Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1089
Peer-review started: April 13, 2020
First decision: June 18, 2020
Revised: August 11, 2020
Accepted: September 3, 2020
Article in press: September 3, 2020
Published online: November 27, 2020
Processing time: 224 Days and 22.3 Hours
Preoperative biliary drainage in patients with presumed resectable perihilar cholangiocarcinoma (PHC) is hypothesized to promote the occurrence of seeding metastases. Seeding metastases can occur at the surgical scars or at the site of postoperative drains, and in case of percutaneous biliary drainage, at the catheter port-site. To prevent seeding metastases after resection, we routinely treated PHC patients with preoperative radiotherapy (RT) for over 25 years until January 2018.
To investigate the incidence of seeding metastases following resection of PHC.
All patients who underwent resection for pathology proven PHC between January 2000 and March 2019 were included in this retrospective study. Between 2000-January 2018, patients received preoperative RT (3 × 3.5 Gray). RT was omitted in patients treated after January 2018.
A total of 171 patients underwent resection for PHC between January 2000 and March 2019. Of 171 patients undergoing resection, 111 patients (65%) were treated with preoperative RT. Intraoperative bile cytology showed no difference in the presence of viable tumor cells in bile of patients undergoing preoperative RT or not. Overall, two patients (1.2%) with seeding metastases were identified, both in the laparotomy scar and both after preoperative RT (one patient with endoscopic and the other with percutaneous and endoscopic biliary drainage).
The incidence of seeding metastases in patients with resected PHC in our series was low (1.2%). This low incidence and the inability of providing evidence that preoperative low-dose RT prevents seeding metastases, has led us to discontinue preoperative RT in patients with resectable PHC in our center.
Core Tip: Routine preoperative radiotherapy (3 times 3.5 Gray) to prevent the occurrence of seeding metastases was used in our tertiary center for 28 years in patients undergoing resection for perihilar cholangiocarcinoma. Seeding metastases occurred in 2 out of 171 patients (1.2%) undergoing resection between 2000 and 2019. Intraoperative bile cytology showed no significant difference in the presence of tumor cells in the bile of patients undergoing preoperative radiotherapy or not. Due to the current low incidence of seeding metastases, preoperative radiotherapy to prevent seeding metastases is now abandoned in our institution.
- Citation: Franken LC, Roos E, Saris J, van Hooft JE, van Delden OM, Verheij J, Erdmann JI, Besselink MG, Busch OR, van Tienhoven G, van Gulik TM. Occurrence of seeding metastases in resectable perihilar cholangiocarcinoma and the role of low-dose radiotherapy to prevent this. World J Hepatol 2020; 12(11): 1089-1097
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1089.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1089
Patients with perihilar cholangiocarcinoma (PHC) have been treated with low-dose radiotherapy (RT) preoperatively at our institution since the early 90s with the aim to prevent seeding metastases. Preoperative radiotherapy was introduced in our practice based on a study published by our institution in 1999, showing that 8 of 41 patients (20%) undergoing resection for PHC who were drained preoperatively, developed implantation metastases within 1 year after resection. In this study, endoscopic biliary drainage was performed in all 41 patients, combined with percutaneous biliary drainage in 4 of these patients. In addition, in 11 patients without biliary drainage, no seeding metastases were found[1]. It was hypothesized that biliary drainage, with perturbation of the tumor, enhances bile contamination with tumor cells, thereby increasing the risk of seeding metastases with bile spill incurred during resection.
In an effort to prevent this complication, all patients with resectable PHC at our tertiary center, received three times 3.5 Gray (Gy) external radiation therapy to the primary tumor on three consecutive days before surgery to kill free-floating tumor cells in the bile and destabilize the tumor cells that might be spilled during the operation. This concept of preventive, preoperative radiation therapy and dose regimen was based on a suggestion of a Mayo clinic study[2] and a study[3] in bladder carcinoma in which 3 times 3.5 Gy external radiation therapy had shown to be effective to reduce scar implementation metastases following surgery. Initial results of twenty-one patients with resected PHC who had undergone preoperative radiation, were promising with none of the patients developing seeding metastases[4].
Seeding metastases resulting from spill of bile during operation may become manifest in the tract of previous abdominal drains or laparotomy scar after resection. In addition, most patients with resectable PHC undergo preoperative biliary drainage which in itself, was thought to promote seeding metastases. Biliary drainage can be performed by two different approaches: Percutaneously or by the endoscopic route. Several studies have suggested that percutaneous biliary drainage carries an additional risk of developing seeding metastases along the catheter tract compared to endoscopic approaches, in which no catheter tract is created. Based on a described incidence of seeding metastases in the catheter tract after percutaneous biliary drainage of 2.6%-6.3%, several Asian authors[5-10] suggested that endoscopic biliary drainage should be the preferred approach.
The aim of this study was to update the occurrence of seeding metastases in our institution and evaluate the role of preventive pre-operative low-dose radiotherapy in patients with resectable PHC.
All consecutive patients who underwent resection for pathology proven PHC at the Amsterdam UMC (location AMC) between January 2000 and March 2019 were included in this retrospective study. The need for ethical approval was waived by the Medical Ethics Review Committee of the Amsterdam UMC, location AMC (W19_155).
Whereas patients with intraductal papillary neoplasm of the bile duct (IPNB) were included in this analysis, patients with other final pathology diagnosis were excluded. Patient and tumor characteristics were obtained from a prospectively maintained database including all patients undergoing resection for suspicion of PHC. Data regarding preoperative work-up, biliary drainage, radiotherapy, type of surgery, postoperative course, follow-up and recurrence was collected. Jaundiced patients were treated by endoscopic biliary drainage or percutaneous biliary drainage, or a combination of both, with a preference at our institution for initial endoscopic biliary drainage.
Patients were stratified according to type of biliary drainage, while patients undergoing both percutaneous and endoscopic biliary drainage were analyzed as a separate group. Surgical procedures generally consisted of resection of the extrahepatic bile duct in combination with (extended) hemihepatectomy. After 2009, intra-operative bile sampling for cytology was routinely performed in the majority of the patients. Results of bile cytology were compared between patients undergoing preoperative radiotherapy or not. Standard follow up after resection was 5 years. Imaging was not standard incorporated in oncological follow-up, but performed when patients showed symptoms of recurrent disease.
Between 2000 and January 2018, patients undergoing resection for PHC were irradiated to 3 times 3.5 Gy to the tumor in the liver hilum, administered on three consecutive days prior to resection. Up to 2004, the hilar area was identified using a conventional simulator, based on the position of the biliary stents. From 2004 onwards, computerized tomography (CT) based planning was used. Throughout, a 3D conformal technique was applied. Patients treated between 2000 and 2012, were also included in the study by Wiggers et al[11]. Primary outcome of this study was the occurrence of seeding metastases, defined as metastases occurring in the laparotomy scar or in tracts of previous abdominal drains. Recurrence was defined as radiologically suspected or pathologically proven recurrence and time of recurrence was documented. Peritoneal recurrence, unlike in other studies, was not considered as seeding metastases. Only the initial location of recurrence was registered.
Descriptive statistics were used to describe the data using statistical product and service solutions version 25 (International Business Machines Corporation, Armonk, NY, United States). The Chi-square test was used to compare the incidence of seeding metastases and the occurrence of tumor cells in bile cytology between groups. Duration of drainage was compared using Mann-Witney U test.
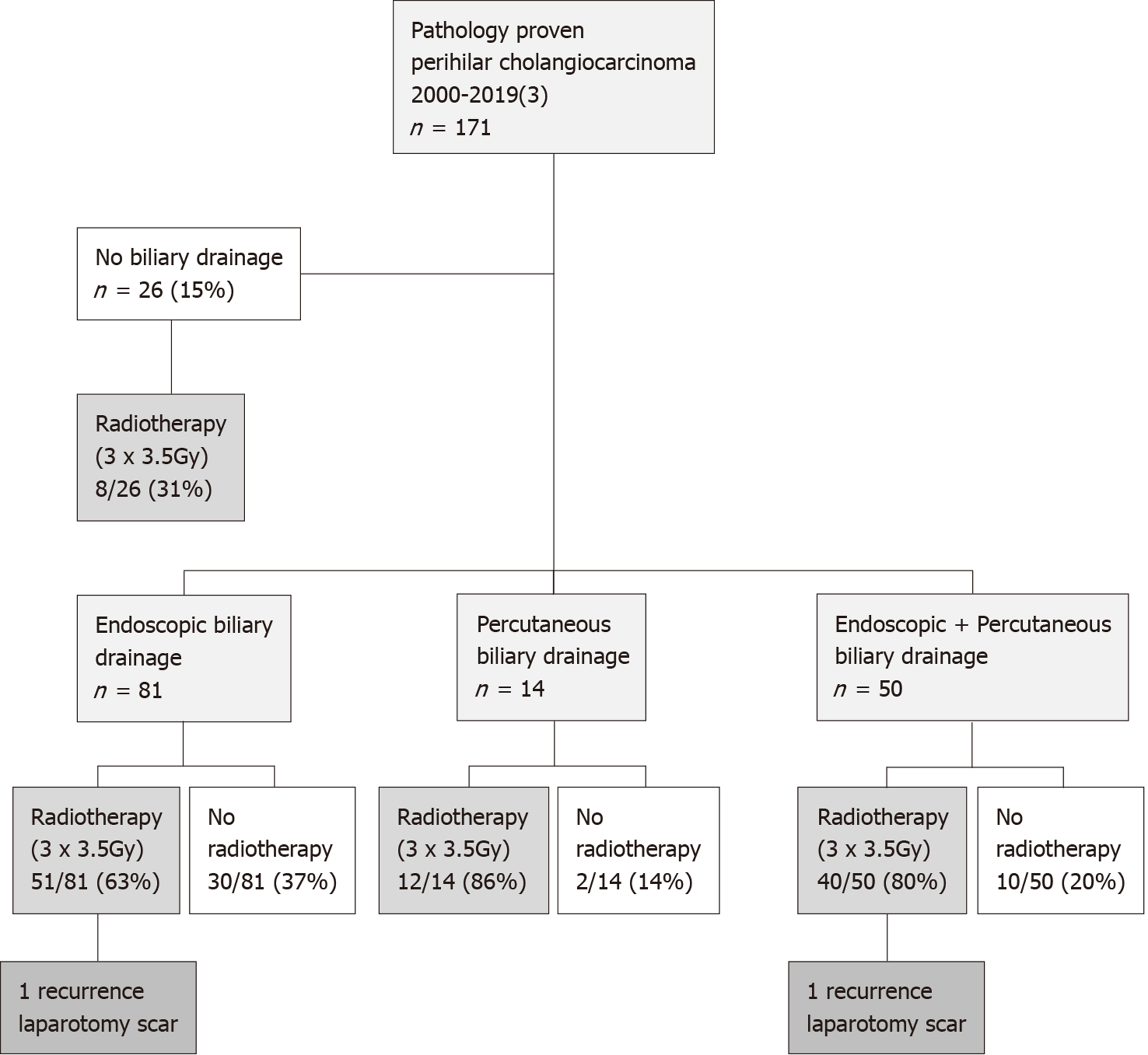
A total of 171 patients who underwent resection for pathology proven PHC between 2000 and March 2019 were identified (Table 1). The median follow-up of survivors was 36 mo [interquartile range (IQR): 16-81]. Biliary drainage prior to resection was performed in 145 patients (85%). In 81 (56%) patients endoscopic biliary drainage took place, 14 (10%) patients underwent percutaneous biliary drainage and 50 (34%) patients underwent both drainage routes, due to the absence of therapeutic success of one route. Median duration between (first) biliary drainage and surgery was 76 d (IQR: 53-101 d) for endoscopic drainage, 53 d (IQR: 36-80 d) for percutaneous biliary drainage and 76 d (range 61-99 d) for both endoscopic and percutaneous biliary drainage, respectively (P = 0.026). Of these 171 patients, there were 161 patients treated between January 2000-January 2018 and ten patients treated between February 2018 and March 2019.
| Characteristics | Patients with PHC (n = 171) |
| Age, mean ± SD | 64 (10) |
| Male, n (%) | 104 (61) |
| ASA, n (%) | |
| 1 | 30 (18) |
| 2 | 112 (65) |
| 3 | 27 (16) |
| Missing | 2 (1) |
| Drainage, n (%) | |
| None | 26 (15) |
| Endoscopic biliary drainage | 81 (48) |
| Percunatenous biliary drainage | 14 (8) |
| Both | 50 (29) |
| Bismuth-Corlette type, n (%) | |
| I | 4 (2) |
| II | 12 (7) |
| IIIa | 85 (50) |
| IIIb | 42 (25) |
| IV | 24 (14) |
| Missing | 4 (2) |
| Portal vein embolization, n (%) | 20 (12) |
| Radiotherapy, n (%) | 111 (65) |
| Resection type, n (%) | |
| Left hemihepatectomy | 61 (36) |
| Extended left hemihepatectomy | 6 (4) |
| Right hemihepatectomy | 40 (23) |
| Extended right hemihepatectomy | 47 (27) |
| Minor liver resection | 4 (2) |
| External bile duct resection only | 13 (8) |
| + Pancreatoduodenectomy | 4 (2) |
| + Portal vein reconstruction | 43 (26) |
| 90-d mortality, n (%) | 22 (13) |
| 90-d morbidity, n (%) | 126 (74) |
| Severe morbidity (Clavien-Dindo ≥ 3), n (%) | 96 (56) |
| Recurrence, n (%) | 61 (36) |
| Local recurrence | 37 (22) |
| Liver metastases | 10 (6) |
| Lung metastases | 2 (1) |
| Peritoneal metastases | 7 (4) |
| Other | 5 (3) |
A total of 111 patients (65%) underwent preoperative radiotherapy (3 times 3.5 Gy) to reduce the risk of seeding metastases (Figure 1). As part of another study[12], 13 patients were additionally treated with postoperative radiotherapy (as well as preoperative radiotherapy) between 2000 and 2001 (20 × 2.2 Gy).
After 2009, intraoperative bile cytology was obtained in 76 of 125 patients (61%). Bile cytology showed (suspicion of) tumor cells in 24 of 76 patients (32%) and was negative in 33 of 76 patients (45%). In the remaining patients, bile cytology was inconclusive in 9 (12%) and not assessable in 10 of 76 patients (14%). Malignant cells were found in the conclusive bile samples of 18 of 41 patients (44%) treated with radiotherapy and in 6 of 16 (37%) patients not treated with radiotherapy. No difference in the distribution of positive or negative bile cytology was observed between patients who were treated with preoperative radiotherapy or not (P = 0.660).
Two patients with metastases in the laparotomy scar (1.2%) were identified (Figure 1). Both patients had been treated with preoperative radiotherapy and one patient was additionally treated with postoperative radiotherapy. Histopathology showed moderately differentiated adenocarcinoma in both cases and both resections were R1 resections. The seeding metastases occurred 21 and 17 mo after resection, respectively. Overall, the incidence of seeding metastases was 1.4% (1 of 81 patients) after endoscopic biliary drainage, 2% (1 of 50 patients) after combined endoscopic and percutaneous biliary drainage, and 0% after both percutaneous biliary drainage (14 patients) and no biliary drainage (26 patients) (P = 0.852).
The hypothesis underlying this study was that low-dose preoperative radiotherapy eradicates free-floating vital tumor cells in bile, thereby reducing the risk of seeding metastases incurred during bile spill at the time of resection. The outcome of this study including 171 patients who had undergone resection for PHC, was that only two patients (1.2%) developed seeding metastases (both at the laparotomy scar). Intraoperative bile cytology showed no significant difference in the presence of tumor cells in bile of patients who underwent preoperative radiotherapy or not.
To our knowledge, literature on application of preoperative radiotherapy in patients with resectable PHC from other centers is lacking. As mentioned above, preoperative radiotherapy was introduced at our institution based on a 20% incidence of seeding metastases reported back in 1999 that has shown a striking decrease to currently 1.2%, suggesting a positive effect of preoperative radiotherapy. However, Wiggers et al[11] published a combined analysis of 234 patients from our institution (Academic Medical Center, Amsterdam and Memorial Sloan Kettering Cancer Center, New York)[11]. This study included 106 patients from the Academic Medical Center (Amsterdam) who were subjected to preoperative radiotherapy and 128 patients from the Memorial Sloan Kettering Cancer Center (New York) were no preoperative radiotherapy is given. The difference in the percentage of seeding metastases between both centers (2 of 106; 1.9% vs 5 of 128; 3.9% P = 0.46) was not significant and the overall incidence was low (3.0%)[11]. Therefore, evidence of a potential benefit of low dose preoperative radiotherapy cannot be delivered, since this would require a randomized study with approximately 1200 patients per arm, which is not feasible for such a rare disease. In view of the incidence of local and distant recurrences and the overall prognosis of PHC, the overall incidence of seeding metastases is clinically less relevant. Hence, in January 2018 we decided to discontinue low dose preoperative radiotherapy. Interestingly, the potential of treatment with stereotactic body radiation therapy for patients with unresectable perihilar cholangiocarcinoma is currently being investigated by multiple group[13,14].
The concept of 3 times 3.5 Gy preoperative radiation therapy to reduce the risk of seeding metastases was based on a study in bladder carcinoma first published in 1969[15]. Follow-up studies on the effect of preoperative low-dose radiotherapy in bladder carcinoma however, are lacking while low-dose radiotherapy seems to have been abandoned in these patients. In current literature, indeed, a trend of a lower seeding metastases rate is observed (Table 2)[5-10,16]. For example, incidence reported by investigators from Hokkaido University Hospital decreased from 6.3% (3 of 48)[5] to 4.5% (3 of 67)[16] in their publications from 2011 and 2014, respectively. Likewise in Nagoya University Hospital, catheter tract recurrence was reported to be 5.6% (19 of 339) in a study performed by Kim et al[10] in 2015[10], whilst this decreased to 3.6% (6 of 168) in a study published in 2017[6]. Surgical techniques for resection of PHC have been refined reducing bile spill, which may have contributed to this decrease.
| Incidence | Patients | |
| Sakata et al[8], 2005 | 3/67 (4.4%), cumulative 6% | Niigata University Graduate School of Medical and Dental Sciences; Percutaneous biliary drainage, 1998-2002 |
| Kang et al[9], 2013 | 6/232 (2.6%) + 8 abdominal wall/wound implant metastases (3.4%) | Seoul National University Hospital; Percutaneous biliary drainage, 1991-2011 |
| Kim et al[10], 2015 | 2/52 (3.8%) | Samsung Medical Center Seoul; Percutaneous biliary drainage, 2000-2012 |
| Kawakami et al[5], 2011 | 3/48 (6.3%) | Hokkaido University Hospital; Percutaneous biliary drainage, 1999-2009 |
| Hirano et al[16], 2014 | 3/67 (4.5%) | Hokkaido University hospital; Percutaneous biliary drainage, 2000-2008 |
| Takahashi et al[7], 2010 | 19/339 (5.6%) | Nagoya University hospital, 1977-2007 |
| Komaya et al[6], 2017 | 6/168 (3.6%) | Nagoya University Hospital Percutaneous biliary drainage, 2003-2012 |
| Ten Hoopen et al[1], 1999 | 8/41 (20%) | AMC Amsterdam; Biliary drainage, 1983-1990 |
| Wiggers et al[11], 2013 | 2/106 (1.9%) | AMC Amsterdam; Percutaenous + endoscopic biliary drainage, 1991-2012 |
Our reported incidence of 1.2%, based one of the largest series on this topic, remains lower than the incidence reported in other studies (up to 6.3%)[5-10,16]. Several factors may contribute to the development of seeding metastases. As mentioned above, preoperative biliary drainage is thought to increase the risk of seeding metastases by enhancing bile contamination with tumor cells. There are some drainage related factors that potentially influence the development of seeding metastases, one of them being the duration of biliary drainage. A recently published study by Komaya et al[6], including 341 resected PHC patients, did not describe the duration of biliary drainage in their patients, although in another study originating from the same group, duration of percutaneous biliary drainage of 60 d or more was identified as an independent risk factor for the development of catheter tract recurrence in distal and perihilar cholangiocarcinoma[7]. In other studies reporting on seeding metastases, duration of biliary drainage was often not reported. In our series, the median duration of biliary drainage of 73 d in case of endoscopic biliary drainage and 77 d after percutaneous biliary drainage. Although this would suggest an increased risk in the majority of our patients, the overall incidence was low (1.2%). Therefore, we cannot fully exclude the possibility that there was an effect of preoperative radiotherapy based on this series alone.
Related to biliary drainage, the type (endoscopic or percutaneous), placement (proximal or transtumoral) may also play a role. Komaya et al[6] showed that the incidence of seeding metastases was higher after percutaneous biliary drainage (44 of 165, 26.7%) compared to endoscopic biliary drainage (25 of 150, 16.7%). Their definition of seeding metastases included right-sided pleural dissemination as well as peritoneal dissemination and catheter tract recurrences, due to the use of the transpleural approach of percutaneous biliary drainage in this cohort, resulting in relatively high seeding metastases rates. Separate analysis of catheter tract recurrence, showed occurrence of catheter tract recurrence in 6 of 164 patients (3.7%) undergoing percutaneous drainage, while recurrence at the laparotomy scar was not described[6]. In contrary, the previously mentioned study by Wiggers including 234 patients showed no significant difference in the occurrence of seeding metastasis between different drainage approaches: 3.4% after percutaneous and 2.7% after endoscopic biliary drainage (P = 0.71)[11].
This study has several limitations, the main limitation being the retrospective design of this single-center study, introducing a significant risk of bias. A direct comparison between patients undergoing preoperative radiation to those who did not is not possible within this study. Before January 2018, patients who did not undergo preoperative radiotherapy (35%) were not randomly assigned and most likely there was a reason not to treat them with preoperative radiotherapy (for example because they did not undergo biliary drainage or for logistic reasons). For some patients treated after stopping preoperative radiotherapy in January 2018, follow-up might be too short for seeding metastases to develop, as seeding metastases occurred after a median of 17 mo in the study by Wiggers et al[11]. In addition, due to the absence of standardized follow-up imaging after resection, not all seeding metastases or recurrences may have been detected. When comparing cytology results of patients with and without radiotherapy, we could not account for viability of the cells. Although cells may stain positively, this does not mean that these cells are still viable and therefore, we could have overlooked an effect of radiotherapy. Due to the overall low number of events (seeding metastases), proper statistical analysis was not feasible in this series and confidence limits are huge.
The incidence of seeding metastases in patients with resected PHC has decreased and is currently low (1.2% in our institution). As the only center in the world reporting on preventive low-dose radiotherapy, we were unable to deliver evidence of a potential benefit of preoperative radiotherapy and therefore preoperative radiotherapy in patients with resectable PHC has been discontinued in our institution.
Routine preoperative radiotherapy (3 times 3.5 Gray) to prevent the occurrence of seeding metastases was used in our tertiary center for 28 years in patients undergoing resection for perihilar cholangiocarcinoma.
Previous research from our department showed that seeding metastases occurred in up to 20% of the patients undergoing resection of perihilar cholangiocarcinoma.
To investigate the occurrence of seeding metastases among patients with resectable perihilar cholangiocarcinoma.
A retrospective study was conducted, including all patients undergoing resection of perihilar cholangiocarcinoma in a larger tertiary center between 2000 and March 2019.
Seeding metastases occurred in 2 out of 171 patients (1.2%) undergoing resection for perihilar cholangiocarcinoma. These seeding metastases occurred at the laparotomy scar in both patients, after 17 and 21 mo, respectively. Intraoperative bile cytology showed no significant difference in the presence of tumor cells in the bile of patients undergoing preoperative radiotherapy or not.
The incidence of seeding metastases in patients with resected perihilar cholangiocarcinoma has decreased. Evidence of a potential benefit of preoperative radiotherapy could not be delivered and therefore preoperative radiotherapy in patients with resectable perihilar cholangiocarcinoma has been discontinued in our institution.
As we are the only center reporting on the use of low-dose radiotherapy to prevent seeding metastases in patients with perihilar cholangiocarcinoma, it is unlikely that other reports on this topic will appear and future research may focus more on the potential of stereotactic body radiation therapy to treat patients with unresectable perihilar cholangiocarcinoma.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coco D, Sperti C S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
| 1. | ten Hoopen-Neumann H, Gerhards MF, van Gulik TM, Bosma A, Verbeek PC, Gouma DJ. Occurrence of implantation metastases after resection of Klatskin tumors. Dig Surg. 1999;16:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Buskirk SJ, Gunderson LL, Schild SE, Bender CE, Williams HJ, McIlrath DC, Robinow JS, Tremaine WJ, Martin JK. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg. 1992;215:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | van der Werf-Messing BH. Cancer of the urinary bladder treated by interstitial radium implant. Int J Radiat Oncol Biol Phys. 1978;4:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Gerhards MF, Gonzalez DG, ten Hoopen-Neumann H, van Gulik TM, de Wit LT, Gouma DJ. Prevention of implantation metastases after resection of proximal bile duct tumours with pre-operative low dose radiation therapy. Eur J Surg Oncol. 2000;26:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kawakami H, Kuwatani M, Onodera M, Haba S, Eto K, Ehira N, Yamato H, Kudo T, Tanaka E, Hirano S, Kondo S, Asaka M. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011;46:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Verification of the oncologic inferiority of percutaneous biliary drainage to endoscopic drainage: A propensity score matching analysis of resectable perihilar cholangiocarcinoma. Surgery. 2017;161:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg. 2010;97:1860-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Sakata J, Shirai Y, Wakai T, Nomura T, Sakata E, Hatakeyama K. Catheter tract implantation metastases associated with percutaneous biliary drainage for extrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:7024-7027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Kang MJ, Choi YS, Jang JY, Han IW, Kim SW. Catheter tract recurrence after percutaneous biliary drainage for hilar cholangiocarcinoma. World J Surg. 2013;37:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Kim KM, Park JW, Lee JK, Lee KH, Lee KT, Shim SG. A Comparison of Preoperative Biliary Drainage Methods for Perihilar Cholangiocarcinoma: Endoscopic vs Percutaneous Transhepatic Biliary Drainage. Gut Liver. 2015;9:791-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Wiggers JK, Groot Koerkamp B, Coelen RJ, Doussot A, van Dieren S, Rauws EA, Schattner MA, van Lienden KP, Brown KT, Besselink MG, van Tienhoven G, Allen PJ, Busch OR, D'Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, Verheij J, Jarnagin WR, van Gulik TM. Percutaneous Preoperative Biliary Drainage for Resectable Perihilar Cholangiocarcinoma: No Association with Survival and No Increase in Seeding Metastases. Ann Surg Oncol. 2015;22 Suppl 3:S1156-S1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Gerhards MF, van Gulik TM, González González D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Kozak MM, Toesca DAS, von Eyben R, Pollom EL, Chang DT. Stereotactic Body Radiation Therapy for Cholangiocarcinoma: Optimizing Locoregional Control with Elective Nodal Irradiation. Adv Radiat Oncol. 2020;5:77-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Koedijk MS, Heijmen BJM, Groot Koerkamp B, Eskens FALM, Sprengers D, Poley JW, van Gent DC, van der Laan LJW, van der Holt B, Willemssen FEJA, Méndez Romero A. Protocol for the STRONG trial: stereotactic body radiation therapy following chemotherapy for unresectable perihilar cholangiocarcinoma, a phase I feasibility study. BMJ Open. 2018;8:e020731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | van der Werf-Messing B. Carcinoma of the bladder treated by suprapubic radium implants. The value of additional external irradiation. Eur J Cancer. 1969;5:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Hirano S, Tanaka E, Tsuchikawa T, Matsumoto J, Kawakami H, Nakamura T, Kurashima Y, Ebihara Y, Shichinohe T. Oncological benefit of preoperative endoscopic biliary drainage in patients with hilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |