Published online Apr 27, 2020. doi: 10.4254/wjh.v12.i4.116
Peer-review started: February 19, 2020
First decision: March 15, 2020
Revised: March 15, 2020
Accepted: March 24, 2020
Article in press: March 24, 2020
Published online: April 27, 2020
Processing time: 63 Days and 21.3 Hours
Primary sclerosing cholangitis (PSC) is a chronic, progressive, hepatobiliary disease characterized by inflammation and fibrosis of the intra- and extra-hepatic bile ducts. Its natural history is one that generally progresses towards cirrhosis, liver failure, cholangiocarcinoma, and ultimately disease-related death, with a median liver transplantation-free survival time of approximately 15-20 years. However, despite its lethal nature, PSC remains a heterogenous disease with significant variability in outcomes amongst different regions of the world. There are also many regions where the outcomes of PSC have not been studied, limiting the overall understanding of this disease worldwide. In this review, we present the geoepidemiologic variations in outcomes of PSC, with a focus on survival pre- and post-liver transplantation as well as the concurrence of inflammatory bowel disease and hepatobiliary neoplasia.
Core tip: There appears to be considerable geoepidemiologic variation in the outcomes of primary sclerosing cholangitis (PSC). Median liver transplantation-free survival in adults with PSC ranges from 14 to 21 years, depending on geographic region. Post-liver transplantation survival for PSC in North America and Europe appears to be nearly twice that found in Asia. The overall average risk of cholangiocarcinoma among patients with PSC is approximately 400 times that of the general population, occurring in roughly 7%-9% of all patients with PSC. However, these rates vary from region to region, with East Asia having rates roughly three-times higher compared to other regions. Studies from North America, Europe, and Oceania generally report worse clinical outcomes for patients with PSC-inflammatory bowel disease compared to patients with only PSC or inflammatory bowel disease; however, this association is less prominent in studies from Asia.
- Citation: Mehta TI, Weissman S, Fung BM, Tabibian JH. Geoepidemiologic variation in outcomes of primary sclerosing cholangitis. World J Hepatol 2020; 12(4): 116-124
- URL: https://www.wjgnet.com/1948-5182/full/v12/i4/116.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i4.116
Primary sclerosing cholangitis (PSC) is a chronic, cholestatic liver disease of unclear etiopathogenesis with a wide spectrum of presentations[1]. The natural history of PSC is one that generally progresses to cirrhosis, liver failure, and death[2-5]. PSC most often affects males in the fourth decade of life, though males as well as females of all ages may be affected. It is also strongly associated with inflammatory bowel disease (IBD)[6-8]. Though a rare disease, PSC is the fifth leading indication for liver transplantation (LT) in the United States and a major indication in other countries[1,3-5]. Moreover, no medical therapy has been shown to significantly delay PSC progression; indeed, it has been suggested that PSC treatments are one of the greatest unmet needs in hepatology[9,10].
Despite the global incidence of PSC, outcomes data are lacking from certain regions of the world. Additionally, few studies have looked at the specific subset of patients with PSC and concurrent IBD (PSC-IBD) with respect to the frequency of their concurrence and the impact on disease-related outcomes. To this end, we queried the PubMed and EMBASE databases on PSC and PSC-IBD related outcomes and abstracted the available relevant data. Based on our findings, we herein review the geoepidemiologic variation in the outcomes of PSC, focusing particularly on LT-free, overall, and post-LT survival, as well as the concurrence of PSC with IBD, and the association of PSC with hepatobiliary and other malignancies.
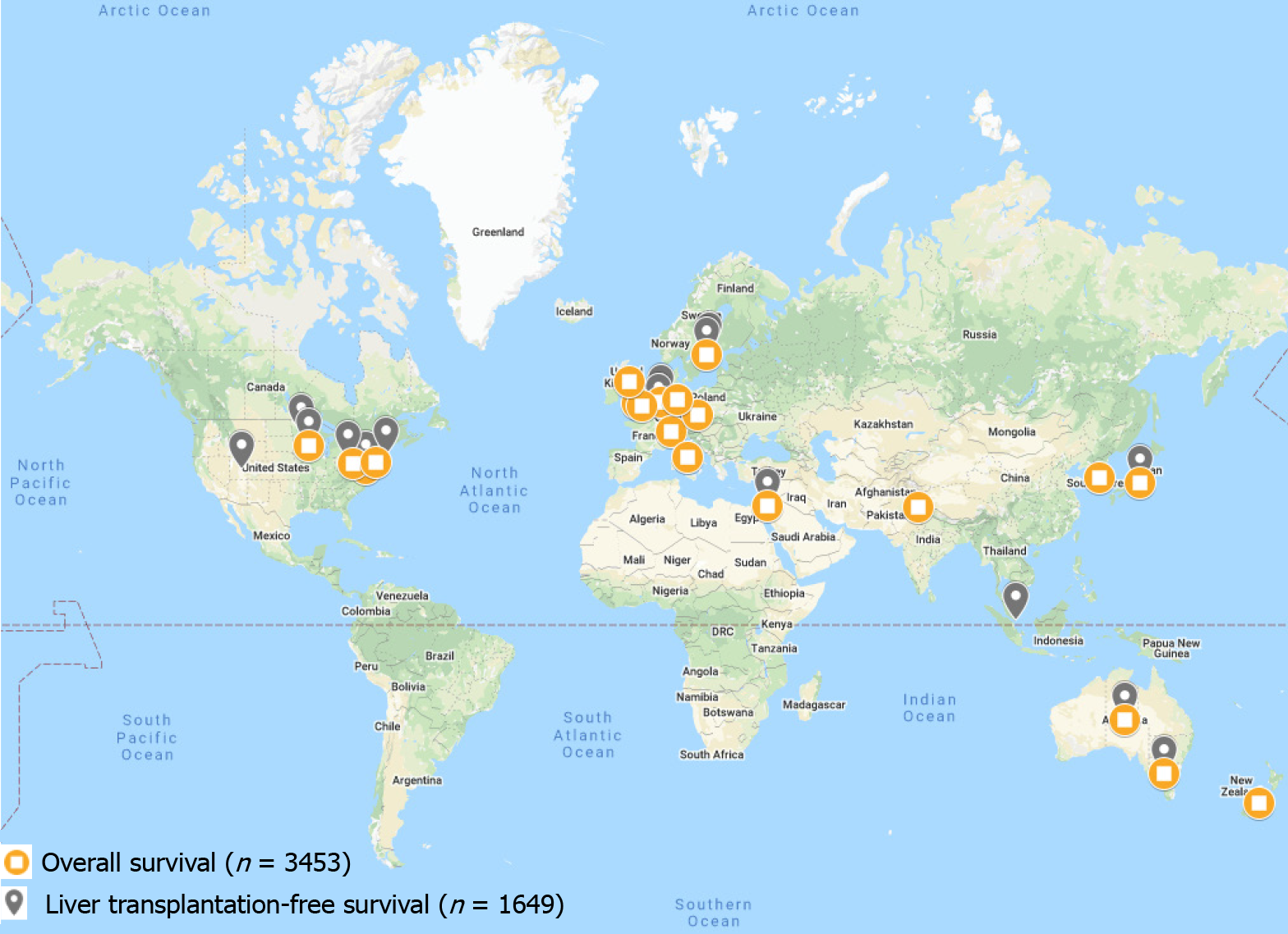
Survival in patients with PSC is highly variable with patient demographics and disease severity playing a large role in this variation. Global LT-free survival (survival free of liver-related death or LT) and global overall survival (OS) among patients with PSC has recently been reported to be 15-20 years from time of diagnosis, though significant variation exists (Table 1). Historically, European and North American studies have reported a median time from diagnosis to death or LT of 10 to 12 years[1,8,11,12]. Recent studies have suggested longer survival times, though this may be due to the fact that until recently, there have only been a small number of outcomes-based studies examining survival in PSC[6,11-14]. Technological advances in LT may also play a role in the metric of LT-free survival (as higher frequencies of LT and/or LT performed at younger ages can decrease LT-free survival). In Europe, Asia, and Oceania, median LT-free survival time appears to be 20 years or more[1,15,16]. However, this statistic does not provide a complete picture. In a Netherlands-based study, Boonstra et al[1] reported the median LT-free survival for all patients with PSC to be 21.3 years[1]. However, median LT-free survival of the subset of patients with PSC treated at LT centers was only 13.2 years[1]. In a Japan-based study, Kumagai et al[17] noted a median LT-free survival and OS of 18 years, though these patients were recruited from a LT center. Median LT-free survival and OS in some regions, such as Israel, even reach as high as 23.5 and 26.3 years, respectively[16]. Indeed, survival among patients with PSC may be increasing, but major confounding factors such as availability of LTs, patient criteria for LTs, as well as competing survival risks and variable ages at disease presentation may disproportionately influence apparent LT-free survival in various regions. Furthermore, few if any studies have been performed in Central and South America, Africa, and much of Asia; thus, trends and comparisons of survival in these regions cannot be accurately performed at this time (Figure 1).
| Region | Studies (n) | Total patients (n) | Age at diagnosis (yr) | PSC-IBD co-incidence (%) | Transplant free survival (yr) | Overall survival (yr) | Time to LT (yr) | Annual incidence CCA1 |
| Africa | 0 | 0 | - | - | - | - | - | - |
| Asia | 9 | 711 | 39 | 39% | 20.8 | 23.6 | 3.5 | 1503 |
| Europe | 18 | 3993 | 35 | 74% | 17.3 | 14.8 | 4.9 | 303 |
| North America | 14 | 1155 | 31 | 66% | 14.5 | 13.8 | 3 | 642 |
| South America | 1 | 21 | 7 | 24% | - | - | - | 433 |
| Oceania | 4 | 416 | 47 | 79% | 23.3 | 10 | 8 | 439 |
| International | 1 | 7121 | 39 | 73% | 14.5 | - | - | - |
| Overall | 47 | 13417 | 37 | 71% | 15.9 | 15.3 | 4.6 | 500 |
Overall, patients with PSC have a three- to four-fold increased risk of all-cause mortality compared to the general population[1,18-20]. Across reported regions, the leading causes of death among patients with PSC are cholangiocarcinoma (CCA), liver failure, LT-related complications, and colorectal cancer[1,8,21-23]. In Asia, liver dysfunction is reported as the most common cause of death in patients with PSC (40%-70%), whereas in Europe and North America the plurality (40%-50%) of PSC-related deaths are due to cancer[16,17,24,25].
LT is the treatment of choice for patients with advanced PSC-related hepatobiliary disease. Current practice guidelines support referral for LT when patients develop a Model for End-stage Liver Disease score of 15 or greater, a Child-Pugh-Turcotte classification of C, or when LT may significantly improve quality of life, such as in the case of intractable pruritis[22,26-29]. However, data regarding time to LT are often difficult to compare between populations because: (1) Studies at referral centers generally have patients with more severe disease, and thus may be more likely to receive a LT (Berkson’s bias); and (2) Patients living in countries/regions with greater health care access may be more likely to receive LTs. For example, the increased availability of LT centers in Europe and North America has significantly altered clinical outcomes such that nearly 50% of patients with PSC treated in these countries receive LTs[30]. In contrast, only approximately 4%-12% of patients in Asian countries receive LTs[17,31]. A major reason for this is that certain countries, such as Japan, have significant shortages of brain death donors and thus rely heavily on living donor LTs[17].
Various European and American studies have reported 1, 3, 5 and 10-year post-LT survival rates in the 70% to 90% range; however, post-LT survival in Asia appears lower with 5 and 10 year post-LT survival rates in the range of 55% to 75%[22,31-38]. Regional differences in post-LT survival may, in part, be due to overall greater clinical experience with LTs in Europe and North America or variations in patient selection criteria across regions. However, other factors may also play a role. Genetic differences, such as human leukocyte antigen profiles, have been associated with LT success rates, and the genetic underpinnings of PSC may help to explain some of the observed differences[32,39]. One North American study explored the risk of LT listing among patients with PSC and identified significantly different HLA associations among various ethnic groups. In particular, European Americans and Hispanics with PSC listed for LT had similar HLA profiles, but African Americans displayed a different HLA profile[40]. In addition, African Americans were more likely to have severe PSC-related disease than other ethnic groups in this study independent of socioeconomic factors, suggesting that genetics may contribute to PSC phenotype[40]. Unfortunately, linkage disequilibrium patterns, associations with HLA-DRB1, HLA-B, and other non-HLA genes as well as varying nomenclature and typing methodologies across regions over time currently preclude the clinical utility of PSC genotyping[41]. Of note, limited data on post-LT survival in pediatric patients with PSC are available; one North American study reported the 5-year LT- free survival among children with PSC to be 78%[42].
Approximately 20%-25% of patients with PSC experience disease recurrence post-LT, though this rate varies by cohort[43]. Recurrent PSC (rPSC) carries the potential need for re-LT and increased risk of mortality. The etiology of rPSC is unknown, but various studies have attempted to identify possible risk factors for recurrent disease. Across regions, pre-LT colectomy has been associated with reduced risk of rPSC, whereas increased age, presence of IBD, increased Model for End-stage Liver Disease score, acute cellular rejection, and pre-LT CCA have been associated with increased risk of rPSC[43]. Time-to-recurrence post-LT also appears to be similar across regions with a median time to recurrence of 5.1 years and a range spanning a few months to multiple decades[43]. Of note, among studies examining rPSC, the median age at LT appears to be younger in Asian studies compared with the global average (approximately 33 years vs 45 years, respectively)[32,36,43,44]. However, as stated previously, most of these data come from European and North American LT centers, possibly limiting prognostication to other regions. Analyses of LT-free survival, OS, and time to LT were conducted using weighted averages of studies from each reported geographic region (Table 1).
Long-established associations and complex interactions exist between PSC and IBD. The PSC-IBD phenotype is distinct with outcomes different from those seen in PSC or IBD alone. Moreover, geographic variations may exist in the PSC-IBD phenotype. In particular, studies from Oceania have noted patients with PSC-IBD to be at an increased risk of death and increased risk of gastrointestinal or hepatobiliary malignancies than patients with PSC alone[45,46]. In contrast, multiple studies from Asia have not identified significant differences in these outcome measures between patients with PSC-IBD and PSC alone[16,17]. A study from Iran even noted favorable outcomes for patients with PSC-UC relative to those with UC alone[47]. However, there are also studies from Asia suggesting worse outcomes in PSC-IBD; one study from South Korea found an increased risk of colorectal neoplasia and a trend towards increased mortality in patients with PSC-UC compared to those with UC alone[48]. Studies from North America generally report worse outcomes for patients with the PSC-IBD phenotype, with most studies suggesting a significantly increased risk of neoplastic disease, rPSC, and potentially earlier onset of rPSC post-LT[49-54]. Studies from Europe appear to have similar findings to that of North America; patients with PSC-IBD appear to have an increased risk of neoplastic disease, particularly colorectal dysplasia, compared to patients with either PSC or IBD alone[1,55]. However, European studies generally have not identified significant survival differences between patients with PSC-IBD and PSC alone[11,55,56].
Differences concerning age at presentation of PSC and PSC-IBD appear to remain highly variable. Multiple studies from various regions have noted that patients with PSC-IBD present at an earlier age than patients with PSC alone, but there are also studies in similar regions that have not identified significant age differences[11,16,17,45,46,49]. Whether this is due to IBD-related symptomatology leading to an earlier age of diagnosis and thus lead-time bias or if the PSC-IBD phenotype itself tends to present at an earlier age is unclear[57].
PSC-IBD concurrence rates appear to vary between regions. Roughly 65% of patients with PSC in Western countries have concurrent IBD, whereas only 30% of patients with PSC in East Asian countries have concurrent IBD[8,17,58,59]. Interestingly, among patients with PSC-IBD in Europe and East Asian countries, the concurrence of PSC-UC was similar at approximately 80%[8,17,58,59]. However, studies from Central Asia and the Middle East have more variable results. Generally, PSC-IBD concurrence rates in these regions are reported as similar to those in Europe, but PSC-UC concurrence rates are much lower, often under 60%[16,60,61]. Lastly, some regions, such as central and southern Europe, Alaska, and northern Canada have identified either very low or even no concurrence of PSC with IBD[62-64].
PSC is a major risk factor for the development of CCA. The risk of CCA among patients with PSC is roughly 400 times that of the general population[1]. The global annual incidence of CCA is approximately 500 per 100000 patients with PSC, or 0.5% annually (Table 1).
The annual incidence of CCA among adult and pediatric patients with PSC is roughly 7%-9% across populations, though estimates in North America vary greatly, with reported incidences as low as 4% and as high as 20%[1,11,42,65-67]. The highest annual incidence of CCA is in Asia, with incidences as high as three times the global average; the reason for this elevated incidence is unknown (Table 1)[16,35,60,68,69]. Interestingly, the highest non-PSC related rates of CCA are also seen in Asia, suggesting another variable (e.g., parasitic infections and chronic viral hepatitis) may be playing a role in the high rate of CCA[70].
Data regarding duration of PSC and risk of CCA are variable, with several studies suggesting PSC increases the risk of CCA over time while other studies have not found the same association[1,65]. This may be due to the fact that the presence of CCA in patients with PSC is often occult (with at least 10% of patients with PSC having “silent” CCA for significant lengths of time), thus the true time to development of carcinoma is unclear[71]. Interestingly, both duration of IBD among PSC-IBD patients and colorectal neoplasia (CRN) among PSC-UC patients increase the risk of CCA development[72].
IBD confers an increased risk of CRN and PSC-IBD further increases the risk of CRN above that of IBD alone[55]. Of note, some studies have reported an increased risk of CRN among PSC-UC patients compared to UC patients but not among PSC-IBD patients relative to IBD patients, implying a specific disease interaction between PSC and UC[72-75].
While regional differences in PSC-IBD associated CRN are difficult to ascertain, it is known that post-LT colorectal neoplasia is of particular concern in patients with PSC and PSC-IBD[22]. Among post-LT PSC-IBD patients, the risk of CRN rises by approximately 1% per year post-LT[22,76]. As such, it is possible that rates of CRN among PSC-IBD patients may be greater in European and North American countries owing to the increased frequency of LT in these regions, though there is little evidence to support this directly. Annual endoscopic monitoring is considered standard of care among PSC-IBD patients[77].
Geographic reporting of PSC-related outcomes is heterogenous, with the majority of studies coming from Europe and North America, a limited number of studies from Asia and Oceania, and very few studies from South America and Africa, hence summary estimates were not amenable to meta-analysis. Moreover, the reporting of results differs even within similar regions, making comparisons challenging. For example, within one region, one study may report LT-free survival while another study may report OS, limiting the ability to make comparisons. Additionally, PSC case identification, outcomes, and other factors may have changed over time. Therefore, when comparing studies from one region to another we may be comparing them not only based on where the studies took place, but when they took place, potentially confounding results. Lastly, our search was limited to studies available in English, which may have left out studies from non-English speaking regions.
Studies on global PSC-related outcomes have increased over the years allowing for novel analyses of regional differences. Causes of PSC-related death vary globally, with liver dysfunction being the primary cause of PSC-related death in Asia, and cancer being the primary cause in both Europe and North America. Although notably, there is a significantly greater rate of CCA in East Asia than the rest of the world. Interestingly, PSC-IBD concurrence rates vary across regions, yet the proportions of PSC-IBD subtypes are largely consistent across regions. Likewise, PSC-IBD related outcomes appear largely consistent across regions. As most studies of PSC have been conducted in the United States and Western European countries, with a paucity of data from other regions, the need for large population-based studies in under-reported regions is imperative to better understand global and regional PSC-related outcomes.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report´s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Tajiri K, Shinkawa H S-Editor: Zhang L L-Editor: A E-Editor: Wu YXJ
| 1. | Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, Naber AH, Kingma PJ, van Buuren HR, van Hoek B, Vleggaar FP, van Geloven N, Beuers U, Ponsioen CY; EpiPSCPBC Study Group. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 504] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 2. | Molodecky NA, Kareemi H, Parab R, Barkema HW, Quan H, Myers RP, Kaplan GG. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53:1590-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Bjøro K, Brandsaeter B, Foss A, Schrumpf E. Liver transplantation in primary sclerosing cholangitis. Semin Liver Dis. 2006;26:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Karlsen TH, Boberg KM. Update on primary sclerosing cholangitis. J Hepatol. 2013;59:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Tabibian JH, Bowlus CL. Primary sclerosing cholangitis: A review and update. Liver Res. 2017;1:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 306] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Schrumpf E, Abdelnoor M, Fausa O, Elgjo K, Jenssen E, Kolmannskog F. Risk factors in primary sclerosing cholangitis. J Hepatol. 1994;21:1061-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Dyson JK, Webb G, Hirschfield GM, Lohse A, Beuers U, Lindor K, Jones DE. Unmet clinical need in autoimmune liver diseases. J Hepatol. 2015;62:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Laborda TJ, Jensen MK, Kavan M, Deneau M. Treatment of primary sclerosing cholangitis in children. World J Hepatol. 2019;11:19-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, Fleming TR, Fisher LD, Beaver SJ, LaRusso NF. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 330] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, Loftus EV, Yawn BP, Dickson ER, Melton LJ. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Freeman E, Majeed A, Kemp W, Roberts SK. Long-term outcomes of primary sclerosing cholangitis: an Australian non-transplant tertiary hospital perspective. Intern Med J. 2019;49:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Yanai H, Matalon S, Rosenblatt A, Awadie H, Berdichevski T, Snir Y, Kopylov U, Katz L, Stein A, Mlynarsky L, Tulchinsky H, Konikoff FM, Horin SB, Braun M, Ben-Ari Z, Chowers Y, Baruch Y, Shibolet O, Dotan I. Prognosis of primary sclerosing cholangitis in israel is independent of coexisting inflammatory bowel Disease. J Crohns Colitis. 2015;9:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Kumagai J, Taida T, Ogasawara S, Nakagawa T, Iino Y, Shingyoji A, Ishikawa K, Akizue N, Yamato M, Takahashi K, Ohta Y, Hamanaka S, Okimoto K, Nakamura M, Ohyama H, Saito K, Kusakabe Y, Maruoka D, Yasui S, Matsumura T, Sugiyama H, Sakai Y, Mikata R, Arai M, Katsuno T, Tsuyuguchi T, Kato N. Clinical characteristics and outcomes of primary sclerosing cholangitis and ulcerative colitis in Japanese patients. PLoS One. 2018;13:e0209352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol. 2008;48:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Liang H, Manne S, Shick J, Lissoos T, Dolin P. Incidence, prevalence, and natural history of primary sclerosing cholangitis in the United Kingdom. Medicine (Baltimore). 2017;96:e7116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Cheung KS, Seto WK, Fung J, Lai CL, Yuen MF. Prognostic Factors for Transplant-Free Survival and Validation of Prognostic Models in Chinese Patients with Primary Biliary Cholangitis Receiving Ursodeoxycholic Acid. Clin Transl Gastroenterol. 2017;8:e100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 568] [Article Influence: 71.0] [Reference Citation Analysis (35)] |
| 22. | Chapman MH, Thorburn D, Hirschfield GM, Webster GGJ, Rushbrook SM, Alexander G, Collier J, Dyson JK, Jones DE, Patanwala I, Thain C, Walmsley M, Pereira SP. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. 2019;68:1356-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 23. | Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA, Marschall HU, Thorburn D, Weersma RK, Fevery J, Mueller T, Chazouillères O, Schulze K, Lazaridis KN, Almer S, Pereira SP, Levy C, Mason A, Naess S, Bowlus CL, Floreani A, Halilbasic E, Yimam KK, Milkiewicz P, Beuers U, Huynh DK, Pares A, Manser CN, Dalekos GN, Eksteen B, Invernizzi P, Berg CP, Kirchner GI, Sarrazin C, Zimmer V, Fabris L, Braun F, Marzioni M, Juran BD, Said K, Rupp C, Jokelainen K, Benito de Valle M, Saffioti F, Cheung A, Trauner M, Schramm C, Chapman RW, Karlsen TH, Schrumpf E, Strassburg CP, Manns MP, Lindor KD, Hirschfield GM, Hansen BE, Boberg KM; International PSC Study Group. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152:1975-1984.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 364] [Article Influence: 45.5] [Reference Citation Analysis (1)] |
| 24. | Chapman RW. Update on primary sclerosing cholangitis. Clin Liver Dis (Hoboken). 2017;9:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Tanaka A, Takamori Y, Toda G, Ohnishi S, Takikawa H. Outcome and prognostic factors of 391 Japanese patients with primary sclerosing cholangitis. Liver Int. 2008;28:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646-59; quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 27. | Bittencourt PL, Cançado EL, Couto CA, Levy C, Porta G, Silva AE, Terrabuio DR; Brazilian Society of Hepatology on the Diagnosis and Management of Autoimmune Diseases of the Liver, Carvalho Filho RJ, Chaves DM, Miura IK, Codes L, Faria LC, Evangelista AS, Farias AQ, Gonçalves LL, Harriz M, Lopes Neto EP, Luz GO, Oliveira P, Oliveira EM, Schiavon JL, Seva-Pereira T, Parise ER. Brazilian society of hepatology recommendations for the diagnosis and management of autoimmune diseases of the liver. Arq Gastroenterol. 2015;52 Suppl 1:15-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1202] [Article Influence: 75.1] [Reference Citation Analysis (1)] |
| 29. | Isayama H, Tazuma S, Kokudo N, Tanaka A, Tsuyuguchi T, Nakazawa T, Notohara K, Mizuno S, Akamatsu N, Serikawa M, Naitoh I, Hirooka Y, Wakai T, Itoi T, Ebata T, Okaniwa S, Kamisawa T, Kawashima H, Kanno A, Kubota K, Tabata M, Unno M, Takikawa H; PSC guideline committee Members: Ministry of Health, Labour and Welfare (Japan) Research Project, The Intractable Hepatobiliary Disease Study Group. Clinical guidelines for primary sclerosing cholangitis 2017. J Gastroenterol. 2018;53:1006-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Takakura WR, Tabibian JH, Bowlus CL. The evolution of natural history of primary sclerosing cholangitis. Curr Opin Gastroenterol. 2017;33:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Tanaka A, Tazuma S, Nakazawa T, Isayama H, Tsuyuguchi T, Inui K, Takikawa H. No negative impact of serum IgG4 levels on clinical outcome in 435 patients with primary sclerosing cholangitis from Japan. J Hepatobiliary Pancreat Sci. 2017;24:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Ueda Y, Kaido T, Okajima H, Hata K, Anazawa T, Yoshizawa A, Yagi S, Taura K, Masui T, Yamashiki N, Haga H, Nagao M, Marusawa H, Seno H, Uemoto S. Long-term Prognosis and Recurrence of Primary Sclerosing Cholangitis After Liver Transplantation: A Single-Center Experience. Transplant Direct. 2017;3:e334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Rossi RE, Conte D, Massironi S. Primary sclerosing cholangitis associated with inflammatory bowel disease: an update. Eur J Gastroenterol Hepatol. 2016;28:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Tamura S, Sugawara Y, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Recurrence of primary sclerosing cholangitis after living donor liver transplantation. Liver Int. 2007;27:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Ang TL, Fock KM, Ng TM, Teo EK, Chua TS, Tan JY. Clinical profile of primary sclerosing cholangitis in Singapore. J Gastroenterol Hepatol. 2002;17:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Egawa H, Ueda Y, Ichida T, Teramukai S, Nakanuma Y, Onishi S, Tsubouchi H. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation in Japanese registry. Am J Transplant. 2011;11:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Emek E, Serin A, Sahin T, Yazici P, Yuzer Y, Tokat Y, Bozkurt B. Experience in Liver Transplantation Due to Primary Sclerosing Cholangitis: A Single Center Experience. Transplant Proc. 2019;51:2439-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Ravikumar R, Tsochatzis E, Jose S, Allison M, Athale A, Creamer F, Gunson B, Iyer V, Madanur M, Manas D, Monaco A, Mirza D, Owen N, Roberts K, Sen G, Srinivasan P, Wigmore S, Fusai G, Fernando B, Burroughs A. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J Hepatol. 2015;63:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 39. | Neumann UP, Guckelberger O, Langrehr JM, Lang M, Schmitz V, Theruvath T, Schonemann C, Menzel S, Klupp J, Neuhaus P. Impact of human leukocyte antigen matching in liver transplantation. Transplantation. 2003;75:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Bowlus CL, Li CS, Karlsen TH, Lie BA, Selmi C. Primary sclerosing cholangitis in genetically diverse populations listed for liver transplantation: unique clinical and human leukocyte antigen associations. Liver Transpl. 2010;16:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14:279-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 42. | Deneau M, Jensen MK, Holmen J, Williams MS, Book LS, Guthery SL. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013;58:1392-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 43. | Steenstraten IC, Sebib Korkmaz K, Trivedi PJ, Inderson A, van Hoek B, Rodriguez Girondo MDM, Maljaars PWJ. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment Pharmacol Ther. 2019;49:636-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Gelley F, Zádori G, Görög D, Kóbori L, Fehérvári I, Gámán G, Gerlei Z, Nagy P, Sárváry E, Nemes B. Recurrence of primary sclerosing cholangitis after liver transplantation - The Hungarian experience. Interv Med Appl Sci. 2014;6:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Ngu JH, Gearry RB, Frampton CM, Stedman CA. Mortality and the risk of malignancy in autoimmune liver diseases: a population-based study in Canterbury, New Zealand. Hepatology. 2012;55:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Liu K, Wang R, Kariyawasam V, Wells M, Strasser SI, McCaughan G, Corte C, Leong RW. Epidemiology and outcomes of primary sclerosing cholangitis with and without inflammatory bowel disease in an Australian cohort. Liver Int. 2017;37:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Moayyeri A, Daryani NE, Bahrami H, Haghpanah B, Nayyer-Habibi A, Sadatsafavi M. Clinical course of ulcerative colitis in patients with and without primary sclerosing cholangitis. J Gastroenterol Hepatol. 2005;20:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Ye BD, Yang SK, Boo SJ, Cho YK, Yang DH, Yoon SM, Kim KJ, Byeon JS, Myung SJ, Yu CS, Yun SC, Kim JH. Clinical characteristics of ulcerative colitis associated with primary sclerosing cholangitis in Korea. Inflamm Bowel Dis. 2011;17:1901-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Navaneethan U, Venkatesh PG, Lashner BA, Shen B, Kiran RP. The impact of ulcerative colitis on the long-term outcome of patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;35:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Jeyarajah DR, Netto GJ, Lee SP, Testa G, Abbasoglu O, Husberg BS, Levy MF, Goldstein RM, Gonwa TA, Tillery GW, Crippin JS, Klintmalm GB. Recurrent primary sclerosing cholangitis after orthotopic liver transplantation: is chronic rejection part of the disease process? Transplantation. 1998;66:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Graziadei IW. Recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2002;8:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Kugelmas M, Spiegelman P, Osgood MJ, Young DA, Trotter JF, Steinberg T, Wachs ME, Bak T, Kam I, Everson GT. Different immunosuppressive regimens and recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2003;9:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Narumi S, Roberts JP, Emond JC, Lake J, Ascher NL. Liver transplantation for sclerosing cholangitis. Hepatology. 1995;22:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Gulamhusein AF, Eaton JE, Tabibian JH, Atkinson EJ, Juran BD, Lazaridis KN. Duration of Inflammatory Bowel Disease Is Associated With Increased Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis and IBD. Am J Gastroenterol. 2016;111:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Mertz A, Nguyen NA, Katsanos KH, Kwok RM. Primary sclerosing cholangitis and inflammatory bowel disease comorbidity: an update of the evidence. Ann Gastroenterol. 2019;32:124-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Fevery J, Van Steenbergen W, Van Pelt J, Laleman W, Hoffman I, Geboes K, Vermeire S, Nevens F. Patients with large-duct primary sclerosing cholangitis and Crohn's disease have a better outcome than those with ulcerative colitis, or without IBD. Aliment Pharmacol Ther. 2016;43:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Palmela C, Peerani F, Castaneda D, Torres J, Itzkowitz SH. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver. 2018;12:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 58. | Takikawa H, Takamori Y, Tanaka A, Kurihara H, Nakanuma Y. Analysis of 388 cases of primary sclerosing cholangitis in Japan; Presence of a subgroup without pancreatic involvement in older patients. Hepatol Res. 2004;29:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, Rajaram R, Rauws EA, Mulder CJ, Reitsma JB, Heisterkamp SH, Tytgat GN. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Parlak E, Kosar Y, Ulker A, Dagli U, Alkim C, Sahin B. Primary sclerosing cholangitis in patients with inflammatory bowel disease in Turkey. J Clin Gastroenterol. 2001;33:299-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Kochhar R, Goenka MK, Das K, Nagi B, Bhasin DK, Chawla YK, Vaiphei K, Singh K, Dilawari JB. Primary sclerosing cholangitis: an experience from India. J Gastroenterol Hepatol. 1996;11:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Hurlburt KJ, McMahon BJ, Deubner H, Hsu-Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002;97:2402-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Katsanos KH, Stamou P, Tatsioni A, Tsianos VE, Zoumbas S, Kavvadia S, Giga A, Vagias I, Christodoulou DK, Tsianos EV; Northwest Greece IBD Study Group. Prevalence of inflammatory bowel disease related dysplasia and cancer in 1500 colonoscopies from a referral center in northwestern Greece. J Crohns Colitis. 2011;5:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300-2307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 212] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 65. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 66. | Toy E, Balasubramanian S, Selmi C, Li CS, Bowlus CL. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011;11:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Lutz HH, Tischendorf JJ. Management of primary sclerosing cholangitis. World J Hepatol. 2011;3:137-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Geramizadeh B, Ghavvas R, Kazemi K, Shamsaeefar A, Nikeghbalian S, Malekhosseini SA. Cholangiocarcinoma Secondary to Primary Sclerosing Cholangitis in Explanted Livers: A Single-Center Study in the South of Iran. Hepat Mon. 2015;15:e33626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Yoon J, Oh SH, Kim HJ, Park SH, Ye BD, Yang SK, Kim KM. Primary Sclerosing Cholangitis with Inflammatory Bowel Disease in Korean Children. Pediatr Gastroenterol Hepatol Nutr. 2015;18:268-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Kirstein MM, Vogel A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc Med. 2016;32:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 71. | Marsh JW, Iwatsuki S, Makowka L, Esquivel CO, Gordon RD, Todo S, Tzakis A, Miller C, Van Thiel D, Starzl TE. Orthotopic liver transplantation for primary sclerosing cholangitis. Ann Surg. 1988;207:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 74. | Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 75. | Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 76. | Altwegg R, Combes R, Laharie D, De Ledinghen V, Radenne S, Conti F, Chazouilleres O, Duvoux C, Dumortier J, Leroy V, Treton X, Durand F, Dharancy S, Nachury M, Goutorbe F, Lamblin G, Boivineau L, Peyrin-Biroulet L, Pageaux GP. Effectiveness and safety of anti-TNF therapy for inflammatory bowel disease in liver transplant recipients for primary sclerosing cholangitis: A nationwide case series. Dig Liver Dis. 2018;50:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Peyrin-Biroulet L, Bonnaud G, Bourreille A, Chevaux JB, Faure P, Filippi J, Laharie D, Vuitton L, Bulois P, Gonzalez F, Trang C, Koch S, Bernardini D, Cellier C; IBD Committee of the French Society of Digestive Endoscopy. Endoscopy in inflammatory bowel disease: recommendations from the IBD Committee of the French Society of Digestive Endoscopy (SFED). Endoscopy. 2013;45:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |