Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1046
Peer-review started: June 23, 2020
First decision: July 30, 2020
Revised: August 10, 2020
Accepted: September 2, 2020
Article in press: September 2, 2020
Published online: November 27, 2020
Processing time: 154 Days and 7.3 Hours
In hepatocellular carcinoma (HCC), detection and treatment prior to growth beyond 2 cm are relevant as a larger tumor size is more frequently associated with microvascular invasion and/or satellites.
To examine the impact of the tumor marker alpha-fetoprotein (AFP) or PIVKA-II in detecting very small HCC nodules (≤ 2 cm in maximum diameter, Barcelona stage 0) in the large number of very small HCC. The difference in the behavior of these tumor markers in HCC development was also examined.
A total of 933 patients with single-nodule HCC were examined. They were subdivided into 394 patients with HCC nodules ≤ 2 cm in maximum diameter and 539 patients whose nodules were > 2 cm. The rates of patients whose AFP and PIVKA-II showed normal values were examined.
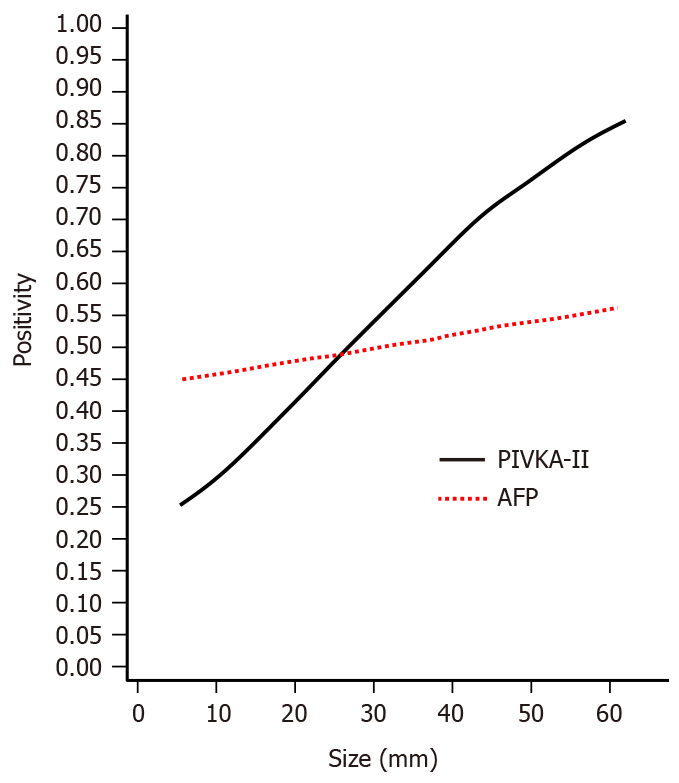
The positive ratio of the marker PIVKA-II was significantly different (P < 0.0001) between patients with nodules ≤ 2 cm in diameter and those with nodules > 2 cm, but there was no significant difference in AFP (P = 0.4254). In the patients whose tumor was ≤ 2 cm, 50.5% showed normal levels in AFP and 68.8% showed normal levels in PIVKA-II. In 36.4% of those patients, both AFP and PIVKA-II showed normal levels. The PIVKA-II-positive ratio was markedly increased with an increase in the tumor size. In contrast, the positivity in AFP was increased gradually and slowly.
In the surveillance of very small HCC nodules (≤ 2 cm in diameter, Barcelona clinical stage 0) the tumor markers AFP and PIVKA-II are not so useful.
Core Tip: In hepatocellular carcinoma, detection and treatment prior to nodule growth of 2 cm (Barcelona stage 0) are relevant as a larger tumor size is more frequently associated with microvascular invasion and/or satellites. We surveyed the real impact of the tumor markers alpha-fetoprotein (AFP) or PIVKA-II in detecting very small hepatocellular carcinoma with a large number of cases (≤ 2 cm in diameter 394 cases) and found in AFP that 50.5% and in PIVKA-II that 68.8% showed normal levels. Moreover, 36.4% of the patients showed normal levels in both AFP and PIVKA-II. In the surveillance of very small hepatocellular carcinoma nodules, the tumor markers are not so useful.
- Citation: Tarao K, Nozaki A, Komatsu H, Komatsu T, Taguri M, Tanaka K, Chuma M, Numata K, Maeda S. Real impact of tumor marker AFP and PIVKA-II in detecting very small hepatocellular carcinoma (≤ 2 cm, Barcelona stage 0) - assessment with large number of cases. World J Hepatol 2020; 12(11): 1046-1054
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1046.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1046
For the detection of hepatocellular carcinoma (HCC) from various liver diseases, especially liver cirrhosis, surveillance with the tumor markers, alpha-fetoprotein (AFP) and PIVKA-II, or detection with the imaging modalities, ultrasonography (US) or magnetic resonance imaging (MRI) [computed tomography (CT)], is usually performed.
Detection and treatment prior to growth beyond 2 cm are relevant as a larger tumor size is more frequently associated with microvascular invasion and/or satellites, which are major predictors of recurrence after initial effective treatment[1]. The same tendency was observed by Stravitz et al[2], and they reported that the early detection of HCC improves the prognosis.
Therefore, we must identify minute HCC nodules (≤ 2 cm in diameter) in the surveillance of HCC. Previous reports concerning the tumor markers AFP and PIVKA-II in very small HCCs included a relatively small number of cases. In this retrospective analysis, we examined the precise levels of these markers in a large number of very small HCC cases (< 2 cm in diameter, 394 cases) and whether the tumor marker AFP or PIVKA-II is useful to find very small HCC (≤ 2 cm in maximum diameter, Barcelona clinic liver cancer staging 0)[3,4]. Also, we examined the difference in the behavior of these tumor markers in relation to the tumor size of HCC nodules.
This was a retrospective study that included 933 patients with single HCC nodules who entered the following three hospitals in Yokohama City for the first time, between January 2008 and January 2019: Gastroenterological Center, Yokohama City University Medical Center, Department of Gastroenterology, Yokohama Municipal Citizen's Hospital, Department of Clinical Research, National Hospital Organization Yokohama Medical Center. HCCs were diagnosed chiefly by dynamic CT and abdominal angiography, which showed early enhancement and early wash out. This work was performed in accordance with the Declaration of Helsinki.
Previously diagnosed HCC was excluded from the protocol. This study was performed after approval by the respective institutional review boards.
The patients were classified according to etiologies of liver diseases: 72 with hepatitis B (presence of hepatitis B surface antigen in serum), 540 with hepatitis C (presence of hepatitis C antibody in serum), 10 with primary biliary cholangitis, five with autoimmune hepatitis, 70 with alcoholic liver diseases, and others (Table 1).
| Maximum diameter of nodules | |||
| ≤ 20 mm | > 20 mm | ||
| Number of patients | 394 | 539 | |
| Sex | Male | 230 (58.4%) | 392 (72.7%) |
| Female | 164 (41.6%) | 147 (22.3%) | |
| Age in yr | 71.3 ± 8.8 | 72.5 ± 10.1 | |
| Etiology | HBV | 33 (8.4%) | 39 (7.2%) |
| HCV | 274 (69.5%) | 266 (49.4%) | |
| PBC | 4 (1.0%) | 6 (1.1%) | |
| Alcohol | 17 (4.3%) | 53 (9.8%) | |
| NASH | 4 (1.0%) | 8 (1.5%) | |
| Autoimmune hepatitis | 3 (0.8%) | 2 (0.4%) | |
| Unknown | 23 (5.8%) | 62 (11.5%) | |
Samples were collected before the treatment for HCC. Concentrations of PIVKA-II and AFP in serum samples were determined by the chemiluminescent enzyme immunoassay in all three hospitals, and the cutoff values for PIVKA-II and AFP were 40 mAU/mL and 10 ng/mL, respectively, in every hospital. For PIVKA-II and AFP, ≤ 40 mAU/mL and ≤ 10 ng/mL were set as normal values, respectively.
The diagnosis of HCC was confirmed by US, MRI, CT, enhanced dynamic CT, and abdominal angiography. All patients underwent abdominal angiography to confirm the single nodules. The maximum diameter of the HCC nodules was scaled by US or MRI.
Helical dynamic CT and abdominal angiography were performed in almost all patients except the patients with hypersensitivity to iodine and with advanced kidney disease. In the helical dynamic CT, an intravenous bolus injection of contrast material and sequential scanning were performed, and intense homogenous arterial-phase (early enhancement) and early washout were thought to be characteristic of HCC[5-7]. Abdominal angiography was also performed to exclude the benign nodular lesions and to exclude the HCC patients with macrovascular invasion.
The patients with macrovascular invasion or extrahepatic metastasis were excluded. In the hepatectomy performed patients, final decision of HCC was made by pathological diagnosis and cases of benign nodules were excluded.
For the comparisons of test-positive proportions between > 2 cm and ≤ 2 cm tumors, we conducted chi-squared tests for AFP and PIVKA-II, respectively. To understand the relationships between the tumor size and test-positive proportions for AFP and PIVKA-II, we applied logistic regression models using the tumor size as an independent variable and test results (positive or not) as the dependent variable. All reported P values correspond to two-sided tests, and P < 0.05 was considered significant. All analyses were performed with Statistics Analysis System, version 9.4 (Statistics Analysis System Institute, Cary, NC, United States).
The clinical characteristics of the patients are summarized in Table 1. Our study included 933 HCC patients with a single nodule. In total, 622 patients were male, and 311 patients were female. The average age was 72.0 ± 9.6 years. Concerning the tumor size at diagnosis, 394 patients had HCC nodules ≤ 2 cm in maximum diameter, and 539 patients had nodules larger than 2 cm in maximum diameter.
The positive rates of AFP and PIVKA-II in patients whose tumor was ≤ 2 cm and those whose tumor size was more than 2 cm are shown in Table 2. The level of PIVKA-II showed a significant difference (P < 0.0001), but there was no difference in AFP (P = 0.4254).
| Maximum diameter of HCC nodules | P value1 | |||
| ≤ 2 cm, n = 394 | > 2 cm, n = 539 | |||
| AFP | (+) | 195 (49.5%) | 281 (52.1%) | 0.4254 |
| (-) | 199 (50.5%) | 258 (47.9%) | ||
| PIVKA | (+) | 123 (31.2%) | 385 (71.4%) | < 0.0001 |
| (-) | 271 (68.8%) | 154 (28.6%) | ||
Table 3 shows the rates of patients whose AFP and PIVKA-II exhibited normal values in those with a maximum tumor size of ≤ 2 cm. In AFP, 50.5% showed normal levels, and in PIVKA-II, 68.8% showed normal levels. A more important finding was that, in 36.4% of the patients, both AFP and PIVKA-II showed normal levels.
| HCC tumor marker | No. of cases (%) |
| AFP, normal cases | 199 (50.5) |
| PIVKA-II, normal cases | 271 (68.8) |
| Both AFP and PIVKA-II, normal cases | 142 (36.4) |
Table 4 shows the treatment methods of all HCC patients. In the very small HCC patients (≤ 2 cm), the radiofrequency ablation group occupied the majority. In the relatively large HCC group (> 2 cm), treatment by transcatheter arterial chemoembolization was the most frequent, followed by hepatectomy and radiofrequency ablation.
| Therapy | No of treated patients | |
| Group | ||
| ≤ 2 cm, n = 394 | > 2 cm, n = 539 | |
| Hepatectomy | 45 | 110 |
| RFA | 223 | 107 |
| TACE | 56 | 136 |
| TACE + RFA | 6 | 32 |
| TAI | 2 | 10 |
| Chemotherapy | 9 | 21 |
| BSC | 10 | 60 |
| Others | 13 | 63 |
Figure 1 shows the relationship between the tumor size and PIVKA-II and AFP positivity. The PIVKA-II positive ratio was markedly increased with an increase in tumor size. In contrast, the positivity in AFP was increased gradually and slowly.
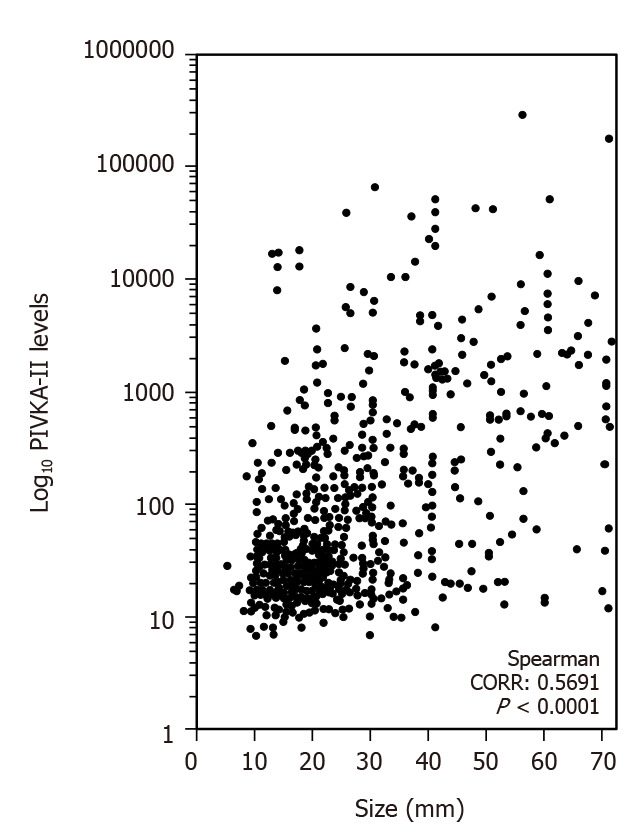
Figure 2 shows the correlation between the tumor size and PIVKA-II levels. The correlation ratio was 0.5691 (P < 0.0001).
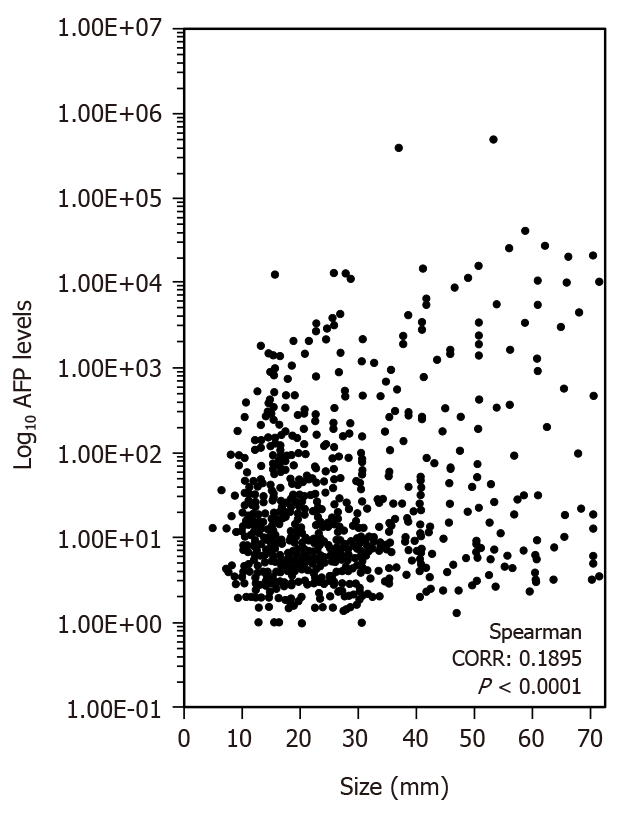
Figure 3 shows the correlation between the tumor size and AFP levels. The correlation ratio was 0.1895 (P < 0.0001).
Although the early detection of HCC with imaging modalities has been developed in recent years, tumor markers are still commonly used in HCC detection and follow-up.
We demonstrated in this study that 36.4% of the patients whose maximum diameter of HCC nodules was equal to or less than 2.0 cm (Barcelona stage 0) showed normal levels of both AFP and PIVKA-II. In support of our results, it was reported that about 30% of HCC patients show false-negative results regarding tumor markers, especially in its early stage[4-10]. Moreover, it was demonstrated that AFP has a sensitivity of about 68% in the diagnosis of HCC, but the sensitivity decreased to about 59% in its early stage[11,12].
In recent years, Huang et al[13] demonstrated that PIVKA-II combined with AFP showed a better diagnostic ability than AFP alone for HCC diagnosis. However, our study confirmed the limitation in detecting HCC in patients with very small single HCC nodules (≤ 2 cm) (Barcelona clinic liver cancer staging 0)[3,4], even in combination with AFP and PIVKA-II.
We demonstrated that more than a third of the patients with minute HCC nodules (≤ 2 cm in diameter) were dropped from surveillance using the tumor markers AFP and PIVKA-II alone. Based on the results, we must depend on imaging modalities such as US or MRI (CT) for the surveillance of minute HCC.
Colli et al[14] conducted a systemic review on this issue and found that pooled estimates of 14 US studies were 60.5% (95% confidence interval (CI): 44-76) for sensitivity[13-29], and that nine MRI studies were 80.6% (95%CI: 70-91) for sensitivity[27,30-37]. The difference in sensitivity between US and MRI may be due to the fact that MRI is less influenced by the operator’s technique and patient's body type.
More recently, Kim et al[38] compared MRI and US in a cohort of 407 patients with cirrhosis who underwent 1100 surveillance examinations and found that MRI had a sensitivity of 83.7% (95%CI: 69.7%-92.2%) for early HCC detection, which was significantly higher than US (25.6%, 95%CI: 14.8%-49.4%). Thus, we must follow-up patients with liver disease, especially liver cirrhosis, at regular intervals (at least every 6 mo) with MRI to detect very small HCC (diameter ≤ 2 cm).
Furthermore, we demonstrated that the PIVKA-II positive ratio was markedly increased with an increase in tumor size. In support of this phenomenon, previous studies established the correlation between the PIVKA-II level and tumor size[16], and that PIVKA-II maintains the growth of HCC[17]. Moreover, Ma et al[18] reported direct clinical evidence of the correlation between PIVKA-II and cell proliferation.
More than one third of the patients with very small HCC nodule (≤ 2 cm in diameter, Barcelona stage 0) were dropped from the surveillance using the tumor markers AFP and PIVKA-II. So, we must survey patients with liver diseases by MRI at regular intervals to detect very small HCC nodules.
In hepatocellular carcinoma (HCC), detection and treatment prior to growth of 2 cm are relevant as a larger tumor size is more frequently associated with microvascular invasion and/or satellites. However, we often experience cases whose tumor size was ≤ 2 cm and who showed normal values in both AFP and PIVKA-II.
Previous reports concerning the tumor markers AFP or PIVKA-II in very small HCC included relatively small number of cases, and a larger study is necessary in order to elucidate the precise levels of these markers.
In the present study, we surveyed the levels of AFP and PIVKA-II in a large number of very small HCC cases (≤ 2 cm in diameter, 394 cases).
We analyzed 933 patients with single HCC nodules and surveyed the limitation of these tumor markers in the surveillance of very small HCC (≤ 2 cm, Barcelona stage 0, 394 cases).
It was found in patients with very small HCC (≤ 2 cm in diameter) that AFP and PIVKA-II levels were normal in 50.5% and 68.8%, respectively. Moreover, 36.4% of the patients showed normal levels of both AFP and PIVKA-II. We examined the difference in behavior of these tumor markers in relation to the size of HCC nodules and found that PIVKA-II positive ratio was markedly increased with an increase in tumor size, whereas the positivity in AFP was increased gradually and slowly.
More than one third of the patients with very small HCC nodule (≤ 2 cm in diameter, Barcelona stage 0) were dropped from the surveillance using the tumor markers AFP and PIVKA-II.
We propose that for detecting very small HCC nodules, we must survey patients with liver diseases by imaging modalities, especially by magnetic resonance imaging.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang GY, Zhong JH S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3597] [Article Influence: 276.7] [Reference Citation Analysis (4)] |
| 2. | Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, Maluf DG, Cotterell AH, Posner MP, Fisher RA. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327-10335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 131] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 4. | Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S. Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol. 2008;15:1670-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Nonomura A, Nakanuma Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999;172:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Ueda K, Terada T, Nakanuma Y, Matsui O. Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Hum Pathol. 1992;23:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 109] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Roncalli M, Roz E, Coggi G, Di Rocco MG, Bossi P, Minola E, Gambacorta M, Borzio M. The vascular profile of regenerative and dysplastic nodules of the cirrhotic liver: implications for diagnosis and classification. Hepatology. 1999;30:1174-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Matsuda M, Asakawa M, Amemiya H, Fujii H. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol. 2011;26:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Cui R, He J, Zhang F, Wang B, Ding H, Shen H, Li Y, Chen X. Diagnostic value of protein induced by vitamin K absence (PIVKA-II) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer. 2003;88:1878-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, Oh EJ. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol. 2013;19:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 12. | Song P, Feng X, Inagaki Y, Song T, Zhang K, Wang Z, Zheng S, Ma K, Li Q, Kong D, Wu Q, Zhang T, Zhao X, Hasegawa K, Sugawara Y, Kokudo N, Tang W; Japan-China Joint Team for Medical Research and Cooperation on HCC. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-γ-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Biosci Trends. 2014;8:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Huang S, Jiang F, Wang Y, Yu Y, Ren S, Wang X, Yin P, Lou J. Diagnostic performance of tumor markers AFP and PIVKA-II in Chinese hepatocellular carcinoma patients. Tumour Biol. 2017;39:1010428317705763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (4)] |
| 16. | Shirabe K, Toshima T, Kimura K, Yamashita Y, Ikeda T, Ikegami T, Yoshizumi T, Abe K, Aishima S, Maehara Y. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int. 2014;34:937-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Cui SX, Zhang YS, Chu JH, Song ZY, Qu XJ. Des-gamma-carboxy prothrombin (DCP) antagonizes the effects of gefitinib on human hepatocellular carcinoma cells. Cell Physiol Biochem. 2015;35:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Ma XL, Zhu J, Wu J, Tian L, Gao YY, Zhang CY, Zhou Y, Dai Q, Wang BL, Pan BS, Zhou J, Fan J, Yang XR, Guo W. Significance of PIVKA-II levels for predicting microvascular invasion and tumor cell proliferation in Chinese patients with hepatitis B virus-associated hepatocellular carcinoma. Oncol Lett. 2018;15:8396-8404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Okazaki N, Yoshida T, Yoshino M, Matue H. Screening of patients with chronic liver disease for hepatocellular carcinoma by ultrasonography. Clin Oncol. 1984;10:241-246. [PubMed] |
| 20. | Maringhini A, Cottone M, Sciarrino E, Marcenò MP, La Seta F, Rinaldi F, Pagliaro L. Ultrasonographic and radionuclide detection of hepatocellular carcinoma in cirrhotics with low alpha-fetoprotein levels. Cancer. 1984;54:2924-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Kobayashi K, Sugimoto T, Makino H, Kumagai M, Unoura M, Tanaka N, Kato Y, Hattori N. Screening methods for early detection of hepatocellular carcinoma. Hepatology. 1985;5:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Tanaka S, Kitamura T, Ohshima A, Umeda K, Okuda S, Ohtani T, Tatsuta M, Yamamoto K. Diagnostic accuracy of ultrasonography for hepatocellular carcinoma. Cancer. 1986;58:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Dodd GD 3rd, Miller WJ, Baron RL, Skolnick ML, Campbell WL. Detection of malignant tumors in end-stage cirrhotic livers: efficacy of sonography as a screening technique. AJR Am J Roentgenol. 1992;159:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 92] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Saada J, Bhattacharya S, Dhillon AP, Dick R, Burroughs AK, Rolles K, Davidson BR. Detection of small hepatocellular carcinomas in cirrhotic livers using iodised oil computed tomography. Gut. 1997;41:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Chalasani N, Horlander JC Sr, Said A, Hoen H, Kopecky KK, Stockberger SM Jr, Manam R, Kwo PY, Lumeng L. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988-2993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95:1535-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL, Ducerf C. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 28. | Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med. 2001;20:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Bennett GL, Krinsky GA, Abitbol RJ, Kim SY, Theise ND, Teperman LW. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol. 2002;179:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, Brown JJ, McFarland EG, Middleton WD, Balfe DM, Ritter JH. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Born M, Layer G, Kreft B, Schwarz N, Schild H. [MRI, CT and CT arterial portography in the diagnosis of malignant liver tumors in liver cirrhosis]. Rofo. 1998;168:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, Brú C, Bruix J; Barcelona Clínic Liver Cancer Group. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | de Lédinghen V, Laharie D, Lecesne R, Le Bail B, Winnock M, Bernard PH, Saric J, Couzigou P, Balabaud C, Bioulac-Sage P, Drouillard J. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? Eur J Gastroenterol Hepatol. 2002;14:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR Am J Roentgenol. 2003;180:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Mori K, Scheidler J, Helmberger T, Holzknecht N, Schauer R, Schirren CA, Bittmann I, Dugas M, Reiser M. Detection of malignant hepatic lesions before orthotopic liver transplantation: accuracy of ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 2002;179:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, Lee SJ, Lee HC, Lee YS. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |