Published online Oct 27, 2020. doi: 10.4254/wjh.v12.i10.754
Peer-review started: June 13, 2020
First decision: July 30, 2020
Revised: August 1, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: October 27, 2020
Processing time: 132 Days and 20.9 Hours
Hepatitis C virus (HCV) infection is an excellent immunological model for understanding the mechanisms developed by non-cytopathic viruses and tumors to evade the adaptative immune response. The antigen-specific cytotoxic T cell response is essential for keeping HCV under control, but during persistent infection, these cells become exhausted or even deleted. The exhaustion process is progressive and depends on the infection duration and level of antigenemia. During high antigenic load and long duration of infection, T cells become extremely exhausted and ultimately disappear due to apoptosis. The development of exhaustion involves the impairment of positive co-stimulation induced by regulatory cytokines, such as transforming growth factor beta 1. This cytokine downregulates tumor necrosis factor receptor (TNFR)-associated factor 1 (TRAF1), the signal transducer of the T cell co-stimulatory molecule TNFR superfamily member 9 (known as 4-1BB). This impairment correlates with the low reactivity of T cells and an exhaustion phenotype. Treatment with interleukin-7 in vitro restores TRAF1 expression and rescues T cell effector function. The process of TRAF1 loss and its in vitro recovery is hierarchical, and more affected by severe disease progression. In conclusion, TRAF1 dynamics on T cells define a new pathogenic model that describes some aspects of the natural history of HCV, and sheds light on novel immunotherapy strategies for chronic viral infections and cancer.
Core Tip: Tumor necrosis factor receptor-associated factor 1 (TRAF1) is the signal transducer of the positive checkpoint tumor necrosis family receptor superfamily member 9 (4-1BB), essential in the activation of adaptive immune response. During persistent hepatitis C virus (HCV) infection, this transducer is down-regulated via transforming growth factor beta 1, linked to T cell exhaustion. Interleukin-7 can restore TRAF1 expression and improve T cell reactivity but only in patients with mild evolution, while cases with a more aggressive progression also need the modulation of other negative co-stimulatory molecules. Therefore, TRAF1 dynamics defines a new pathogenic model that explains the different level of T cell exhaustion and progression during HCV infection and supports the rationale for immunotherapeutic strategies in chronic viral infections.
- Citation: Peña-Asensio J, Sanz-de-Villalobos E, Miquel J, Larrubia JR. Tumor necrosis family receptor superfamily member 9/tumor necrosis factor receptor-associated factor 1 pathway on hepatitis C viral persistence and natural history. World J Hepatol 2020; 12(10): 754-765
- URL: https://www.wjgnet.com/1948-5182/full/v12/i10/754.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i10.754
Hepatitis C virus (HCV) evolution is heterogenous as a result of the particular interplay between the virus and the immune system[1]. The outcome of the fight between host and pathogen depends on the balance of the host-microbe interaction, which causes varying degrees of progressive liver damage[2-5]. The fine-tuning of this equilibrium can induce either rapid or slow disease progression, which depends on the degree of impairment of the adaptive immune system[6]. During persistent non-cytopathic viral infection, the antigen (Ag)-specific T cell response is exhausted and unable to clear infection despite achieving partial viral control[7,8]. The correct activation of this response relies on the interaction with Ag-presenting cells (commonly known as APCs) in the proper cytokine environment with the right co-stimulation[1,9,10]. Non-cytopathic viruses manipulate T cell co-stimulation for their own benefit, favoring the induction of negative co-stimulatory receptors and inhibiting positive co-stimulatory pathways[11-13]. Tumor necrosis factor receptor (TNFR) superfamily member 9 (4-1BB) is a TNFR-associated factor 1 (TRAF1)-binding checkpoint molecule that is normally absent from resting cells but is induced by T cell receptor (TCR) signaling[14]. It is a positive activator of the T cell response, which is key during viral infection and cancer. TRAF1 is the major signal transducer after 4-1BB triggering[15], and its downregulation on T cells is used by pathogens as a mechanism to evade specific adaptive immune responses[2,16].
In this review, we present an update on the current knowledge of the role of the 4-1BB/TRAF1 pathway in the outcome of HCV infection, and how it can be manipulated to overcome T cell exhaustion. Although this immunotherapeutic strategy is no longer needed in the era of direct acting anti-viral (commonly referred to as DAA) medications[17,18], lessons obtained from this persistent infection model can be extrapolated to other viral infections, such as hepatitis B virus (known as HBV) and human immunodeficiency virus (HIV), or cancer.
HCV is a highly variable, positive-sense, single-stranded hepatotropic non-cytopathic RNA virus of the family Flaviviridae[19,20], with parenteral, vertical, and sexual transmission capacities[21]. HCV induces progressive liver damage that can lead to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma[3,22]. About one-third of patients spontaneously clear the virus but in the remaining two-thirds, the infection persists unless an anti-viral treatment is administered[5]. Currently, the infection is easily controlled by using DAA drugs[17]. Nevertheless, it is still possible to learn from HCV about the host-pathogen interaction in chronic viral diseases, which can be applied to other chronic viral infections and cancer.
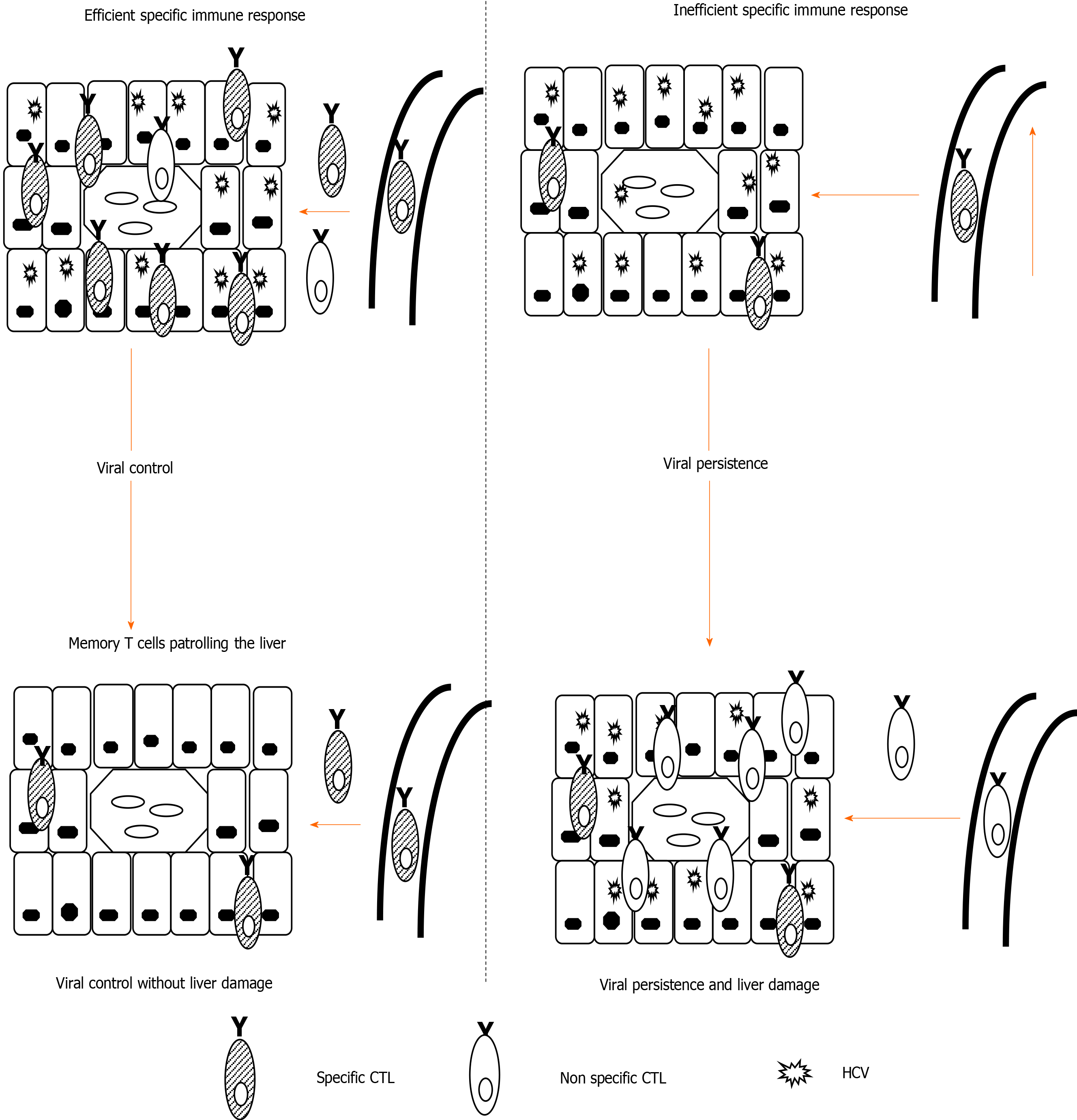
During the natural history of untreated, persistent HCV infection, there are three different progression groups: Slow, mild, and rapid fibrosers. Slow fibrosers do not develop significant fibrosis during their life, whereas rapid fibrosers can progress to cirrhosis, portal hypertension, or hepatocellular carcinoma in as quickly as 10-20 years after primoinfection[23]. Host factors such as sex, age of infection, alcohol consumption, co-infection with HIV or HBV, steatosis, and insulin resistance[23,24], as well as the quality of the adaptive immune response[1], are involved in the different evolution patterns of HCV. HCV-specific cytotoxic T cells play a central role in controlling HCV infection[25,26]. During persistent HCV infection, however, the cytotoxic T cell response becomes dysfunctional, with cells presenting markers of exhaustion and apoptosis[27-30]. Nevertheless, these HCV-specific CD8 T cells can still partially control viral replication[31].
Interestingly, it is not HCV-specific CD8 T cells but other inflammatory cells recruited to the infected liver that are ultimately responsible for persistent liver damage[32,33] (Figure 1). Therefore, long-lasting infection linked to a weak CD8-specific T cell response can induce permanent non-specific inflammatory infiltrates that can promote the rapid progression of liver fibrosis[33,34]. In fact, a high level of prolonged antigenemia induces a hierarchical loss of effector functions and ultimate apoptosis of T cells[35]. During persistent HCV infection, the level of specific T cell impairment positively correlates with the speed of liver fibrosis progression. These data suggest that stronger T cell exhaustion may facilitate rapid fibrosis progression. In support, rapid fibrosers with long-lasting infection lack detectable peripheral HCV-specific cytotoxic T cells, which although exhausted, are present in slow fibrosers and short-term disease[2]. Consequently, it may be possible to restore specific T cell responses to improve viral control, and in addition, to prevent liver damage by reducing pro-inflammatory chemokines and cytokines secreted in the infected liver.
During chronic hepatitis C, some pro-fibrogenic and immunoregulatory cytokines, such as transforming growth factor beta 1 (TGF-β1) are increased. In vitro analysis has shown that after Ag encounter, HCV-specific CD8 T cells secrete TFG-b1, which is linked to effector dysfunction and can be rescued by anti-TGF-β1 blocking antibodies[36]. Moreover, HCV itself is able to induce liver cells to express TGF-β1, and the number of TGF-β1-secreting regulatory T cells is also enhanced during chronic hepatitis C infection[37,38]. Among its immunoregulatory properties, TGF-β1 has been linked with the negative modulation of the positive co-stimulatory checkpoint 4-1BB/TRAF1 in some chronic viral infections, such as those by HIV, HCV, and lymphocoriomeningitis virus[2,16].
In the next sections of this review, this specific pathogenic axis will be discussed in detail.
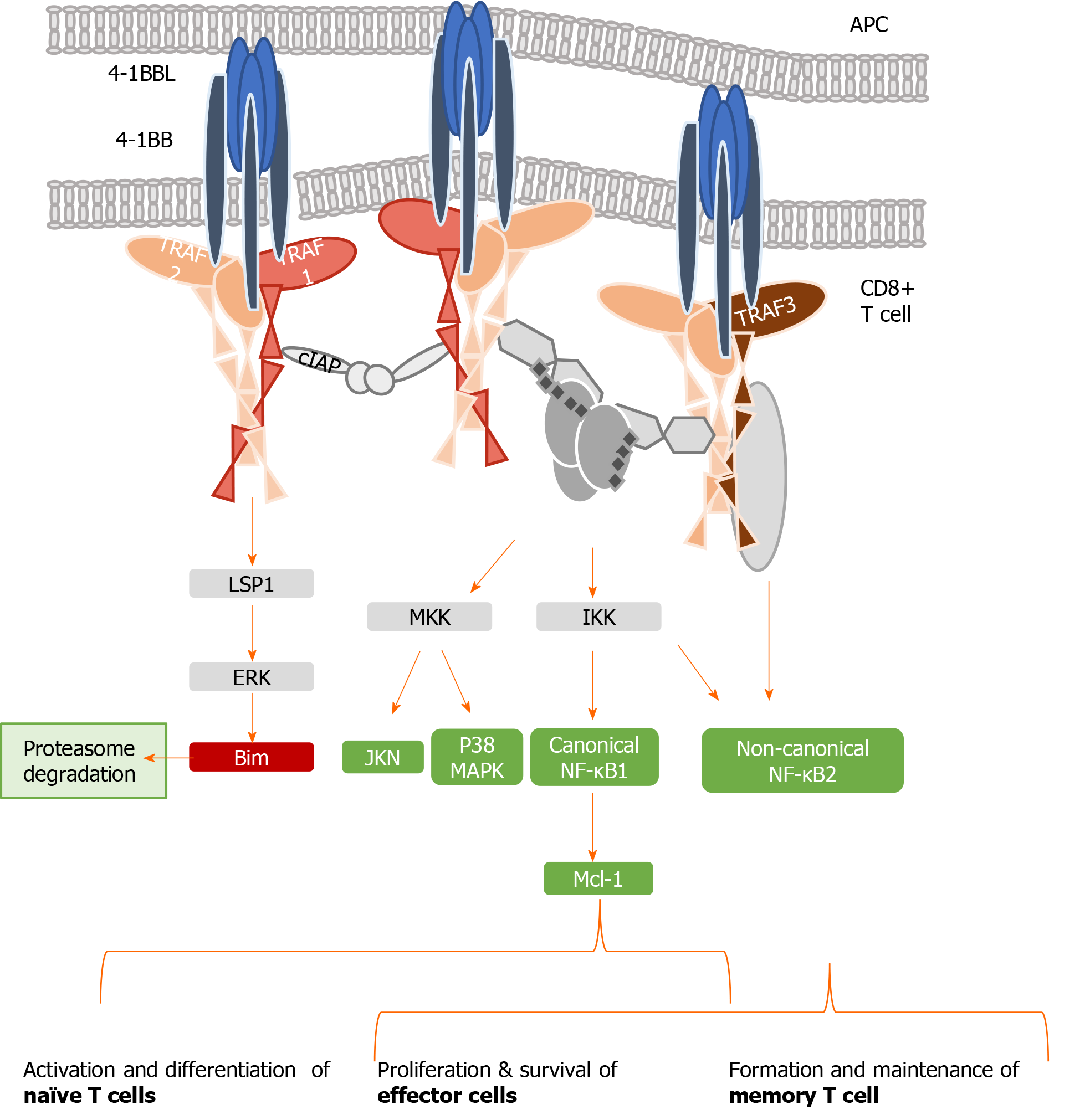
4-1BB, also called CD137, is a co-stimulatory checkpoint that is predominantly expressed on activated CD8 T cells and natural killer cells[39], and in lower levels on CD4 T cells, dendritic cells, granulocytes, and mast cells[40]. It binds to 4-1BB-ligand (4-1BBL, CD137L, or L/TNFR9), which is present on such APCs as activated B cells, dendritic cells, and macrophages[41]; the 4-1BB/TRAF1 pathway is shown in Figure 2. 4-1BBL trimer has a three-bladed propeller structure and binds to three 4-1BB receptor monomers[42]. 4-1BB translocates to the membrane after Ag encounter on CD8+ T cells[43], recruiting the TRAF family members TRAF1, 2, and 3[44]. Signaling through the 4-1BB receptor depends on the association with TRAF1 and 2 molecules, as evidence shows that the lack of any of them blocks 4-1BB/4-1BBL downstream transduction[16,45].
TRAF 1, 2, and 3 can form heterodimers and interact with adaptor proteins (i.e., ubiquitin ligases, proteases, kinases), creating a three-dimensional structure complex where enzymatic processes can be carried out[46]. TRAF1 differs from the other members of its family, as it lacks the N-terminal RING finger domain, which prevents it from acting as an E3 ubiquitin ligase. However, TRAF1 acts as a bridge between a wide range of adaptor proteins, regulating their activity[47] and interacting with several TNFR members, prompting their stimulation or inhibition. TRAF1 has a role in T cell activation through the canonical nuclear factor-kappa B (NF-κB) pathway and an alternate pathway. These two different mechanisms of action regulate the physiology of T cells. In the canonical pathway, TRAF1 is inducible after cell activation through NF-κB[48], and is present in a restricted group of cells in which activated lymphocytes are included[49]. TRAF1 regulates survival signals mediated by TRAF2, modulating their ability to mediate sustained activation of NF-κB and c-Jun N-terminal kinase[50]. Specifically, TRAF1 is implicated in extracellular signal-regulated kinase (ERK) activation mediated by leukocyte-specific protein 1[51].
ERK phosphorylates Bim, eliciting its elimination by the proteasome and abrogating its anti-apoptotic effects[52]. The formation of two heterotrimers TRAF1:TRAF2 results in the recruitment of cellular inhibitor of apoptosis protein (cIAP) as well as the interaction with other adaptor proteins and protein kinases, which leads to activation of the NF-κB pathway[53]. TRAF2 can also dimerize to activate E3 ubiquitin ligases through their RING finger domains. Evidence indicates that the interactions among different TRAFs heterodimers allow them to adopt an octagonal superstructure where many 4-1BB/4-1BBL act simultaneously. This structure has been called the 4-1BB signalosome and could provide a model to design novel 4-1BB analogues as immunotherapeutic strategy[46]. Downstream signaling leads to the phosphorylation of inhibitor of kappa B kinase subunit β and subsequent activation of canonical NF-κB[54], ERK1/2[55], and p38 mitogen-activated protein kinase[56]. Collectively, this 4-1BB-dependent modulation results in CD8 T cell proliferation and survival.
When TNFR signaling is active, TRAF1 also engages the non-canonical NF-κB pathway by degrading TRAF3[54,57,58]. Initiation of the non-canonical NF-κB pathway is delayed with respect to the canonical one, which may play a role in T cell activation and memory differentiation[56]. Thus, in contrast to the rapid and transient activation of the canonical NF-κB pathway, activation of the non-canonical NF-κB pathway is characteristically slow and persistent. On the other hand, TRAF1 also regulates the canonical pathway by preventing TRAF2 degradation or enhancing cIAP recruitment, degrading NF-κB-inducing kinase, which is necessary for activation of the alternate NF-κB pathway[14,58]. Therefore, TRAF1 is a key transducer involved in initial T cell activation and proliferation by the canonical NF-κB pathway, but also in the generation of the memory and effector pool in a delayed manner through the non-canonical NF-κB pathway[54,56].
Figure 2 summarizes the different pathways involved in 4-1BB signaling.
Cytotoxic T cells carry out an essential task in non-cytopathic virus control[59,60]. This population is able to recognize infected cells and clear the virus by cytopathic and non-cytopathic mechanisms. Follow-up of healthcare workers after accidental needlestick HCV exposure showed that in those who naturally controlled the virus, HCV-specific CD8 T cells initially destroyed some hepatocytes but later removed the virus by releasing interferon-g[60]. These immune cells become activated by the combination of three different signals. First of all, the interaction between the APC and the TCR is necessary[61]. Thereafter, the interleukin (IL)-2 receptor is upregulated and its subsequent activation promotes T cell proliferation[62]. These two signals must be combined with the activation of early and late positive co-stimulatory checkpoints. Early positive co-stimulatory CD27 and CD28 counteract the inhibitory effects of negative checkpoints such as programmed cell death protein-1 (PD-1)[63-65]. Late positive co-stimulatory molecules such as 4-1BB play an important role in boosting the T cell response and inducing memory generation[14,66].
The 4-1BB/TRAF1 pathway promotes T cell memory formation[67] and survival[55,68] but also regulates effector T cell trafficking into the infected organ[69]. The triggering of this pathway can also improve T cell effector function by mitochondrial morphological and functional reprogramming[12,70,71]. Noteworthy, 4-1BB co-stimulation activates glucose and fatty acid metabolism to enhance CD8 T cell reactivity[72]. As noted above, the role of 4-1BB in T cell survival is mainly mediated via ERK by the downregulation of the pro-apoptotic protein Bim[55,73,74]. Thus, pharmacological intervention of this pathway can improve the T cell response by increasing survival and reactivity.
Tumors and persistent viral infections counter positive co-stimulation by early induction of negative checkpoints and inhibition of the positive checkpoints[7]. During non-cytopathic persistent viral infections, specific CD8 T cells are characterized by the expression of negative co-stimulatory molecules such as PD-1, T cell immunoglobulin and mucin-domain containing-3, and cytotoxic T-lymphocyte protein 4[11,27,75]. In addition, these viruses can impair downstream signaling of 4-1BB by causing the loss of its signal transducer TRAF1[16], which explains why positive immunotherapeutic modulation of 4-1BB has failed to boost the virus-specific CD8 T cell response[76]. During chronic lymphocytic choriomeningitis virus infection in mice, TRAF1 Loss on specific CD8 T cells is caused by TGF-β1-induced TRAF1 degradation, and this effect can be counter-regulated by common-g chain receptor cytokines, such as IL-7[16].
Interestingly, similar data have been reported for some human infections. Particularly, in chronic progressors during HIV infection, TRAF1 expression is lower than in elite controllers[16]. T cells from those elite controllers are more active in controlling HIV-infected cells and the process is correlated with TRAF1-mediated Bim downregulation. Indeed, the T cell response during HCV infection shares many features with HIV, and consequently, TRAF1 signaling could also be involved in HCV-specific T cell exhaustion, as will be discussed in the next section.
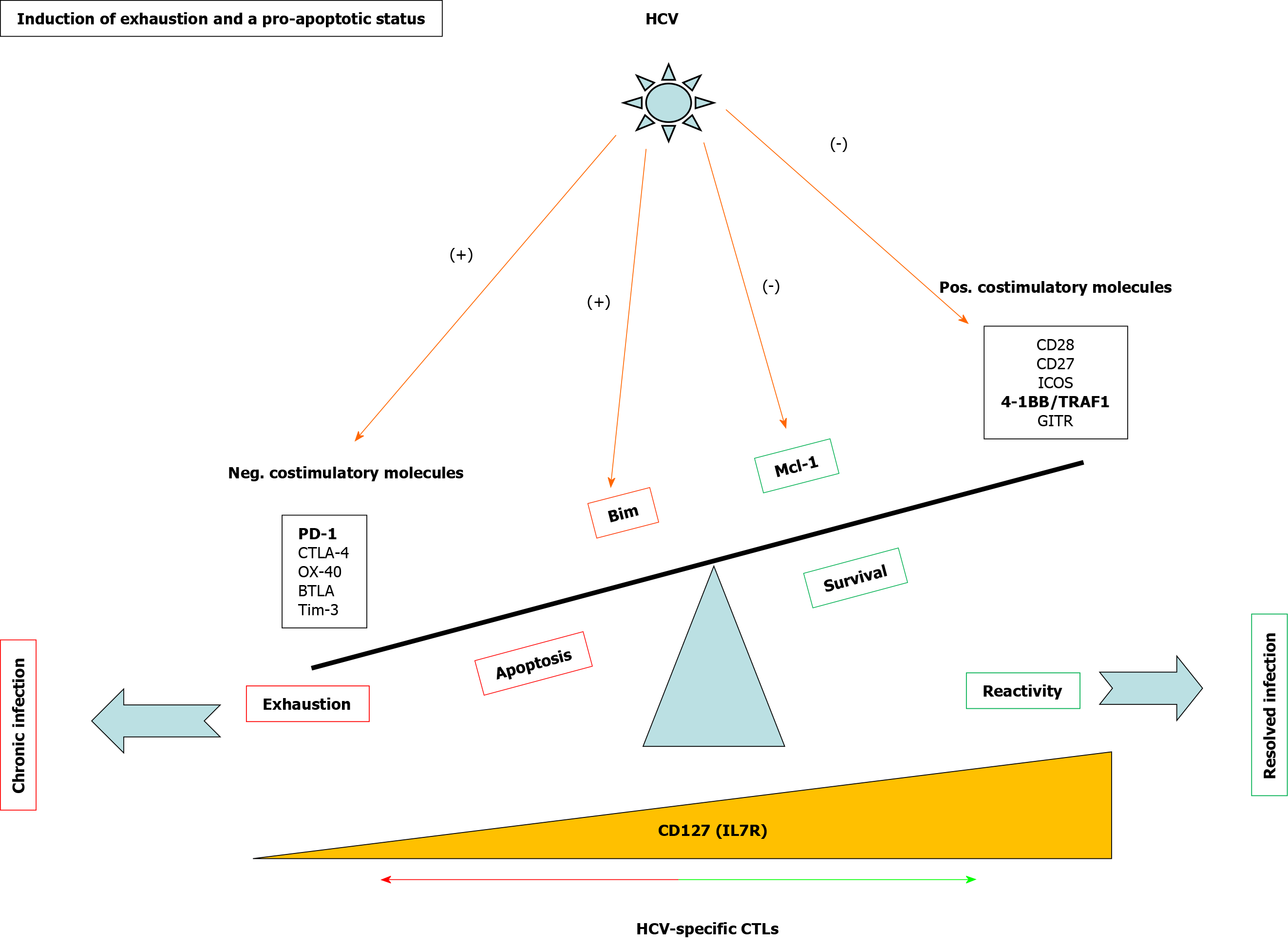
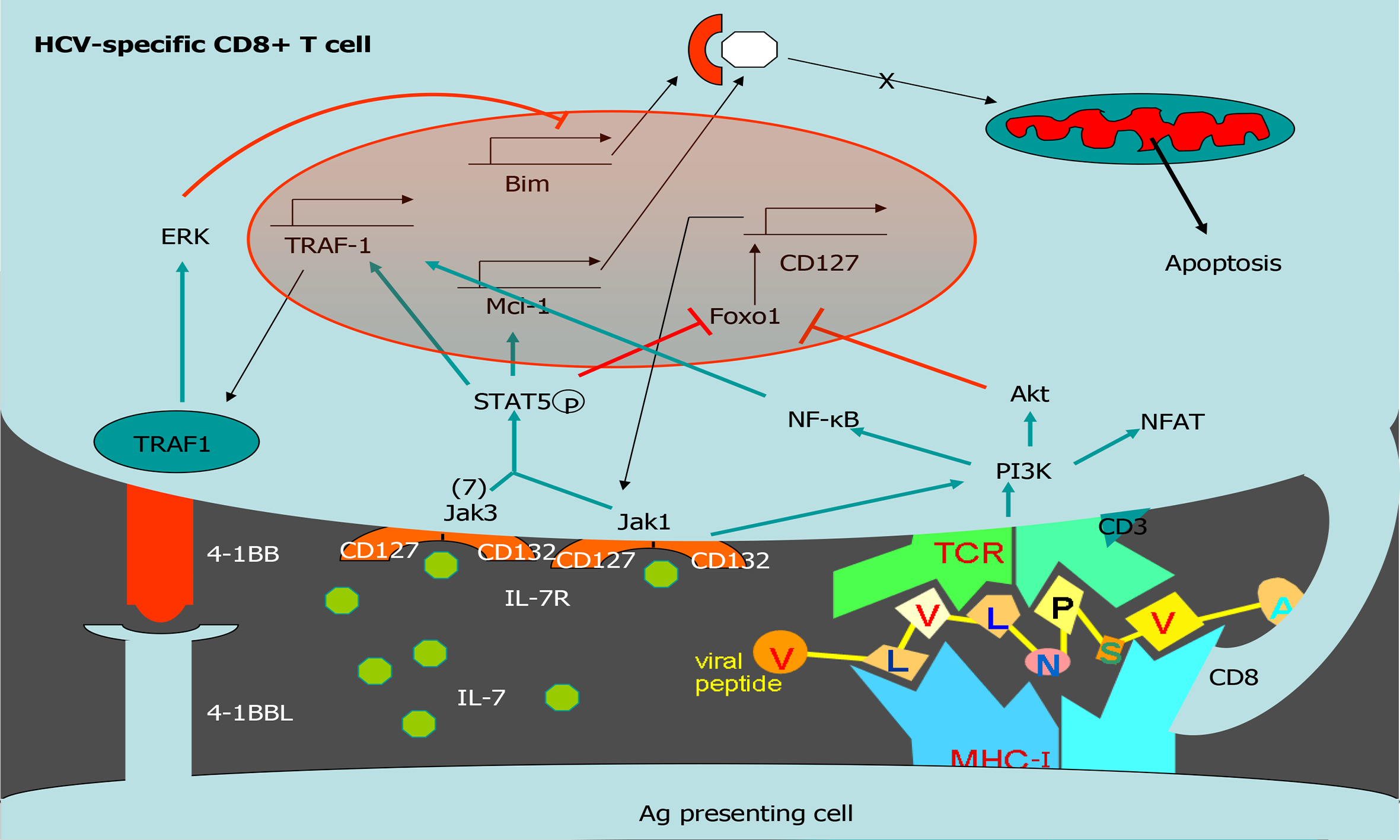
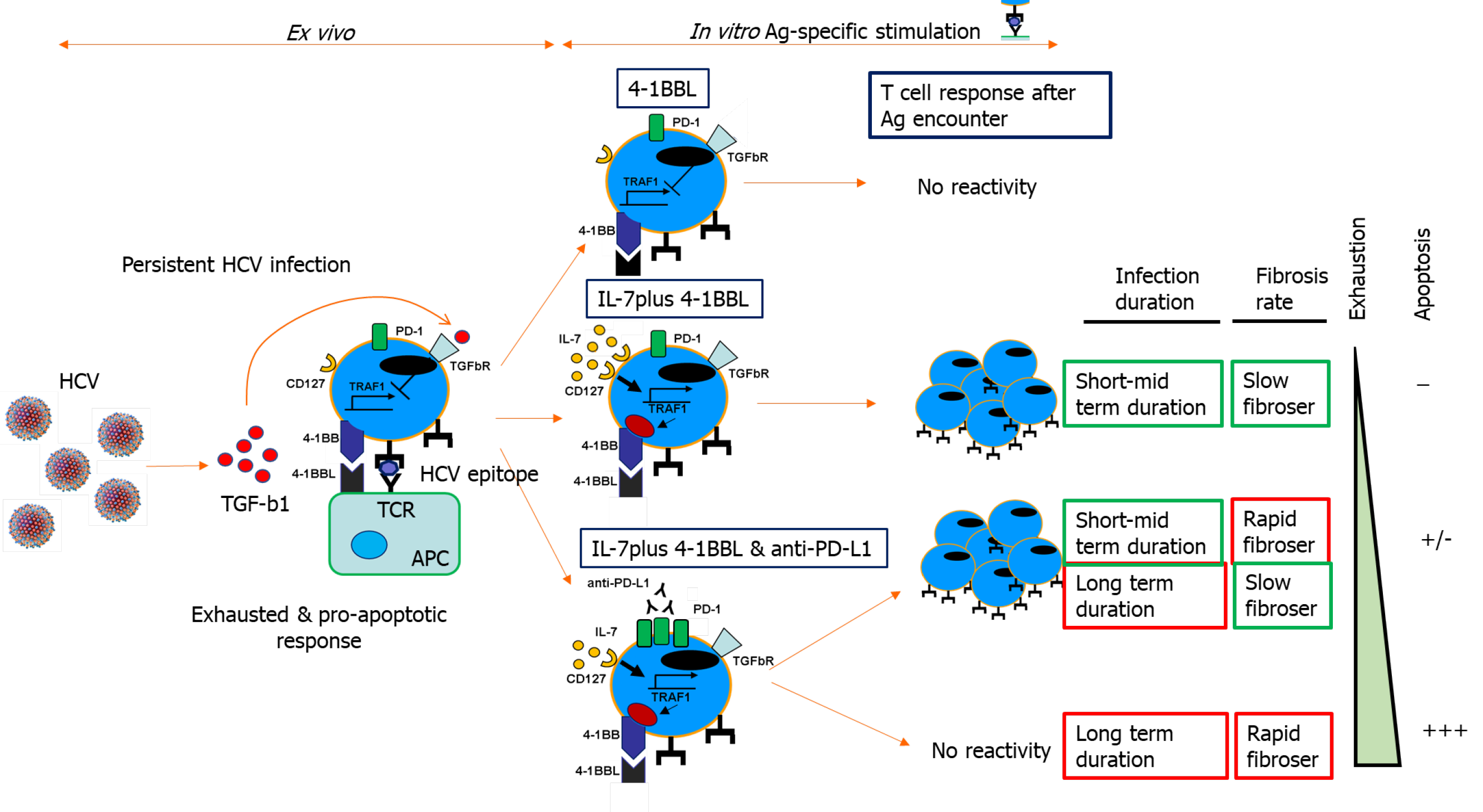
Exhausted HCV-specific cytotoxic T cells are characterized by the high expression of negative checkpoint proteins, such as PD-1, and low expression of the IL-7 receptor CD127[27] (Figure 3). Lack of CD127 makes these cells less sensitive to the pro-survival cytokine IL-7, which stabilizes the anti-apoptotic protein myeloid leukemia cell differentiation protein (Mcl-1)[28] (Figure 3). IL-7/IL-7R signaling positively regulates Mcl-1 via signal transducer and activator of transcription 5[77] but also increases TRAF1 level[16] (Figure 4). As previously stated, 4-1BB/TRAF1 also counters Bim via ERK signaling[55] (Figure 4). Moreover, during persistent HCV infection, TGF-β1 Level is increased, and this cytokine downregulates TRAF1 expression on T cells. Hence, during HCV infection, the combination of low IL-7 sensitivity linked to the higher TGF-β1 Level could be the “perfect storm” to desensitize 4-1BB signaling via TRAF1 Loss. This suggests that, as in HIV infection[16], the loss of TRAF1 in HCV-specific CD8 T cells during chronic hepatitis C is central to the aforementioned imbalance between Bim and Mcl-1[28] (Figures 2 and 3). Therefore, HCV-specific T cells could be poorly reactive and prone to apoptosis due to the lack of signaling by IL-7 and 4-1BB.
TGF-β1 Levels are increased during persistent HCV infection[2,36,37] and there is low IL-7 receptor expression on T cells. TRAF1 is positively and negatively regulated by IL-7 and TGF-β1, respectively[16]. With this in mind, we hypothesize that high TGF-β1 Level during HCV infection could downregulate TRAF1, impairing 4-1BB signaling and upregulating Bim. Furthermore, low CD127 expression on HCV-specific CD8 T cells would also reduce Mcl-1 Levels. The combination of low Mcl-1 and high Bim levels would synergize to negatively affect T cell proliferation, cytotoxicity, and survival (Figure 4).
To test this hypothesis, our group detected TRAF1 expression directly ex vivo on HCV-specific CD8 T cells from chronically-infected and treated patients. As was expected, those individuals with persistent viral replication had lower TRAF1 expression than HCV controllers[2]. Moreover, TRAF1 expression was inversely correlated with the exhausted and pro-apoptotic phenotypes and directly correlated with T cell reactivity. Low TRAF1 expressing T cells were PD-1high, Mcl-1low, and CD127low, and did not expand after Ag encounter. Analysis of the supernatants of Ag-specific T cell cultures showed that those cases with less proliferative potential had higher levels of TGF-β1. Moreover, a negative correlation was also observed between serum TGF-β1 Level and TRAF1 expression on Ag-specific CD8 T cells. Furthermore, TGF-β1 in vitro treatment of HCV-specific CD8 T cells from resolvers induced TRAF1 downregulation, and this effect was counteracted by IL-7 treatment. Although the CD127 expression level is low in the effector progeny subset, the low frequency progenitor pool still maintains this receptor, and it is this population that is suitable for immunotherapy[78,79]. Moreover, IL-7 at a therapeutic dose can antagonize multiple cellular and molecular networks[80]. These data suggest that during persistent HCV infection, TGF-β1 downregulates TRAF1 in T cells, which can be reversed by ex vivo IL-7 treatment.
Consequently, we developed an IL-7 and 4-1BBL combination treatment to improve T cell reactivity; IL-7-dependent upregulation of TRAF1 restored 4-1BB signaling to fully enable the agonist actions of 4-1BBL over 4-1BB. We observed a hierarchical response that was dependent on the stage of HCV infection; only cases with less severe fibrosis and lower evolution responded favorably to the 4-1BBL/IL-7 combination[2]. We speculated that cases with worse progression probably had higher burden of exhausted T cells with increased PD-1 expression, leading us to add anti-PD-L1 treatment to the IL-7/4-1BBL combination[81]. After the combined treatment, we were able to restore two other groups of cases: Those with low fibrosis progression but long-term infection, and those with rapid-progression and short-lasting disease. Unfortunately, those cases with less favorable factors, specifically rapid fibrosis progressors with long-term infection, were not responsive to the treatment[2]. This may have been due to the loss of these T cell populations from apoptosis (Figure 5).
The HCV-specific T cell response impacts infection outcomes. Mid-slow fibrosis progressors have less exhausted T cells, but the length of infection also influences the impairment of the T cell response. Worse T cell reactivity is observed the longer the infection lasts, and the faster liver fibrosis takes place. T cell response impairment is mediated by an exhausted and pro-apoptotic status that is characterized by the upregulated expression of negative checkpoints and the inhibition of positive co-stimulatory molecules. Among the latter is 4-1BB signaling via its effector TRAF1. This pathway regulates downstream Bim via ERK and is involved in T cell activation and survival. TRAF1 is induced by IL-7 and downregulated by TGF-β1. During persistent HCV infection, TGF-β1 Level is increased and can contribute to T cell exhaustion by TRAF1 loss. Depending on the stage of the infection, IL-7 ex vivo treatment can restore TRAF1 expression and T cell reactivity (Figure 5).
4-1BB/TRAF1 has a pathogenic role in chronic HCV infection that describes a new mechanism of T cell exhaustion and explains different infection outcomes. Modulation of 4-1BB/TRAF1 can be useful as an immunotherapeutic strategy in chronic viral infections and cancer.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver, No. 1340.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lendvai G S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol. 2014;20:3418-3430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 2. | Moreno-Cubero E, Subirá D, Sanz-de-Villalobos E, Parra-Cid T, Madejón A, Miquel J, Olveira A, González-Praetorius A, García-Samaniego J, Larrubia JR. According to Hepatitis C Virus (HCV) Infection Stage, Interleukin-7 Plus 4-1BB Triggering Alone or Combined with PD-1 Blockade Increases TRAF1low HCV-Specific CD8+ Cell Reactivity. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 740] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 5. | Poynard T, Ratziu V, Benhamou Y, Opolon P, Cacoub P, Bedossa P. Natural history of HCV infection. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O'Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol. 2019;37:457-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 1272] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 8. | Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2189] [Cited by in RCA: 3190] [Article Influence: 319.0] [Reference Citation Analysis (0)] |
| 9. | Nandi D, Pathak S, Verma T, Singh M, Chattopadhyay A, Thakur S, Raghavan A, Gokhroo A, Vijayamahantesh. T cell costimulation, checkpoint inhibitors and anti-tumor therapy. J Biosci. 2020;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Funsten JR, Murillo Brizuela KO, Swatzel HE, Ward AS, Scott TA, Eikenbusch SM, Shields MC, Meredith JL, Mitchell TY, Hanna ML, Bingham KN, Rawlings JS. PKC signaling contributes to chromatin decondensation and is required for competence to respond to IL-2 during T cell activation. Cell Immunol. 2020;347:104027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 12. | Fisicaro P, Boni C, Barili V, Laccabue D, Ferrari C. Strategies to overcome HBV-specific T cell exhaustion: checkpoint inhibitors and metabolic re-programming. Curr Opin Virol. 2018;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Scharf L, Tauriainen J, Buggert M, Hartogensis W, Nolan DJ, Deeks SG, Salemi M, Hecht FM, Karlsson AC. Delayed Expression of PD-1 and TIGIT on HIV-Specific CD8 T Cells in Untreated HLA-B*57:01 Individuals Followed from Early Infection. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Edilova MI, Abdul-Sater AA, Watts TH. TRAF1 Signaling in Human Health and Disease. Front Immunol. 2018;9:2969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Kim CM, Jeong JH, Son YJ, Choi JH, Kim S, Park HH. Molecular basis for TANK recognition by TRAF1 revealed by the crystal structure of TRAF1/TANK complex. FEBS Lett. 2017;591:810-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Wang C, McPherson AJ, Jones RB, Kawamura KS, Lin GH, Lang PA, Ambagala T, Pellegrini M, Calzascia T, Aidarus N, Elford AR, Yue FY, Kremmer E, Kovacs CM, Benko E, Tremblay C, Routy JP, Bernard NF, Ostrowski MA, Ohashi PS, Watts TH. Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J Exp Med. 2012;209:77-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 18. | Alazard-Dany N, Denolly S, Boson B, Cosset FL. Overview of HCV Life Cycle with a Special Focus on Current and Possible Future Antiviral Targets. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61:S3-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Chevaliez S, Pawlotsky JM. HCV Genome and Life Cycle. In: Tan SL, editor Hepatitis C Viruses: Genomes and Molecular Biology. Norfolk, United Kingdom: Horizon Bioscience, 2006. |
| 21. | Pradat P, Virlogeux V, Trépo E. Epidemiology and Elimination of HCV-Related Liver Disease. Viruses. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 518] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 23. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 24. | Sarin SK, Kumar M. Natural history of HCV infection. Hepatol Int. 2012;6:684-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645-1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 494] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Spada E, Mele A, Berton A, Ruggeri L, Ferrigno L, Garbuglia AR, Perrone MP, Girelli G, Del Porto P, Piccolella E, Mondelli MU, Amoroso P, Cortese R, Nicosia A, Vitelli A, Folgori A. Multispecific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut. 2004;53:1673-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, González-Praetorius A, Albertos S, García-Garzón S, Lokhande M, Parra-Cid T. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(-) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection. Cell Immunol. 2011;269:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Larrubia JR, Lokhande MU, García-Garzón S, Miquel J, González-Praetorius A, Parra-Cid T, Sanz-de-Villalobos E. Persistent hepatitis C virus (HCV) infection impairs HCV-specific cytotoxic T cell reactivity through Mcl-1/Bim imbalance due to CD127 down-regulation. J Viral Hepat. 2013;20:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, Freeman GJ, Lennox JL, Workowski KA, Hanson HL, Grakoui A. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808-9822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398-11403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 453] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 31. | Seigel B, Bengsch B, Lohmann V, Bartenschlager R, Blum HE, Thimme R. Factors that determine the antiviral efficacy of HCV-specific CD8(+) T cells ex vivo. Gastroenterology. 2013;144:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Larrubia JR, Calvino M, Benito S, Sanz-de-Villalobos E, Perna C, Pérez-Hornedo J, González-Mateos F, García-Garzón S, Bienvenido A, Parra T. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Larrubia JR, Benito-Martínez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14:7149-7159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Bertoletti A, Maini MK. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr Opin Immunol. 2000;12:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911-4927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1281] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 36. | Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882-5892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Hall CH, Kassel R, Tacke RS, Hahn YS. HCV+ hepatocytes induce human regulatory CD4+ T cells through the production of TGF-beta. PLoS One. 2010;5:e12154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Kim JH, Lee CH, Lee SW. Hepatitis C virus infection stimulates transforming growth factor-β1 expression through up-regulating miR-192. J Microbiol. 2016;54:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 621] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 40. | Vinay DS, Kwon BS. 4-1BB signaling beyond T cells. Cell Mol Immunol. 2011;8:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Pollok KE, Kim YJ, Hurtado J, Zhou Z, Kim KK, Kwon BS. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-mu-primed splenic B cells. Eur J Immunol. 1994;24:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Won EY, Cha K, Byun JS, Kim DU, Shin S, Ahn B, Kim YH, Rice AJ, Walz T, Kwon BS, Cho HS. The structure of the trimer of human 4-1BB ligand is unique among members of the tumor necrosis factor superfamily. J Biol Chem. 2010;285:9202-9210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4-1BB. J Immunol. 1993;150:771-781. [PubMed] |
| 44. | Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 291] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, Watts TH. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849-1862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 253] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Zapata JM, Perez-Chacon G, Carr-Baena P, Martinez-Forero I, Azpilikueta A, Otano I, Melero I. CD137 (4-1BB) Signalosome: Complexity Is a Matter of TRAFs. Front Immunol. 2018;9:2618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 47. | Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene. 2001;20:6482-6491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 514] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 48. | Schwenzer R, Siemienski K, Liptay S, Schubert G, Peters N, Scheurich P, Schmid RM, Wajant H. The human tumor necrosis factor (TNF) receptor-associated factor 1 gene (TRAF1) is up-regulated by cytokines of the TNF ligand family and modulates TNF-induced activation of NF-kappaB and c-Jun N-terminal kinase. J Biol Chem. 1999;274:19368-19374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Zapata JM, Krajewska M, Krajewski S, Kitada S, Welsh K, Monks A, McCloskey N, Gordon J, Kipps TJ, Gascoyne RD, Shabaik A, Reed JC. TNFR-associated factor family protein expression in normal tissues and lymphoid malignancies. J Immunol. 2000;165:5084-5096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Arron JR, Pewzner-Jung Y, Walsh MC, Kobayashi T, Choi Y. Regulation of the subcellular localization of tumor necrosis factor receptor-associated factor (TRAF)2 by TRAF1 reveals mechanisms of TRAF2 signaling. J Exp Med. 2002;196:923-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Sabbagh L, Andreeva D, Laramée GD, Oussa NA, Lew D, Bisson N, Soumounou Y, Pawson T, Watts TH. Leukocyte-specific protein 1 Links TNF receptor-associated factor 1 to survival signaling downstream of 4-1BB in T cells. J Leukoc Biol. 2013;93:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 53. | Zheng C, Kabaleeswaran V, Wang Y, Cheng G, Wu H. Crystal structures of the TRAF2: cIAP2 and the TRAF1: TRAF2: cIAP2 complexes: affinity, specificity, and regulation. Mol Cell. 2010;38:101-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 54. | McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative vs classical NF-κB pathway in T cells. J Biol Chem. 2012;287:23010-23019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180:8093-8101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 1326] [Article Influence: 165.8] [Reference Citation Analysis (0)] |
| 57. | Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 534] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 58. | Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 521] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 59. | Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol. 2014;61:S14-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 60. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 61. | Liu QJ, Gao B. Manipulation of MHC-I/TCR interaction for immune therapy. Cell Mol Immunol. 2008;5:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Abbas AK, Trotta E, R Simeonov D, Marson A, Bluestone JA. Revisiting IL-2: Biology and therapeutic prospects. Sci Immunol. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 63. | Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, Sica GL, Sharpe AH, Freeman GJ, Blazar BR, Turka LA, Owonikoko TK, Pillai RN, Ramalingam SS, Araki K, Ahmed R. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 760] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 64. | Krueger J, Rudd CE. Two Strings in One Bow: PD-1 Negatively Regulates via Co-receptor CD28 on T Cells. Immunity. 2017;46:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2271] [Article Influence: 189.3] [Reference Citation Analysis (0)] |
| 66. | Sanchez-Paulete AR, Labiano S, Rodriguez-Ruiz ME, Azpilikueta A, Etxeberria I, Bolaños E, Lang V, Rodriguez M, Aznar MA, Jure-Kunkel M, Melero I. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur J Immunol. 2016;46:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 67. | Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, Benedict CA, Ware CF, Croft M. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur J Immunol. 2010;40:2762-2768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Zhou AC, Batista NV, Watts TH. 4-1BB Regulates Effector CD8 T Cell Accumulation in the Lung Tissue through a TRAF1-, mTOR-, and Antigen-Dependent Mechanism to Enhance Tissue-Resident Memory T Cell Formation during Respiratory Influenza Infection. J Immunol. 2019;202:2482-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Teijeira A, Labiano S, Garasa S, Etxeberria I, Santamaría E, Rouzaut A, Enamorado M, Azpilikueta A, Inoges S, Bolaños E, Aznar MA, Sánchez-Paulete AR, Sancho D, Melero I. Mitochondrial Morphological and Functional Reprogramming Following CD137 (4-1BB) Costimulation. Cancer Immunol Res. 2018;6:798-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 71. | Menk AV, Scharping NE, Rivadeneira DB, Calderon MJ, Watson MJ, Dunstane D, Watkins SC, Delgoffe GM. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J Exp Med. 2018;215:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 72. | Choi BK, Lee DY, Lee DG, Kim YH, Kim SH, Oh HS, Han C, Kwon BS. 4-1BB signaling activates glucose and fatty acid metabolism to enhance CD8+ T cell proliferation. Cell Mol Immunol. 2017;14:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Sabbagh L, Srokowski CC, Pulle G, Snell LM, Sedgmen BJ, Liu Y, Tsitsikov EN, Watts TH. A critical role for TNF receptor-associated factor 1 and Bim down-regulation in CD8 memory T cell survival. Proc Natl Acad Sci USA. 2006;103:18703-18708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J Immunol. 2007;179:8252-8263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215-4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 736] [Article Influence: 40.9] [Reference Citation Analysis (1)] |
| 76. | Fisicaro P, Valdatta C, Massari M, Loggi E, Ravanetti L, Urbani S, Giuberti T, Cavalli A, Vandelli C, Andreone P, Missale G, Ferrari C. Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology. 2012;143:1576-1585.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 78. | Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 735] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 79. | Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1496] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 80. | Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, Assouline B, Lahl K, Sparwasser T, Ohashi PS, Mak TW. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 81. | Vezys V, Penaloza-MacMaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol. 2011;187:1634-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |