Published online Jun 27, 2019. doi: 10.4254/wjh.v11.i6.553
Peer-review started: February 13, 2019
First decision: April 11, 2019
Revised: June 11, 2019
Accepted: June 17, 2019
Article in press: June 17, 2019
Published online: June 27, 2019
Processing time: 136 Days and 22.7 Hours
Hepatitis E virus (HEV) infection is a cause of chronic hepatitis in immunosuppressed patients. Sustained virologic response rates to a 12-wk course of ribavirin therapy were reported to be > 70% in the West. This study describes the outcome of HEV treatment in a transplant center in Singapore.
To study the outcome of ribavirin treatment in a series of chronic HEV patients, and the cause of treatment failure.
We studied all of the transplant recipients who were diagnosed with HEV infection between 2012 to 2015. The outcome of therapy and virologic relapse are monitored for three years after the end of therapy.
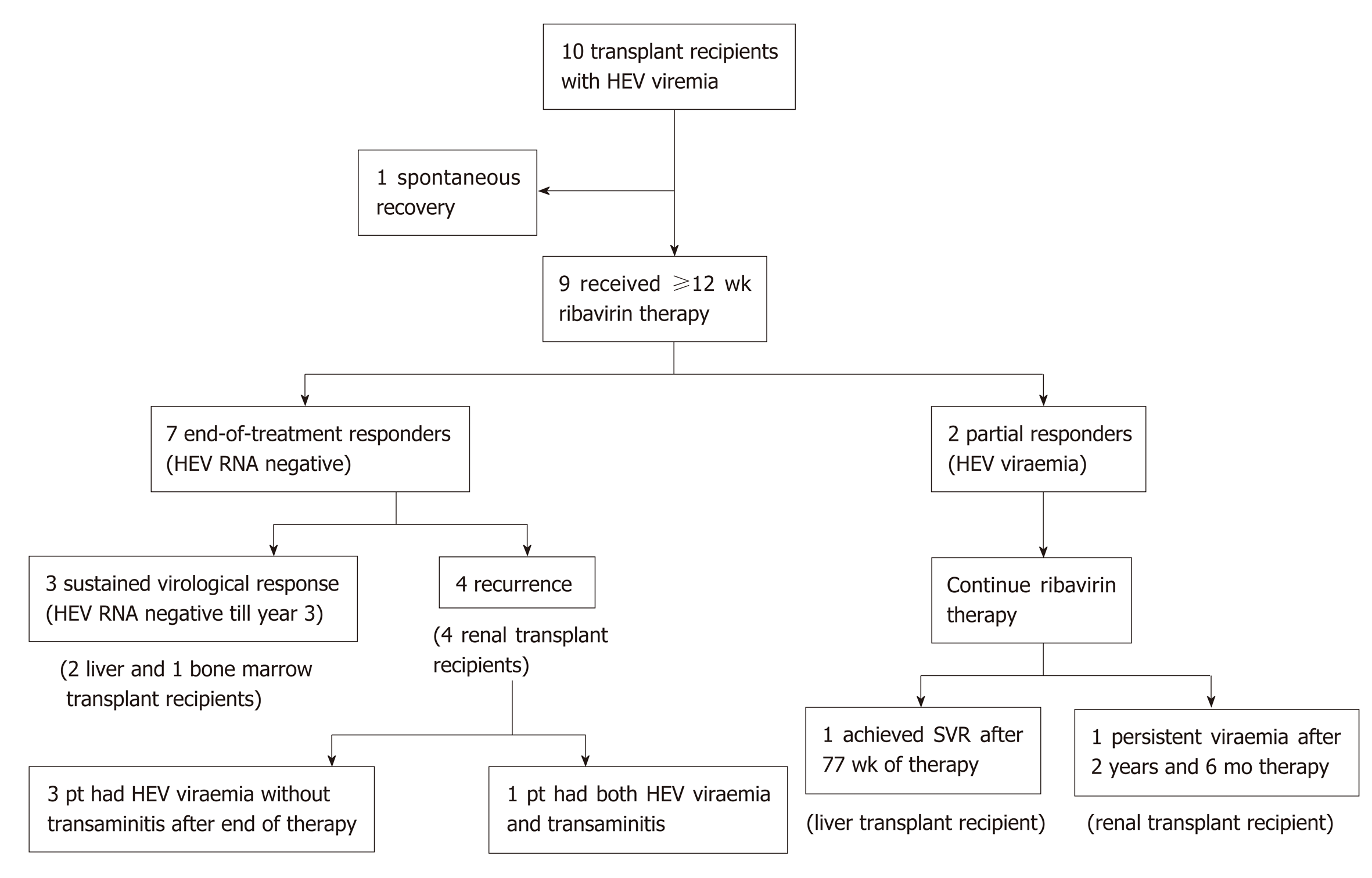
Ten transplant recipients (4 liver, 5 kidney, and 1 bone marrow transplantation) with positive HEV RNA were studied. Nine patients received at least 12 wk of ribavirin therapy, and the remaining patient resolved after reducing immunosuppression therapy. Two subjects had prolonged viremia that lasted more than one year, despite continuous ribavirin therapy. Four ribavirin-treated patients (44.4%) had HEV RNA relapse after achieving a virologic response by the end of treatment. The overall failure rate is 66.7%. Being a kidney transplant recipient is the strongest risk factor for not achieving an initial sustained virologic response (0/5 treated, Chi-Square test, P < 0.05). The most common side effect of ribavirin is anemia (100%) (haemoglobin reduction of 3-6.2 g/dL). Seven patients required either a blood transfusion or erythropoietin therapy.
The sustained virologic response rate of 12-wk ribavirin therapy for HEV infection in this Asian series was lower than expected. Kidney transplant recipients had a higher rate of treatment failure due to higher immunosuppression requirements and adverse effects.
Core tip: Hepatitis E virus (HEV) infection is a cause of chronic hepatitis in immunosuppressed patients. Sustained virologic response (SVR) rate to a 12-wk course of ribavirin therapy was reported to be > 70% in the West. This study describes the outcome of HEV treatment in a transplant centre in Singapore. Ten transplant recipients (liver, kidney, bone marrow transplantation) with positive HEV RNA were studied. The SVR rate of 12-wk ribavirin therapy for HEV infection in this Asian series was lower than expected; an overall failure rate of 66.7%. Kidney transplant recipients had a higher treatment failure due to higher immunosuppression requirements and adverse effects.
- Citation: Low EXS, Tripon E, Lim K, Tan PS, Low HC, Dan YY, Lee YM, Muthiah M, Loo WM, Koh CJ, Phyo WW, Pang J, Lim SG, Lee GH. Risk factors for ribavirin treatment failure in Asian organ transplant recipients with chronic hepatitis E infection. World J Hepatol 2019; 11(6): 553-561
- URL: https://www.wjgnet.com/1948-5182/full/v11/i6/553.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i6.553
In the last decade, our understanding of hepatitis E virus (HEV) infection has changed from that of an acute self-limiting disease into one that has "two-faces". In immunosuppressed or immunocompromised hosts, some HEV genotypes may cause chronic hepatitis and sometimes accelerated liver decompensation, especially in post-organ transplant recipients[1]. While it was previously thought to be limited to developing countries with a few sporadic cases in industrialized nations secondary to migration, it has been clear that autochthonous infection is also found in developed countries[2,3]. A Singapore study done with the Communicable Diseases Division of the Ministry of Health showed that between 2000-2011, 45.5% (n = 481) of reported acute hepatitis E cases diagnosed by ELISA for Anti-HEV immunoglobulin M were considered to be autochthonous[4]. Singapore is unique, in that it is an industrialized country with a population of 5.6 million that serves as a port and travel hub for many countries deemed endemic for HEV. The ethnic Chinese in Singapore make up a majority of the population (76%), while ethnic Malays make up 15%, and ethnic Indians 7%.
The characteristics of genotypes 1-4 HEV have been well-described, with genotype 1 and 2 causing waterborne outbreaks and only infecting humans, and genotypes 3 and 4 recognized as zoonotic infections carried by mainly domestic pigs, boar, and deer[2]. More recently, additional genotypes have been identified. Genotypes 5 and 6 HEV are found to occur in wild boar in Japan, and genotype 7 have been identified in dromedary camels in Dubai[5]. Chronic infections of HEV have been predominantly caused by HEV genotype 3, although case reports on chronic infection due to HEV genotypes 4 and 7 in liver transplant recipients[6] have been published.
Studies from France have reported a rate of chronicity of about 58% in post-transplant patients diagnosed with HEV[3]. Ribavirin is considered a safe and effective treatment option for chronic HEV. A study from the same group in France reported a sustained virologic response (SVR) rate of 78% (n = 59)[7], while a study in Germany gave an SVR rate of 75% (n = 4) in a group of liver transplant patients given riba-virin[8].
While a reasonable strategy for a trial of decreasing immunosuppression doses and eventual starting of ribavirin has been suggested, there have been some issues with regards to the management of HEV in transplant patients, and no guidelines have been established. In 2013, Kamar et al[9,10] proposed that carriage of HEV for more than 3 mo can be called the threshold for identifying chronicity. Their group further suggests that persistence of HEV replication for 3 mo to be the cutoff point for considering treating HEV with ribavirin. Others, however, disagree and believe that HEV conforms to the convention of a 6-mo cut-off, as in hepatitis B and C[11]. A systematic review has concluded that ribavirin is an effective and reasonably safe first-line option for the treatment of chronic hepatitis E, especially in organ transplant recipients[12].
Most clinical studies on chronic HEV cohorts come from studies based in Europe and the United States. There is a need to characterize and investigate the chronic HEV infection in Asian populations, including those in Southeast Asia.
We report on the first 10 consecutive cases of hepatitis E diagnosed in im-munosuppressed post-transplant patients in an organ transplant center in Singapore. The aim is to describe and characterize hepatitis E infection in this multi-ethnic Asian series, and how the outcome differs from the experience of our Western counterparts.
This retrospective case series study includes all the immunosuppressed patients, solid organ, and haematological transplant recipients who were diagnosed with hepatitis E virus infection in the National University Hospital, a tertiary referral center in Singapore, between May 2012 to September 2015. All patients with newly diagnosed HEV infection based on positive HEV RNA assay, who were on immunosuppression therapy for prevention of graft rejection, were followed up. In one patient, the time of infection was determined with the use of stored, frozen serum. During the study period, the subjects who had transaminitis that were caused by persistent HEV replication were treated with ribavirin. The study was approved by the Domain-Specific Review Board of the National Healthcare Group (DSRB Number 2016/00250), and all the patients gave written informed consent.
Medical records including all available laboratory assays for HEV, including RNA and serology, were reviewed. Pertinent data, like the type of organ transplantation, time of organ transplantation and types and doses of immunosuppression at the time of HEV infection diagnosis, were included. The management of the HEV infection was also reviewed, including the length of time from onset of unexplained transaminitis, to diagnosis and subsequent resumption of ribavirin. The outcome of ribavirin therapy and virologic relapse were monitored for three years after the end of therapy.
All samples were analysed at the Molecular Diagnostic Center at the National University Hospital. HEV RNA was detected and quantified with the use of a commercial real-time PCR assay for HEV (RealStar® HEV RT-PCR Kit 2.0, Altona Diagnostics, Hamburg, Germany). The limit of detection for HEV RNA was 10 IU/μL. HEV genotyping was determined by sequencing and analysis as described in a previous study[5]. An SVR is defined as an undetectable level of HEV RNA in the serum at least 6 mo after completion of ribavirin therapy. A non-responder is defined as persistently detectable HEV RNA after 12-wk of treatment with ribavirin. Relapse is defined as recurrent HEV RNA-positive viremia after the completion of a 12-wk ribavirin therapy, despite an initial response and HEV RNA negativity at the end of treatment.
Proportions were compared with the use of the Fisher’s exact test. Quantitative variables were compared with the use of the non-parametric Wilcoxon test. Independent factors associated with non-responder or relapse after initial SVR were analysed with the use of SPSS version 21 software. In this analysis, non-responder/ relapser (as defined above) were compared with those with a durable SVR for three years. A P value (two-sided) of less than 0.05 was considered as statistically significant.
The data of the first 10 HEV RNA-positive patients who had received a solid organ transplant (5 kidney transplant recipients, four liver-transplant recipients, and one bone marrow transplant recipients) were analysed. They were all diagnosed with HEV infections between 2012 to 2015, based on positive HEV RNA assays. Nine patients (Singapore resident) were infected with HEV genotype 3, and the remaining patient who came from United Arab Emirates had HEV genotype 7 (a ribavirin responder). All patients were investigated for HEV after presenting with transaminitis, and all the other viral causes and other primary hepatic causes of transaminitis were ruled out.
The demographics and clinically relevant characteristics of the HEV-infected patients are summarized in Table 1. The time between organ transplantation and the diagnosis of HEV infection ranged from 2 wk to 23 years after organ transplantation. Immunosuppression was tacrolimus-based in 6 patients (60%), and tacrolimus levels at the time of diagnosis of HEV infection were 2.8-9.5 μg/mL. Most patients (80%) had mild to moderate elevation of liver enzymes (< 400 IU/mL) at the time of diagnosis. One patient, who was 2 wk post-liver transplantation, had extremely high transaminase levels (ALT 8127 U/L and AST 2591 U/L) at the time HEV RNA was found to be positive. This result was later deemed mainly due to liver reperfusion injury. The liver enzyme and HEV RNA levels of this patient spontaneously resolved within the next 12 wk, with the progressive reduction of immunosuppression therapy.
| Variables | Value |
| Age, yr | |
| Mean | 47 |
| Range | 34-59 |
| Male gender, n (%) | 8 (80) |
| Type of organ transplant, n (%) | |
| Liver | 4 (40) |
| Kidney | 5 (50) |
| Bone Marrow | 1 (10) |
| Time from transplant to unexplained transaminitis in mo | |
| Median | 41.5 |
| Range | 0.5-276 |
| Time from transplant to diagnosis of HEV in mo | |
| Median | 43.0 |
| Range | 0.5-276 |
| Immunosuppressive therapy at the diagnosis of HEV, n (%) | |
| Tacrolimus | 6 (60) |
| Cyclosporin | 1 (10) |
| Mycophenolate | 6 (60) |
| Azathioprine | 3 (30) |
| Prednisolone | 7 (70) |
| Sirolimus | 1 (10) |
| BEAM-R | 1 (10) |
| (carmustine, etoposide, cytarabine, and melphalan – rituximab) | |
| Peak alanine transaminase, as U/L | |
| Median | 213 |
| Range | 133-8127 |
| Peak aspartate aminotransferase, as U/L | |
| Median | 203 |
| Range | 84-2591 |
| Baseline estimated glomerular filtration rate, as mL/min | |
| Median | 63 |
| Range | 27-108 |
| Baseline creatinine, as μmol/L | |
| Median | 141 |
| Range | 66-323 |
In the remaining nine patients, a decision to start ribavirin therapy was made by the clinician based on the trajectory of the HEV viral load and extent of HEV-related liver injury (Figure 1). The median time between the onset of transaminitis due to HEV and the initiation of ribavirin therapy was 3 mo (range, 1 to 9). In 7 patients, the period of observation was under 3 mo.
The median starting dose of ribavirin was 600 mg per day (range, 400 to 800), which was equivalent to 9.7 mg per kilogram of body weight per day (range, 2.7 to 13.5). The dosages were subsequently adjusted based on the estimated glomerular filtration rate (GFR). In more than half of patients (5 out of 9), dose reductions of ribavirin were necessary due to clinically significant anemia.
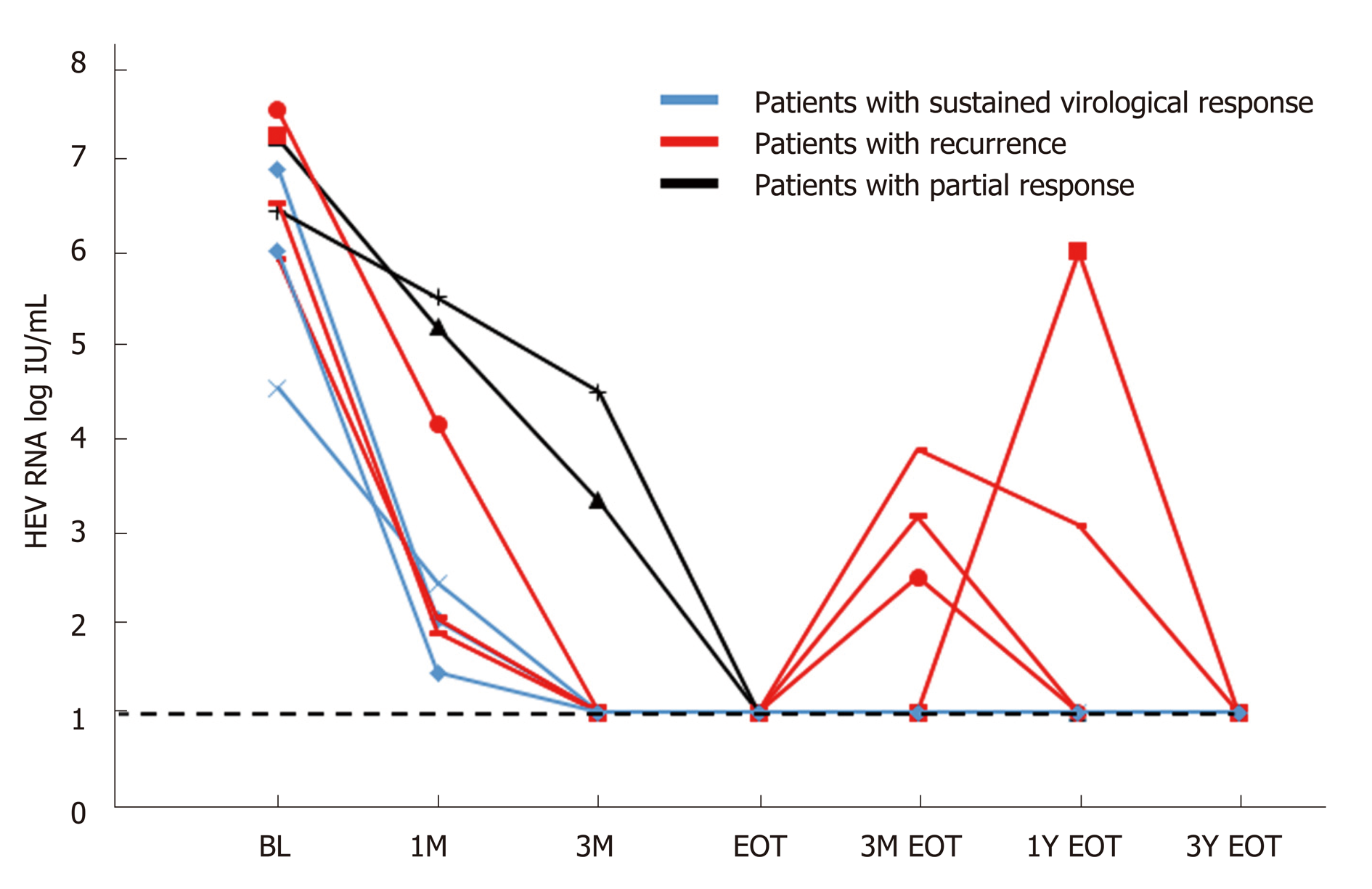
All the patients had positive HEV RNA at the start of ribavirin therapy (Figure 2). Peak viral load ranged from 1.78 x 104 to 3.36 x 107 IU/mL. At the end of the first month, HEV RNA levels were assessed in 7 patients, and the level remained detectable in all patients (range, 1.4 to 5.5 log IU/mL). At mo 3 and the end of therapy, 7 of the 9 patients undetectable HEV RNA, and two patients remained detectable with prolonged viremia despite continuous ribavirin therapy. Both these patients were given reduced doses of ribavirin due to side effects (mainly symptomatic anemia) before achieving SVR (1.5 and 3.7 years, respectively). One of them was later found to be non-compliant to therapy due to fear of adverse effects. After resuming ribavirin therapy, four patients (44%, all are kidney transplant recipients) had good responses during treatment, but developed HEV recurrence after initial complete virologic response at the end of therapy.
At the beginning of ribavirin therapy, all nine patients had an elevation of liver enzymes. Eight patients had transaminitis greater than two times the normal upper limit. All treated subjects had a resolution of transaminitis within 10 wk of starting ribavirin. Eight patients had normal liver tests after just 4 wk of therapy.
The overall failure rate of achieving sustained viral clearance for a 12-wk course of ribavirin was 66.67% (delayed virologic response plus viral recurrence after completing treatment). All four patients with viremia recurrence were kidney transplant recipients. One of them had a late relapse, which occurred one year after the end of therapy. During the HEV recurrence, transient transaminitis was noted. An HEV reinfection cannot be excluded. The rest of the other relapsers did not have accompanying transaminitis during the recurrence of HEV viremia. All 4 of them responded to the second course of ribavirin (12-16 wk) and achieved durable SVR.
We analysed the factors that could potentially influence the rate of SVR (Table 2). Given the limited sample size, the only statistically significant predictive factor found to be associated with SVR was being a kidney transplant recipient (0 of 5 treated, χ2 test, P < 0.05). The most common side effect of ribavirin was anemia, with many patients requiring dose adjustments, erythropoietin or blood transfusions. Of the nine treated patients, all had a decrease in haemoglobin (Hb) levels (range, 3 to 6.2 g/dL). Five patients required blood transfusions, and seven required erythropoietin injections during ribavirin therapy.
| Sustained responders, n = 4 | Relapsers/ delayed responders, n = 5 | |
| Type of transplant | 3 liver transplants, 1 bone marrow transplant | Kidney transplant |
| Mean age in yr | 42 (15–58) | 48 (17–66) |
| Males | 3 | 4 |
| Mean Time from transplant to HEV infection | 40.3 mo (7-132 mo) | 130.7 mo (3.5-204 mo) |
| Mean HEV viral load peak, as IU/mL | 6.0 x 106 | 1.2 x 107 |
| Mean daily dose of Prednisolone at time of infection, n = 6 | 13.5 mg (n = 2) | 9.6 mg (n = 4) |
| Mean level of Tacrolimus at time of infection, as μg/L for n = 5 | 5.5 (6.4, 7.4, 2.8) n = 3 | 8.6 (9.5, 7.7) n = 2 |
| Mean peak ALT, as U/L | 402 U/L (223-766) | 169 U/L (133–202) |
| Mean peak AST, as U/L | 374 U/L (186-818) | 132 U/L (84-219) |
| Lowest eGFR, as mL/min | 49 (34 to 85) | 32 (11-68) |
| Mean ribavirin dose, as mg/kg body weight, at initiation | 10.3 mg/kg body weight (6.9- 13.5) | 7.8 mg/kg body weight (2.7-11.9) |
| Haemoglobin drop | 3.3 g/dL | 3.5 g/dL |
| Resolution of Transaminitis after starting treatment | 15 d | 35 d |
| (12-21) | (14-70) | |
| Mean time to viral clearance after starting ribavirin | 8.5 wk (8-11) | 9.3 wk (5-12), n = 4 |
| HEV recurrence | None in all 4 patients (including the non-adherent subject after she became compliant to ribavirin Rx) | Yes in 4 patients + 1 patient with persistent viremia |
Since the landmark paper by Kamar et al[7] in 2014, a 12-wk course of ribavirin monotherapy has been widely used to treat chronic hepatitis E virus infection in transplant recipients, if a reduction in immunosuppression fails to eradicate the virus. Our study aims to assess the effects of ribavirin therapy in this Asian series, and compare the results with prior studies. Our study has two main findings: the SVR rate of ribavirin therapy for chronic HEV infection in our immunosuppressed Asian hosts may be lower than reported earlier, and kidney transplant recipients are associated with a higher risk of HEV recurrence or partial response to ribavirin monotherapy.
In this study, all five consecutive kidney transplant recipients (50%) had either partial response (HEV remains detectable) or had a recurrence of HEV after an initial virologic response. Statistically and clinically, this is unlikely to be coincidental. In Kamar et al[7] 2014, 42 out of 59 patients (71%) had a kidney transplant or kidney and pancreas transplantation, and they responded to treatment almost immediately from the beginning. The ability of the patients to tolerate the full dose of ribavirin therapy is one of the main reasons. Kidney transplant patients tend to have lower GFR than the other patients, and thus develop more haemolytic anemia on the same dose of ribavirin. On average, our kidney transplant patients received 7.8 mg/kg body weight of ribavirin, which was comparable to 8.1 mg/kg body weight in the study, but still significantly lower than the liver and bone marrow transplant recipients in our study (10.3 mg/kg). Our kidney transplant recipients were also slightly older (48 vs 42 years), had more years between transplantation and HEV infection, roughly double the peak viral load, and were on higher doses of calcineurin inhibitors than liver transplant recipients.
At the end of the 12-wk therapy, one patient (a kidney transplant recipient) continued to have high viremia of > 7 log IU/mL, whose HEV viremia persisted for another 3 years, during which he continued to receive reduced-dose, maintenance ribavirin therapy due to his renal graft impairment. Another patient had borderline HEV RNA levels at the end of treatment. Her HEV infection subsequently recurred due to non-adherence to ribavirin therapy. Hence the rate of virologic response at the end of therapy was 78%. Adding the number of virological recurrences (n = 4), the overall failure rate of a 12-wk ribavirin therapy for this study is 66.7%, far higher than that reported in the Western population. For those who had a recurrence of HEV, a second course of ribavirin for 12 wk resulted in a complete virologic response at the end of therapy and SVR at wk 24. In retrospect, a longer course of ribavirin therapy may be considered in kidney transplant patients who could not tolerate a full dose of ribavirin therapy.
It needs to be pointed out that in this study, most patients received ribavirin therapy between 1 mo to 3 mo after the first HEV RNA positive result on clinical grounds, based on the discretion of the physicians. Thus, these were not technically chronic HEV infection by the original definition that requires viral persistence of 6 mo or more. It is possible that some of these patients (the three patients who had SVR) may spontaneously clear the virus if ribavirin had not been started. However, we are focusing on the group of immunosuppressed subjects who had persistent viremia or HEV recurrence beyond 6 mo, thus qualifying them as chronic HEV infections. It is interesting to note that as HEV infection in transplant recipients are diagnosed increasingly earlier, and there is little reason to continue observing deranged liver function tests when treatment is available, the definition and cut-off for treatment of chronic/persistent HEV infection in this group are due for revision.
Anemia was the main limiting side effect. The mean hemoglobin fall was 3.4 g/dL for the patients who were treated with ribavirin. Three patients (33.3%) required a transient interruption of ribavirin therapy due to severe anemia. Five patients need transfusions, and seven patients require erythropoietin.
This uncontrolled case series has several limitations. (1) The number of patients studied was small; (2) The immunosuppression regimen was not controlled; and (3) The dosage of ribavirin and the monitoring protocol for each patient were individualized based on clinical assessment.
In conclusion, this Asian single-centre case series shows that the SVR rate of HEV infection treated with a 12-wk course of ribavirin may be lower than reported earlier. Kidney transplant recipients are at higher risk of relapse, possibly due to higher immunosuppression requirements and reduced tolerance for higher ribavirin dosages. A 3-mo regimen seems to be sufficient for the remaining organ transplant recipients. Whether a longer therapy for all Asian kidney transplant recipients, and whether the extended therapy should be guided by the on-treatment response, remains uncertain. Larger prospective studies are required to determine the most beneficial dose and duration of ribavirin therapy.
There has been an increase in the amount of literature and our understanding of Hepatitis E virus (HEV) infection since the landmark paper by Kamar et al in 2014. This study describes the outcome of HEV treatment in a transplant center in Singapore, where immunosuppressed Asian hosts appear to have lower sustained virologic response (SVR) rates after a 12-wk course of ribavirin than reported earlier.
Singapore is a unique industrialized country where, although ethnic Chinese make up 76% of the population, it is a thriving hub with many international visitors. Seven genotypes of HEV have been described so far, and studies have reported a rate of chronicity of 50%-60%. Ribavirin is considered an effective treatment option for chronic HEV in post-transplant patients, where success rates > 75% have been reported in France and Germany alongside a reduction in immunosuppression dose. Since most clinical studies come from Europe and the United States, there is a pressing need to characterize and investigate the state of chronic HEV infection in Asian populations.
Our report describes the first 10 consecutive cases of hepatitis E diagnosed in post-transplant patients in an organ transplant center in Singapore.
This is a retrospective case series that studied all newly diagnosed HEV infections in post-transplant patients from May 2012 to September 2015. Subjects who had transaminitis that were caused by persistent HEV replication were treated with ribavirin, and the results were collected and tabulated. Data from the first 10 HEV RNA-positive patients who had received a solid organ transplant (5 kidney transplant recipients, 4 liver-transplant recipients, and 1 bone marrow transplant recipient) were analysed.
One of the patients was from United Arab Emirates, and the other nine were Singapore residents. The median starting dose of ribavirin was 600 mg per day. The dosages were subsequently adjusted based on the estimated GFR. In more than half of patients (5 out of 9), dose reductions of ribavirin were necessary due to clinically significant anemia. The overall failure rate of achieving sustained viral clearance for a 12-wk course of ribavirin was 66.67% (delayed virologic response plus viral recurrence after completing treatment) – far higher than that reported in Western populations. All four patients with viremia recurrence were kidney transplant recipients, which was found to be the only statistically significant predictive factor. The most common side effect of ribavirin was anemia.
This study proposes that kidney transplant recipients, particularly those with poorer renal function, are more susceptible to the adverse effects of ribavirin. Asian patients with lower body weight may be even more likely to suffer from the side effects. This Asian single-centre case series shows that the SVR rate of HEV infection treated with a 12-wk course of ribavirin may be lower than reported earlier. Kidney transplant recipients are at higher risk of relapse, possibly due to higher immunosuppression requirements and reduced tolerance for higher ribavirin dosages.
More effective therapy for chronic HEV infection may be needed, including more accurate markers to predict ribavirin response. A large, prospective, controlled study comparing kidney transplants and other groups of chronic HEV patients will be useful to confirm the results of this study and minimise bias.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cunha C S-Editor: Cui LJ L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 999] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 2. | Khuroo MS, Khuroo MS. Hepatitis E: an emerging global disease - from discovery towards control and cure. J Viral Hepat. 2016;23:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Legrand-Abravanel F, Kamar N, Sandres-Saune K, Garrouste C, Dubois M, Mansuy JM, Muscari F, Sallusto F, Rostaing L, Izopet J. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis. 2010;202:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Tan LT, Tan J, Ang LW, Chan KP, Chiew KT, Cutter J, Chew SK, Goh KT. Epidemiology of acute hepatitis E in Singapore. J Infect. 2013;66:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lee GH, Tan BH, Teo EC, Lim SG, Dan YY, Wee A, Aw PP, Zhu Y, Hibberd ML, Tan CK, Purdy MA, Teo CG. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:355-357.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 411] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 6. | Wu CH, Ho CM, Tsai JH, Sun HY, Hu RH, Lee PH. First Case Genotype 4 Hepatitis E Infection After a Liver Transplant. Exp Clin Transplant. 2017;15:228-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 8. | Galante A, Pischke S, Polywka S, Luetgehethmann M, Suneetha PV, Gisa A, Hiller J, Dienes HP, Nashan B, Lohse AW, Sterneck M. Relevance of chronic hepatitis E in liver transplant recipients: a real-life setting. Transpl Infect Dis. 2015;17:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Kamar N, Rostaing L, Legrand-Abravanel F, Izopet J. How should hepatitis E virus infection be defined in organ-transplant recipients? Am J Transplant. 2013;13:1935-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Kamar N, Marion O, Izopet J. When Should Ribavirin Be Started to Treat Hepatitis E Virus Infection in Transplant Patients? Am J Transplant. 2016;16:727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Meisner S, Polywka S, Memmler M, Nashan B, Lohse AW, Sterneck M, Pischke S. Definition of chronic hepatitis E after liver transplant conforms to convention. Am J Transplant. 2015;15:3011-3012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat. 2015;22:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |