Published online Jan 27, 2020. doi: 10.4254/wjh.v12.i1.10
Peer-review started: July 1, 2019
First decision: August 7, 2019
Revised: September 30, 2019
Accepted: December 8, 2019
Article in press: December 8, 2019
Published online: January 27, 2020
Processing time: 186 Days and 11.3 Hours
Hepatic encephalopathy (HE) is a major complication of cirrhosis with independent prognostic significance. The current management of HE is mainly based on lactulose. Rifaximin has been shown to decrease the risk of HE recurrence in patients with episodic forms. HE can also be persistent. However, there is no drug support recommendation for rifaximin use in this setting.
To assess the effectiveness of rifaximin in the management of recurrent episodes of HE and recurrent acute exacerbations on persistent HE, in “real life conditions”.
In this retrospective study, using a within-subjects design, we collected data of patients treated with rifaximin for HE in two liver diseases centers, during the six-month period before and during the six-month period after the initiation of rifaximin. The primary effectiveness endpoint was the total number of HE events involving hospitalization.
Rifaximin was introduced for prevention of recurrent HE episodes in 29 out of 62 patients with normal mental status between episodes and for prevention of recurrent acute exacerbations on persistent HE in 33 out of 62 patients. In the “prevention of recurrent HE episodes” group, fewer HE events (0.79 vs 1.78; P = 0.013) were reported during the period of time when rifaximin was used. In the “prevention of recurrent acute exacerbations on persistent HE” group, there was no significant difference in the number of HE-events (1.48 vs 1.77; P = 0.582).
In this real-life experience, the effectiveness of rifaximin was confirmed in the prevention of HE episodes recurrence but was not proved in the prevention of acute exacerbations recurrence on persistent HE.
Core tip: The clinical and economic burdens of hepatic encephalopathy (HE) are tremendous and growing worldwide. Therapies to improve the quality of life of patients and to decrease the rate of hospitalizations and economic consequences are needed. Rifaximin was proved effective to reduce the risk of HE recurrence in patients with episodic forms. However, real-life data are still scarce, particularly concerning persistent HE. In this real-life experience, the effectiveness of rifaximin was confirmed in the prevention of HE episodes recurrence but was not proved in the management of persistent HE.
- Citation: Chautant F, Guillaume M, Robic MA, Cadranel JF, Peron JM, Lison H, Cool C, Bureau C, Duhalde V. Lessons from “real life experience” of rifaximin use in the management of recurrent hepatic encephalopathy. World J Hepatol 2020; 12(1): 10-20
- URL: https://www.wjgnet.com/1948-5182/full/v12/i1/10.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i1.10
Cirrhosis is the final stage of most chronic liver diseases and is the fourth most common cause of death in Europe, accounting for 170000 deaths per year against 33539 in the United States and 1.03 million worldwide[1]. The exact prevalence of cirrhosis is unknown. It is estimated between 0.1% to 0.27% in the European[2] and United States populations[3].
Hepatic encephalopathy (HE) is a frequent and major complication of cirrhosis. HE is estimated to affect 30%-40% of patients with cirrhosis at some time during their clinical course[4]. HE impairs daily functioning and health-related quality of life (HRQL) of cirrhotic patients[5] and is associated with a high risk of recurrence. Episodes of HE are associated with residual effects on cognitive functions[6] and are frequently associated with hospitalizations[7]. Frequent occurrence of HE and high hospital admission rates result in a significant economic burden to healthcare systems[8]. HE affects the patients’ survival and constitutes an important prognostic factor[9,10] with a 1-year survival rate of 40% to 50% and a 3-year survival rate of approximately 20%[11,12]. Therefore, the prevention of HE is a major objective in the management of cirrhotic patients.
Current therapeutic approaches for HE treatment and prevention aim at reducing the production or intestinal absorption of ammonia. Lactulose, a nonabsorbable disaccharide, is the first line treatment of HE[4]. Some poorly absorbed antibiotics, such as neomycin or vancomycin, have proven to be effective. Their use has been limited by an increased risk of antimicrobial resistance and/or severe adverse effects[13,14].
Rifaximin, a minimally absorbed oral antibiotic, appears to have a better profile and aims at decreasing intestinal ammonia production. This antibiotic may also reduce the bacterial translocation involved in inflammation in HE[15]. An antiprotozoal, nitazoxanide also showed interesting results in association with lactulose[16]. Furthermore, rifaximin treatment could be an alternative to norfloxacin in secondary prevention of spontaneous bacterial peritonitis, another complication of liver cirrhosis[17].
In 2010, the efficacy of rifaximin has been reported for preventing episodes of HE in patients with an history of recurrent HE[18]. The use of rifaximin also improved HRQL in patients with cirrhosis and recurrent HE[19]. On the contrary, the literature data are inconclusive concerning the management of persistent HE and are scarce in real life conditions. In this study we described the use of rifaximin and assessed the effectiveness of rifaximin in the management of HE in “real life”.
This approach is very important as real-life data are relatively scarce and can be much different compared to randomized studies as underlined by Krag et al[20] in the PROSPER multicenter observational study.
We performed a retrospective study conducted in two liver diseases centers in France; one is an academic hepatology center (Toulouse) and the other is a primary referral hospital with high expertise in chronic liver diseases and cirrhosis (Creil).
All cirrhotic patients with HE treated with rifaximin were considered for inclusion. The inclusion period ranged from July 1, 2010 to September 13, 2013, according to the French temporary authorization for rifaximin use. For each patient, the date of inclusion corresponded to the initiation of rifaximin treatment.
The following parameters were collected at inclusion. We considered anamnestic data: Gender, age, etiology of cirrhosis, medical history (HE, gastrointestinal bleeding, ascites, renal failure and hepatocellular carcinoma), presence of a transjugular intrahepatic portosystemic shunt (TIPS), smoking and/or active alcoholism; anthropometric and biochemical data.
The study period included the six months before the initiation of treatment with rifaximin (period 1: “without rifaximin”) up to six months or until the end of treatment (period 2: “with rifaximin”). This treatment duration was chosen from the pivotal study schedule[18]. Each patient served as his or her own control. The period “without rifaximin” was used as the reference period for assessing the effectiveness of rifaximin.
For each period, clinical characteristics were recorded: The number of hospitalizations for HE, number of HE events during hospitalization, cumulative time of HE-related hospitalization, grade of HE (according to the West Haven Criteria[21]), the presence or absence of the main precipitating factors of HE described in the literature factors, occurrence of bacterial infections. The recurrent and persistent character of HE was specified, according to the time course of HE.
Recurrent HE is a term used when episodes occur within a time frame of 6 mo or less. Persistent HE denotes a pattern of behavioral alterations such as depressive mood, mild anxiety and/or difficulties to sleep at night that are always present interspersed with relapses of HE[4]. Therefore, in addition to persistent HE, acute exacerbations are noticed[22]. We have considered as recurrent HE either when confronted to a recurrence of HE episodes or to a recurrence of acute exacerbations on persistent HE. The characteristics related to rifaximin treatment were also considered: Indication, dosage, duration, cause of interruption, occurrence of adverse effects, and concomitant use of lactulose. The indication was based on data collected during the period without rifaximin and was not modified during the 6-mo follow up with rifaximin.
Data were collected for the two periods using hospitalization and consultation reports via electronic medical records in the same way. The clinical description of HE is based on a non-standardized evaluation. It depends on the physical examination by a senior hepato gastroenterologist according to the West-Haven criteria.
The primary effectiveness endpoint of the study was the total number of HE events during the period of hospitalization; the HE events corresponded to episodes of HE and acute exacerbations in patients with a persistent form.
The secondary endpoints were the number of HE-related hospitalizations, the length of HE-related hospitalization, the probability of HE recurrence among patients in remission from HE episodes, the probability of persistent HE cessation, occurrence of bacterial infections, safety and predictive factors of response to treatment. HE-related hospitalizations corresponded to hospitalizations with a diagnosis of HE; length of hospitalization: Total duration of hospital stay. The presence of predictive factors of response to treatment was assessed by comparing clinical and biological parameters collected at baseline between patients who responded to rifaximin therapy and patients who did not. The response to treatment was defined as the cessation of persistent HE or maintenance of remission from recurrent HE among patients in remission from episodes of HE. Some results were considered for the entire cohort and for two subgroups of patients, “prevention of recurrent HE episodes” and “prevention of recurrent acute exacerbations on persistent HE”.
In order to ensure optimal comparison to evaluate the effectiveness and tolerance of rifaximin between the two periods, all data presented were assessed by taking into account the follow-up time. The results were expressed within 100 d of follow-up. As a retrospective study, the approval of the ethics committee was not mandatory (i.e., according to the Jardé Law in France). It did not require a consent from patients as the study analyzed data from usual care during hospitalization. The patients’ data were made anonymous prior to analysis.
Qualitative variables have been described by the number and the percentage of each modality. They were compared using McNemar’s chi-square tests or with Fisher exact tests (for expected values < 5). Quantitative variables were described by mean ± SD and were compared using Student’s t-test for paired samples. For all tests, a significant level of 0.05 was considered. Kaplan-Meier methods were used to estimate the probability of HE recurrence and cessation of persistent HE. A statistical analysis was performed with SPSS software, version 19.0 (Chicago, IL, United States).
Sixty-two patients were included, with a sex ratio male/female of 2.6 and an average age of 62 ± 10 years. The cause of cirrhosis was alcohol in 41 (66%), hepatitis B or C in 16 (26%), non-alcoholic fatty-liver disease in 10 (16%), other causes in 7 (11%); combined etiologies were found in 12 patients (19%). The HE events occurring six months before the introduction of rifaximin therapy were described. A recurrent HE was observed for about half of the patients (55%). The majority of the patients (68%) has been hospitalized several times for HE (Table 1).
| Demographic characteristics | |
| Age, mean ± SD, yr | 62 ± 10 |
| Male sex, n (%) | 45 (73) |
| Cirrhosis etiology, n (%) | |
| Alcohol | 41 (66) |
| Alcohol withdrawal, n (%) | 34/41 (83) |
| Hepatitis-B or C virus | 16 (26) |
| Non-alcoholic fatty liver disease | 10 (16) |
| Other | 7 (11) |
| Previous liver related complication, n (%) | |
| HE | 62 (100) |
| Gastrointestinal bleeding | 28 (45) |
| Ascites | 39 (63) |
| Renal failure | 24 (39) |
| Hepatocellular carcinoma | 14 (23) |
| Presence of oesophageal varices | 48 (77) |
| TIPS, n (%) | 25 (40) |
| Scores | |
| Child class, n | |
| A/B/C | 2/36/24 |
| Child score, mean ± SD | 9.2 ± 2.0 |
| MELD, mean ± SD | 16.8 ± 6.4 |
| Total number of HE events occurred in hospitalization or requiring hospitalization, n (%) | |
| 0 | 1 (2) |
| 1 | 16 (26) |
| 2 | 17 (27) |
| > 2 | 28 (45) |
| The highest West Haven grade1, n (%) | |
| 1 | 6 (10) |
| 2 | 20 (32) |
| 3 | 24 (39) |
| 4 | 12 (19) |
| Repeated HE-related hospitalizations (> 1), n (%) | 42 (68) |
| Recurrent HE, n (%) | 34 (55) |
During period 1, 53 patients (85.5%) received lactulose therapy (average dose, 37 ± 18 g/d; range, 10-90 g/d) compared to 52 patients (83.9%) during period 2, (average dose, 42 ± 22 g/d; range, 10-90 g/d). The average dose of rifaximin therapy during period 2 was 1000 mg/d for 54 patients (87%) and 1200 mg/d for 8 patients (13%). Rifaximin was prescribed according to two indications: Prevention of recurrent acute exacerbations on persistent HE in 33 patients (53%) and prevention of recurrent HE episodes in 29 patients (47%). Thirty-four patients (55%) received rifaximin therapy for at least six months. A treatment discontinuation was observed in 24 patients (39%). The causes were: death (n = 7), liver transplantation (n = 5), improvement of symptoms (n = 4), lack of clinical improvement (n = 3), non-compliance (n = 3), adverse effect (n = 1) and palliative care (n = 1). A temporary and voluntary interruption of treatment was observed in 4 patients with an average duration of 33.2 d. During this 6-mo follow-up, the average duration of rifaximin therapy was 143 d (range, 7-180 d).
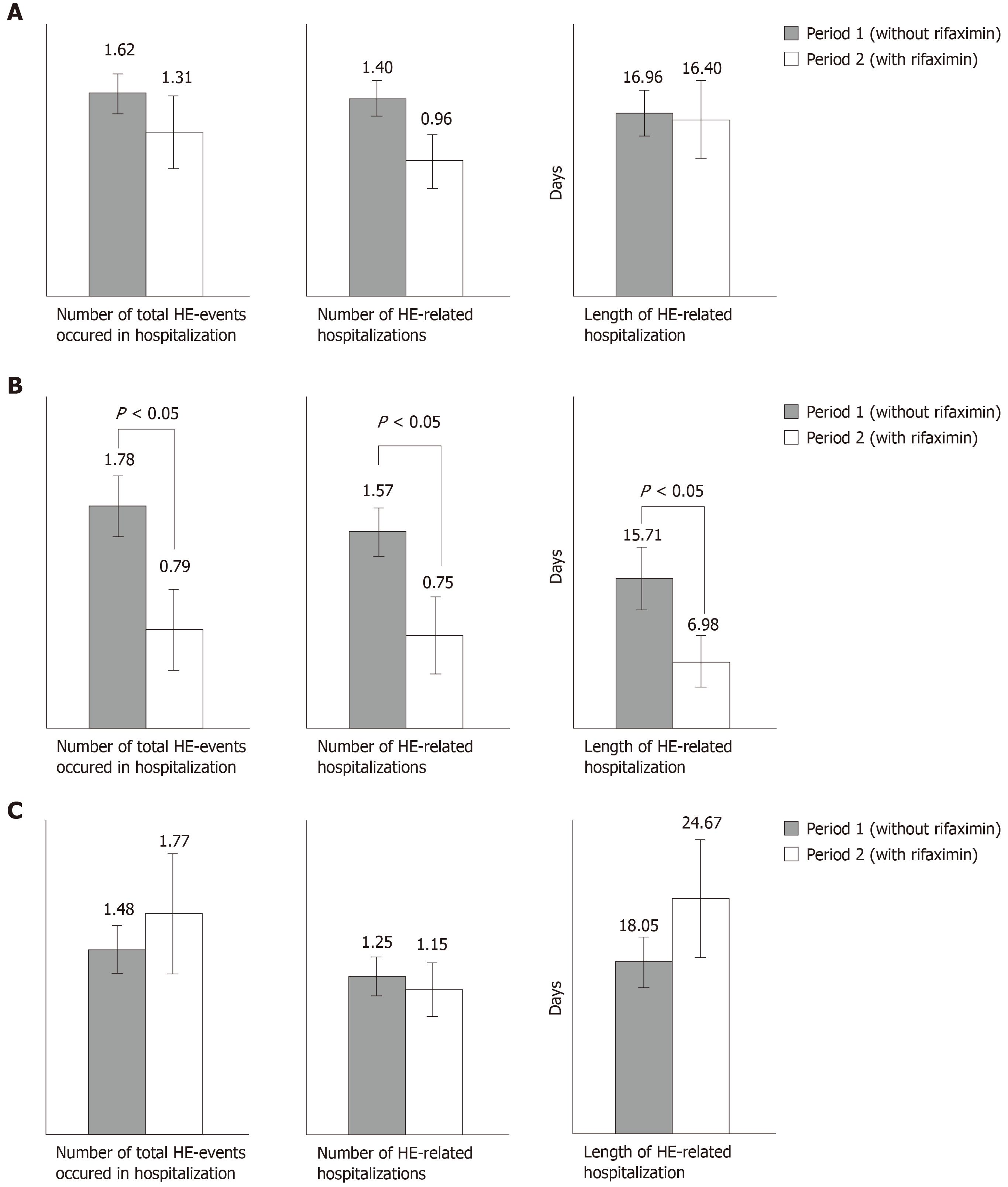
In the whole cohort, we observed a downward trend in the total number of HE-events, in the number of HE-related hospitalizations, and in the length of hospital stay between periods 1 and 2 (Figure 1A).
For the subgroup “prevention of recurrent HE episodes” (Figure 1B), the total number of HE events (0.79 vs 1.78; P = 0.013), the number of HE-related hospitalizations (0.75 vs 1.57; P = 0.030) and the length of HE-related hospitalization (6.98 d vs 15.71 d; P = 0.027) were significantly lower during the period with rifaximin compared to the period without rifaximin.
When rifaximin was given for prevention of recurrent acute exacerbations on persistent HE, there was a non-significant increase in the number of HE events (1.77 vs 1.48) and in the length of hospitalization (24.67 d vs 18.05 d). The number of HE-related hospitalizations decreased between the two periods (1.15 vs 1.25; P = 0.728) but the difference was not significant (Figure 1C).
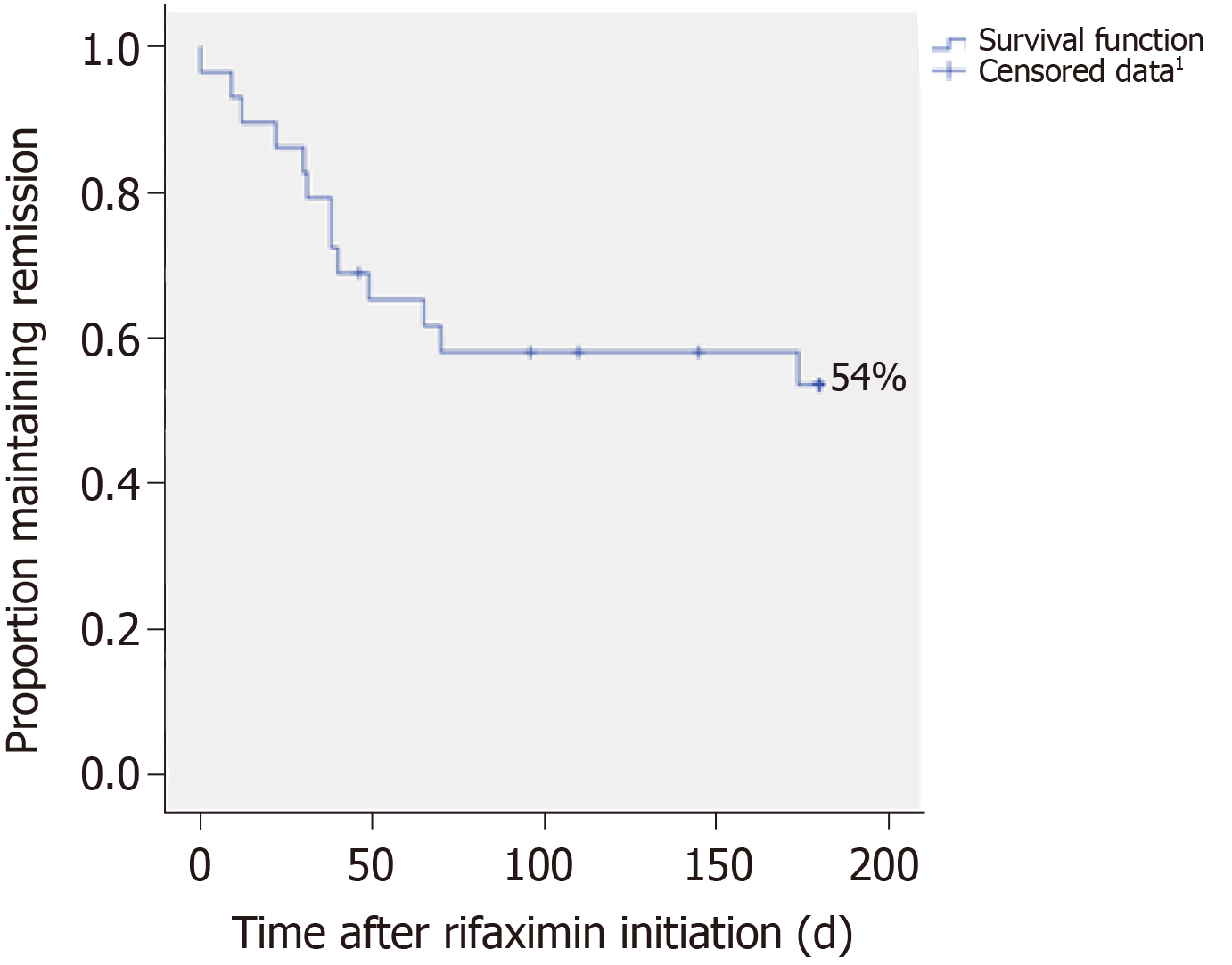
The probability of HE recurrence was 46% among patients in remission from HE episodes during rifaximin therapy. The median time to the first HE occurrence was 118.5 d (95%CI: 91.8-145.3) (Figure 2).
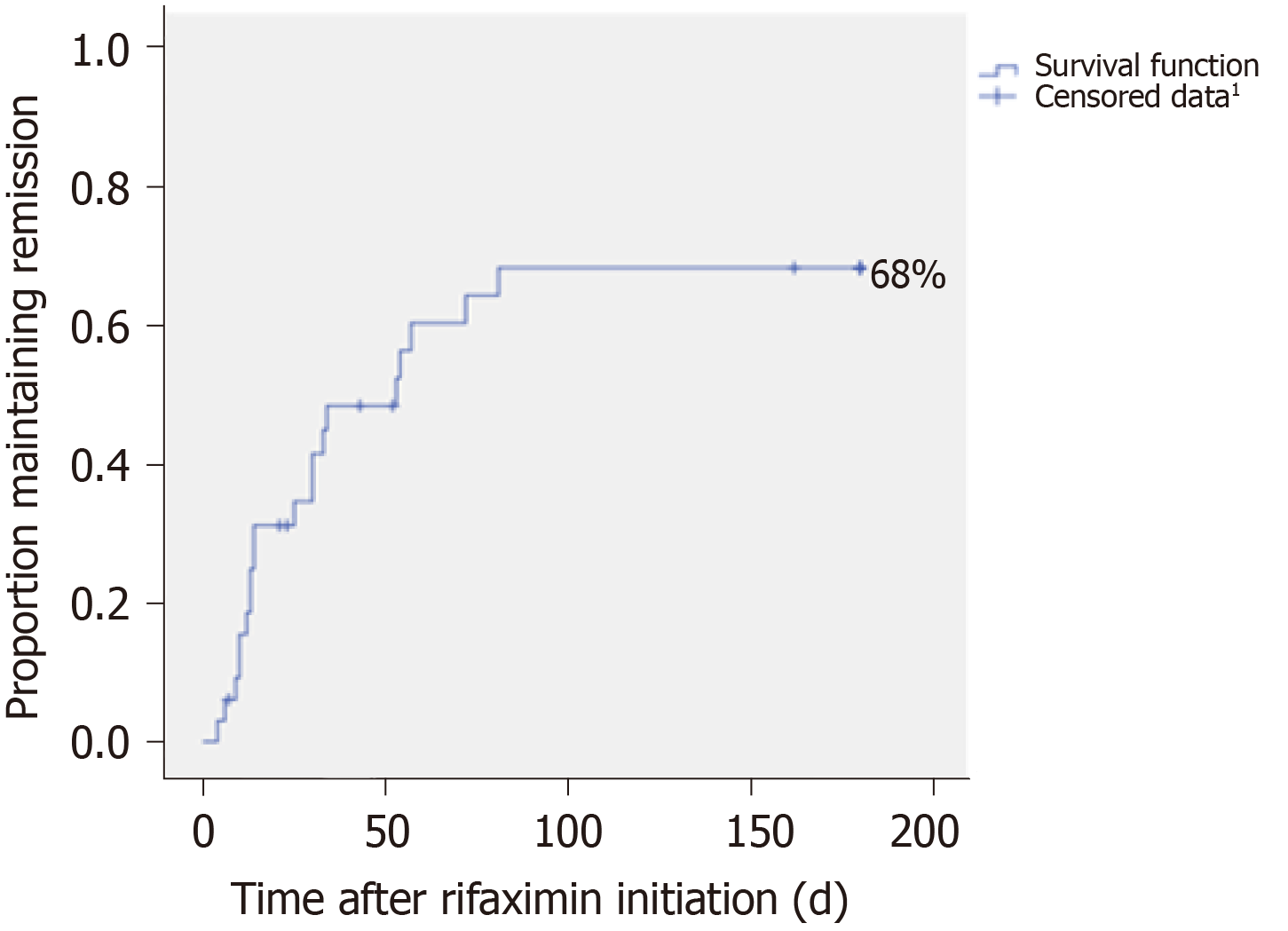
The probability of persistent HE cessation was 68%. The median time to disappearance was 78.3 d (95%CI: 52.0-104.7). After two months of treatment, more than 50% of patients concerned were free of persistent HE (Figure 3).
Twenty-six patients had one or more bacterial infections during period 1 compared to 13 patients during period 2. The rate of infectious events per patient was similar before and after the introduction of rifaximin treatment (0.35 ± 0.47 vs 0.34 ± 0.54; P = 0.95). Rifaximin was well tolerated. Only 2 patients experienced adverse events leading to the discontinuation of the drug for one of them (pruritus and drug induced liver injury with no real correlation shown associated with the intake of rifaximin).
All variables collected at the inclusion were analyzed and no significant difference was found. The main characteristics of both groups are summarized in Table 2.
| Parameter | Non-responder (n = 26) | Responder (n = 36) | P value |
| Indication, n (%) | |||
| Prevention of recurrent HE episodes/prevention of recurrent acute exacerbations on persistent HE | 13 (50)/13 (50) | 16 (45)/20 (55) | 0.66 |
| Sex (M/F), n (%) | 21 (81)/5 (19) | 24 (67)/12 (33) | 0.22 |
| TIPS, n (%) | 8 (31) | 17 (47) | 0.19 |
| Cirrhosis etiology, n (%) | |||
| Alcohol | 20 (77) | 21 (58) | 0.13 |
| Hepatitis-B or C virus | 7 (27) | 9 (25) | 0.86 |
| Lactulose therapy (period 1) | |||
| Patients treated, n (%) | 22 (85) | 31 (86) | 1.0 |
| Mean dose, mean ± SD (g) | 35 ± 17 | 39 ± 19 | 0.48 |
| Age | 63 ± 10 | 62 ± 10 | 0.89 |
| HE (period 1) | |||
| HE events, mean ± SD | 1.41 ± 1.0 | 1.62 ± 1.16 | 0.23 |
| HE-related hospitalizations, mean ± SD | 1.3 ± 1.0 | 1.40 ± 0.95 | 0.16 |
| Length of HE-related hospitalization, mean ± SD | 14 ± 13 | 17 ± 16 | 0.51 |
| Repeated HE-related hospitalizations (> 1), n (%) | 16 (62) | 26 (72) | 0.37 |
| Infectious events | 0.31 ± 0.44 | 0.35 ± 0.47 | 0.92 |
| Biochemical | |||
| INR | 1.7 ± 0.8 | 1.6 ± 0.4 | 0.71 |
| Serum albumin (g/L) | 28 ± 5 | 29 ± 5 | 0.63 |
| Serum bilirubin (μmol/L) | 63 ± 99 | 35 ± 20 | 0.11 |
| Serum sodium (mmol/L) | 134 ± 7 | 135 ± 5 | 0.62 |
| Serum creatinine (μmol/L) | 97 ± 50 | 107 ± 111 | 0.65 |
| AST (UI/L) | 59 ± 31 | 80 ± 135 | 0.45 |
| ALT (UI/L) | 33 ± 19 | 44 ± 30 | 0.11 |
| Hemoglobin (g/dL) | 10.4 ± 1.9 | 10.5 ± 1.7 | 0.79 |
| CRP (mg/L) | 13 ± 19 | 11 ± 11 | 0.67 |
| Scores | |||
| Child Pugh score | 9 ± 2 | 9 ± 2 | 1.0 |
| MELD score | 16 ± 6 | 17 ± 6 | 0.53 |
In this retrospective study conducted in two liver diseases centers, we found that rifaximin was efficient in secondary prevention in patients presenting recurrent episodes of HE by reducing the risk of HE recurrence associated with hospitalization. In fact, we observed a reduction of the total number of HE events, the number of HE-related hospitalizations, and the length of HE-related hospitalization by up to 50%. Conversely, we were not able to show any effectiveness of rifaximin in patients with recurrent acute exacerbations on persistent HE. We observed an upward trend in the number of HE events and in the duration of HE-related hospitalization as well as a downward trend in the number of HE-related hospitalizations. Likewise, a cessation of persistent HE in more than 50% of patients after two months of treatment has been observed. Nevertheless, this result does not take into account any subsequent recurrences and does not ensure the maintenance of remission. Indeed, in this real-life study, rifaximin was efficient in patients receiving HE according to the manufacturer’s recommendations.
Similarly, the PROSPER study which should enroll approximately 550 patients with HE, is ongoing under ‘real world’ clinical practice conditions. Results are still unavailable but the controlled design of this study could avoid some bias related to uncontrolled study[20]. Actually, it can be argued that in our study the sample size was relatively small, however we found results in accordance with the literature when rifaximin was used in patients with recurrent HE and without persistent HE[18]. The limited sample (n = 33) of patients suffering from recurrent acute exacerbations on persistent HE precludes any conclusions about the absence of rifaximin effectiveness in this subgroup of patients. Moreover, although not evaluated in our study, savings costs could be made due to a decrease in the number of rehospitalization. In the study of Orr et al[23], real world data from seven United Kingdom liver centers of patients with cirrhosis treated with rifaximin were analyzed (n = 326). Following the beginning of rifaximin treatment, the total hospital length of stay was reduced by between 31 and 53% resulting in an average saving of £4858-£6607 per patient per year in hospital admission costs.
Our results in the subgroup “prevention of recurrent HE episodes” are in accordance with those of Orr et al[23]. In this retrospective study, comparing the 6 mo pre-rifaximin and post-rifaximin initiation in living patients at the end of the observation period (n = 114), there were significant reductions in the average number of hospitalizations per patient (liver-related 1.3 to 0.5, P < 0.001), hospital bed days per patient (liver-related 17.8 to 6.8, P < 0.001), 30-day hospital re-admissions per patient (liver-related 0.5 to 0.2, P = 0.039) and emergency department attendances per patient (all-cause, 1.0 to 0.5, P < 0.001).
In our real life conditions, an off-label use of rifaximin was also found in more than half of the cases: the prevention of acute exacerbations in patients with persistent HE, i.e., another form of recurrent HE. This use was associated with a lack of therapeutic alternatives and followed the failure of high-dose lactulose therapy. The literature data are relatively poor and unclear in the area of persistent HE; to our knowledge, an effectiveness assessment from this perspective is the first attempt. A few studies comparing rifaximin to nonabsorbable disaccharides demonstrated its efficacy in the management of HE events. However, baseline characteristics and the type of HE are not always clearly described. Most studies indicate an efficacy of rifaximin in the treatment of HE; some use the term “chronic” HE[24-31]. It can also refer to patients with recurrent episodes of HE as well as patients with continuous abnormalities of the mental state[32]. This underlines the difficulty to characterize HE and to appreciate its time course and its severity both in clinical practice as well as in clinical trials. Indeed, there is no standardized and reproducible tool to identify and grade HE[33]: The clinical examination can be based on different clinical scales, especially the West Haven criteria, the current gold standard[34]. However, this is a subjective and a non-standardized grading system with limited inter-observer reliability[4] which depends e.g., on the clinician’s own experience, the knowledge of the patient’s previous condition and education and involvement of spouses or family members because many episodes or exacerbations of HE occur at home. Recently, Bajaj et al[33] therefore developed a standardized and reproducible clinical tool, the HE Grading Instrument, requiring further prospective applications[33]. The persistent form of HE makes us raise more questions, which are connected to a definition which is not specific enough: e.g., how long does it take to consider an episode as persistent HE. The most recent proposed nomenclature of HE classifies the recurrent HE as a distinct entity; no details were provided concerning the acute exacerbations on persistent HE[4].
Considering the conflicting results in terms of effectiveness, some predictive factors of response have been researched. No significant results were obtained. It would probably have been more relevant to consider alcohol withdrawal and not the alcoholic etiology of cirrhosis as predictive factor because of the impact of alcohol on bacterial overgrowth and bacterial translocation involved in the pathogenesis of HE[35].
The main strengths of this study are: (1) The ability of the patients to serve as their own controls to overcome inter-individual variability and get results from small samples; and (2) The originality of its subject which is particularly interesting in the management of persistent HE. The main limitations of this study are acknowledged: (1) The small sample size especially in the subgroup of patients suffering from recurrent acute exacerbations on persistent HE precludes any conclusions about the absence of rifaximin effectiveness in this indication; (2) The effectiveness assessment is based on the most serious HE-events involving hospitalizations and does not take into account the outpatients events; (3) The data are based on non-standardized information obtained from hospitalization and consultation reports and depended on the evaluation of each clinician supporting cirrhotic patients; (4) The data from “TIPS patients” (n = 25; 40%) have not been analyzed as a separate entity. Because TIPS is a known risk factor of HE, the actual effectiveness of rifaximin on HE could have been underestimated[4]; and (5) The lack of a control group may limit the interpretation of the results. Numerous factors are involved in improving the patient’s health status, including specific drug effect, placebo effect, other therapeutic measures introduced, and the natural history of the disease.
In conclusion, in this first “real life” experience, rifaximin was used for two indications in a similar way. In secondary prevention of recurrent episodes of HE, rifaximin prescription is validated by a consistent study and widely prescribed in this indication. For the prevention of acute exacerbations recurrence on persistent HE, this use is much more nebulous. After at least six months of therapy, the effectiveness of rifaximin was confirmed in preventing the recurrence of HE episodes; but we cannot conclude that rifaximin is effective in the management of persistent HE. However, we noticed some encouraging results with a certain probability of persistent HE cessation. Some randomized controlled trials are needed to assess the efficacy of rifaximin in this type of HE. Nevertheless, first of all, it seems necessary to develop standardized and reproducible tools to improve the patient’ selection in clinical trials and allow results’ comparisons among studies. It seems to be an essential step to achieve further progress in the management of HE in clinical practice.
Hepatic encephalopathy (HE) is a major complication of cirrhosis with independant prognostic significance. The clinical and economic burdens of HE is tremendous and growing worldwide. Therapies are needed to improve the quality of life of patients and to decrease the rate of hospitalizations and the economic consequences.
The current management of HE is mainly based on lactulose. Rifaximin has been shown to decrease the risk of HE recurrence in patients with episodic forms. HE can also be persistent. However, there is no drug support recommendation for rifaximin use in this setting.
The study aimed at assessing the effectiveness of rifaximin in the management of recurrent episodes of HE and recurrent acute exacerbations on persistent HE, in “real life conditions”.
This is a retrospective study using a within-subjects design. The data of patients treated with rifaximin for HE is collected in two liver diseases centers, during the six-month period before and during the six-month period after the initiation of rifaximin. The primary effectiveness endpoint was the total number of HE events involving hospitalization.
In the case of patients presenting recurrent episodes of HE, we observed a significantly reduction of the total number of HE-events by up to 50%. Conversely, in the prevention of acute exacerbations in patients with persistent HE, an off-label use which has been found in more than half of the studied population, there was no significant difference in the number of HE-events.
The effectiveness of rifaximin was confirmed in the prevention of HE episodes recurrence but was not proved in the prevention of acute exacerbations recurrence on persistent HE.
We noticed some encouraging results with a certain probability of persistent HE cessation. Randomized controlled trials are needed to assess rifaximin efficacy in this type of HE. It seems necessary to develop standardized and reproducible tools to improve the patients’ selection in clinical trials and allow results comparison among studies. It seems to be an essential step to achieve further progress in the management of HE in clinical practice.
The authors would like to acknowledge Mrs Fanny Hadad for reviewing the English version.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd-Elsalam S, García-Compeán D, Thompson RR S-Editor: Ma YJ L-Editor: A E-Editor: Wu YXJ
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 2. | Zatoński WA, Sulkowska U, Mańczuk M, Rehm J, Boffetta P, Lowenfels AB, La Vecchia C. Liver cirrhosis mortality in Europe, with special attention to Central and Eastern Europe. Eur Addict Res. 2010;16:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 504] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 4. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 5. | Moscucci F, Nardelli S, Pentassuglio I, Pasquale C, Ridola L, Merli M, Riggio O. Previous overt hepatic encephalopathy rather than minimal hepatic encephalopathy impairs health-related quality of life in cirrhotic patients. Liver Int. 2011;31:1505-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT, Luketic V, White MB, Sanyal AJ. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332-2340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Neff G, Zachry W III. Systematic Review of the Economic Burden of Overt Hepatic Encephalopathy and Pharmacoeconomic Impact of Rifaximin. Pharmacoeconomics. 2018;36:809-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 10. | Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl. 2007;13:1366-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 368] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Fichet J, Mercier E, Genée O, Garot D, Legras A, Dequin PF, Perrotin D. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care. 2009;24:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Greenberg LH, Momary H. Audiotoxicity and nephrotoxicity due to orally administered neomycin. JAMA. 1965;194:827-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med. 1999;340:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 729] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 15. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Abd-Elsalam S, El-Kalla F, Elwan N, Badawi R, Hawash N, Soliman S, Soliman S, Elkhalawany W, ElSawaf MA, Elfert A. A Randomized Controlled Trial Comparing Nitazoxanide Plus Lactulose With Lactulose Alone in Treatment of Overt Hepatic Encephalopathy. J Clin Gastroenterol. 2019;53:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2016;28:1450-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, Teperman L, Hillebrand D, Huang S, Merchant K, Shaw A, Bortey E, Forbes WP. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 19. | Sanyal A, Younossi ZM, Bass NM, Mullen KD, Poordad F, Brown RS, Vemuru RP, Mazen Jamal M, Huang S, Merchant K, Bortey E, Forbes WP. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Krag A, Schuchmann M, Sodatonou H, Pilot J, Whitehouse J, Strasser SI, Hudson M. Design of the Prospective Real-world Outcomes Study of hepatic encephalopathy Patients' Experience on Rifaximin-α (PROSPER): an observational study among 550 patients. Hepatol Med Policy. 2018;3:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Dharel N, Bajaj JS. Definition and nomenclature of hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S37-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 22. | Swaminathan M, Ellul MA, Cross TJ. Hepatic encephalopathy: current challenges and future prospects. Hepat Med. 2018;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Orr JG, Currie CJ, Berni E, Goel A, Moriarty KJ, Sinha A, Gordon F, Dethier A, Dillon JF, Clark K, Richardson P, Middleton P, Patel V, Shawcross D, Preedy H, Aspinall RJ, Hudson M. The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-α. Liver Int. 2016;36:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Loguercio C, Federico A, De Girolamo V, Ferrieri A, Del Vecchio Blanco C. Cyclic treatment of chronic hepatic encephalopathy with rifaximin. Results of a double-blind clinical study. Minerva Gastroenterol Dietol. 2003;49:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, Castells L, Rodríguez-Martínez D, Fernández-Rodríguez C, Coll I, Pardo A; Spanish Association for the Study of the Liver Hepatic Encephalopathy Cooperative Group. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Paik YH, Lee KS, Han KH, Song KH, Kim MH, Moon BS, Ahn SH, Lee SJ, Park HJ, Lee DK, Chon CY, Lee SI, Moon YM. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Jiang Q, Jiang XH, Zheng MH, Jiang LM, Chen YP, Wang L. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2008;20:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Eltawil KM, Laryea M, Peltekian K, Molinari M. Rifaximin vs. conventional oral therapy for hepatic encephalopathy: a meta-analysis. World J Gastroenterol. 2012;18:767-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Wu D, Wu SM, Lu J, Zhou YQ, Xu L, Guo CY. Rifaximin versus Nonabsorbable Disaccharides for the Treatment of Hepatic Encephalopathy: A Meta-Analysis. Gastroenterol Res Pract. 2013;2013:236963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 32. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1411] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 33. | Bajaj JS, Frederick RT, Bass NM, Ghabril M, Coyne K, Margolis MK, Santoro M, Coakley DF, Mokhtarani M, Jurek M, Scharschmidt BF. Overt hepatic encephalopathy: development of a novel clinician reported outcome tool and electronic caregiver diary. Metab Brain Dis. 2016;31:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Ferenci P. Hepatic encephalopathy. Gastroenterol Rep (Oxf). 2017;5:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, Del Vecchio Blanco C, Loguercio C. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis. 2011;43:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |