Published online May 27, 2019. doi: 10.4254/wjh.v11.i5.477
Peer-review started: March 12, 2019
First decision: April 10, 2019
Revised: April 12, 2019
Accepted: April 19, 2019
Article in press: April 19, 2019
Published online: May 27, 2019
Processing time: 79 Days and 8.5 Hours
Congenital dyserythropoietic anemia type 1 (CDA1) is an autosomal recessive disorder of ineffective erythropoiesis, resulting in increased iron storage. CDA1 is usually diagnosed in children and adolescents but can rarely present in the neonatal period with severe anemia at birth. There are no prior reports of neonatal liver histologic findings of CDA1. We report a case of CDA1 in a newborn presenting with severe anemia, cholestasis and liver failure, where liver biopsy helped confirm the diagnosis.
A term infant, born via emergency Cesarean section, presented with cholestasis, hepatosplenomegaly, multiorgan failure and severe anemia at birth. A prior pregnancy was significant for fetal demise at 35 wk without autopsy or known etiology for the fetal demise. Parents are both healthy and there is no history of consanguinity. On further evaluation, the patient was found to have severe ferritin elevation and pulmonary hypertension. An extensive infectious and metabolic work-up was negative. Salivary gland biopsy was negative for iron deposition. At 2 wk of age, a liver biopsy showed findings consistent with CDA1. A genome rapid sequencing panel revealed novel variants in the CDAN1 gene. The patient’s liver dysfunction, cholestasis and organomegaly resolved, however she remains transfusion-dependent.
We report liver pathology findings of CDA1 with a novel genetic mutation for the first time in a newborn.
Core tip: Congenital dyserythropoietic anemia type 1 (commonly known as CDA1) is an autosomal recessive disorder of ineffective erythropoiesis, resulting in increased iron storage. We report a rare case of CDA1 with novel genetic mutations in a newborn presenting with severe anemia, cholestasis and liver failure. This case highlights how liver histology helped confirm the diagnosis.
- Citation: Jaramillo C, Ermarth AK, Putnam AR, Deneau M. Neonatal cholestasis and hepatosplenomegaly caused by congenital dyserythropoietic anemia type 1: A case report. World J Hepatol 2019; 11(5): 477-482
- URL: https://www.wjgnet.com/1948-5182/full/v11/i5/477.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i5.477
Congenital dyserythropoietic anemia type 1 (CDA1) is an autosomal recessive disorder of ineffective erythropoiesis, resulting in increased iron storage, and considered a form of secondary hemochromatosis[1]. Most CDA1 patients have a mutation in the CDAN1 gene[2]. CDA1 is usually diagnosed in children and adolescents with moderate to severe macrocytic anemia. However, it can rarely present in the neonatal period with severe anemia at birth[2]. Additional clinical findings include hepatosplenomegaly (HSM), jaundice, cholestasis, liver dysfunction, transient thrombocytopenia and persistent pulmonary hypertension of the newborn[2]. The diagnosis is based on hematologic abnormalities and positive genetic testing[3]. Bone marrow biopsy findings include spongy heterochromatin, enlargement of nuclear pores and invagination of cytoplasm into the nuclear area[4].
Prior reports have described liver biopsy findings of extramedullary hematopoiesis and iron accumulation in autopsies and adult patients[5-8]. There have been no prior reports of neonatal liver histologic findings of CDA1. We report a case of CDA1 in a newborn presenting with severe anemia, cholestasis and liver failure, where liver biopsy helped confirm the diagnosis.
This is a former 37 wk and 3 d old female transferred to our institution due to respira-tory failure.
The patient was delivered by emergency Cesarean section due to non-reassuring fetal heart rate tracings at an outside hospital to a 28-year-old, Caucasian, gravida 4, para 2, 0, 1, 2 with an unremarkable pregnancy. A prior pregnancy was significant for fetal demise at 35 wk without autopsy or known etiology for the fetal demise. Parents are both healthy and there is no history of consanguinity. Perinatal laboratory results included maternal blood type O (+) with negative antibody screen, negative venereal disease research laboratory, hepatitis B, and human immunodeficiency virus and rubella. Apgar scores were 7 and 8. Birth weight was 3070 g (21st percentile), length 18 inches (16th percentile), occipital frontal circumference 32.5 cm (3rd percentile).
At birth, this patient had no facial or limb dimorphism. She was started on supplemental oxygen due to duskiness 10 min after birth. Subsequently, she required endotracheal intubation and initiation of inhaled nitric oxide. She was then transferred to our institution due to respiratory failure on day of life (DOL) 1. On arrival, she was found to have HSM.
On admission to our institution, she was found to have liver dysfunction with an International normalized ratio (commonly referred to as INR) of 2.1, total bilirubin of 9 mg/dL, direct bilirubin of 2.1 mg/dL, aspartate aminotransferase 655 U/L and alanine aminotransferase 65 U/L. Partial thromboplastin time was within normal limits, with mildly low fibrinogen and elevated D-dimers. Anemia and thrombocytopenia were also present. The anemia was present since birth with a hemoglobin of 7.4 g/dL and hematocrit of 23.7%. Her platelets were initially normal but soon started to decline, with a nadir of 54 k/μL on DOL 1. She was also found to have pulmonary hypertension, right ventricular hypertrophy and required high frequency oscillator ventilation due to hypoxemic respiratory failure.
Additional laboratory work-up included serum ferritin of 40664 ng/mL and normal soluble interleukin 2 receptor. Initially her gamma-glutamyltransferase (commonly referred to as GGT) was normal and then peaked at 390 U/L on DOL 25. Infectious studies included negative herpes simplex virus, Epstein-Barr virus, cytomegalovirus, adenovirus, parvovirus, enterovirus, echovirus, parechovirus and human herpesvirus 6 PCR. Multiple blood cultures and urine cultures were also negative. She also had negative work-up for inborn errors of metabolism: normal serum plasma amino acids, urine organic acids, ammonia and very long/branched-chain fatty acids.
Imaging studies included an initial echocardiogram on DOL 1, which showed a large patent ductus arteriosus, small patent foramen ovale and dilated and hypertrophied right ventricle with suprasystemic pressures. An abdominal ultrasound showed HSM with minimal ascites, and a liver Doppler was normal.
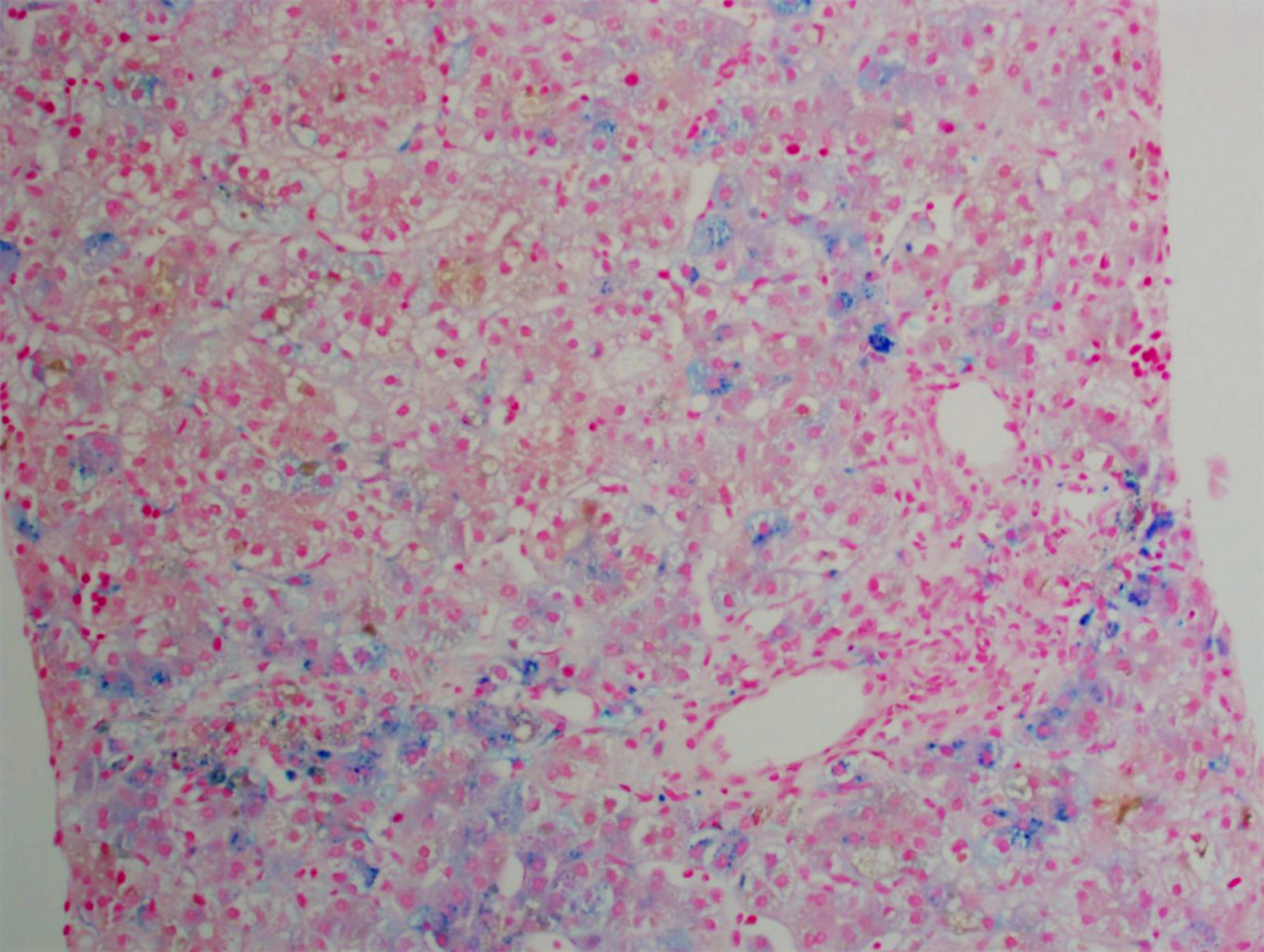
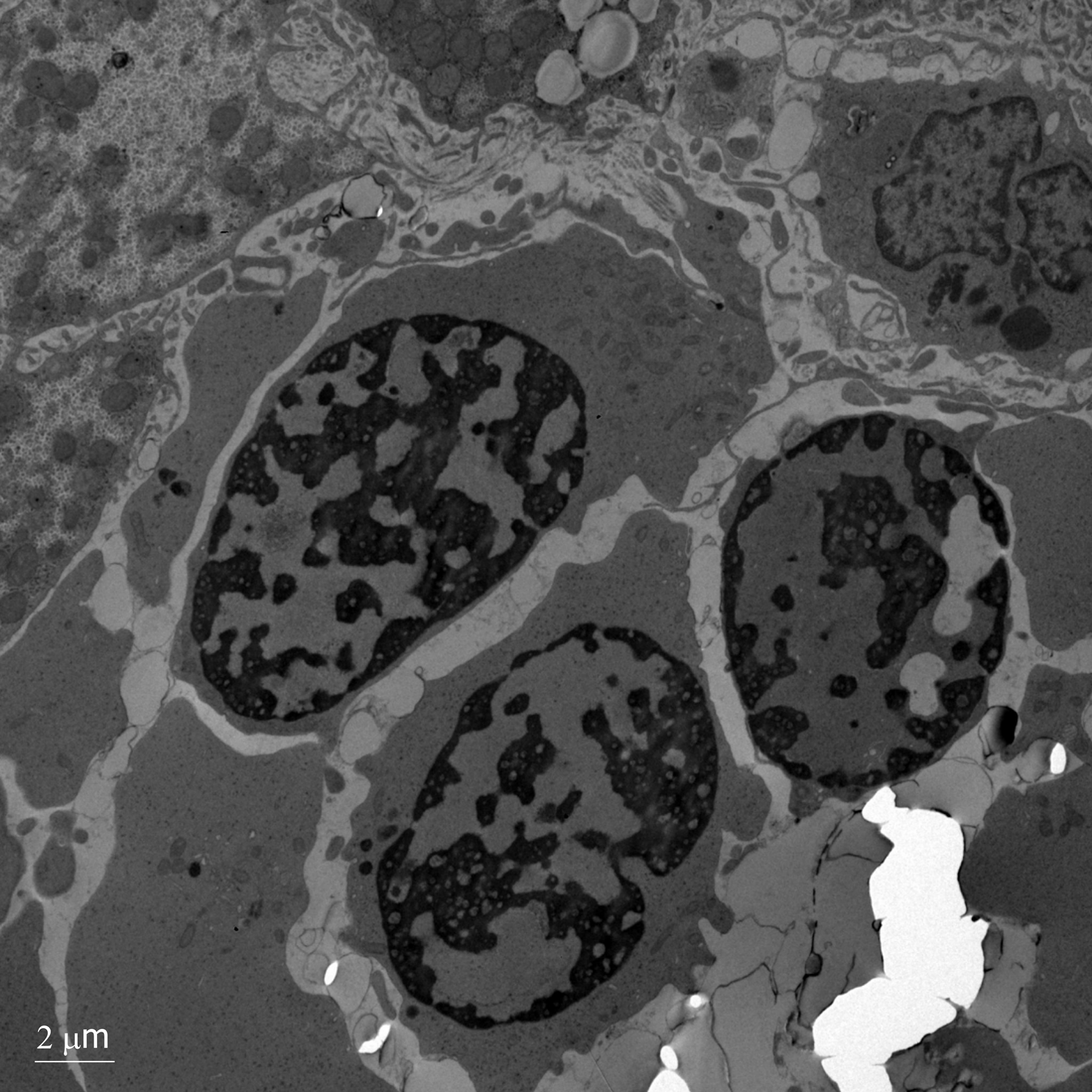
A salivary gland biopsy performed on DOL 5 did not show any evidence of iron deposition. On DOL 15, a liver biopsy was performed, which showed iron deposition (Figure 1) and erythroblasts with spongy appearance (Figure 2). Immunohisto-chemical staining for cytomegalovirus and HSV were negative. A genome rapid sequencing panel of over 4500 genes was performed (ARUP Laboratories, Salt Lake City, UT, United States) and revealed novel compound heterozygous variants in CDAN1, c.2174G>A (p.Arg725Gln) and c.1003C>T (p.Arg335Trp), each variant inherited from an asymptomatic parent.
The final diagnosis of the presented case is CDAN1 resulting from c.2174G>A (p.Arg725Gln) and c.1003C>T (p.Arg335Trp) mutations.
The infant remained on broad spectrum antibiotics, antivirals and required multiple packed red blood cell, fresh frozen plasma and platelet transfusions. She also received intravenous immunoglobulin. She was weaned off mechanical ventilation on DOL 16 and was discharged from the neonatal intensive care unit (known as the NICU) at DOL 43.
Her INR normalized by DOL 2. Her ferritin levels remained elevated but were declining with a level of 4133 ng/mL at NICU discharge. She had improving liver enzymes, bilirubin and thrombocytopenia throughout her NICU stay. At discharge, she was on nasal cannula and sildenafil for persistent pulmonary hypertension. By 7 wk of age, her bilirubin had normalized, and by 4 mo of age, her liver enzymes and GGT had normalized. At her first gastroenterology follow-up 4 wk after discharge, her organomegaly had resolved. At 1 year of age, her ferritin level had decreased to 1139 ng/mL and had 9.3 mg of iron/g of liver tissue determined by magnetic resonance hepatic iron quantification, still consistent with iron overload[5]. She remains transfusion-dependent.
CDA1 is a rare disorder of ineffective erythropoiesis that leads to severe anemia[6]. It has been mainly described in European countries and in the Bedoiun Israeli population[2]. The diagnosis is more commonly suspected in patients presenting with hematologic abnormalities such as moderate-severe macrocytic anemia (MCV > 90), inappropriately low reticulocytes for degree of anemia, macrocytosis, elliptocytes and basophilic stippling on peripheral blood smear, bone marrow aspirate findings of erythroid hyperplasia with interchromatic bridges on light microscopy and erythroblasts with spongy appearance of heterochromatin and invaginations of the nuclear membrane on electron microscopy[3]. Other common findings may include jaundice, splenomegaly, limb dimorphism, hypoplastic nails and syndactyly[3].
Diagnosis in the newborn period is rare[2]. The patient described above presented with multiple clinical characteristics previously described in the literature. HSM and early jaundice are commonly encountered (65% and 53%, respectively). Neonates can also present with direct hyperbilirubinemia in up to 20% of cases. Thrombocytopenia has been described as transient, which is consistent with this patient's presentation[2]. Persistent fetal circulation/pulmonary hypertension has also been reported in up to 15% of patients; the reported cases have had pulmonary hypertension without any underlying cardiopulmonary abnormalities requiring high pressure ventilation[2,7]. It is also reported that patients who have clinical manifestations of CDA1 in the neonatal period have severe intrauterine anemia at birth, and there have been cases of hydrops fetalis[8,9].
In this case, a liver biopsy supported evidence for the diagnosis of CDA1 before genetic testing was performed, with identification of typical siderosis and extramedullary hematopoiesis[6,10]. Both of these findings are consistent with prior adult liver pathology reports. Most recently in 2016, Salihoglu et al[10] reported a case of CDA1 in an adult in whom a liver biopsy was performed to exclude Wilson’s disease and was found to have extramedullary hematopoiesis. Another case report describes a 28-year-old diagnosed with CDA1 with a liver biopsy that showed massive siderosis and early cirrhosis[6]. Autopsy reports have also been described with findings of extramedullary hematopoiesis of the liver and spleen[8].
The initial therapeutic modality for this disease is intermittent blood transfusions, however if patients become transfusion-dependent, there have been cases of successful treatment with interferon alpha[6,11,12]. Up to 80% of affected neonates require blood transfusions in the first month of life, with reported transfusion independence by 4 mo of age in 88% of patients[2]. There are also three reported cases of bone marrow transplantation in patients resistant to interferon therapy[3,13]. Our patient is currently receiving intermittent blood transfusions approximately every 4 wk.
From a gastrointestinal and hepatology standpoint, CDA1 patients have a future risk of gallstones (reported in patients as young as 4 years of age)[4] and secondary hemochromatosis; the latter develops with age due to increased iron absorption even in patients who are not chronically transfused[3]. Hence, it is suggested that patients are periodically monitored (every 3 mo) for iron overload starting at age 10[3].
Liver biopsy can be a helpful tool in the diagnosis of infants with unexplained liver dysfunction. This case report describes the liver histopathology and electron microscopy findings of CDA1 caused by a novel genetic mutation in the pediatric age group. CDA1 is in the differential diagnosis of infants with unexplained anemia, hyperbilirubinemia and HSM.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karatza AA S-Editor: Cui LJ L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Beutler E, Felitti V, Gelbart T, Ho N. Genetics of iron storage and hemochromatosis. Drug Metab Dispos. 2001;29:495-499. [PubMed] |
| 2. | Shalev H, Kapelushnik J, Moser A, Dgany O, Krasnov T, Tamary H. A comprehensive study of the neonatal manifestations of congenital dyserythropoietic anemia type I. J Pediatr Hematol Oncol. 2004;26:746-748. [PubMed] |
| 3. | Tamary H, Dgany O. Congenital Dyserythropoietic Anemia Type I. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors. GeneReviews. Seattle, WA, United States 1993; . |
| 4. | Bader-Meunier B, Leverger G, Tchernia G, Schischmanoff O, Cynober T, Bernaudin F, Leblanc T, Munzer M, Roda L, Soler C, Thuret I, Delaunay J. Clinical and laboratory manifestations of congenital dyserythropoietic anemia type I in a cohort of French children. J Pediatr Hematol Oncol. 2005;27:416-419. [PubMed] |
| 5. | Sarigianni M, Liakos A, Vlachaki E, Paschos P, Athanasiadou E, Montori VM, Murad MH, Tsapas A. Accuracy of magnetic resonance imaging in diagnosis of liver iron overload: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13:55-63.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Goede JS, Benz R, Fehr J, Schwarz K, Heimpel H. Congenital dyserythropoietic anemia type I with bone abnormalities, mutations of the CDAN I gene, and significant responsiveness to alpha-interferon therapy. Ann Hematol. 2006;85:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Shalev H, Moser A, Kapelushnik J, Karplus M, Zucker N, Yaniv I, Tamary H. Congenital dyserythropoietic anemia type I presenting as persistent pulmonary hypertension of the newborn. J Pediatr. 2000;136:553-555. [PubMed] |
| 8. | Kato K, Sugitani M, Kawataki M, Ohyama M, Aida N, Koga N, Ijiri R, Imaizumi K, Kigasawa H, Tanaka Y, Itani Y. Congenital dyserythropoietic anemia type 1 with fetal onset of severe anemia. J Pediatr Hematol Oncol. 2001;23:63-66. [PubMed] |
| 9. | Tekinalp G, Sarici SU, Erdinç AS, Gögüş S, Balci S, Gürgey A. Lethal hydrops fetalis due to congenital dyserythropoietic anemia in a newborn: association of a new skeletal abnormality. Pediatr Hematol Oncol. 2001;18:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Salihoglu A, Elverdi T, Eskazan AE, Eyice D, Bavunoglu I, Ar MC, Ongoren S, Guzel E, Baslar Z, Tunckale A, Tuzuner N, Soysal T. Congenital Dyserythropoietic Anemia Type 1: Report of One Patient and Analysis of Previously Reported Patients Treated with Interferon Alpha. Indian J Hematol Blood Transfus. 2016;32:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Marwaha RK, Bansal D, Trehan A, Garewal G. Interferon therapy in congenital dyserythropoietic anemia type I/II. Pediatr Hematol Oncol. 2005;22:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Parez N, Dommergues M, Zupan V, Chambost H, Fieschi JB, Delaunay J, Miélot F, Cramer EM, Dommergues JP, Wickramasinghe SN, Tchernia G. Severe congenital dyserythropoietic anaemia type I: prenatal management, transfusion support and alpha-interferon therapy. Br J Haematol. 2000;110:420-423. [PubMed] |
| 13. | Ayas M, al-Jefri A, Baothman A, al-Mahr M, Mustafa MM, Khalil S, Karaoui M, Solh H. Transfusion-dependent congenital dyserythropoietic anemia type I successfully treated with allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29:681-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |