also been approved for use in patients with HCV genotype 2 in Japan[22,23]. These therapies have shown no adverse effects due to ribavirin and have thus become first-line treatments. These therapies also represent rescue treatments for patients with sofosbuvir plus ribavirin failure. However, in consensus statements and recommendations on the treatment of hepatitis C from the Asian-Pacific Association for the Study of the Liver, sofosbuvir plus weight-based ribavirin for 12 wk is recommended as a first-line treatment, and ledipasvir and sofosbuvir for 12 wk is recommended for treatment-naïve HCV genotype 2 patients who cannot tolerate ribavirin[24]. In addition, SVR rates from Asian real-world data were similar to those of the phase III trial[25]. Sofosbuvir plus ribavirin therapy offers advantages in terms of both cost and real-world evidence.
Some limitations need to be considered for the present study. First, some selection biases would be present. Second, the number of patients may not have been sufficient to reach definitive conclusions regarding safety and effectiveness in patients ≥ 75-years-old. Third, reasons for failure of this therapy could not be clarified by our analysis because the number of patients who did not achieve SVR was too small. Furthermore, sofosbuvir-specific resistance-associated substitutions (RASs) were not tested for in this study. The prevalence of the naturally occurring RAS·S282T, as the only known variant conferring sofosbuvir resistance in vitro, is reportedly rare in genotype 2 (0.22%)[26]. However, RAS·A150V has recently been found to be associated with reduced response to treatment with sofosbuvir and ribavirin and has appeared in genotype 2a (13.8%) and genotype 2b (1.03%)[26]. In addition, the naturally occurring nucleoside inhibitor-specific RASs (E237G, M289I/L, L320 F, and V321A/I) are also found in genotype 2[26]. The influence of these preexisting RASs on SVR should be analyzed using a larger number of cases with treatment failure.
Sofosbuvir and ribavirin represent an acceptable and effective treatment even for patients ≥ 75-years-old in a real-world setting. An extremely high SVR rate can be achieved when adequate management for adverse effects is performed to avoid discontinuation of treatment, irrespective of age.
In real-world settings, elderly patients infected with hepatitis C virus (HCV) represent a substantial and growing population and carry a high risk of advanced liver diseases such as cirrhosis and hepatocellular carcinoma. Therefore, these patients should be treated as soon as possible. Clinical trials of interferon-free direct-acting antiviral agent regimens using sofosbuvir and ribavirin for patients with genotype 2 HCV have been reported since 2013. However, patients ≥ 75-years-old were not included in the sofosbuvir plus ribavirin regimen of those clinical trials. The safety and effectiveness of sofosbuvir plus ribavirin for elderly patients ≥ 75-years-old has thus remained unclear.
In recent HCV treatment guidelines from Western countries, sofosbuvir plus ribavirin therapy is no longer recommended because of the adverse effects of ribavirin and the relatively lower sustained viral response (SVR) rate compared to other direct-acting antiviral agent therapies. However, in consensus statements and recommendations on the treatment of hepatitis C from the Asian-Pacific Association for the Study of the Liver, sofosbuvir plus weight-based ribavirin for 12 wk is recommended as a first-line treatment, and SVR rates from Asian real-world data were similar to those of the phase III trial. Sofosbuvir plus ribavirin therapy also offers advantages in terms of both cost and real-world evidence. The real-world safety and efficacy of sofosbuvir plus ribavirin for elderly patients ≥ 75-years-old can provide useful information regarding treatment strategy for elderly patients with HCV in the Asia-Pacific region.
The aim of the present study is to evaluate the real-world safety and efficacy of sofosbuvir plus ribavirin for elderly patients ≥ 75-years-old compared to non-elderly patients
This is a multicenter post-marketing prospective cohort study of sofosbuvir plus ribavirin therapy for patients infected with genotype 2 HCV in a real-world clinical setting. We treated 265 patients with genotype 2 HCV using standard approved doses of sofosbuvir (400 mg/d) plus ribavirin adjusted by body weight, administered orally for 12 wk. In the present study, 31% of patients were ≥ 75-years-old, and 12% had a history of hepatocellular carcinoma (HCC) treatment. Furthermore, 34% of enrolled patients had cirrhosis, and 20% had moderate chronic kidney disease. The primary end point of the present study was SVR at 24 wk after the end of therapy.
Regarding efficacy, SVR rates for the overall cohort, patients < 65-years-old, ≥ 65-years-old but < 75-years-old, and ≥ 75-years-old were 97% (258/265), 98% (93/95), 97% (84/87), and 98% (81/83), respectively (P = 0.842). Among patients ≥ 75-years-old with a history of interferon plus ribavirin therapy, the SVR rate was 100% (14/14). Furthermore, the SVR rate of cirrhotic patients ≥ 75-years-old was also extremely high (98%, 40/41). From these results, a high SVR rate (> 95%) would be expected even in patients ≥ 75-years-old, irrespective of cirrhosis or history of interferon treatment. Logistic regression analyses identified history of HCC treatment and alpha-fetoprotein as factors significantly associated with SVR. SVR rate was significantly lower for patients with HCC treatment (91%) than for patients without history of HCC treatment (98%, P = 0.004). Regarding safety, although dose reduction or interruption of ribavirin due to anemia was required in 21.7% of patients ≥ 75-years-old and 7.7% of patients < 75-years-old, treatment discontinuation was required for only one patient (0.4%). Therefore, this treatment appears tolerable even in patients ≥ 75-years-old.
Sofosbuvir and ribavirin represent an acceptable and effective treatment even for patients ≥ 75-years-old in a real-world setting. An extremely high SVR rate can be achieved when adequate management for adverse effects is performed to avoid discontinuation of treatment, irrespective of age.
Sofosbuvir plus ribavirin might be less effective in patients with a history of HCC treatment or high alpha-fetoprotein level, irrespective of age. These patients should probably be treated using some other ribavirin-free direct-acting antiviral agent therapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FEM, Hussien A, Tuna N S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 2. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, Schiff E, Al-Assi MT, Subramanian GM, An D, Lin M, McNally J, Brainard D, Symonds WT, McHutchison JG, Patel K, Feld J, Pianko S, Nelson DR; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 3. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT, Subramanian GM, McHutchison JG, Weiland O, Reesink HW, Ferenci P, Hézode C, Esteban R; VALENCE Investigators. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 638] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, Nelson D, Nader F, Hunt S. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C). J Hepatol. 2014;60:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T, Umemura T, Takehara T, Sakamoto N, Nishigaki Y, Nakane K, Toda N, Ide T, Yanase M, Hino K, Gao B, Garrison KL, Dvory-Sobol H, Ishizaki A, Omote M, Brainard D, Knox S, Symonds WT, McHutchison JG, Yatsuhashi H, Mizokami M. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Dusheiko G, Main J, Thomas H, Reichard O, Lee C, Dhillon A, Rassam S, Fryden A, Reesink H, Bassendine M, Norkrans G, Cuypers T, Lelie N, Telfer P, Watson J, Weegink C, Sillikens P, Weiland O. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996;25:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 255] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Bodenheimer HC, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology. 1997;26:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 263] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Rose L, Bias TE, Mathias CB, Trooskin SB, Fong JJ. Sofosbuvir: A Nucleotide NS5B Inhibitor for the Treatment of Chronic Hepatitis C Infection. Ann Pharmacother. 2014;48:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Tamai H, Shingaki N, Mori Y, Moribata K, Kawashima A, Maeda Y, Niwa T, Deguchi H, Inoue I, Maekita T, Iguchi M, Kato J, Ichinose M. Low-Dose Pegylated Interferon α-2b Plus Ribavirin for Elderly and/or Cirrhotic Patients with Genotype 2 Hepatitis C Virus. Gut Liver. 2016;10:617-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ogawa E, Furusyo N, Nomura H, Takahashi K, Higashi N, Kawano A, Dohmen K, Satoh T, Azuma K, Nakamuta M, Koyanagi T, Kato M, Shimoda S, Kajiwara E, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Effectiveness and safety of sofosbuvir plus ribavirin for HCV genotype 2 patients 65 and over with or without cirrhosis. Antiviral Res. 2016;136:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Atsukawa M, Tsubota A, Kondo C, Shimada N, Abe H, Kato K, Okubo T, Arai T, Itokawa N, Iio E, Tanaka Y, Iwakiri K. Effectiveness and safety of community-based treatment with sofosbuvir plus ribavirin for elderly patients with genotype 2 chronic hepatitis C. Dig Liver Dis. 2017;49:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Nishida N, Kono M, Minami T, Chishina H, Arizumi T, Takita M, Yada N, Ida H, Hagiwara S, Minami Y, Ueshima K, Sakurai T, Kudo M. Safety, Tolerability, and Efficacy of Sofosbuvir Plus Ribavirin in Elderly Patients Infected with Hepatitis C Virus Genotype 2. Dig Dis. 2016;34:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Hirosawa T, Morimoto N, Miura K, Tahara T, Murohisa T, Okamura Y, Sato T, Numao N, Imai M, Tano S, Murayama K, Kurata H, Ozawa I, Fukaya Y, Yoshizumi H, Watanabe S, Tsukui M, Takaoka Y, Nomoto H, Isoda N, Yamamoto H. No Regional Disparities in Sofosbuvir Plus Ribavirin Therapy for HCV Genotype 2 Infection in Tochigi Prefecture and Its Vicinity. Intern Med. 2019;58:477-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Welzel TM, Nelson DR, Morelli G, Di Bisceglie A, Reddy RK, Kuo A, Lim JK, Darling J, Pockros P, Galati JS, Frazier LM, Alqahtani S, Sulkowski MS, Vainorius M, Akushevich L, Fried MW, Zeuzem S; HCV-TARGET Study Group. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66:1844-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Akahane T, Kurosaki M, Itakura J, Tsuji K, Joko K, Kimura H, Nasu A, Ogawa C, Kojima Y, Hasebe C, Wada S, Uchida Y, Sohda T, Suzuki H, Yoshida H, Kusakabe A, Tamada T, Kobashi H, Mitsuda A, Kondo M, Shigeno M, Ide Y, Morita A, Kitamura T, Abe T, Izumi N. Real-world efficacy and safety of sofosbuvir + ribavirin for hepatitis C genotype 2: A nationwide multicenter study by the Japanese Red Cross Liver Study Group. Hepatol Res. 2019;49:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Sho T, Suda G, Nagasaka A, Yamamoto Y, Furuya K, Kumagai K, Uebayashi M, Terashita K, Kobayashi T, Tsunematsu I, Onodera M, Meguro T, Kimura M, Ito J, Umemura M, Izumi T, Kawagishi N, Ohara M, Ono Y, Nakai M, Natsuizaka M, Morikawa K, Ogawa K, Sakamoto N; NORTE Study Group. Safety and efficacy of sofosbuvir and ribavirin for genotype 2 hepatitis C Japanese patients with renal dysfunction. Hepatol Res. 2018;48:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Hu KQ, Kyulo NL, Lim N, Elhazin B, Hillebrand DJ, Bock T. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol. 2004;99:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Tada T, Tanaka J, Yoshizawa H. Predictive value of tumor markers for hepatocarcinogenesis in patients with hepatitis C virus. J Gastroenterol. 2011;46:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 991] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 20. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1210] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 21. | Tacke F, Günther R, Buggisch P, Klinker H, Schober A, John C, Lutz T, Pfeiffer-Vornkahl H, Niederau C, Cornberg M, Sarrazin C, Mauss S. Treatment of HCV genotype 2 with sofosbuvir and ribavirin results in lower sustained virological response rates in real life than expected from clinical trials. Liver Int. 2017;37:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Toyoda H, Chayama K, Suzuki F, Sato K, Atarashi T, Watanabe T, Atsukawa M, Naganuma A, Notsumata K, Osaki Y, Nakamuta M, Takaguchi K, Saito S, Kato K, Pugatch D, Burroughs M, Redman R, Alves K, Pilot-Matias TJ, Oberoi RK, Fu B, Kumada H. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection. Hepatology. 2018;67:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Asahina Y, Itoh Y, Ueno Y, Matsuzaki Y, Takikawa Y, Yatsuhashi H, Genda T, Ikeda F, Matsuda T, Dvory-Sobol H, Jiang D, Massetto B, Osinusi AO, Brainard DM, McHutchison JG, Kawada N, Enomoto N. Ledipasvir-sofosbuvir for treating Japanese patients with chronic hepatitis C virus genotype 2 infection. Liver Int. 2018;38:1552-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, Lesmana CR, Sollano J, Kumar M, Jindal A, Sharma BC, Hamid SS, Dokmeci AK, Mamun-Al-Mahtab, McCaughan GW, Wasim J, Crawford DH, Kao JH, Yokosuka O, Lau GK, Sarin SK. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 25. | Wei B, Ji F, Yeo YH, Ogawa E, Zou B, Stave CD, Dang S, Li Z, Furusyo N, Cheung RC, Nguyen MH. Real-world effectiveness of sofosbuvir plus ribavirin for chronic hepatitis C genotype 2 in Asia: a systematic review and meta-analysis. BMJ Open Gastroenterol. 2018;5:e000207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Wu R, Geng D, Chi X, Wang X, Gao X, Xu H, Shi Y, Guan Y, Wang Y, Jin J, Ding Y, Niu J. Computational analysis of naturally occurring resistance-associated substitutions in genes NS3, NS5A, and NS5B among 86 subtypes of hepatitis C virus worldwide. Infect Drug Resist. 2019;12:2987-3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |