Published online Oct 27, 2020. doi: 10.4254/wjh.v12.i10.766
Peer-review started: February 27, 2020
First decision: April 25, 2020
Revised: June 2, 2020
Accepted: August 15, 2020
Article in press: August 15, 2020
Published online: October 27, 2020
Processing time: 239 Days and 19.6 Hours
Angiogenesis plays an important role in the occurrence and development of tumors. Registered tyrosine kinase inhibitors targeting vascular endothelial growth factor reduce angiogenesis. Apatinib, a tyrosine kinase inhibitor, can specifically inhibit vascular endothelial growth factor receptor 2, showing encouraging anti-tumor effects in a variety of tumors including advanced hepatocellular carcinoma (HCC). This article intends to review the clinical research and application prospects of apatinib in the field of HCC.
Core Tip: Apatinib, as a tyrosine kinase inhibitor, has a good inhibitory effect on advanced hepatocellular carcinoma (HCC). In this article, we will introduce the role of apatinib in advanced HCC from the aspects of structure and mechanism, pharmacokinetics, preclinical studies, clinical trials, side effects, and combined drug use.
- Citation: Zhang XH, Cao MQ, Li XX, Zhang T. Apatinib as an alternative therapy for advanced hepatocellular carcinoma. World J Hepatol 2020; 12(10): 766-774
- URL: https://www.wjgnet.com/1948-5182/full/v12/i10/766.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i10.766
Hepatocellular carcinoma (HCC) is the third most common malignant tumor in China. Its 5-year survival rate is only 14.1%, which seriously threatens people's health and life[1]. Asymptomatic or insignificant symptoms are common in the early course of the disease. About 70%-85% of patients are in advanced stage at the time of diagnosis[2], and the natural survival time is only 4.2 mo in the Asia-Pacific region and 7.9 mo in Europe[3,4]. For patients who have no opportunity for surgery or metastasis after treatment, effective systemic treatment is necessary.
In the "Guidelines Insights: Hepatobiliary Cancers, Version 2.2019", first-line targeted drugs for palliative systemic therapy include sorafenib and lenvatinib[5]. As a multi-target kinase inhibitor, sorafenib can inhibit the proliferation of HCC cells through the RAF/MEK/ERK signaling pathway and block the angiogenesis by inhibiting vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptors (PDGFRs)[6]. Two phase III clinical trials confirmed that sorafenib prolonged the overall survival (OS) by 2.3-3.2 mo, while the objective response rate (ORR) was 2% to 3.3%[3,4]. The effect of lenvatinib is not inferior to sorafenib, while OS and progression free survival (PFS) are improved compared with the latter. However, the therapeutic effect is still not very satisfying[7].
Apatinib mesylate (YN968D1) is a highly specific small molecule VEGFR-2 tyrosine kinase inhibitor, preventing its downstream signaling pathways, blocking the migration and proliferation of vascular endothelial cells, reducing tumor microvessel density, and inhibiting tumor angiogenesis[8-11]. With the announcement of the results of phase I and phase II clinical trials, the China Food and Drug Administration (CFDA) approved apatinib as the third-line treatment for advanced gastric cancer or adenocarcinoma of the gastroesophageal junction in October 2014.
In this review, we summarize the structure, mechanism and pharmacokinetic characteristics of apatinib, overview the current data of apatinib in clinical studies, and propose future development directions of HCC.
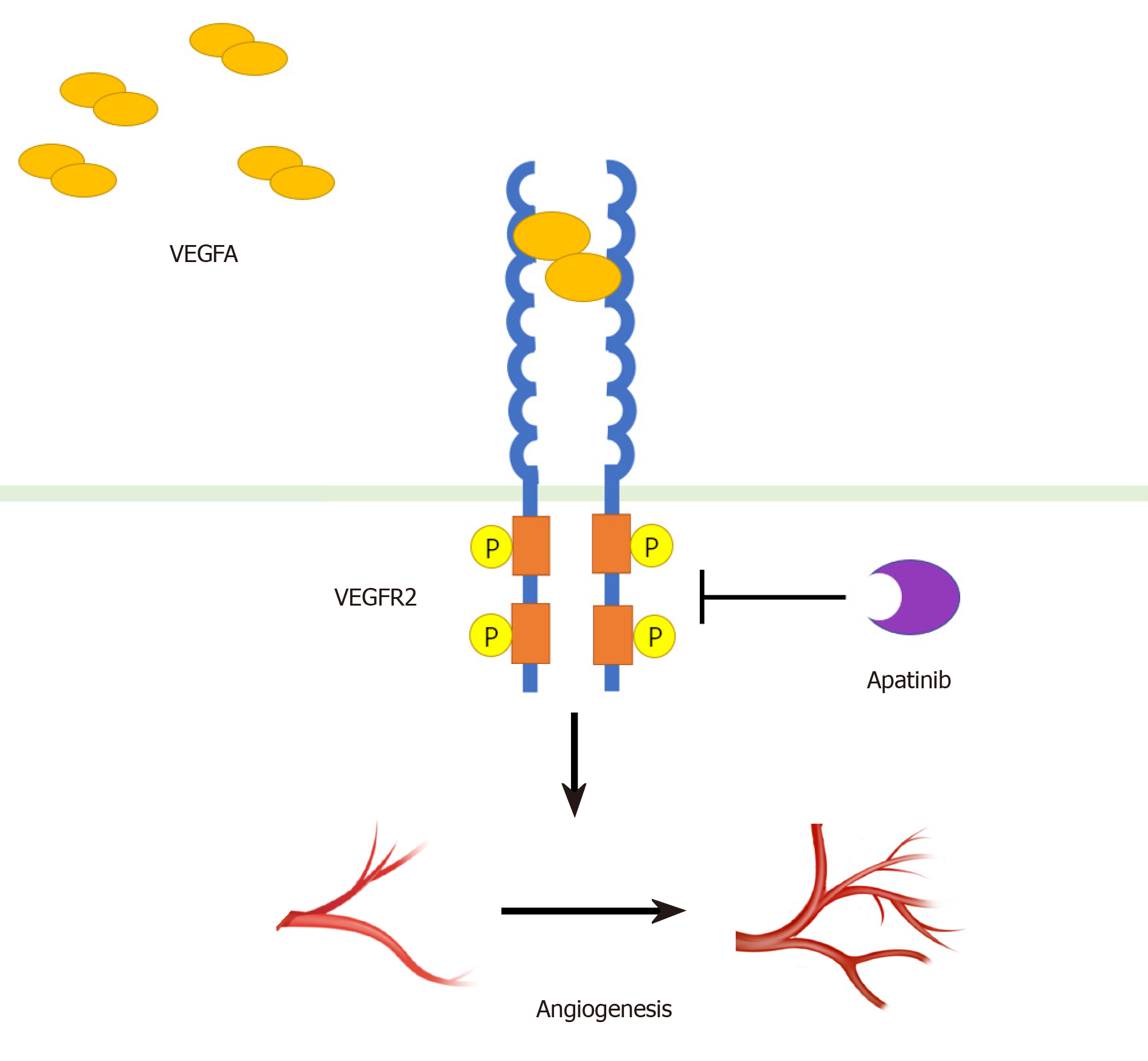
Angiogenesis plays an important role in the occurrence and development of tumors[12]. Vascular endothelial growth factor (VEGF) and its receptor VEGFR have been thought to play a central role in angiogenesis and tumor growth[13]. The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PLGF). Similarly, there are three subtypes of receptor family, including VEGFR-1, VEGFR-2, and VEGFR-3[14]. The combination of VEGF-A and VEGFR-2 is considered to be mainly involved in the generation of blood vessels in solid tumors[15-18]. VEGF-A binds to the Ig-like domains 2 and 3 of VEGFR-2 to dimerize the receptor, which in turn causes the tyrosine kinase of receptor to undergo autophosphorylation[15] (Figure 1). Subsequently, several different molecular pathways are activated simultaneously: The RAF/MEK/ERK pathway promotes endothelial cell proliferation and survival; the p38-MAPK pathway increases the migration and invasion of endothelial cells, and enhances chemotactic and homing of bone marrow-derived vascular precursor cells; and the PI3K/AKT/mTOR pathway improves endothelial cell survival and vascular permeability[14,15,18-21].
Apatinib mesylate is a derivative of valatinib. Its predecessor is YN968D11 (N-[4-(1-cyano-cyclopentyl) phenyl]-2-(4-pyridylmethyl) amino-3-pyridine carboxamide mesylate). It highly specifically binds to the intracellular ATP binding site of VEGFR-2, preventing receptor phosphorylation. Apatinib has a strong affinity for VRGFR2 (IC50 = 2), which is ten times that of other anti-angiogenic drugs such as sorafenib (IC50 = 90)[8,9,22,23].
The pharmacokinetic analysis showed that the time to maximum plasma concentration level after administration was about 3-4 h with an average half-life of 9 h[9]. There are many main pathways of apatinib biotransformation, in which M1-1 is the main metabolite and shows the strongest inhibitory effect on VEGFR-2, and it is most closely related to the anti-angiogenic effect of apatinib. In contrast, M9-2 has no obvious inhibitory effect on the above enzymes. The oxidative metabolites of apatinib are mainly formed in the liver in a NADPH-dependent manner. The process is mainly mediated by the CYP3A4/5 enzyme, followed by CYP2D6, CYP2C9, and CYP2E1. After 96 h of oral apatinib, drug excretion rate was 76.8%, including 69.8% in stool and 7.0% in urine[24].
Apatinib can effectively inhibit the activity of VEGFR-2 kinase and block its downstream signaling by specifically competing for the ATP binding site in the cell[8]. Apatinib also inhibits the proliferation, migration, and tube formation of human umbilical vein endothelial cells (HUVEC), blocking the germination of rat aortic rings[8,25].
In HCC, apatinib can induce cell cycle arrest at the G2/M phase, promoting apoptosis of HCC cells in vitro, and its inhibitory effect is related to the expression level of VEGFR[26]. Li et al[27] have found that apatinib promotes tumor cell apoptosis and inhibits metastasis, which may be related to the down-regulation of PDGFR-α, IGF-IR, and AKT phosphorylation levels. Similar results have been observed in SMMC-7721 cells, in which apatinib promoted apoptosis by inhibiting the phosphorylation level of PI3K/AKT[28]. In pancreatic cancer, apatinib promotes apoptosis of pancreatic cells by down-regulating hypoxia inducible factor-1α (HIF-1α) and increasing reactive oxygen levels[29]. In thyroid cancer, apatinib inhibits the expression of angiopoietin through tumor cell AKT/GSK3β/ANG pathway, thereby inhibiting tumor angiogenesis[25]. Apatinib inhibits cell invasion and migration by inhibiting the RET/SRC signaling pathway, suggesting a potential role in treating KIF5B-RET-driven tumors[30]. Apatinib can also promote the apoptosis of tumor cells of extrahepatic bile duct cancer[31], esophageal cancer[32], colon cancer[33], osteosarcoma and glioma[34], and B and T cell acute lymphoblastic leukemia[33].
In an immunodeficiency mouse xenograft model of HCC, apatinib was administered orally three times a week, and the inhibition rate of tumor growth was 71% after 30 d, and no significant weight loss or treatment-related death was observed[27]. Liang et al[35] evaluated the therapeutic effect of apatinib and sorafenib in HCC by multimodal molecular imaging. The results showed that apatinib inhibits the growth and angiogenesis of HCC, which is equivalent to sorafenib but has fewer side effects[35]. Apatinib can also cause metabolomics changes. After apatinib treatment, 3-hydroxybutyric acid (3-HB) is significantly increased in serum, tumor, and the liver, which aids antitumor effect of apatinib[36].
Apatinib alone or in combination with chemotherapeutics can effectively inhibit a variety of established human tumor xenograft models with less toxicity. The combination of apatinib with docetaxel and adriamycin significantly inhibits the growth of transplanted lung cancer, which is significantly different from the apatinib group and the chemotherapy drug group. In addition, the combination of apatinib with oxaliplatin and fluorouracil also showed a significant inhibitory effect in colon cancer[8]. Tong et al[37] selected a subset of K562 leukemia cells with higher doxorubicin resistance as the object of observation. The experimental results showed that apatinib could significantly reduce the IC50 value of doxorubicin in this subgroup of cells and significantly increase the sensitivity to chemotherapy drugs. It was also confirmed in a tumor xenograft model that apatinib could reverse ABCB1 and ABCG2-mediated multidrug resistance (MDR) by directly inhibiting ABCB1 and ABCG2 function, leading to the rise of intracellular concentrations of chemotherapeutic drugs. The reversal of MDR further supports the potential role of combining apatinib with other conventional anticancer drugs in overcoming clinical resistance[38].
Qin et al[39] reported a prospective, randomized, open label, nationwide, multicenter, phase II clinical trial of apatinib as second-line therapy for advanced HCC. The primary endpoint of the study was time to disease progression (TTP). The secondary endpoints included OS, ORR, disease control rate (DCR), quality of life, and serum alpha-fetoprotein (AFP) levels. A total of 121 patients with advanced HCC were enrolled and randomly assigned 1:1 to the 850 mg dose group and the 750 mg dose group. The results confirmed that the clinical efficacy of apatinib (850 mg and 750 mg) in different dose groups was basically the same for advanced HCC with initial treatment and good basic conditions: mTTP and mOS were not significantly different between the two groups (4.2 mo vs 3.3 mo, P > 0.05; 9.7 mo vs 9.8 mo, P > 0.05). The DCRs of the two groups were 48.6% and 37.3% (P > 0.05), and the ORRs were 8.6% and 0 (P > 0.05), respectively. The incidence of adverse events was also similar between these two groups. In terms of safety, the drug-related toxicities in the 850 mg dose group were more than those in the 750 mg dose group, but the differences were not statistically significant, including hand-foot skin reaction (HFSR), elevated aminotransferase, and elevated bilirubin. Grade 3 and above drug-related side effects included hypertension, proteinuria, HFSR, fatigue, and peripheral blood cell reduction. Considering that most patients with liver cancer have basic liver diseases, they recommended that 750 mg qd as dose for subsequent studies.
Kong et al[40] retrospectively evaluated the efficacy and safety of apatinib in 22 patients with advanced HCC who were resistant to sorafenib or could not afford sorafenib. Apatinib was administered continuously at 500 mg/d or 250 mg/d with clinical emphasis on TTP, OS, and safety. Until the last follow-up, the median disease progression time for these 22 patients was 10.4 mo, and 50% of patients survived longer than 11.4 mo. The percentages of patients achieving complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were 0%, 40.9%, 40.9%, and 18.2%, respectively, and the ORR and DCR were 40.9% and 81.8%, respectively. At the same time, 14 of the 22 cases had decreased alpha-fetoprotein levels, of which seven had fallen by half or more. Adverse events mainly included HFSR (81.8%), diarrhea (77.3%), hypertension (63.6%), fatigue (59.1%), hoarseness (54.5%), and nausea (50%). Grade 3 or 4 drug-related adverse events mainly included hypertension (27.3%), HFSR (13.6%), and thrombocytopenia (9.1%). In view of the side effects of advanced patients and the high-dose treatment, patients receiving low-dose treatment (250 mg/d) had fewer and less adverse events and achieved good responses.
A prospective study by Yu et al[41] evaluated the efficacy and safety of apatinib in advanced HCC. A total of 31 patients participated in the study, including four in the intermediate stage and 27 in the advanced stage. The dose was 500 mg/d. According to the first follow-up CT and MRI after 6 wk of treatment, the numbers of patients achieving PR, SD, and PD in 31 patients were 10 (32.3%), 15 (48.4%), and 6 (19.4%). The ORR and DCR were 32.3% and 80.7% respectively. The mPFS was 4.8 mo, and the 6- and 12-mo survival rates were 73.8% and 55.4% respectively. The most common grade 3 adverse effects were hypertension (48.4%), thrombocytopenia (6.5%), and an increase in total bilirubin or transaminase (6.5%). By adjusting drug dosage and symptomatic treatment, all toxic reactions could be controlled.
Liu et al[42] retrospectively reviewed the efficacy and safety of apatinib in the treatment of unresectable or recurrent HCC. A total of 32 patients with HCC or intrahepatic bile duct cancer were included in the study[42]. No CR occurred, PR, SD, and PD were observed in 5 (16%), 14 (44%), and 13 (41%) patients, respectively, and DCR was 60%. The mPFS for HCC was 5 mo, and the mPFS for intrahepatic cholangiocarcinoma was 3 mo. The mOS for HCC and bile duct carcinoma were 13 mo and 5 mo, respectively. The most common adverse effects were proteinuria (31%), hypertension (28%), and liver dysfunction (13%).
Zhang et al[43] evaluated the efficacy and safety of apatinib for sorafenib refractory advanced hepatitis B virus-associated HCC. A total of 43 patients were retrospectively analyzed[43]. ORR and DCR were 25.6% and 67.4%, respectively. mPFS and mOS were 3 mo and 8 mo, respectively. The 1-year and 2-year survival rates were 34.9% and 9.3%, respectively. The most common toxicities were weight loss, HFSR, and hypertension.
Apatinib shows a therapeutic effect on advanced HCC with lung metastasis[44]. In a retrospective and multicenter study, 61 patients with advanced HCC were enrolled in the study, including 41 patients with lung metastases, three with multiple organ metastases, and 20 with no pulmonary metastases. The main focus was on metastasis specificity and PFS. All patients had a median PFS of 3.37 mo and an ORR of 11.6%. The median mPFS of 41 patients with pulmonary metastases was 5 mo, and the mORR was 22.0%. Compared with patients without lung metastases, patients with only lung metastases had better mPFS (hazard ratio/HR = 0.316), although mORR was similar.
In a series of clinical studies of apatinib, common adverse events include hematological toxicity (leukopenia, granulocytopenia, and thrombocytopenia) and non-hematological toxicity (hypertension, proteinuria, HFSR, etc.). Among the common important adverse events are hypertension, proteinuria, and HFSR.
In the phase I study of apatinib, the overall incidence of hypertension reached 69.5%, of which grade 3 to 4 reached 6.5%. Hypertension is the most common adverse reaction of anti-angiogenic drugs, especially VEGF/VEGFR inhibitors. Current research suggests that reduction of nitric oxide (NO) and increase of endothelin (ET) are the main causes of hypertension in anti-VEGF treatment[45,46]. Both methods can cause vasodilation dysfunction and strengthen systolic function. In addition, abnormal blood vessel density and reduced capillaries are also the cause of hypertension[47]. In the current treatment plan, besides reducing the drug dose, another effective treatment is the use of antihypertensive drugs.
The overall incidence of proteinuria in the phase I study was 34.8%, and the incidence of grade 3 to 4 was 13%. The occurrence of proteinuria is related to the inhibition of VEGF signaling by apatinib, whereas adequate VEGF is needed to maintain the integrity of glomerular structure and function. In animal experiments, podocyte specific VEGF gene knockout can cause structural and functional changes, which in turn affects glomerular filtration rate and causes proteinuria[48]. Although the persistence of high blood pressure can cause kidney damage[49], in clinical practice, many patients have proteinuria without hypertension, suggesting that proteinuria caused by apatinib may not be related to hypertension, and the specific mechanism needs further exploration.
The overall incidence of HFSR in phase I clinical studies was 45.6%, and the incidence in grade 3 to 4 was 13%, which can be alleviated by reducing the dose of the drug. Its mechanism is unknown. Possible reasons include: Decreased renewal and dysfunction of endothelial cells; damage to sweat ductal epithelial cells due to inhibition of PDGF and c-Kit; keratinocyte dysfunction due to c-Kit inhibition; and broken balance between vascular and epidermal damage[50,51].
In addition to the common adverse events mentioned above, other adverse events include bleeding, fatigue, diarrhea, infection, dyspnea, hoarseness, skin albinism, and rash. However, most of these events are mild and controllable, and can be relieved with supportive treatment. Remarkably, clinical trials have shown that adverse events caused by apatinib are often associated with better efficacy and longer survival benefits[52].
The combination of apatinib with other treatments has yielded interesting results in advanced HCC. In the combination with trans-artery chemo-embolization (TACE), Zhu et al[53] reported that after 9 mo of TACE combined with apatinib for advanced HCC, DCR and ORR in the TACE group were 81.82% and 36.36%, and they were 95.45% and 63.64% in the TACE plus apatinib group. The PFS was 11.15 and 16.5 mo, respectively[53]. DCR, ORR, and PFS were significantly improved. There was no significant difference in the incidence of adverse events after embolization between the two groups of patients. However, the incidence of hypertension, HFSR, and proteinuria in the combined group was significantly higher (P < 0.05). Adverse effects were alleviated after symptomatic treatment.
Xu et al[54] studied the effect of carrelizumab (PD-1 mAb, SHR-1210) and apatinib in the treatment of advanced HCC, gastric cancer, and esophagogastric junction cancer in a phase I clinical study[54]. Of the 16 evaluable HCC patients, eight achieved PR, in whom one was in the apatinib 125 mg cohort and seven received apatinib 250 mg. ORR and DCR were 50.0% and 93.8%, respectively. Patients receiving apatinib had a 6-mo PFS rate of 51.3% and a 9-mo PFS rate of 41%. A phase III study on the combined use of the two drugs is underway (NCT02329860).
Apatinib, as a new type of small molecule tyrosine kinase inhibitor, shows high selective affinity for VEGFR-2, blocking its downstream signal transduction. Although there is no sufficient evidence, from the primary research and exploration, apatinib may have potential advantages, such as better ORR, survival benefits, and less toxic and side effects, which is still waiting for further research and confirmation. Combined therapy shows a prominent role by working through different mechanisms and will hold an important position in the future[55]. Apatinib, as an alternative targeted drug, will be likely to have a promising effect in combination therapy. A number of clinical trials of combination therapy including apatinib are currently underway (NCT03793725, NCT03839550, NCT03463876, and NCT03764293). Current research still has certain limitations. Most of the studies are small in size. The mechanisms need further exploration to ensure a higher level of evidence. With the development of basic and clinical research, apatinib alone or in combination with other therapy may benefit more patients with HCC.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: El-Bendary M, Eskens F S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Li JH
| 1. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3429] [Article Influence: 489.9] [Reference Citation Analysis (1)] |
| 2. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [PubMed] |
| 3. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10272] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 5. | Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, Anders R, Are C, Brown D, Chang DT, Cloyd J, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Hammond LJ, Darlow SD. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw. 2019;17:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (1)] |
| 6. | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 833] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 7. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [PubMed] |
| 8. | Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 9. | Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, Yv K, Wang F, Li C, Qian J, Zheng C, Zuo Y. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075-6081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Rivera LB, Bergers G. CANCER. Tumor angiogenesis, from foe to friend. Science. 2015;349:694-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69 Suppl 3:4-10. [PubMed] |
| 14. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [PubMed] |
| 15. | Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003-2012. [PubMed] |
| 16. | Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004;2:315-326. [PubMed] |
| 17. | Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 18. | Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1083] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 19. | Gimbrone MA, Leapman SB, Cotran RS, Folkman J. Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumors. J Natl Cancer Inst. 1973;50:219-228. [PubMed] |
| 20. | Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826-835. [PubMed] |
| 21. | Longo R, Gasparini G. Challenges for patient selection with VEGF inhibitors. Cancer Chemother Pharmacol. 2007;60:151-170. [PubMed] |
| 22. | Nienhüser H, Schmidt T. Angiogenesis and Anti-Angiogenic Therapy in Gastric Cancer. Int J Mol Sci. 2017;19. [PubMed] |
| 23. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [PubMed] |
| 24. | Ding J, Chen X, Gao Z, Dai X, Li L, Xie C, Jiang H, Zhang L, Zhong D. Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor apatinib in humans. Drug Metab Dispos. 2013;41:1195-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Jin Z, Cheng X, Feng H, Kuang J, Yang W, Peng C, Shen B, Qiu W. Apatinib Inhibits Angiogenesis Via Suppressing Akt/GSK3β/ANG Signaling Pathway in Anaplastic Thyroid Cancer. Cell Physiol Biochem. 2017;44:1471-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Yang C, Qin S. Apatinib targets both tumor and endothelial cells in hepatocellular carcinoma. Cancer Med. 2018;7:4570-4583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Li X, Xu A, Li H, Zhang B, Cao B, Huang J. Novel role of apatinib as a multi-target RTK inhibitor in the direct suppression of hepatocellular carcinoma cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Zhang H, Cao Y, Chen Y, Li G, Yu H. Apatinib promotes apoptosis of the SMMC-7721 hepatocellular carcinoma cell line via the PI3K/Akt pathway. Oncol Lett. 2018;15:5739-5743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | He K, Wu L, Ding Q, Haider F, Yu H, Wang H, Xiang G. Apatinib Promotes Apoptosis of Pancreatic Cancer Cells through Downregulation of Hypoxia-Inducible Factor-1α and Increased Levels of Reactive Oxygen Species. Oxid Med Cell Longev. 2019;2019:5152072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Lin C, Wang S, Xie W, Zheng R, Gan Y, Chang J. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget. 2016;7:59236-59244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Peng S, Zhang Y, Peng H, Ke Z, Xu L, Su T, Tsung A, Tohme S, Huang H, Zhang Q, Lencioni R, Zeng Z, Peng B, Chen M, Kuang M. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett. 2016;373:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Hu L, Sun F, Sun Z, Ni X, Wang J, Wang J, Zhou M, Feng Y, Kong Z, Hua Q, Yu J. Apatinib enhances the radiosensitivity of the esophageal cancer cell line KYSE-150 by inducing apoptosis and cell cycle redistribution. Oncol Lett. 2019;17:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Deng M, Zha J, Jiang Z, Jia X, Shi Y, Li P, Chen XL, Fang Z, Du Z, Xu B. Apatinib exhibits anti-leukemia activity in preclinical models of acute lymphoblastic leukemia. J Transl Med. 2018;16:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, Zheng B, Guo W. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8:e3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 35. | Liang Q, Kong L, Du Y, Zhu X, Tian J. Antitumorigenic and antiangiogenic efficacy of apatinib in liver cancer evaluated by multimodality molecular imaging. Exp Mol Med. 2019;51:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Feng S, Wang H, Wang Y, Sun R, Xie Y, Zhou Z, Wang H, Aa J, Zhou F, Wang G. Apatinib induces 3-hydroxybutyric acid production in the liver of mice by peroxisome proliferator-activated receptor α activation to aid its antitumor effect. Cancer Sci. 2019;110:3328-3339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Tong XZ, Wang F, Liang S, Zhang X, He JH, Chen XG, Liang YJ, Mi YJ, To KK, Fu LW. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol. 2012;83:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, Ma XX, To KK, Ambudkar SV, Chen ZS, Fu LW. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981-7991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 286] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 39. | Qin S. Apatinib in Chinese patients with advanced hepatocellular carcinoma: A phase II randomized, open-label trial. J Clin Oncol. 2014;32:4019-4019. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Kong Y, Sun L, Hou Z, Zhang Y, Chen P, Cui Y, Zhu X, Song T, Li Q, Li H, Zhang T, Qin L. Apatinib is effective for treatment of advanced hepatocellular carcinoma. Oncotarget. 2017;8:105596-105605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Yu WC, Zhang KZ, Chen SG, Liu WF. Efficacy and Safety of apatinib in patients with intermediate/advanced hepatocellular carcinoma: A prospective observation study. Medicine (Baltimore). 2018;97:e9704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Zhen L, Jiali C, Yong F, Han X, Hongming P, Weidong H. The Efficacy and Safety of Apatinib Treatment for Patients with Unresectable or Relapsed Liver Cancer: a retrospective study. J Cancer. 2018;9:2773-2777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Fan W, Wang Y, Huang G, Li J. Apatinib for Patients With Sorafenib-Refractory Advanced Hepatitis B Virus Related Hepatocellular Carcinoma: Results of a Pilot Study. Cancer Control. 2019;26:1073274819872216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Du X, Chen D, Lin Z, Dong Z, Lu Y, Liu L, Wu D. Efficacy of apatinib in advanced hepatocellular carcinoma with lung metastasis: a retrospective, multicenter study. J BUON. 2019;24:1956-1963. [PubMed] |
| 45. | Lankhorst S, Kappers MH, van Esch JH, Danser AH, van den Meiracker AH. Hypertension during vascular endothelial growth factor inhibition: focus on nitric oxide, endothelin-1, and oxidative stress. Antioxid Redox Signal. 2014;20:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Touyz RM, Lang NN, Herrmann J, van den Meiracker AH, Danser AHJ. Recent Advances in Hypertension and Cardiovascular Toxicities With Vascular Endothelial Growth Factor Inhibition. Hypertension. 2017;70:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Bossaer JB, Geraci SA, Chakraborty K. Cardiovascular Toxicity and Management of Targeted Cancer Therapy. Am J Med Sci. 2016;351:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707-716. [PubMed] |
| 49. | Lankhorst S, Kappers MH, van Esch JH, Smedts FM, Sleijfer S, Mathijssen RH, Baelde HJ, Danser AH, van den Meiracker AH. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension. 2014;64:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | McLellan B, Ciardiello F, Lacouture ME, Segaert S, Van Cutsem E. Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Ann Oncol. 2015;26:2017-2026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Ding F, Liu B, Wang Y. Risk of hand-foot skin reaction associated with vascular endothelial growth factor-tyrosine kinase inhibitors: A meta-analysis of 57 randomized controlled trials involving 24,956 patients. J Am Acad Dermatol. 2019;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai Y, Wang Z, Yang Y, Sun G, Wang L, Zheng L, Xu N, Cheng Y, Guo W, Yu H, Liu T, Lagiou P, Li J. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol. 2017;10:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Zhu Y, Feng B, Mei L, Sun R, Guo C, Zhu J. Clinical efficacy of TACE combined with Apatinib in the treatment of advanced hepatocellular carcinoma. J BUON. 2019;24:608-614. [PubMed] |
| 54. | Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 55. | Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, Ochiai H, Bardeesy N, Huang P, Cobbold M, Zhu AX, Jain RK, Duda DG. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology. 2020;71:1247-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |