Published online Jul 27, 2020. doi: 10.4254/wjh.v12.i7.406
Peer-review started: March 9, 2020
First decision: April 3, 2020
Revised: May 26, 2020
Accepted: May 28, 2020
Article in press: May 28, 2020
Published online: July 27, 2020
Processing time: 135 Days and 6 Hours
Since the first living donor liver transplantation (LDLT) was performed by Raia and colleagues in December 1988, LDLT has become the gold standard treatment in countries where cadaveric organ donation is not sufficient. Adequate hepatic venous outflow reconstruction in LDLT is essential to prevent graft congestion and its complications including graft loss. However, this can be complex and technically demanding especially in the presence of complex variations and congenital anomalies in the graft hepatic veins.
Herein, we aimed to present two cases who underwent successful right lobe LDLT using a right lobe liver graft with rudimentary or congenital absence of the right hepatic vein and describe the utility of a common large opening drainage model in such complex cases.
Thanks to this venous reconstruction model, none of the patients developed postoperative complications related to venous drainage. Our experience with venous drainage reconstruction models shows that congenital variations in the hepatic venous structure of living liver donors are not absolute contraindications for LDLT.
Core tip: In this study, we aimed to present two cases who underwent successful right lobe living donor liver transplantation using a right lobe liver graft with rudimentary or congenital absence of the right hepatic vein and describe the utility of a common large opening drainage model in such complex cases. Thanks to this venous reconstruction model, none of the patients developed postoperative complications related to venous drainage.
- Citation: Demyati K, Akbulut S, Cicek E, Dirican A, Koc C, Yilmaz S. Is right lobe liver graft without main right hepatic vein suitable for living donor liver transplantation? World J Hepatol 2020; 12(7): 406-412
- URL: https://www.wjgnet.com/1948-5182/full/v12/i7/406.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i7.406
Since the first successful liver transplantation (LT) performed in 1967, LT has become the gold standard treatment for many liver diseases in adult and pediatric patients[1]. In socioculturally developed western countries, most of the liver graft requirements are provided from the cadaveric organ pool, while in Asian and Middle Eastern countries, a significant portion of the organ requirements are provided from the living donor pool[1,2]. In deceased donor liver transplantation, whole size liver graft is harvested with the inferior vena cava (IVC) and then venous anastomosis can be performed easily between the IVC of the liver graft and IVC of the recipient using conventional, piggyback, or modified piggyback techniques[1,2]. In contrast, variations in the vascular structure of the liver graft obtained from a living liver donor (LLD) cause difficulties during vascular reconstruction in LDLT, especially hepatic venous reconstruction. Venous drainage of the right lobe (RL) is more complex compared to the left lobe of the liver. To both benefit from liver graft optimally and avoid congestion-related complications, all large venous structures including inferior right hepatic vein (IRHV), segment 5 vein (V5) and segment 8 vein (V8) should be integrated into the venous drainage system[3]. In other words, meticulous assessment of the vascular structures of the LLD candidates by preoperative radiological instruments and thus identification of variations is critical for both LLDs safety and planning of graft implantation techniques.
To evaluate the hepatic vascular structures of LLD candidates, Doppler ultrasonography (US), multidetector computed tomography (MDCT) and, if necessary, conventional hepatic angiography are the most commonly used techniques[2]. Variations detected in the liver vascular anatomy of the potential LLD candidates either result in rejecting the candidate or the surgical team considers alternative surgical techniques such as various venous drainage models.
Congenital absence of the right hepatic vein (RHV) is one of the rarest hepatic vascular anomalies. This anomaly is usually associated with multiple large IRHVs or wider middle hepatic vein (MHV) tributaries. To our knowledge, no clinical studies or case reports related to this RHV anomaly have been published in the English literature except autopsy studies. To our knowledge, a successful LDLT using the RL liver graft without the RHV was performed by our clinic for the first time in the world[2]. After that, Ray and colleagues reported that they performed successful LDLT using a RL liver graft without a RHV orifice. Herein, we present hepatic venous drainage reconstruction models of RL liver grafts obtained from two LLDs with congenital RHV anomalies.
Case 1: A 25-year-old healthy male (BMI: 20.2 kg/m2, total liver volume: 1136 cc, RL: 786 cc, remnant liver: 34%) was admitted to our liver transplant institute to give a part of his liver to his 26-year-old sister with Budd Chiari Syndrome. He had no chronic disease.
Case 2: A 31-year-old healthy male (BMI: 23.7 kg/m2, total liver volume: 1428 cc, RL: 1000 cc, remnant liver: 30%) was admitted to our liver transplant institute to give a part of his liver to his 56-year-old uncle with alcoholic liver cirrhosis.
Case 1 and Case 2: Physical examination revealed that vital signs were within normal limits. The LLD candidates were examined according to the donor evaluation algorithm applied in our liver transplant institute.
Case 1: Biochemical blood tests and viral markers were within normal limits. Contrast-enhanced MDCT showed that the RHV was rudimentary and that the RL was drained by three IRHVs, one of them was located in the hepatocaval ligament. As our institute is experienced in RL drainage models, cadaveric organ donation was insufficient, and the recipient could not provide another potential donor candidate; thus, we decided to accept the LLD candidate.
Case 2: The potential LLD was examined according to the donor evaluation algorithm applied in our institute. Contrast-enhanced MDCT showed congenital absence of the RHV and that the RL was drained by two large IRHVs.
The healthy individual who had a rudimentary RHV was accepted as a suitable LLD candidate.
The healthy individual who had no RHV was accepted as a suitable LLD candidate.
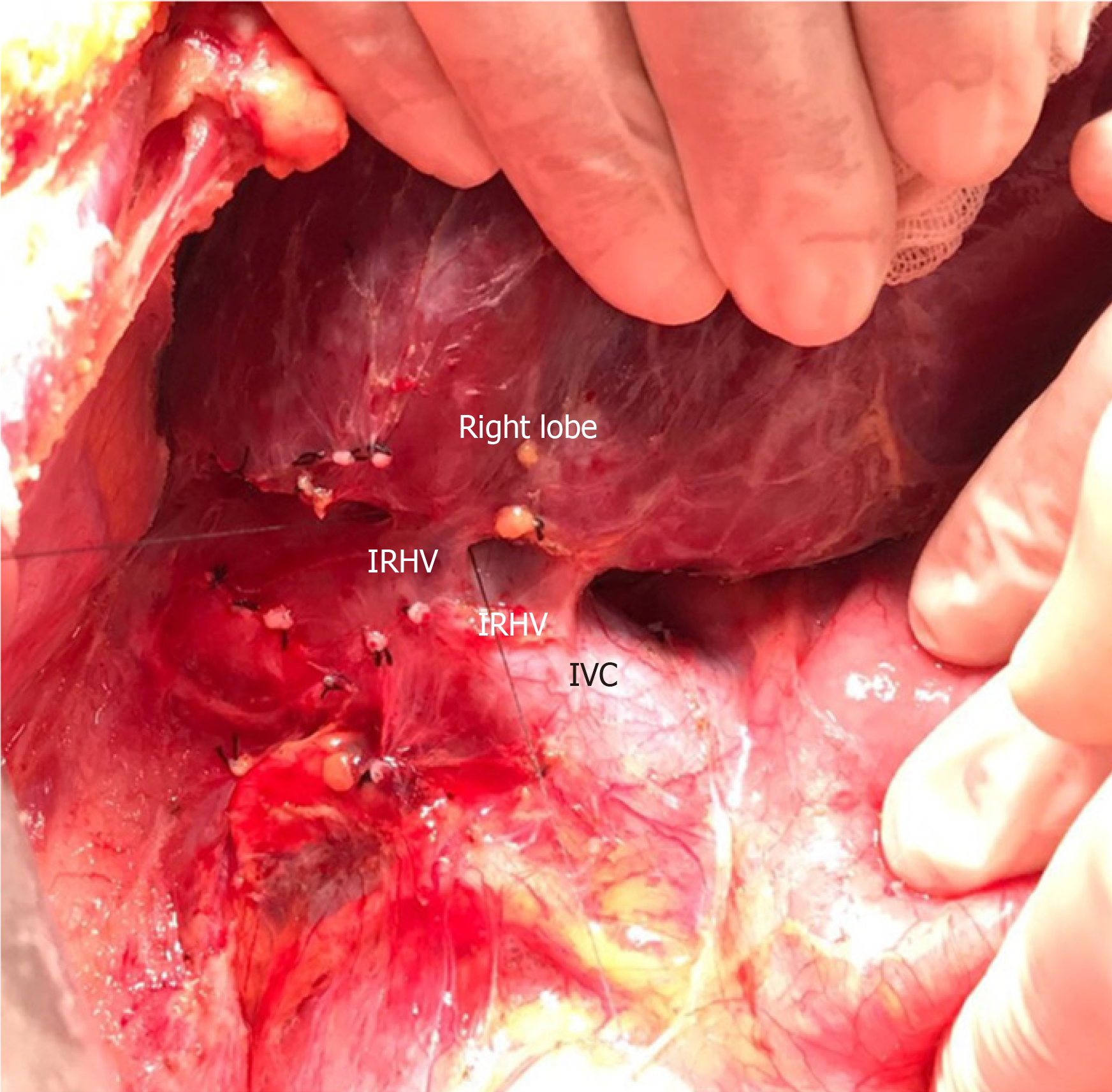
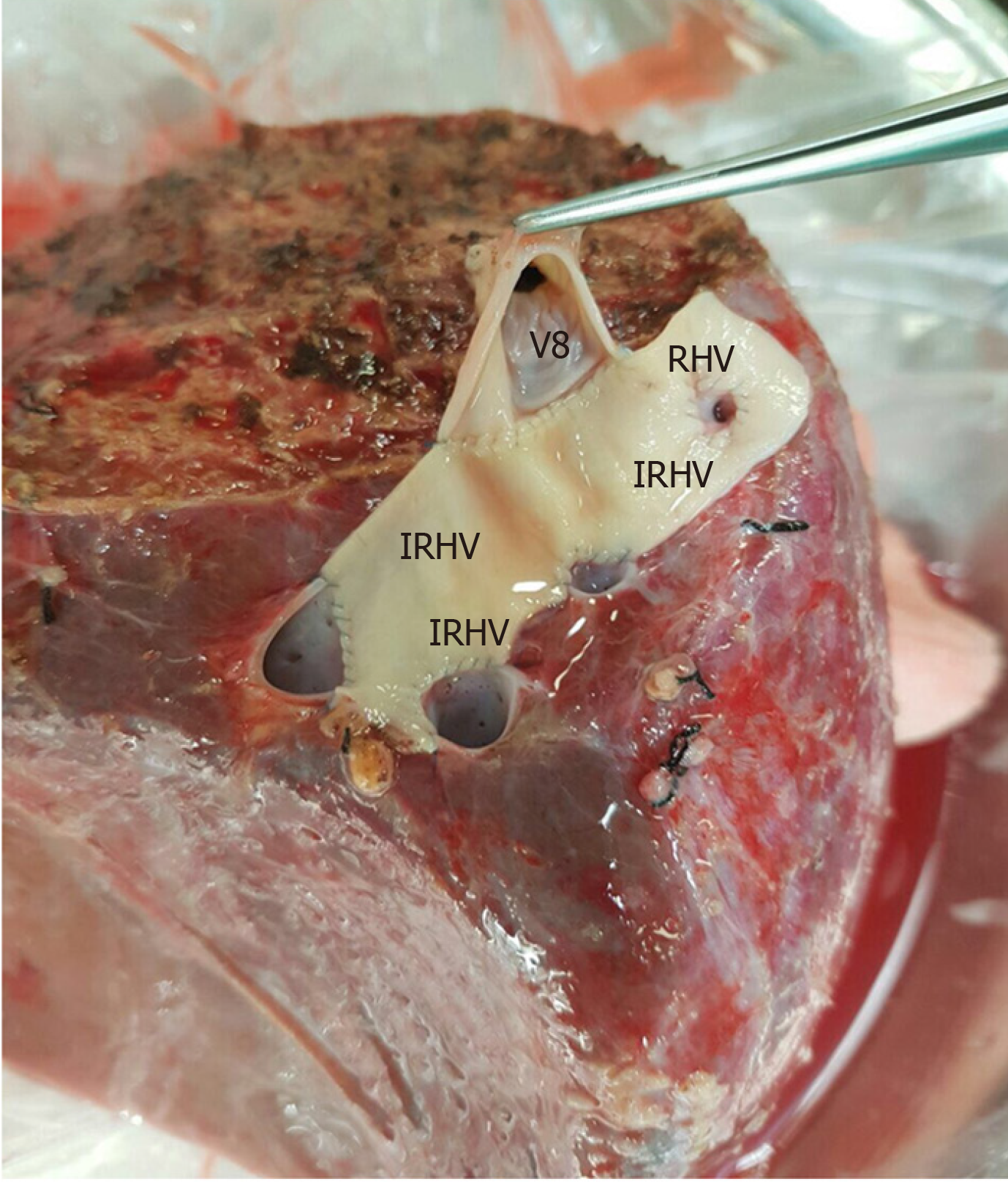
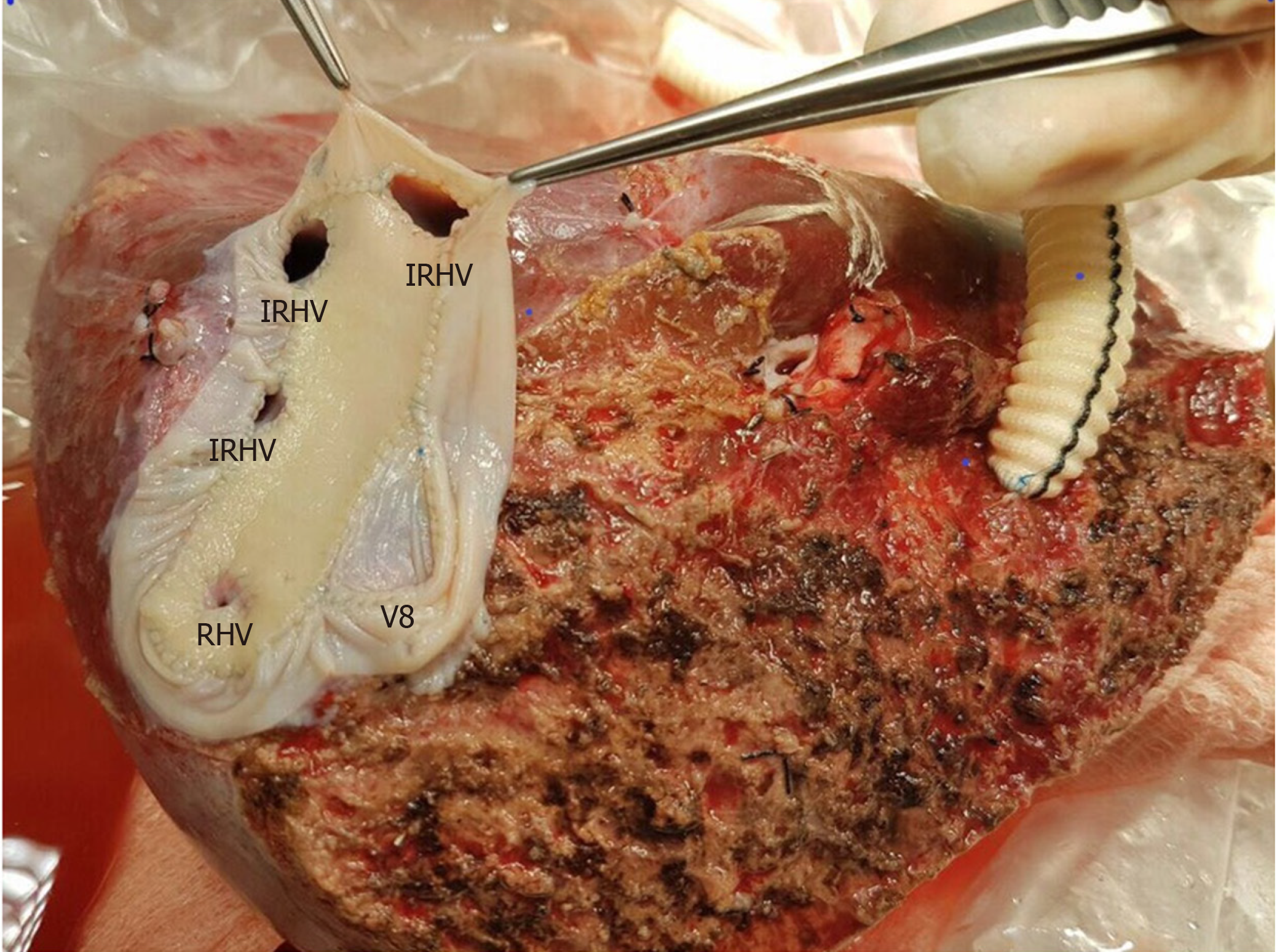
RL hepatectomy was performed as previously described in our institute. Three IRHVs of 5-6 mm diameter, which drained the RL into the IVC, were preserved until the parenchymal transection was completed. Parenchymal transection was performed using the CUSA (Cavtron Ultrasonic Surgical Aspirator, Integra, United States) without Pringles maneuver. During transection, two V5 and one V8 were marked and preserved to be integrated into the venous drainage model. Bloodless RL graft volume and graft-recipient weight ratio were measured as 765 g and 1.03%, respectively.
RL hepatectomy was performed as previously described in our institute, all three IRHVs were preserved until the parenchymal transection was completed and transection was performed using the CUSA without Pringles maneuver (Figure 1). During transection, two V5 and two V8 were marked and preserved to be integrated into the venous drainage model. Bloodless RL graft volume and graft-recipient weight ratio were measured as 1000 g and 1.02%, respectively. The drainage model was found to be successful by postoperative MDCT. Finally, the recipient was discharged without postoperative complications.
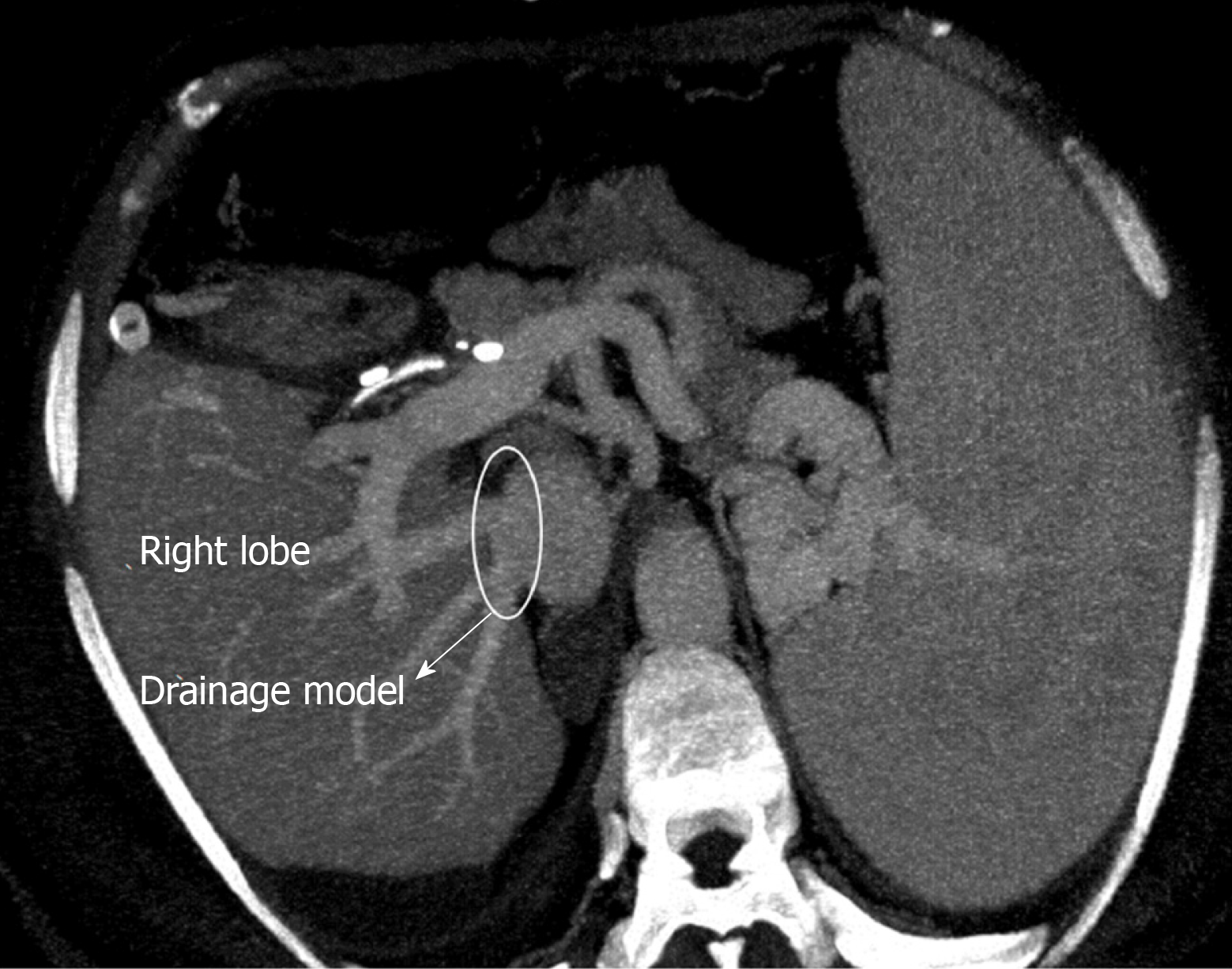
The LLD had an uneventful postoperative clinical course. The drainage model was found to be successful by postoperative MDCT. Finally, the recipient was discharged on postoperative day 21 without complications.
The LLD had an uneventful postoperative clinical course. The drainage model was found to be successful by postoperative MDCT. Finally, the recipient was discharged with minimal biliary complications.
The perfusion and washing of the liver grafts with preservation solutions on the back-table stage were performed as described previously[2]. Using the cryopreserved vascular graft materials, a common large opening drainage model was created to include three IRHVs and V8. The rudimentary RHV was also integrated into the drainage model. For this common large opening drainage model, an aortic vascular graft was used as a quilt, while a saphenous vein graft was used to both create a circumferential fence and extend V8 to the main drainage model. Both V5 orifices on the cut surface were first created as a single orifice and then anastomosed directly to the recipient's left hepatic vein stump using an expanded polytetrafluoroethylene vascular graft (Figures 2 and 3). The liver implantation techniques used in both cases were not different from other LT recipients with normal RHV. Postoperative US and MDCT were performed to determine whether the venous drainage model was successful in both recipients (Figure 4).
LDLT has been expanded to overcome the graft shortage and disparity between supply and demand in patients on the LT waiting list. However, unlike a whole size deceased donor liver graft, most of the living liver grafts require reconstruction of the venous structures including RHV, IRHVs and MHV tributaries to restore venous drainage of the corresponding segments to prevent any postoperative congestion. Hepatic venous structures may be delineated using modern imaging techniques: Doppler US, MDCT, and conventional angiography are particularly useful for observing the venous structures. Variations or congenital anomalies in hepatic venous structure in LLD candidates can disqualify the candidate or alter surgical choice. One such hepatic venous anomaly is congenital absence of the RHV or a rudimentary RHV. As vascular anomalies and variations in LLD candidates may cause unexpected complications and difficulties, these vascular anomalies and variations must be evaluated and documented clearly by imaging techniques before surgery. In our cases, the rudimentary or congenitally absent RHV and presence of the IRHVs were identified easily on preoperative MDCT, which allowed us to plan the surgery.
Difficulties in hepatic venous drainage in LDLT has been addressed by many studies with many technical considerations and modifications investigated[1-12]. While controversy exists regarding the ideal criteria and method of incorporating the IRHVs into the graft’s drainage system and the ideal method of draining segments 5 and 8, it is agreed that venous congestion due to inadequate outflow reconstruction impairs regeneration, and is associated with increased complications including graft loss[8,12].
In our patients with absent or rudimentary main RHV and the presence of multiple major IRHVs, a common large opening drainage model allowed for a wider ostium, which achieved faster and easier anastomosis with the IVC reducing the warm ischemia time with an ostium tolerating compression with less risk of compression and obstruction. We reported a similar case from the same center in 2013, but did not find similar reported cases in the English literature[2]. One case was reported with a liver transplant in the absence of a RHV ostium with the right hepatic vein present and drained into the IVC through a single ostial opening by the middle and left hepatic veins, in that case a subtotal MHV was to be taken leaving behind the proximal MHV with drainage of the segment 4b and RHV vein into it, as the patient’s RHV joined the MHV intra-hepatically[13].
The venous outflow reconstruction is technically challenging for RL liver grafts with an undrained anterior sector, along with the presence of multiple IRHVs with vulnerability of congestion if not adequately reconstructed. Adding to the complexity is the presence of a wide variability in the pattern of branching of hepatic veins, difficulty in determining the optimal anastomotic site and direction especially in the presence of major IRHVs to anastomose which requires further time[14,15]. Authors recommend that short hepatic veins with a diameter ≥ 4 mm should be integrated into the drainage system, which is our approach[2,3,15]. The need for IRHVs to be integrated into the drainage system is even more essential in the absence of adequate drainage through the RHV due to its absence or in cases where it is rudimentary, where in these cases the major IRHVs dominate the venous outflow.
A common large opening reconstruction technique diminishes morbidity as well as potential mortality associated with compromised graft outflow and has been proved to be safe[1,4-6]. A single, wide orifice is achieved by various venoplasty techniques during back-table procedures using cryopreserved conduits, or the recipient's saphenous vein, or synthetic vascular grafts[3,5-7]. The technique used to perform a back-table venoplasty to form a single, large orifice remains an easy procedure without added risks[5]. Also, in the presence of dense adhesions due to previous surgeries, reduced available length of IVC, and multiple collaterals, the outflow reconstruction becomes technically less complex with this technique in addition to reducing the warm ischemia time with one single anastomosis to the IVC.
With regard to the MHV tributaries, which drain the central region of the liver, our approach is to leave the MHV in the donor’s side in cases without a segment 4b vein. In cases with a segment 4b vein, the decision to include the MHV in the graft is made with respect to the remnant liver volume. If the remnant liver volume is ≤ 30%, the MHV should be left in the donor’s side. If the remnant liver volume is > 30%, the decision is made with respect to the diameters of veins draining segment 5 and 8[2,6].
In conclusion, rudimentary or congenital absence of RHV is not an absolute contraindication for RL-LDLT in centers with experience in venous outflow reconstruction and various drainage models. However, it is important to meticulously examine the vascular structures of donor candidates using preoperative radiological instruments.
Manuscript Source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Akamatsu N, Yi PS, Zheng H S-Editor: Zhang L L-Editor: Webster JR E-Editor: Ma YJ
| 1. | Ara C, Akbulut S, Ince V, Aydin C, Gonultas F, Kayaalp C, Unal B, Yilmaz S. Circumferential Fence With the Use of Polyethylene Terephthalate (Dacron) Vascular Graft for All-in-One Hepatic Venous Reconstruction in Right-Lobe Living-Donor Liver Transplantation. Transplant Proc. 2015;47:1458-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Akbulut S, Yilmaz M, Eris C, Kutlu R, Yilmaz S. Living-donor liver transplant using the right hepatic lobe without the right hepatic vein: solving the drainage problem. Exp Clin Transplant. 2013;11:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Hwang S, Ha TY, Ahn CS, Moon DB, Kim KH, Song GW, Jung DH, Park GC, Namgoong JM, Jung SW, Yoon SY, Sung KB, Ko GY, Cho B, Kim KW, Lee SG. Reconstruction of inferior right hepatic veins in living donor liver transplantation using right liver grafts. Liver Transpl. 2012;18:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Thorat A, Jeng LB, Yang HR, Li PC, Li ML, Yeh CC, Chen TH, Hsu SC, Poon KS. Outflow reconstruction for right liver allograft with multiple hepatic veins: "V-plasty" of hepatic veins to form a common outflow channel versus 2 or more hepatic vein-to-inferior vena cava anastomoses in limited retrohepatic space. Liver Transpl. 2016;22:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Jeng LB, Thorat A, Li PC, Li ML, Yang HR, Yeh CC, Chen TH, Hsu CH, Hsu SC, Poon KS. "V-Plasty" technique using dual synthetic vascular grafts to reconstruct outflow channel in living donor liver transplantation. Surgery. 2015;158:1272-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Thorat A, Jeng LB, Li PC, Li ML, Yang HR, Yeh CC, Chen TH, Hsu SC. "Single Oval Ostium Technique" using Polytetrafluoroethylene Graft for Outflow Reconstruction in Right Liver Grafts with venous Anomalies in Living Donor Liver Transplantation. Hepatogastroenterology. 2015;62:698-702. [PubMed] |
| 7. | Barut B, Akbulut S, Kutluturk K, Koc C, Gonultas F, Kayaalp C, Kutlu R, Yilmaz S. Eligibility of Circumferential Fence With the Autologous Peritoneal Patch for Venous Reconstruction in Right Lobe Living-Donor Liver Transplant: A Case Control Study. Exp Clin Transplant. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Ozbilgin M, Unek T, Egeli T, Agalar C, Ozkardeşler S, Altay C, Astarcioglu I. Comparison of Patients With and Without Anterior Sector Venous Drainage in Right Lobe Liver Transplantation From Live Donors in Terms of Complications, Rejections, and Graft Survival: Single-Center Experience. Transplant Proc. 2019;51:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Sakamoto K, Ogawa K, Matsui T, Utsunomiya T, Honjo M, Ueno Y, Tamura K, Inoue H, Nakamura T, Watanabe J, Takai A, Tohyama T, Takada Y. Reconstruction of Middle Hepatic Vein Tributaries With Artificial Vascular Grafts in Living Donor Liver Transplant Using Right Lobe Grafts: A Case Series. Transplant Proc. 2019;51:1506-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Pravisani R, Soyama A, Takatsuki M, Hidaka M, Adachi T, Ono S, Hara T, Hamada T, Kanetaka K, Eguchi S. Relationship Between Venous Drainage Patterns and Regeneration of Segments 5 and 8 in Right Lobe Grafts in Adult Living-Donor Liver Transplant Recipients. Exp Clin Transplant. 2019;17:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Ali MA, Yong CC, Eng HL, Wang CC, Lin TL, Li WF, Wang SH, Lin CC, Yap A, Chen CL. Cryopreserved arterial grafts as a conduit in outflow reconstruction in living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2015;22:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Pravisani R, Soyama A, Takatsuki M, Hidaka M, Adachi T, Ono S, Hara T, Hamada T, Eguchi S. Impact of the Inferior Right Hepatic Veins on Right Liver Lobe Regeneration in Living-Donor Liver Transplant: 3-Dimensional Computed Tomography Scan Analyses in Donors and Recipients. Exp Clin Transplant. 2019;17:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ray S, Anila T, Jha SK, Rawat S, Rawat KS, Singhvi SK. Is the absence of Right Hepatic Vein opening into Inferior Vena Cava a contraindication for right lobe liver donation in Living Donor Liver Transplantation? Common hepatic venous trunk-A rare hepatic vein anomaly: A case report and review. Int J Surg Case Rep. 2017;30:159-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Shilal P, Tuli A. Anatomical variations in the pattern of the right hepatic veins draining the posterior segment of the right lobe of the liver. J Clin Diagn Res. 2015;9:AC08-AC12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY. Quilt venoplasty using recipient saphenous vein graft for reconstruction of multiple short hepatic veins in right liver grafts. Liver Transpl. 2005;11:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |