Published online Jul 27, 2020. doi: 10.4254/wjh.v12.i7.363
Peer-review started: February 13, 2020
First decision: April 29, 2020
Revised: May 12, 2020
Accepted: May 29, 2020
Article in press: May 29, 2020
Published online: July 27, 2020
Processing time: 160 Days and 20.1 Hours
Aloe vera exerts several biological activities, such as, anti-inflammatory, antioxidant, and antimicrobial effects. It was recently shown to reduce insulin resistance and triglyceride level. We hypothesized that aloe vera would have beneficial effects in alleviating non-alcoholic steatohepatitis (NASH) in rats.
To examine the therapeutic effects of aloe vera in NASH rats.
All rats were randomly divided into 3 groups (n = 6 in each group). Rats in the control group were fed ad libitum with a standard diet for 8 wk. Rats in the NASH group were fed ad libitum with a high-fat high-fructose diet (HFHFD) for 8 wk. Rats in the aloe vera group were fed ad libitum with a HFHFD and aloe vera in dimethylsulfoxide (50 mg/kg) by gavage daily for 8 wk. Liver samples were collected at the end of the treatment period.
Hepatic malondialdehyde (MDA) levels increased significantly in the NASH group as compared with the control group (377 ± 77 nmol/mg vs 129 ± 51 nmol/mg protein, respectively, P < 0.001). Glutathione (GSH) levels were significantly lower in the NASH group than the control group (9 ± 2 nmol/mg vs 24 ± 8 nmol/mg protein, respectively, P = 0.001). The expression of interleukin-18 (IL-18), nuclear factor-kappa β, and caspase-3 increased, while peroxisome proliferator-activated receptor gamma decreased in the NASH group compared with the controls. Following aloe vera administration, MDA levels decreased (199 ± 35 nmol/mg protein) and GSH increased (18 ± 4 nmol/mg protein) markedly. Steatosis, hepatocyte ballooning, lobular inflammation and increased hepatocyte apoptosis were observed in the NASH group. Aloe vera treatment attenuated these changes in liver histology.
Aloe vera attenuated oxidative stress, hepatic inflammation and hepatocyte apoptosis, thus improving liver pathology in rats with NASH.
Core tip: To the best of our knowledge, this is the first study to evaluate the therapeutic effects of aloe vera in non-alcoholic steatohepatitis (NASH). In this animal model of NASH, we found that aloe vera decreased oxidative stress markers, replenished natural antioxidants, and reduced hepatic inflammation and hepatocyte apoptosis. Thus, aloe vera can alleviate the pathologic changes seen in NASH.
- Citation: Klaikeaw N, Wongphoom J, Werawatganon D, Chayanupatkul M, Siriviriyakul P. Anti-inflammatory and anti-oxidant effects of aloe vera in rats with non-alcoholic steatohepatitis. World J Hepatol 2020; 12(7): 363-377
- URL: https://www.wjgnet.com/1948-5182/full/v12/i7/363.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i7.363
Due to the obesity epidemic, non-alcoholic fatty liver disease (NAFLD) has become the most common liver disease worldwide with an estimated prevalence of 24%[1]. In the United States, NAFLD has now surpassed alcoholic liver disease as the leading indication for liver transplantation in women[2]. A subset of patients with NAFLD develop non-alcoholic steatohepatitis (NASH) which can lead to fibrosis progression and cirrhosis[3,4]. Currently, there are no Food and Drug Administration approved medications for the treatment of NASH. Weight loss, the mainstay of treatment for NASH, is difficult to achieve and hardly sustainable. Alternative therapies that are safe, effective and inexpensive are attractive options for the management of lifelong diseases such as NASH.
Indigenous to Africa, Asia and Mediterranean regions, aloe vera has long been used as a medicinal plant for various conditions[5,6]. Aloe vera contains at least 75 potentially active constituents such as vitamins, enzymes, minerals, sugars, plant steroids, hormones and amino acids[7]. Aloe vera and its constituents exert several biological activities, for instance, anti-inflammatory (salicylic acid, campesterol, β-sitosterol and C-glucosyl chromone), antioxidant (vitamin A, C and E), antitumor (anthraquinones and phorbol myristic acetate), and antimicrobial effects (aloin and emodin)[5,7,8]. Aloe vera has never been directly studied in NASH but it has shown potential benefits in other liver conditions such as amelioration of acetaminophen-induced liver damage[9]. Moreover, aloe vera has been shown to reduce insulin resistance and hepatic triglyceride levels which are major components of NASH[10,11]. With the aforementioned evidence, we hypothesized that aloe vera could alleviate NASH via its anti-inflammatory and antioxidant properties. To the best of our knowledge, this is the first study to evaluate the effects of aloe vera on NASH development in an animal model.
The study protocol was approved by the Institutional Review Board for Animal Research Studies, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Male Sprague-Dawley® rats weighing 220-260 g were obtained from the National Laboratory Animal Center, Mahidol University, Nakorn Pathom, Thailand. The animals were kept in a controlled temperature room at 25 ± 1ºC under standard conditions with a normal 12 h light-12 h dark cycle. All rats had free access to drinking water. The animals were allowed to acclimate to the new environment for 1 wk prior to initiation of the experiment.
Leaves of 1-year-old aloe vera plants were cut and washed thoroughly with water to cleanse the aloin-containing juice. The spiked edges were sliced off to extract the pulp. The pulp was then mixed in a blender and sieved through fine gauze. Aloe vera gel was turned into powder by freeze drying using a lyophilizer. Before use, the aloe vera powder was reconstituted into gel form and dispensed in distilled water (DW).
A total of 18 rats were randomly divided into 3 groups as follows: (1) Group 1 (control group, n = 6): Rats were fed ad libitum with standard laboratory chow (National Laboratory Animal Center, Mahidol University, Nakorn Pathom, Thailand) which contained 35% of total energy from fat, 47% from carbohydrate, and 18% from protein for 8 wk; (2) Group 2 (NASH group, n = 6): Rats were fed ad libitum with a made-in-house high-fat high-fructose diet (HFHFD) which contained 55% of total energy from fat, 35% from carbohydrate (20% from fructose and 15% from starch), and 10% from protein for 8 wk; and (3) Group 3 (aloe vera group, n = 6): Rats were fed ad libitum with the HFHFD plus daily administration of aloe vera (50 mg/kg) dissolved in DW by gavage for 8 wk. Aloe vera powder was supplied by Lipo Chemical Co., United States.
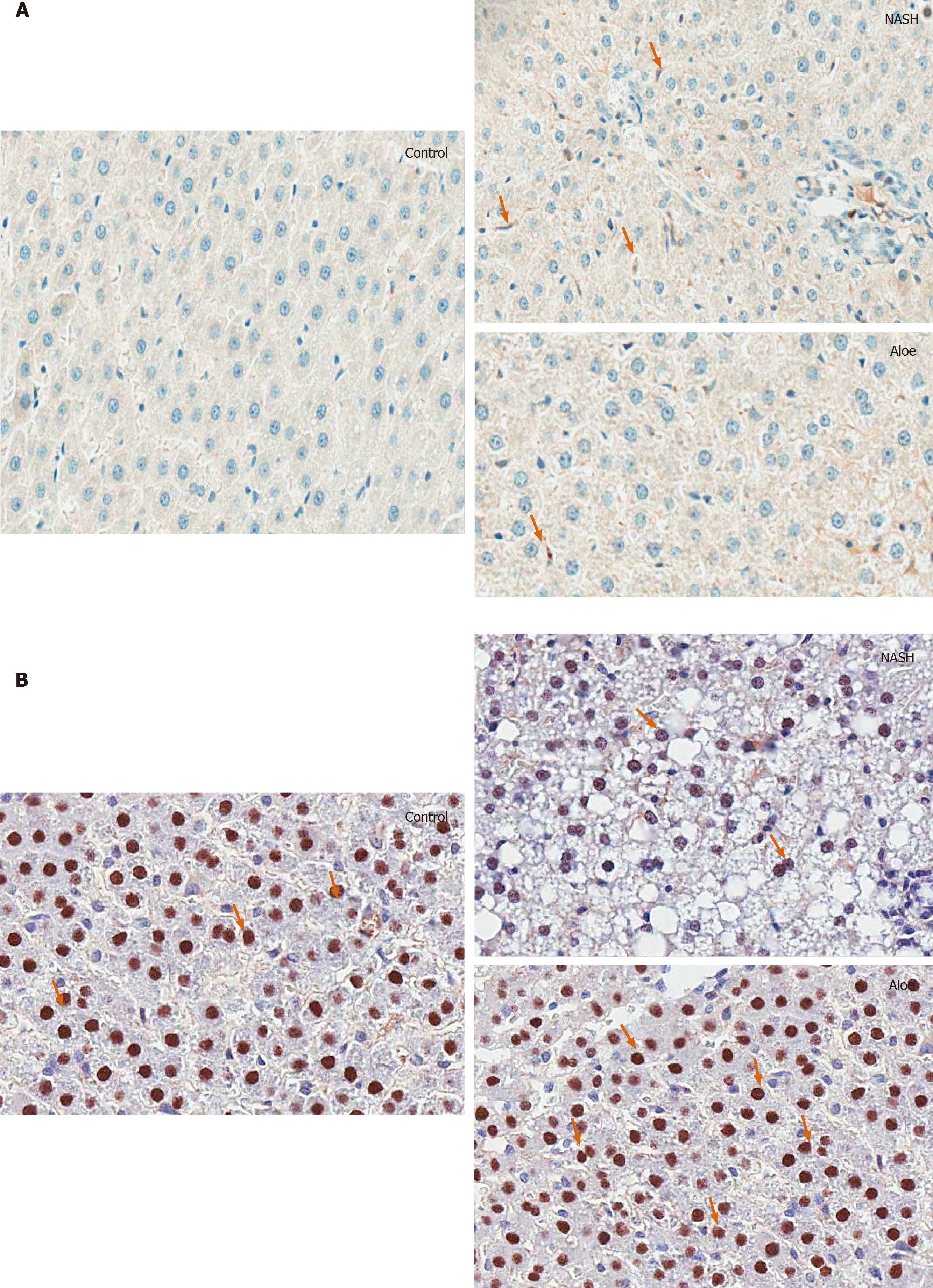
Animals were weighed weekly during the experimental period. At the end of 8 wk, all rats were euthanized with sodium thiopental overdose after a 12-h fast. The liver was surgically removed and cut into several pieces. Three small pieces of liver were immediately frozen in liquid nitrogen and stored at -80ºC until malondialdehyde (MDA) and glutathione (GSH) analysis. The remaining liver specimen was fixed in 10% formaldehyde for histopathological examination and the expression of IL-18, PPARγ, caspase-3, cytochrome-C and NF-kβ was analyzed using an immunohistochemistry technique.
MDA level was measured from homogenized tissue using a commercial assay kit (Cayman Chemical Company, Ann Arbor, MI, United States). The test involved measuring the rate of production of thiobarbituric acid-reactive substances under high-temperature and acidic conditions. The process is described as follows: One gram of liver tissue was homogenized in radioimmunoprecipitation assay buffer (RIPA buffer) containing protease inhibitor and sonicated on ice for 15 s. Supernatants were obtained after centrifugation at 1600 × g for 10 min at 4°C. The absorbance of the supernatant fraction was read at a wavelength of 532 nm. MDA levels were calculated from a standard curve and expressed as nmol/mg protein.
GSH level was quantified using a commercial assay kit (Cayman Chemical Company, Ann Arbor, MI, United States). Liver tissues were washed with phosphate buffered saline (PBS) solution. Tissues were then homogenized with cold MES buffer before being centrifuged at 10000 × g for 15 min at 4°C. The supernatants were collected and deproteinated. The absorbance of the supernatant fraction was read at a wavelength of 405 nm and GSH values were calculated from a standard curve and expressed as nmol/mg protein.
After being fixed in formaldehyde, liver samples were embedded in paraffin and sliced at a thickness of 3 μm. The tissue sections were then deparaffinized with xylene and ethanol for 10 min. Antigen retrieval was achieved by treating the slides with citrate buffer at pH 6.0 and heating in a microwave for 13 min. The slides were incubated with 3% hydrogen peroxide to block endogenous peroxidase activity for 5 min and with 3% normal horse serum to block nonspecific binding for 20 min. Tissues were then washed with PBS solution. The sections were subsequently incubated with primary antibodies for IL-18 (Gene Tex, CA, United States), PPAR-γ (Santa Cruz Biotechnology, CA, United States), NF-kβ (Abcam, MA, United States), caspase-3, and cytochrome-C (R and D, United States) for 30 min at room temperature and washed again with PBS solution. The slides were then incubated with specific secondary antibodies for 30 min at room temperature. When color development with diaminobenzidine was detected, the sections were counterstained with hematoxylin.
Under light microscopy, IL-18-positive cells were defined as Kupffer cells with dark brown-stained nuclei. Hepatocytes with PPAR-γ, NF-kβ, caspase-3 and cytochrome-C expression were characterized as liver cells with brownish nuclei. Images of each sample were taken at high-magnification (40 ×). The numbers of positive stained cells were counted using Aperio ImageScope software (Leica Biosystems Imaging, Inc., MD, United States) and expressed as the percentage of immunoreactive cells or average intensity (pixel).
Liver samples were processed using a standard technique. Collected liver tissue was fixed in 10% formalin at room temperature for 24-48 h, embedded in paraffin and sectioned at 3 μm using a microtome. Each tissue section was stained with hematoxylin and eosin and placed on glass slides for light microscopic examination. An experienced pathologist blinded to the experiment evaluated all samples. All fields in each section were examined and graded for steatosis (0-3), hepatocyte ballooning (0-3) and lobular inflammation (0-3) according to the criteria described by Brunt et al[12]. The percentage of apoptotic hepatocytes was determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method using the ApopTag® Peroxidase In Situ Apoptosis Detection kit (Millipore, CA, United States). The procedure was performed according to the manufacturer’s instructions.
Continuous data are presented as mean ± SD. One-way ANOVA and the post-hoc Tukey HSD were used to compare results between the groups. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics for Windows version 17 (SPSS, Inc., Chicago, IL, United States).
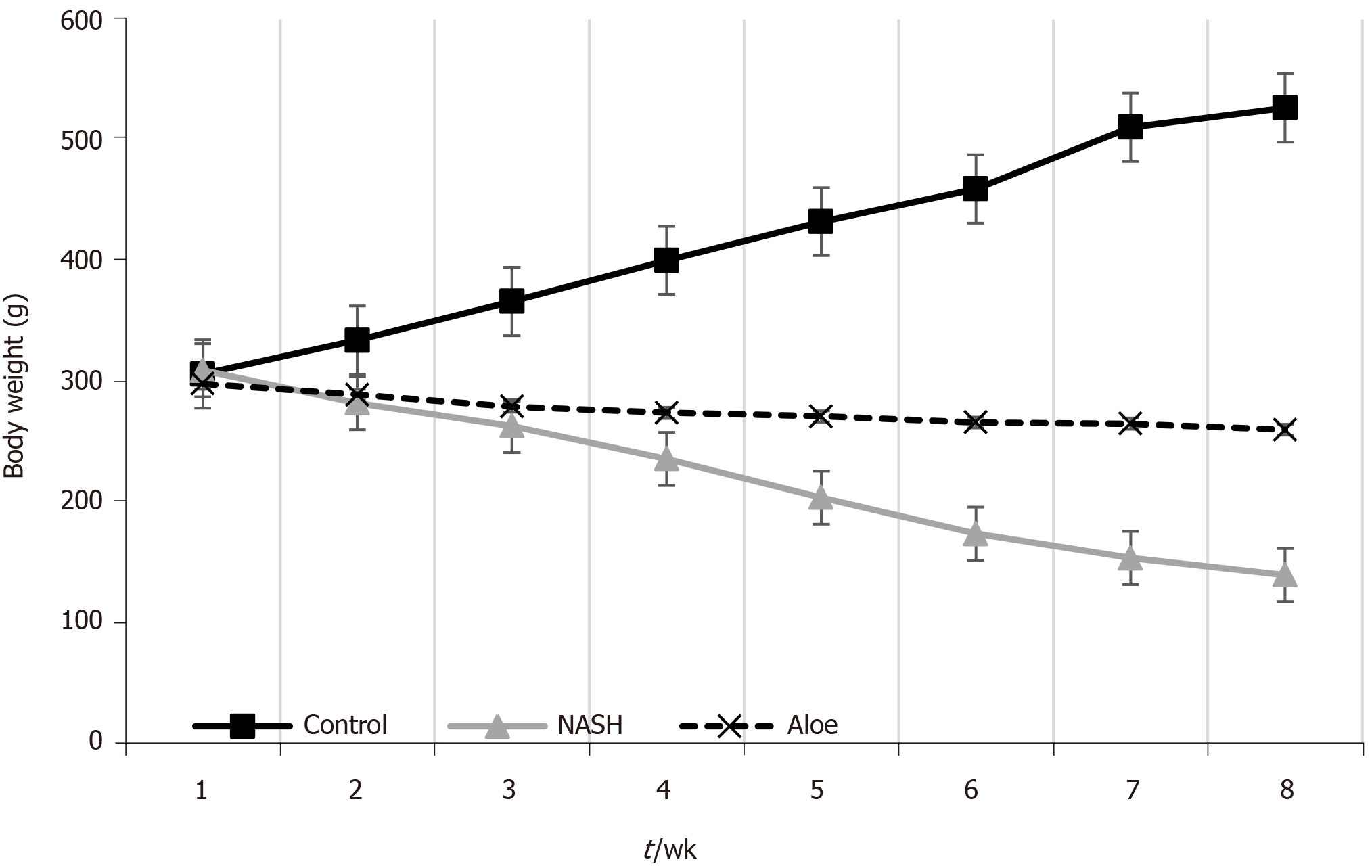
There were no differences in body weight among the groups at the beginning of the experiment. After eight weeks, rats fed with the HFHFD (NASH group) had lower body weight than those in the control group (223 ± 14.0 g vs 417 ± 11.2 g, respectively, P < 0.001). Following aloe vera administration, rats in the treatment arm gained more weight than those in the NASH group (276 ± 3.6 g vs 223 ± 14.0 g, respectively, P < 0.001) (Figure 1).
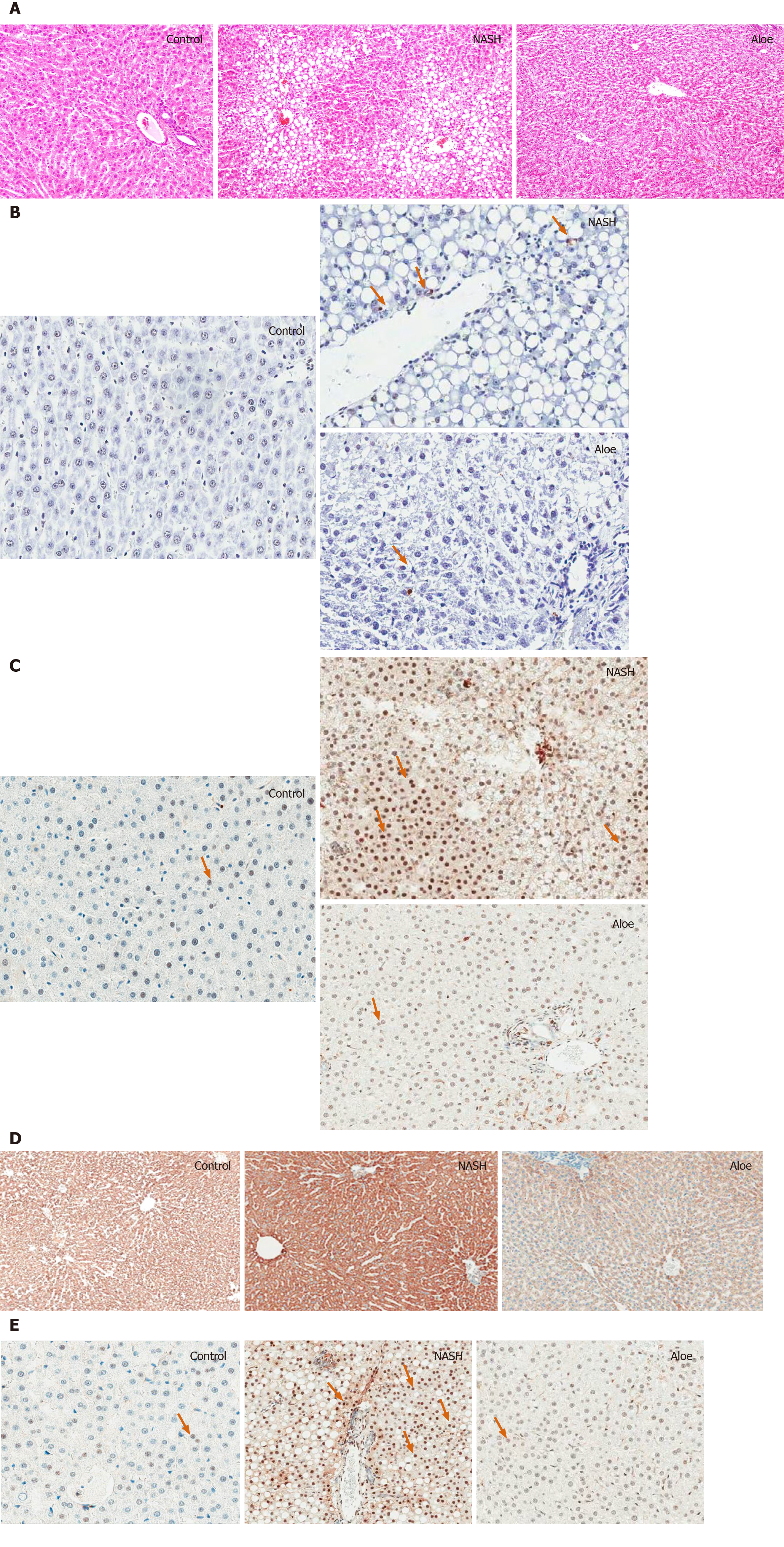
The histologic scores in each group are summarized in Table 1. Liver histology was normal in the control group. In contrast, liver pathology in the NASH group revealed significant macrovesicular and microvesicular steatosis, hepatocyte ballooning and lobular inflammation. Following aloe vera treatment, liver pathology significantly improved with only mild steatosis, minimal hepatocyte ballooning and lobular inflammation present (Figure 2A).
| Group | Steatosis | Inflammation | Ballooning | ||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | |
| Control | - | - | - | - | - | - | - | - | - | - | - |
| Non-alcoholic steatohepatitis | - | - | 3 | 3 | 0 | 5 | 1 | - | - | 4 | 2 |
| Aloe vera | 3 | 3 | - | - | 2 | 4 | - | - | 3 | 3 | - |
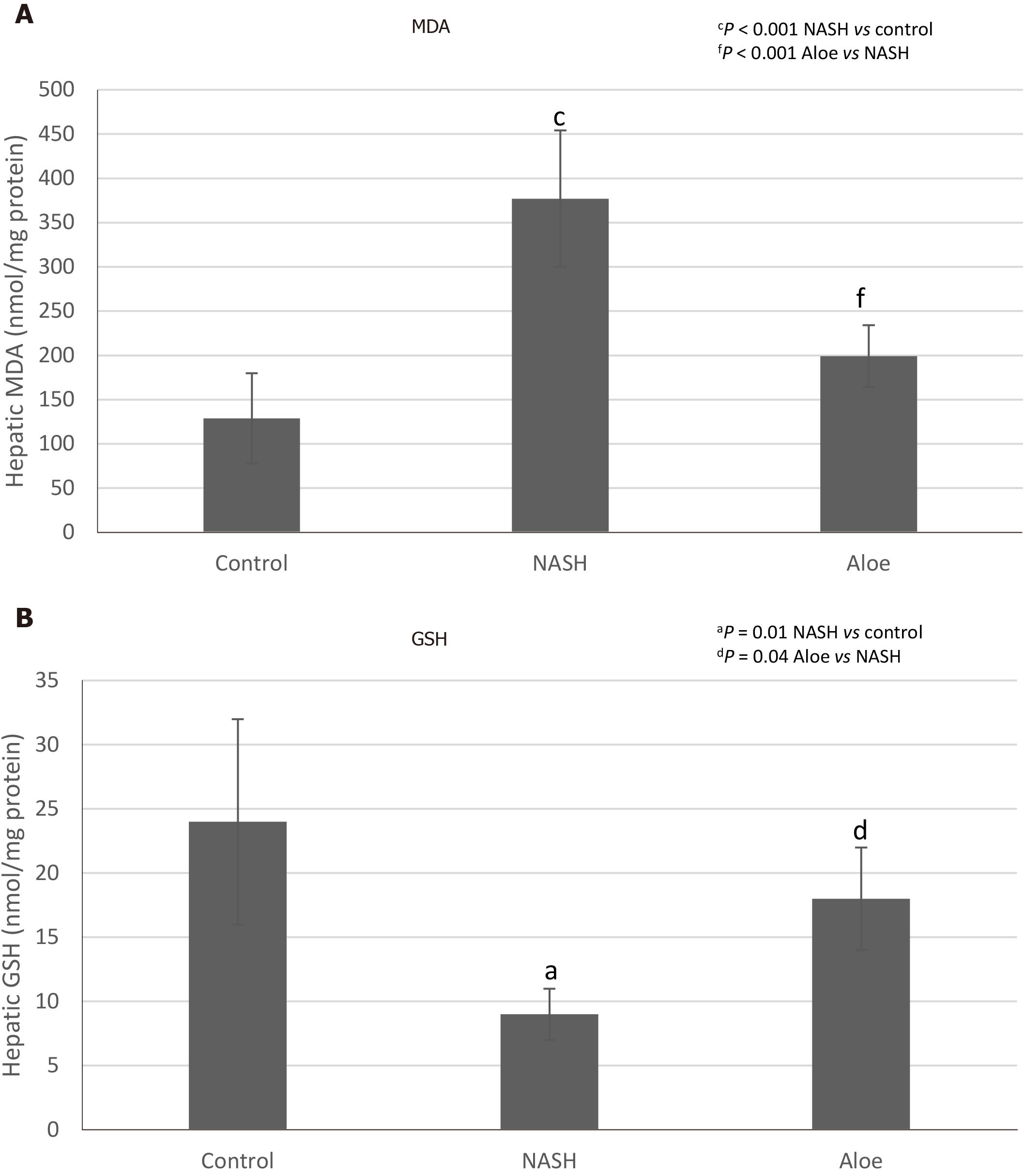
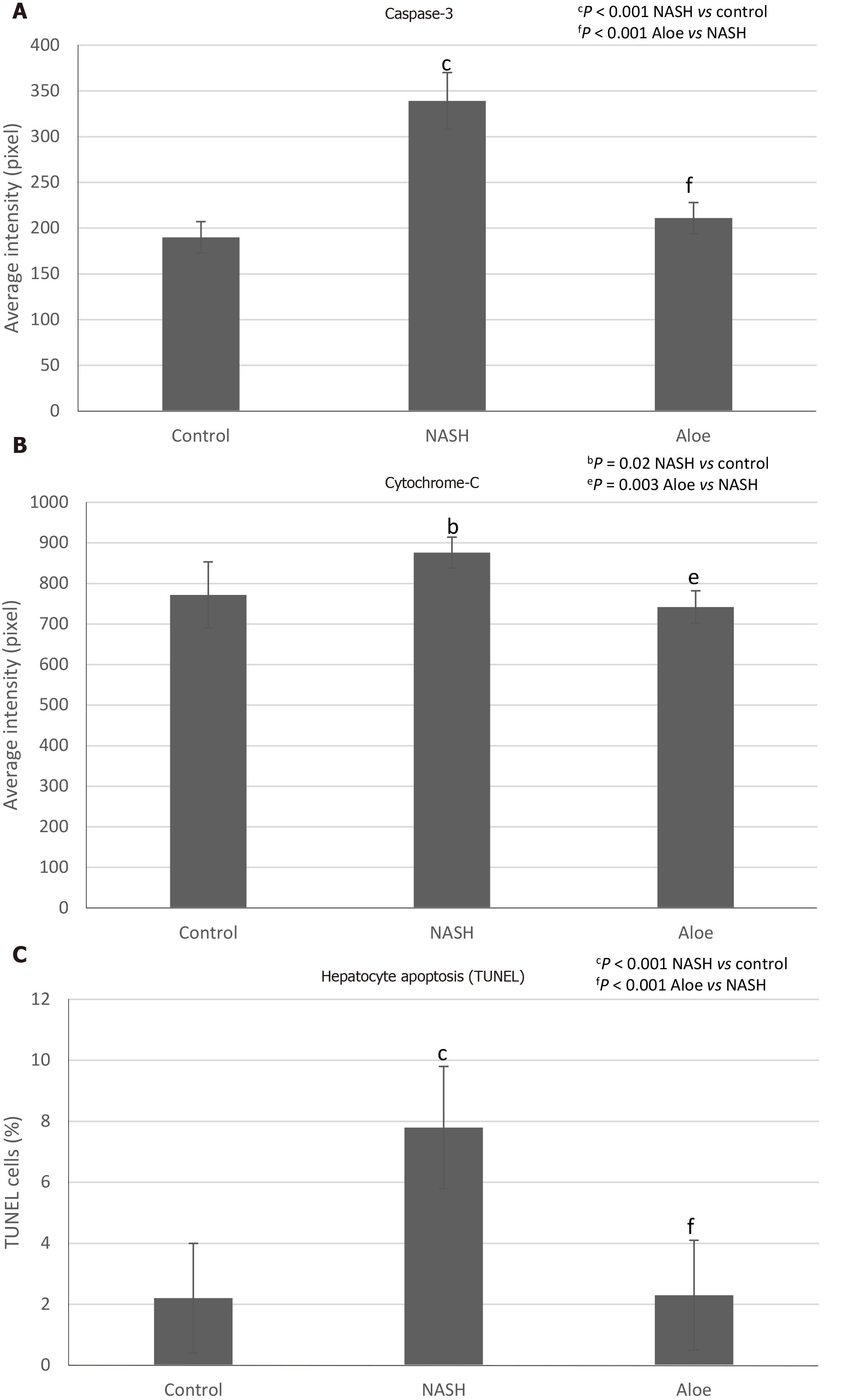
As shown in Figure 3A, MDA levels in the NASH group were significantly higher than those in the control group (377 ± 77 nmol/mg vs 129 ± 51 nmol/mg protein, P < 0.001). MDA levels declined significantly in rats receiving aloe vera along with HFHFD compared to those receiving HFHFD alone (199 ± 35 nmol/mg vs 377 ± 77 nmol/mg protein, P < 0.001). As demonstrated in Figure 3B, GSH levels in the NASH group were significantly lower than those in the control group (9 ± 2 nmol/mg vs 24 ± 8 nmol/mg protein, P < 0.001). Aloe vera treatment led to a notable rise in hepatic GSH levels (18 ± 4 nmol/mg vs 9 ± 2 nmol/mg protein in the aloe vera and NASH groups, respectively, P = 0.04).
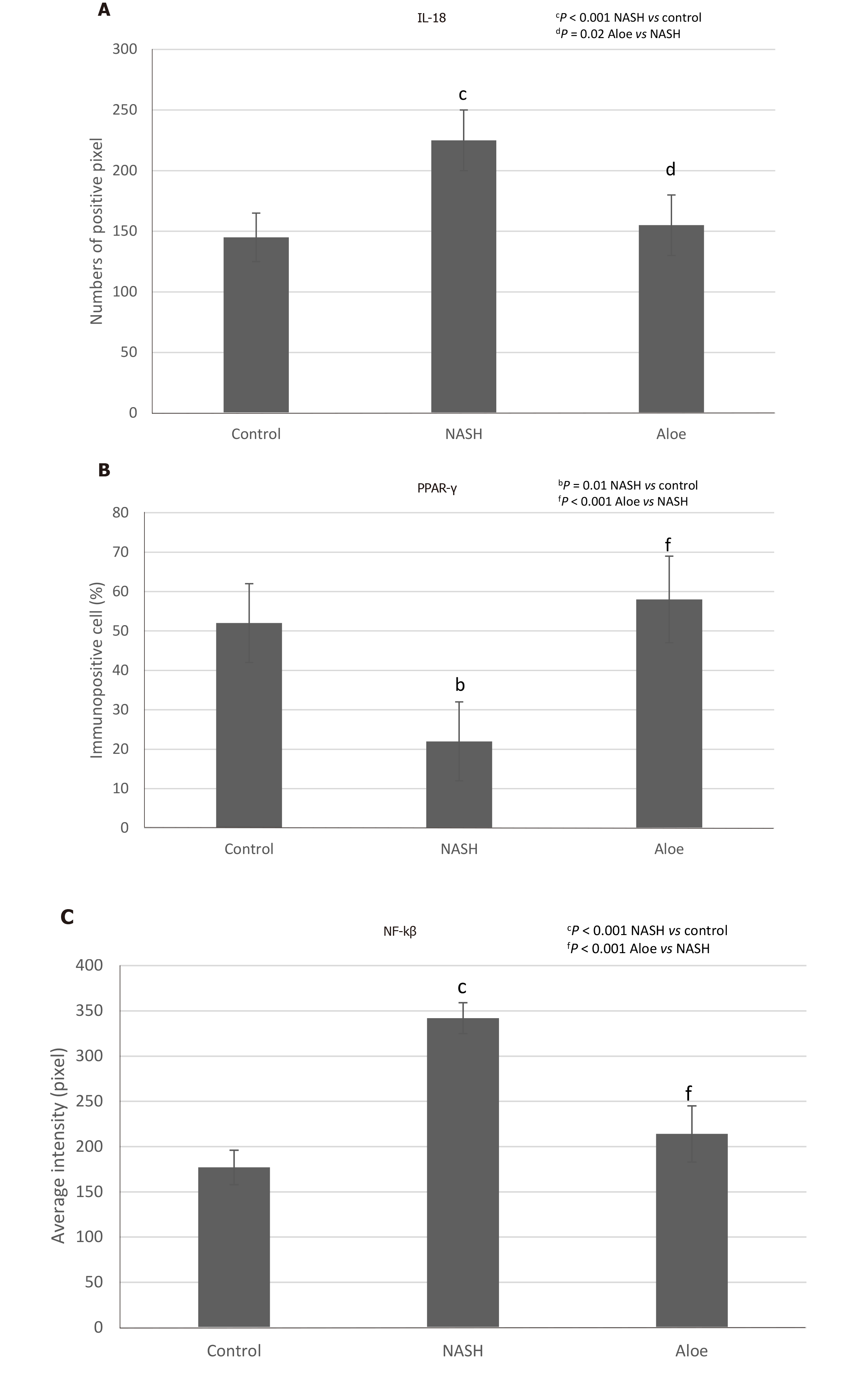
As illustrated in Figures 2, 4, and 5, the expression of IL-18 and NF-kβ increased, while the percentage of PPAR-γ positive cells decreased in the NASH group as compared to controls. In contrast, aloe vera treatment restored the changes in hepatic IL-18, PPAR-γ, and NF-kβ expression to the levels close to those observed in the control group.
Using the TUNEL method, we found that the degree of hepatocyte apoptosis was significantly higher in the NASH group as compared with the control and aloe vera groups. Similarly, markers of apoptosis such as caspase-3 and cytochrome-C were also higher in the NASH group, while the expression of these 2 markers was similar in the control and aloe vera groups (Figures 2 and 6).
The pathogenesis of NAFLD is a complex process involving insulin resistance and lipid accumulation in the liver followed by lipid peroxidation, oxidative stress, and inflammatory responses[13]. Insulin resistance facilitates adipose tissue lipolysis followed by the release of free fatty acids (FFA) in the serum, and promotes lipogenesis in the liver, thus increasing hepatic fat accumulation. These lipids, especially saturated fatty acids (SFA), lead to lipotoxic stress in the endoplasmic reticulum and mitochondria and subsequently hepatocyte apoptosis. Moreover, SFA can activate toll-like receptor-4 leading to NF-kβ activation and TNF-α and IL-6 production, the important cytokines associated with inflammatory responses in the liver[14].
Our results showed that aloe vera improved liver histopathological changes associated with HFHFD. In this experiment, we used aloe vera crude extract; therefore, we could not pinpoint the actual active ingredient of aloe vera that might have therapeutic effects against NASH. Previous studies suggested that phytosterols were the potential substances of interest. Misawa and colleagues evaluated the effects of lophenol and cycloartanol extracted from aloe vera gel on glucose and lipid metabolism in diabetic, obese rats. The authors found that lophenol and cycloartanol reduced the expression of both gluconeogenic and lipogenic genes in the liver along with the reduction in hepatic fat contents. This study, however, did not evaluate liver histology[15]. Similarly, Nomaguchi et al[16] used five phytosterols isolated from aloe vera gel in mice fed with high fat diet and found that aloe vera phytosterols could reduce body fat and liver triglyceride.
Accumulating evidence supports the implication of lipid peroxidation and oxidative stress in the development of NAFLD[17-19]. In accordance with other research, we found that MDA levels, a marker of oxidative stress, increased in rats with NASH as compared with the control group. Moreover, natural antioxidants, such as GSH, significantly declined in animals receiving HFHFD further perpetuating oxidative stress in the liver. The administration of aloe vera attenuated the increment in MDA levels and restored GSH levels in rats with NASH. Despite not being studied directly in animal models of NASH, aloe vera has been shown to reduce oxidative stress markers such as thiobarbituric acid reactive substances and increase natural antioxidants such as GSH and superoxide dismutase in streptozotocin-induced diabetic rats[20].
PPAR-γ, a member of the nuclear hormone receptor superfamily, is involved in the regulation of adipocyte differentiation, lipid metabolism, and liver inflammation[21-23]. In vitro and in vivo studies suggested that PPAR-γ provided protection against NASH by inhibiting hepatic stellate cell proliferation and migration[24], reducing pro-inflammatory cytokine production, and suppressing fatty acid synthesis[22]. Zhao et al[21] previously demonstrated that the mRNA levels of PPAR-γ were lower in rats fed with high fat diet and the levels were negatively correlated with the degree of hepatic inflammation, necrosis and fibrosis, as well as serum TNF-α and hepatic MDA contents. Similarly, we found that PPAR-γ expression was significantly lower in rats with NASH and this was restored to the level of control rats with aloe vera treatment. Comparable with our results, Nomaguchi et al[16] found that aloe vera could stimulate PPAR-γ and α activities in a dose-dependent manner as well as decrease body fat, hepatic triglyceride levels and serum lipid panels in diet-induced obese mice[16].
Recent data suggested that hepatocyte apoptosis may play a pivotal role in the progression of NAFLD[25,26]. Lipid accumulation, especially saturated FFAs and free cholesterol, may sensitize Fas- and TNF-mediated hepatocyte apoptosis and induce mitochondrial dysfunction, thus activating both extrinsic and intrinsic pathways of apoptosis[25-27]. The activation of both pathways leads to the release of pro-apoptotic proteins such as cytochrome-C, which then triggers the downstream effector caspases 3, 6, and 7 to initiate the apoptotic processes[28]. In this study, we found increases in hepatocyte apoptosis on liver histology, and cytochrome-C and caspase-3 expression in rats with NASH. Conversely, the degree of apoptosis and its markers decreased significantly with aloe vera treatment. To the best of our knowledge, this is the first study to evaluate the effect of aloe vera on hepatocyte apoptosis.
IL-18 has previously been shown to be involved in both innate and acquired immune responses by inducing several cytokines such as interferon-γ, TNF-α and IL-1[29]. However, recent studies demonstrated that IL-18 also played an important role in the regulation of metabolic functions and the development of NAFLD and NASH. Animal studies reported increases in food intake, body weight, insulin resistance, serum glucose and serum lipid levels, and eventually the development of NASH in IL-18 deficient mice[30,31]. The severity of NASH also appeared to be higher in IL-18 knockout mice as compared to wild-type mice[32]. In our study, we found increased expression of IL-18 in rats fed with HFHFD and this was normalized by the administration of aloe vera. These findings could be explained in 2 ways. The elevated IL-18 expression could be an attempt to offset the metabolic derangement due to HFHFD or simply the inflammatory responses from fat accumulation in the liver. Human studies showed similar results of elevated IL-18 levels in patients with NAFLD and these levels were positively correlated with the degree of liver injury[33,34]. Although the presence of IL-18 is crucial in maintaining energy homeostasis, the overexpression of IL-18 could accelerate hepatocyte apoptosis and perpetuate severe liver damage[35]. Aloe vera treatment decreased IL-18 expression through its anti-inflammatory and insulin sensitizing effects[10].
A unique finding in our study was the presence of weight loss in rats fed with HFHFD. This was unexpected given that several experimental models of NASH showed significant weight gain in rats receiving HFHFD compared with control diet[36-38]. This finding of weight loss was previously seen in a NASH model produced by a methionine and choline deficient diet[39,40]. We could not ascertain the amount of methionine and choline in our diet formula but it was possible that both nutrients were at low levels given a protein content of only 10% in our custom diet. Another hypothesis was the presence of high monounsaturated and polyunsaturated fatty acid contents in our diet (19% and 6% of total energy, respectively). Studies have shown that mono- and polyunsaturated fatty acids are associated with higher post-prandial fat oxidation, diet-induced thermogenesis, decreased appetite and less weight gain as compared with SFAs[41-44]. Lastly, we did not measure the total caloric intake of rats in each group; therefore, we could not say with absolute certainty that rats with HFHFD diet received an equal amount of calories compared to control rats. It is important to note, however, that liver histology in our model was consistent with NASH despite weight loss.
Our study, however, was not without limitations. First, rats with NASH in our model were slim, which differed from the usual NASH phenotype in humans. Our findings may be useful in the understanding of “lean” NASH in humans, but translating our results to “obese” NASH should be done with caution. Second, aloe vera and HFHFD were administered simultaneously resulting in our study representing more of a prevention model than treatment model. Further studies are warranted to confirm the therapeutic effects of aloe vera. Third, we only evaluated the gross effects of aloe vera on NASH development in this study. Additional in vitro studies are needed to determine cellular and subcellular targets of aloe vera.
In summary, aloe vera reduced lipid accumulation, oxidative stress, hepatic inflammation, hepatocyte apoptosis and histologic changes in this rat model of NASH.
Non-alcoholic fatty liver disease (NAFLD) is one of the most common liver diseases worldwide. However, there is no Food and Drug Administration (FDA) approved medication for the treatment of NAFLD. Aloe vera has previously been shown to have anti-inflammatory and anti-oxidant properties, which might be beneficial in the treatment of NAFLD.
With the absence of FDA-approved treatment for NAFLD, we attempted to find a safe and effective treatment for NAFLD. Alternative medicines that are safe, effective and inexpensive are attractive options for the management of life-long diseases, such as non-alcoholic steatohepatitis (NASH).
The main objective of this study was to evaluate the effects of aloe vera on NASH development in an animal model.
Rats were divided into 3 groups: Control, NASH [rats received high-fat high-fructose diet (HFHFD) to induce NASH pathology], and NASH + aloe vera. We compared liver histopathology, oxidative stress marker [malondialdehyde (MDA)], anti-oxidant level [glutathione (GSH)], inflammatory marker (IL-18), degree and markers of hepatocyte apoptosis [terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), caspase-3, cytochrome-C], and PPAR-γ expression among the three groups.
We found that by administering aloe vera along with HFHFD, we were able to significantly reduce the severity of NASH pathology in this animal model. In this study, aloe vera treatment increased the level of natural anti-oxidant (GSH), reduced oxidative stress (MDA) and inflammatory markers (IL-18), and decreased the degree of hepatocyte apoptosis (TUNEL). At the subcellular level, we also found that aloe vera increased the expression of PPAR-γ and reduced the expression of NF-kβ, caspase-3 and cytochrome-C.
This is the first study to evaluate the effects of aloe vera in rats with NASH. We found that aloe vera reduced the severity of NASH pathology in rats that received HFHFD. We hypothesized that aloe vera exerted its treatment effects by reducing oxidative stress and inflammation in the liver.
The rats in our model were lean, so our results might not be entirely applicable to obese NASH that is seen more commonly in humans. Further studies with an obese rat model are warranted to confirm the effects of aloe vera in those conditions. Moreover, we used aloe vera crude extract in this study. Additional studies will be needed to identify the active ingredients in aloe vera that have anti-NASH effects.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Inoue K, Tajiri K, Tsukanov V S-Editor: Yan JP L-Editor: Webster JR E-Editor: Wang LL
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3784] [Article Influence: 540.6] [Reference Citation Analysis (2)] |
| 2. | Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 3. | Ekstedt M, Nasr P, Kechagias S. Natural History of NAFLD/NASH. Curr Hepatol Rep. 2017;16:391-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1-16, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Rahmani AH, Aldebasi YH, Srikar S, Khan AA, Aly SM. Aloe vera: Potential candidate in health management via modulation of biological activities. Pharmacogn Rev. 2015;9:120-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Rajeswari R, Umadevi M, Sharmila Rahale C, Pushpa R, Selvavenkadesh S, Sampath Kumar KP, Bhowmik D. Aloe vera: The Miracle Plant Its Medicinal and Traditional Uses in India. J Pharm Phytochem. 2012;1:118-124. |
| 7. | Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Foster M, Hunter D, Samman S, Benzie IFF, Wachtel-Galor S, Herbal Medicine: Biomolecular and Clinical Aspects. Evaluation of the Nutritional and Metabolic Effects of Aloe vera. In: Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Werawatganon D, Linlawan S, Thanapirom K, Somanawat K, Klaikeaw N, Rerknimitr R, Siriviriyakul P. Aloe vera attenuated liver injury in mice with acetaminophen-induced hepatitis. BMC Complement Altern Med. 2014;14:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Shin E, Shim KS, Kong H, Lee S, Shin S, Kwon J, Jo TH, Park YI, Lee CK, Kim K. Dietary Aloe Improves Insulin Sensitivity via the Suppression of Obesity-induced Inflammation in Obese Mice. Immune Netw. 2011;11:59-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2885] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 13. | Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 14. | Fuchs M, Sanyal AJ. Lipotoxicity in NASH. J Hepatol. 2012;56:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Misawa E, Tanaka M, Nomaguchi K, Nabeshima K, Yamada M, Toida T, Iwatsuki K. Oral ingestion of aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in zucker diabetic fatty rats. J Agric Food Chem. 2012;60:2799-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Nomaguchi K, Tanaka M, Misawa E, Yamada M, Toida T, Iwatsuki K, Goto T, Kawada T. Aloe vera phytosterols act as ligands for PPAR and improve the expression levels of PPAR target genes in the livers of mice with diet-induced obesity. Obes Res Clin Pract. 2011;5:e190–e201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Sumida Y, Niki E, Naito Y, Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic Res. 2013;47:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 18. | Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, Varela N, Contreras J, Lazarte R, Csendes A, Rojas J, Maluenda F, Burdiles P, Diaz JC, Smok G, Thielemann L, Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond). 2004;106:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087-26124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1267] [Cited by in RCA: 1088] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 20. | Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 2005;57:90-96. [PubMed] |
| 21. | Zhao CY, Jiang LL, Li L, Deng ZJ, Liang BL, Li JM. Peroxisome proliferator activated receptor-gamma in pathogenesis of experimental fatty liver disease. World J Gastroenterol. 2004;10:1329-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Wu CW, Chu ES, Lam CN, Cheng AS, Lee CW, Wong VW, Sung JJ, Yu J. PPARgamma is essential for protection against nonalcoholic steatohepatitis. Gene Ther. 2010;17:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Everett L, Galli A, Crabb D. The role of hepatic peroxisome proliferator-activated receptors (PPARs) in health and disease. Liver. 2000;20:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 317] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5:201-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 27. | Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 486] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 28. | Thapaliya S, Wree A, Povero D, Inzaugarat ME, Berk M, Dixon L, Papouchado BG, Feldstein AE. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci. 2014;59:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Dinarello CA. Interleukin-18. Methods. 1999;19:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, van de Loo FA, Verschueren I, Pulawa L, Akira S, Eckel RH, Dinarello CA, van den Berg W, van der Meer JW. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 330] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 31. | Yamanishi K, Maeda S, Kuwahara-Otani S, Watanabe Y, Yoshida M, Ikubo K, Okuzaki D, El-Darawish Y, Li W, Nakasho K, Nojima H, Yamanishi H, Hayakawa T, Okamura H, Matsunaga H. Interleukin-18-deficient mice develop dyslipidemia resulting in nonalcoholic fatty liver disease and steatohepatitis. Transl Res. 2016;173:101-114.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1876] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 33. | Vecchiet J, Falasca K, Cacciatore P, Zingariello P, Dalessandro M, Marinopiccoli M, D'Amico E, Palazzi C, Petrarca C, Conti P, Pizzigallo E, Guagnano MT. Association between plasma interleukin-18 levels and liver injury in chronic hepatitis C virus infection and non-alcoholic fatty liver disease. Ann Clin Lab Sci. 2005;35:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Flisiak-Jackiewicz M, Bobrus-Chociej A, Tarasów E, Wojtkowska M, Białokoz-Kalinowska I, Lebensztejn DM. Predictive Role of Interleukin-18 in Liver Steatosis in Obese Children. Can J Gastroenterol Hepatol. 2018;2018:3870454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Finotto S, Siebler J, Hausding M, Schipp M, Wirtz S, Klein S, Protschka M, Doganci A, Lehr HA, Trautwein C, Khosravi-Far R, Strand D, Lohse A, Galle PR, Blessing M, Neurath MF. Severe hepatic injury in interleukin 18 (IL-18) transgenic mice: a key role for IL-18 in regulating hepatocyte apoptosis in vivo. Gut. 2004;53:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Lozano I, Van der Werf R, Bietiger W, Seyfritz E, Peronet C, Pinget M, Jeandidier N, Maillard E, Marchioni E, Sigrist S, Dal S. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab (Lond). 2016;13:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 37. | Jensen VS, Hvid H, Damgaard J, Nygaard H, Ingvorsen C, Wulff EM, Lykkesfeldt J, Fledelius C. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague-Dawley rats. Diabetol Metab Syndr. 2018;10:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Kucera O, Cervinkova Z. Experimental models of non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2014;20:8364-8376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 148] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Veteläinen R, van Vliet A, van Gulik TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J Gastroenterol Hepatol. 2007;22:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol. 2003;39:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Yang SC, Lin SH, Chang JS, Chien YW. High Fat Diet with a High Monounsaturated Fatty Acid and Polyunsaturated/Saturated Fatty Acid Ratio Suppresses Body Fat Accumulation and Weight Gain in Obese Hamsters. Nutrients. 2017;9:1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream). Int J Obes Relat Metab Disord. 2002;26:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Lawton CL, Delargy HJ, Brockman J, Smith FC, Blundell JE. The degree of saturation of fatty acids influences post-ingestive satiety. Br J Nutr. 2000;83:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |