Published online May 27, 2019. doi: 10.4254/wjh.v11.i5.421
Peer-review started: December 29, 2018
First decision: March 5, 2019
Revised: April 5, 2019
Accepted: April 19, 2019
Article in press: April 19, 2019
Published online: May 27, 2019
Processing time: 151 Days and 0.9 Hours
About 10 million people in China are infected with hepatitis C virus (HCV), with the seroprevalence of anti-HCV in the general population estimated at 0.6%. Delaying effective treatment of chronic hepatitis C (CHC) is associated with liver disease progression, cirrhosis, hepatocellular carcinoma, and liver-related mortality. The extrahepatic manifestations of CHC further add to the disease burden of patients. Managing CHC-related advanced liver diseases and systemic manifestations are costly for both the healthcare system and society. Loss of work productivity due to reduced well-being and quality of life in CHC patients further compounds the economic burden of the disease. Traditionally, pegylated-interferon plus ribavirin (PR) was the standard of care. However, a substantial number of patients are ineligible for PR treatment, and only 40%–75% achieved sustained virologic response. Furthermore, PR is associated with impairment of patient-reported outcomes (PROs), high rates of adverse events, and poor adherence. With the advent of direct acting antivirals (DAAs), the treatment of CHC patients has been revolutionized. DAAs have broader eligible patient populations, higher efficacy, better PRO profiles, fewer adverse events, and better adherence rates, thereby making it possible to cure a large proportion of all CHC patients. This article aims to provide a comprehensive evaluation on the value of effective, curative hepatitis C treatment from the clinical, economic, societal, and patient experience perspectives, with a focus on recent data from China, supplemented with other Asian and international experiences where China data are not available.
Core tip: Chronic hepatitis C is a systemic disease that manifests both hepatically and extrahepatically, leading to impaired patient-reported outcomes (PROs) and huge economic burden on the healthcare system and society. Direct acting antivirals are effective hepatitis C therapies that improve PROs and have broad eligible patient populations, good efficacy, few adverse events, and high adherence rates. Sustained virologic response is associated with improved clinical outcomes and increased work productivity. Curative therapies for hepatitis C was of substantial societal and economic value because they reduce productivity loss and avoid the management costs associated with advanced liver disease and extrahepatic manifestations.
- Citation: Xie Q, Xuan JW, Tang H, Ye XG, Xu P, Lee IH, Hu SL. Hepatitis C virus cure with direct acting antivirals: Clinical, economic, societal and patient value for China. World J Hepatol 2019; 11(5): 421-441
- URL: https://www.wjgnet.com/1948-5182/full/v11/i5/421.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i5.421
In recent years, direct acting antiviral (DAA) treatments have replaced pegylated-interferon (PEG-IFN) and ribavirin (RBV) combination therapy (PR) as standard of care for patients with chronic hepatitis C (CHC) globally[1-3]. DAAs are associated with over 90% rates of sustained virologic response (SVR), fewer side effects, shorter treatment durations, and improved adherence compared to PR therapy[4].
Due to the substantial impact of hepatitis C virus (HCV) infection globally on patients, their families, and public health systems, the World Health Organization (WHO) has set HCV elimination goals that include reduction of HCV incidence by 80% and HCV-related mortality by 65% by 2030[5]. As part of the strategy to achieve these goals, the WHO included DAA therapies in its 2017 edition of List of Essential Medicines[6]. Specifically, the latest (2018) WHO guidelines for HCV treatment make an updated recommendation of using pan-genotypic regimens for treating adult patients with chronic HCV infection[5]. Besides their proven high efficacy, pan-genotypic regimens enable cost saving and care pathway simplification by eliminating the need for pre-treatment genotyping, potentially reducing loss to follow up among patients. Furthermore, the recommended pan-genotypic regimens, such as sofosbuvir/velpatasvir (SOF/VEL), enable most HCV patients to be treated with simple treatment strategies regardless of patients’ prior treatment experience or cirrhosis status, with minimal need for regimen adjustment or on-treatment monitoring[5]. As reflected in this recommendation by the WHO, the value of high-impact, curative treatment for HCV infection is wide-ranging and goes beyond clinical efficacy.
In China, the number of individuals infected with HCV is estimated at 10 million, with a seroprevalence of anti-HCV antibodies of 0.6% among the general population[7,8]. In addition to the large and growing number of HCV-infected individuals, there have been a diversification of HCV genotypes (GTs) and a broadening of the age spectrum among Chinese HCV patients; GT1b or GT2a HCV patients historically infected through blood transfusion are aging, while an increasing number of younger patients are becoming infected with GT3a, 3b and 6a HCV through injection drug use[9-11]. The resulting healthcare expenditures, as well as reduction in quality of life and loss of work productivity among the Chinese CHC population, are expected to have wide-ranging economic and societal implications.
In addition to the heavy disease burden, the management of the HCV epidemic has been compounded by a lag in adopting DAAs in China: up to early 2017, no DAA had been approved in China. To improve the availability of innovative treatments (including DAAs), China introduced policies that expedite the regulatory review process for drug registration. To date (March 2019), most of the mainstream DAA regimens used internationally have been approved in China, including daclatasvir plus asunaprevir (DCV + ASV), sofosbuvir plus simeprevir (SOF + SMV), SOF + DCV, the fixed-dose combination ombitasvir/paritaprevir/ritonavir with or without dasabuvir (O/P/r ± D), SOF/VEL, ledipasvir (LDV)/SOF, and elbasvir/grazoprevir (EBR/GZR). Glecaprevir/pibrentasvir (GLE/PIB) and SOF/VEL/voxilaprevir (VOX) are also pending approval.
While the rapid improvement in DAA availability is encouraging, China is still far from universal adoption of DAA-based therapies. In an initial step to address the issue of drug accessibility, the Chinese government included SOF/VEL in the latest (October 2018) National Essential Drug List as the first and only DAA treatment[12]. Nevertheless, DAAs are not covered by the National Medical Insurance scheme for reimbursement, and thus much less affordable compared with IFN-based therapies. As such, a considerable number of patients, such as those in rural and less-developed areas and those with limited financial means, would still have to resort to IFN-based therapies.
In light of how IFN-free DAA regimens have revolutionized treatment for HCV-infected patients, many countries are now aiming for elimination of the disease[13]; the introduction of DAA regimens in China therefore, also provides the opportunity for potential hepatitis C elimination. In the 2017–2020 National Viral Hepatitis Action Plan, the Chinese government emphasized the use of more efficacious treatment as part of the strategy to reduce the spread of HCV[14]. The overarching Healthy China 2030 Plan also showed the Chinese government’s commitment to establish public health as the foundation for future economic and societal development[15]. In line with the country’s strategic approach to healthcare and hepatitis management, this article aims to comprehensively evaluate the value of curative HCV therapies in the dimensions of clinical, patient, economic and societal benefits, in the hope of providing useful references for various stakeholders and policy makers in China. Where possible, data from China have been used, supplemented with data from other Asian countries and from around the world when Chinese data are unavailable.
The principal impact of HCV is on the liver, the predominant site of HCV replication. While initial HCV infection resolves spontaneously in around 15%–25% of cases, the majority of patients will develop CHC[16]. The long-term outcomes of the liver inflammation caused by HCV infection include the development of fibrosis, compensated and decompensated cirrhosis, hepatocellular carcinoma (HCC), and end-stage liver disease (ESLD)[16]. Progression of CHC typically occurs over many years; it is estimated that 10%–20% of patients will develop cirrhosis and the annual risk of HCC in patients with cirrhosis is approximately 1%–4%[16]. If left untreated, CHC patients would progress to more advanced disease stages, which in turn are associated with accelerated disease progression, elevated risks of developing HCC, and consequently lower survival rates[4]. A long-term retrospective cohort study in Japanese CHC patients showed that untreated F0/F1 patients have a 0.5% annual risk for HCC development, while this increased to 7.9% in F4 patients[17]. A systematic review of CHC patients in Asia, including China, reported that the 5-year survival for cirrhotic CHC patients was 73.8%, but falls to 39.2% following progression to ESLD, wherein liver transplantation is required[18].
China has an estimated annual incidence of 53593 cases (95%CI: 16144–92466) of HCV-related HCC[19], with > 93000 cases of HCV-related liver cancer deaths recorded in 2005[20]. The majority of Chinese HCV patients were infected through blood transfusion before 1993–1996, in whom age and duration of infection are significant risk factors for disease progression[21]. As these patients grow older and enter their third or fourth decade of infection, the occurrence of decompensated cirrhosis (DCC), HCC and ELSD will rise. On the other hand, China has seen a recent increase in younger patients with GT3 HCV[10,22]. A study in Shanghai reported evidence that GT3 patients undergo faster disease progression, with GT3 patients < 50 years of age showing significantly more advanced fibrosis than their non-GT3 counterparts[22]. If left untreated, considerable numbers of liver sequelae will develop in these younger GT3 patients in future.
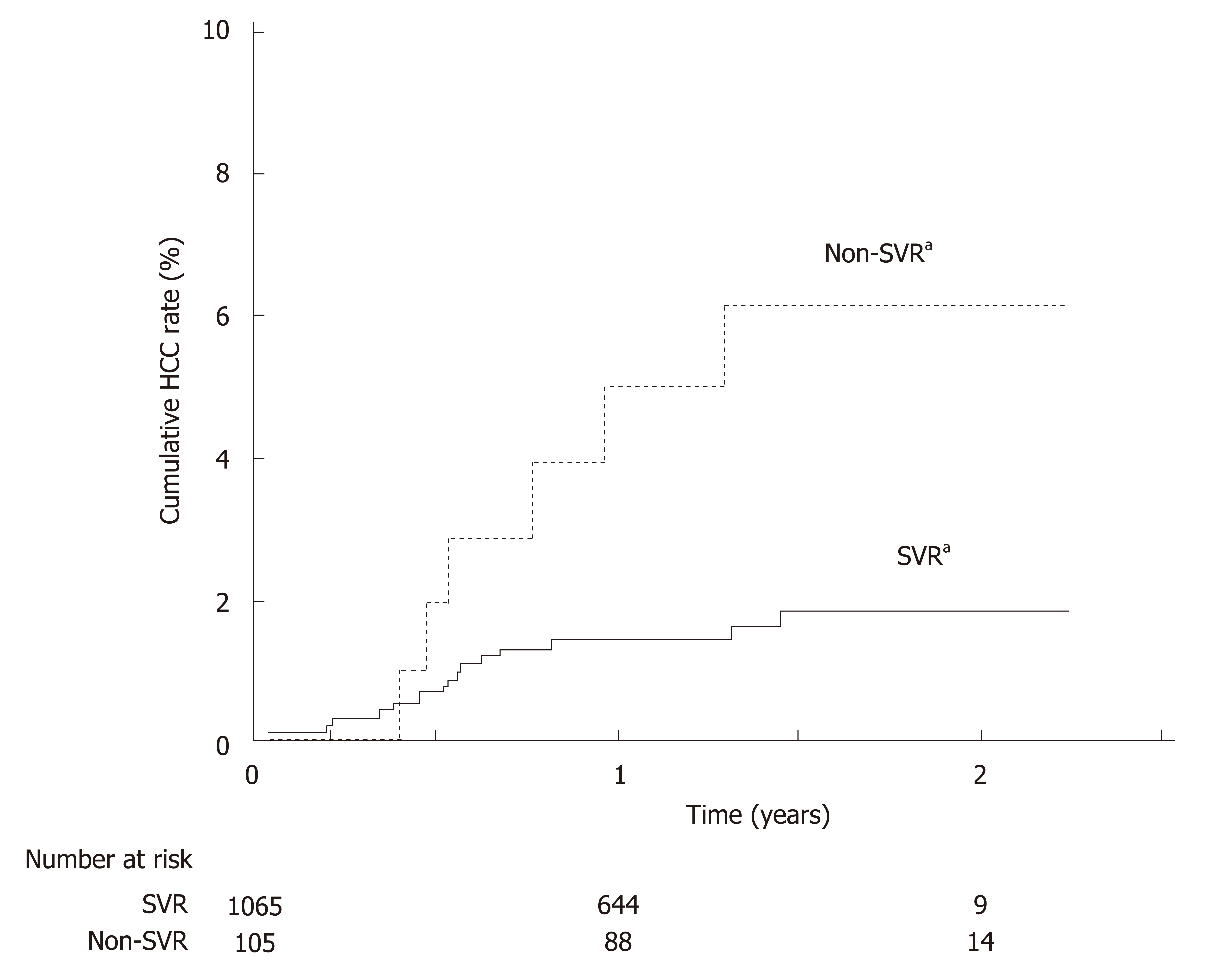
Antiviral treatment and resultant SVR improve the long-term clinical outcome of HCV patients[23-28]. The beneficial effects of IFN-based treatment and SVR on reducing cirrhosis progression, HCC, and mortality have been well documented, and this is reflected by the recently published results from two large-scale studies in Asia and two Chinese cohort studies[29-32]. Similar clinical benefits have been observed with SVR to DAA-based treatment. In a large-scale, retrospective study in Japan, GT1 HCV patients who achieved SVR to all-oral DAA regimens had a lower cumulative incidence of HCC than non-SVR patients at 2 years post-treatment (Figure 1)[27]. Furthermore, a recent Chinese prospective study in DAA-treated and case-matched PR-treated patients showed no difference in the risk of developing HCC post-SVR[33]. DAA treatment was also associated with a 32% reduction in liver-related mortality relative to no treatment[25], and DAA-mediated SVR conferred reductions in all-cause mortality relative to non-SVR in patients with or without advanced liver disease by 79% and 56%, respectively[23,24].
A meta-analysis of 31 studies in predominantly GT1 HCV-infected patients (including Asian patients) showed that achieving SVR conferred survival benefit irrespective of patients’ clinical characteristics, with difficult-to-treat populations such as cirrhotic patients experiencing the largest extent of reduction in 5-year mortality compared to no SVR[34]. Nonetheless, the hepatic and survival benefits of achieving SVR are maximized by treating patients in earlier disease stages[31,35]. For instance, for Asian cirrhotic patients who achieved SVR, despite a reduction in cumulative risk of HCC by 25.5 percentage points relative to cirrhotic patients without SVR, the associated risk of HCC was still higher than non-cirrhotic patients with SVR (0.54 vs 0.37)[31]. Thus, the WHO guidelines recommend treating all HCV-infected patients without disease stage-based restriction or prioritization, with an emphasis on minimizing treatment delay after diagnosis[5].
Although SVR to either IFN- or DAA-based treatment reduces liver disease progression, the impact of IFN-based treatment would be limited due to low SVR rates (40%–75%)[4,36]. In a meta-analysis of 12 studies involving 25497 CHC patients on IFN-based therapy, although SVR achievement led to a 76% reduction in HCC risk, only 36% of patients achieved SVR[37]. PR treatment also has numerous contraindications and side effects, such as RBV-induced hemolytic anemia and various neuropsychiatric, autoimmune, ischemic, and infectious disorders that may be caused or aggravated by IFN[38,39]. In the nationwide CCgenos study, 56.7% of untreated Chinese HCV patients were IFN-ineligible[40]. In contrast, DAA regimens confer high real-world SVR rates of 90%–100%, and extend HCV cure to patient populations that could not be effectively treated in the PR era, such as patients with DCC and/or liver transplant, and patients with concomitant renal impairment or psychiatric disorders (although the eligible patient populations and efficacy profiles do differ among DAA regimens)[41]. As such, DAAs would have a greater impact than IFN-based therapy in significantly reducing HCV-related liver sequelae and deaths at the population level[42]. Evidence for such populational benefits associated with expanded DAA use is emerging internationally, and various countries are promoting the use and reimbursement of DAAs as a key strategy towards the goal of HCV elimination. For example, in England, a national 40% scale-up of DAA provision in 2015 was followed by reductions in the incidence of HCV-related cirrhosis (42%), liver transplantations (32%), and deaths (8%)[43]. In 2018, Canada is progressively removing the eligibility criterion of F2+ fibrosis for DAA reimbursement[44].
In China, DAAs achieved high SVR rates in pivotal clinical studies (Table 1). Evaluated genotype-specific regimens (DCV + ASV, LDV/SOF, EBR/GZR, and O/P/r + D) all achieved SVR12 rates ≥ 92% in GT1 or GT1b patients[45-48]. Pan-genotypic regimens evaluated in clinical studies included SOF + RBV ± PEG-IFN and SOF/VEL. For SOF + RBV ± PEG-IFN, cirrhotic patients achieved lower SVR12 rates than non-cirrhotic patients (Table 1)[49]. For SOF/VEL, the only patient population with an SVR12 rate below 90% was GT3b cirrhotic patients (Table 1), who also exhibited a high prevalence of baseline A30K + L31M substitutions[50,51]. Due to the short period of DAA application, real-world efficacy data in China are only emerging. Majority of the early real-world studies focused on SOF-based regimens (reviewed by An et al[52]); two examples are shown in Table 1, where SOF-based regimens demonstrated high efficacy in difficult-to-treat patients[53,54].
| Study Information | Study regimen | SVR12 | Ref. |
| Pivotal phase 3; TN patients; GT1b | DCV + ASV | Non-cirrhotic: 92% | [45] |
| Cirrhotic: 94% | |||
| Pivotal phase 3b; TN and TE patients; GT1, 2, 3, 6 | SOF + RBV ± PEG-IFN | Non-cirrhotic GT1/2/3/6: 96%/93%/97%/100% | [49] |
| Cirrhotic GT1/2/3/6: 84%/88%/88%/50% | |||
| (GT6 with cirrhosis: n = 2) | |||
| Pivotal phase 3b; TN and TE patients; GT1b | LDV/SOF | 100% | [46] |
| Pivotal phase 3; TN and TE patients; GT1b, 2, 3, 6 | SOF/VEL | GT1b/2/3/6: 100%/100%/83%/100% (Subgroup SVR12: GT3a: 91%; GT3b non-cirrhotic/cirrhotic: 96%/50%) | [50] |
| Pivotal phase 3; TN patients; GT1, 6 | EBR/GZR | GT1/6: 97%/80% (GT1: 140/146 patients were GT1b; GT6: n = 5) | [48] |
| Pivotal phase 3; TN and TE patients; GT1b | O/P/r + D | 99%–100% | [47] |
| Prospective cohort; TE; GT1b | SOF + DCV | 100% (SVR24) | [53] |
| LDV/SOF | 100% (SVR24) | ||
| PEG-IFN + RBV | 28% (SVR24) | ||
| Real-world study; TN and TE HCV patients with DCC | SOF-containing regimens | 90%; with significant improvement in hepatic function among SVR patients | [54] |
Using China-specific SVR data where available, modeling studies predicted that compared to PR, DAA treatment would markedly reduce the HCV-associated long-term disease burden and mortality. A study simulating the national-level disease burden of CHC in China predicted that with no treatment, the prevalence of HCV would continue increasing and reach 28.1 million in 2050, with 2.4 million liver-related deaths, mostly attributable to DCC and HCC[55]. The model predicted that using PR therapy would not be able to revert the trend of increase in HCV prevalence. In contrast, universal adoption of DAAs from 2021 onward would significantly reduce the HCV prevalence. Furthermore, compared to using PR therapy, universal DAA adoption would reduce the cases of incident DCC, HCC, liver transplants, and liver-related deaths by 61%, 45%, 50%, and 61%, respectively[55]. Similarly, simulations of 10000 Chinese CHC patients over a lifetime horizon predicted that various DAA regimens would significantly reduce the incidence of HCV-related liver sequelae and mortality compared to PR therapy[56,57]. These simulations took into consideration the composition of different HCV genotypes, treatment history and cirrhotic status of the patient population, thus, pan-genotypic regimens (SOF/VEL and GLE/PIB) were predicted to achieve higher overall SVR rates and greater reduction in disease progression than genotype-specific DAA regimens (Table 2)[56,57].
| Study Information | Regimens compared | Predicted reduction in liver sequelae relative to PR therapy | |||
| DCC | HCC | Liver transplant | Liver-related death | ||
| Simulating 10000 Chinese CHC patients over a lifetime horizon; | GLE/PIB[57] | -95% | -90% | -95% | -92% |
| SOF/VEL[56,57] | -96% | -91% | -96% | -93% | |
| DCV + ASV[56] | -51% | -48% | -51% | -49% | |
| O/P/r + D[56] | -59% | -55% | -59% | -57% | |
| EBR/GZR[56] | -59% | -55% | -59% | -57% | |
| GT1b, 2, 3, 6 | |||||
Besides the direct impact on the liver, HCV-infected patients may experience liver-unrelated symptoms that, depending on epidemiological evidence, are considered extrahepatic manifestations (EHMs) associated or possibly associated with HCV infection[58,59]. The most documented EHMs are mixed cryoglobulinemia/ cryoglobulinemic vasculitis and B-cell non-Hodgkin’s lymphoma. A diverse range of other conditions also occur at higher prevalence in HCV-infected patients, including type 2 diabetes mellitus, renal diseases, fatigue, cardiovascular disease, and lichen planus (LP), to name a few[58]. EHMs can occur in > 70% of CHC patients, and can be present before advancement into ESLD[59]. Underlining the impact of EHMs on HCV patients, a Taiwanese study reported a cumulative 18-year EHM-related mortality of 19.8% in patients with chronic HCV infection, much higher than the non-liver-related mortality in those without HCV infection (12.2%)[60]. Published studies on EHMs in China are few, and there is currently a lack of clinical data on the prevalence and management of EHMs among Chinese patients[61].
A recent meta-analysis investigated the extrahepatic benefit of antiviral treatment and SVR in HCV patients[62]. Achieving SVR significantly reduced extrahepatic mortality (vs no SVR, OR 0.44, 95%CI: 0.28–0.67), was associated with improvements in cryoglobulinemic vasculitis and B-cell lymphoproliferative diseases, and reduced the incidence of insulin resistance and diabetes[62]. Concordantly, IFN-based treatment induced favorable immunologic response in Chinese cryoglobulinemic patients with HCV infection, and IFN-based SVR reduced the risk for type 2 diabetes mellitus among Japanese HCV patients[63,64]. Of note, IFN-based treatment may exacerbate symptoms of cryoglobulinemic vasculitis, likely due to the immune-stimulatory effects of IFN[65,66]. IFN also induces lichenoid inflammation, and is thus contraindicated to LP[66].
Emerging data support the extrahepatic benefit of successful DAA treatment for HCV patients[67]. In two prospective studies on patients with HCV-related mixed cryoglobulinemia, SOF-based regimens conferred 100% SVR rates and clinical improvement or resolution of mixed cryoglobulinemia-associated vasculitis[68,69]. In a retrospective study on 46 CHC patients with lymphoproliferative disorders, DAA treatment (mostly SOF-based) achieved an SVR rate of 98%, together with a lymphoproliferative disease response rate of 67% and survival benefit[70]. In a prospective Japanese study, 7 patients with HCV-related oral LP all achieved resolution or improvement of oral LP lesions and cutaneous LP upon DAA-based SVR[71].
In short, existing data on DAAs are in line with data from the IFN era, showing that SVR attainment aids the amelioration of HCV-associated EHMs. While further research is needed on the effect of DAAs on EHMs, the higher virologic efficacy, fewer side effects, and shorter treatment durations of DAAs would likely amplify the health benefit of reducing disease burden associated with extrahepatic complications[67].
Effective treatment of diagnosed patients is an integral part of a comprehensive approach to preventing HCV transmission, which also requires public education to raise disease awareness and efficient screening and linkage to care[72]. HCV prevention strategies also need to be aligned with the predominant mode of transmission[72].
There is substantial regional variation in risk factors for HCV infection in China. In regions outside Southern China, blood transfusion is the major route of HCV transmission, accounting for 57.5%–69.3% of existing HCV-infected patients. Infection through surgery or dental treatment is more common in Northern China than in other areas, whereas HCV transmission via high-risk behavior such as intravenous drug abuse is more prevalent in Southern and Western China[10]. Within each broad geographical region, the modes of HCV transmission may also show urban–rural differences. Current HCV prevention measures in China include screening of all blood donors, as well as harm reduction services for high-risk groups like injection drug users (IDUs)[3,73].
In addition to existing prevention measures, treating HCV infection with highly effective therapy can function as a prevention strategy [treatment as prevention, TasP] by essentially removing individuals in key populations from the pool of transmitters. Numerous TasP modelling studies predicted that HCV treatment of sufficient effectiveness and accessibility would help reduce the incidence and chronic prevalence of HCV infection among IDUs, prisoners, and men who have sex with men (MSM)[74]. For example, in Melbourne, with HCV prevalence among IDUs at 50%, increasing uptake of DAA treatment to 40 per 1000 IDUs annually is expected to halve HCV prevalence rates within 15 years; while scaling up treatment to 54 per 1000 IDUs annually could cut prevalence rates by as much as 75%[75].
Empirical evidence verifying these positive projections are currently scarce, but some real-world programs are underway, reflecting confidence in the potential of HCV TasP. In Australia, a world-first HCV surveillance and treatment program assessing the use of SOF/VEL for HCV TasP in prisons is expected to be completed by 2019[76]. Notably, high real-world efficacy of SOF/VEL has been demonstrated among HCV-infected, treatment-adherent IDUs with recent injection, lending confidence to the notion that treatment scale-up and adherence management would effectively control HCV transmission among IDUs[77]. In 2016, Iceland (with a population of 340000) launched the nationwide program of Treatment as Prevention for Hepatitis C (“TraP Hep C”), offering universal access to DAAs for HCV-infected patients, with an emphasis on treating high-risk transmitters such as IDUs[78]. International guidelines also recognized the benefit of reduced transmission with successful HCV treatment[1,2,79]. Guidelines by the European Association for the Study of the Liver and the WHO further highlighted that HCV screening and treatment should be prioritized in individuals at high-risk of transmitting HCV such as IDUs[2,79].
With the regional variations in HCV epidemiology in China, tailored strategies at the provincial or even district/city level will be necessary for HCV prevention and control[80]. Suitable measures targeting specific modes of transmission will facilitate HCV ‘micro-elimination’ within certain populations: such is the strategy adopted by many countries for HCV elimination, and can form part of a realistic approach in China towards accomplishing the goals in the National Viral Hepatitis Action Plan[14]. In particular, China has a documented IDU population of 2.95 million, among whom the estimated HCV prevalence is 50.4%[81,82]. In this key population, efficacious HCV treatments, together with targeted education campaigns, continued harm reduction measures, and efficient diagnosis and linkage to care, would be required to reduce the prevalence and transmission of HCV[14,15].
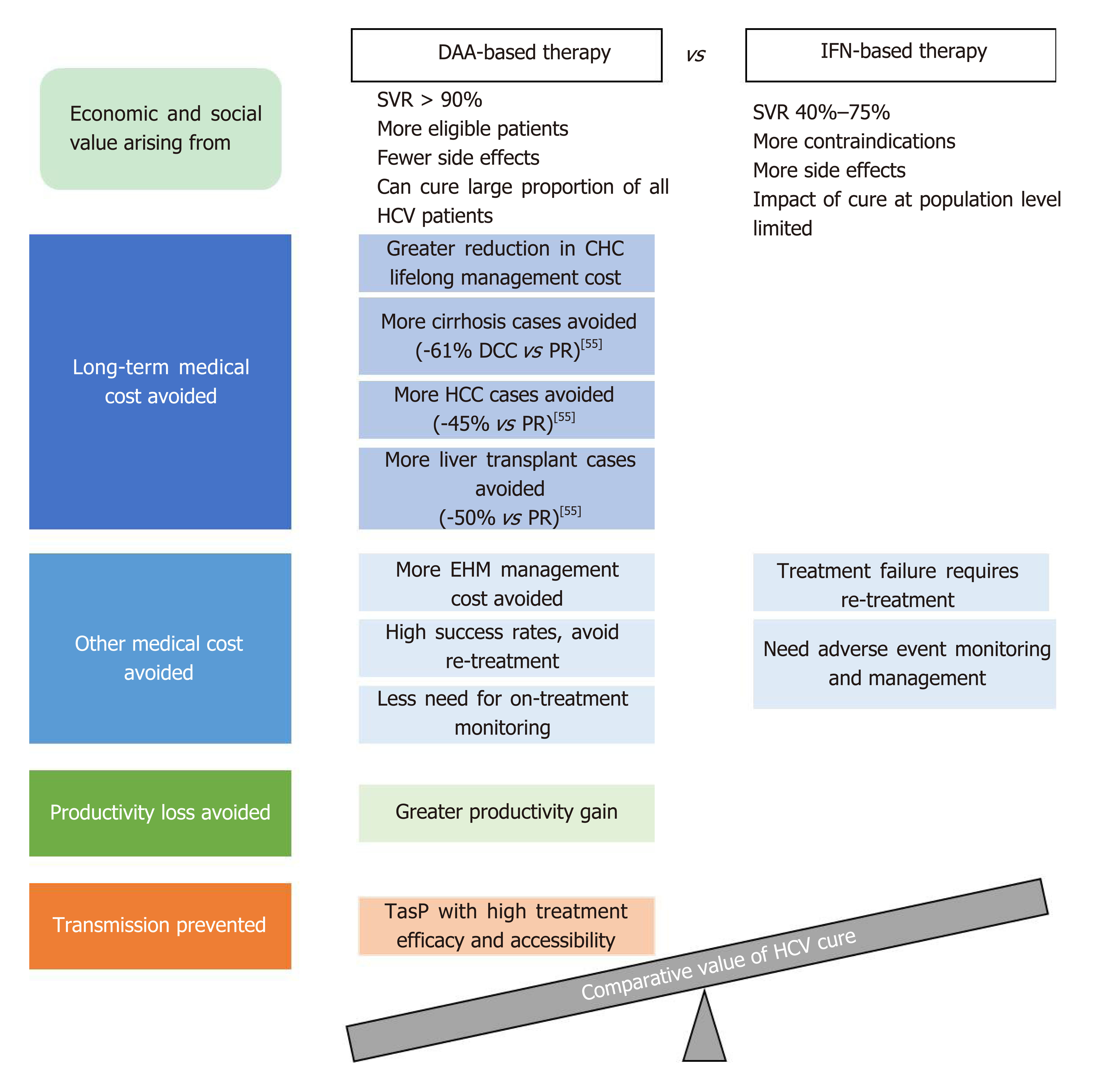
As illustrated in the WHO 2018 guidelines, HCV control strategies are formulated based on not only the clinical efficacy of treatment options, but also their cost-effectiveness and the broader value and benefits they bring to patients and society[5]. Curing HCV infection can generate economic and societal value on many fronts, depending on the efficacy, safety, and other characteristics of the treatment options used. Figure 2 provides an overview of the main factors contributing to the economic and societal value of DAA- and IFN-based HCV treatment. It is challenging for any existing value assessment model to incorporate all the factors shown in Figure 2; the following sections seek to provide relevant information in these areas to enable a holistic discussion on the value of curing HCV infection.
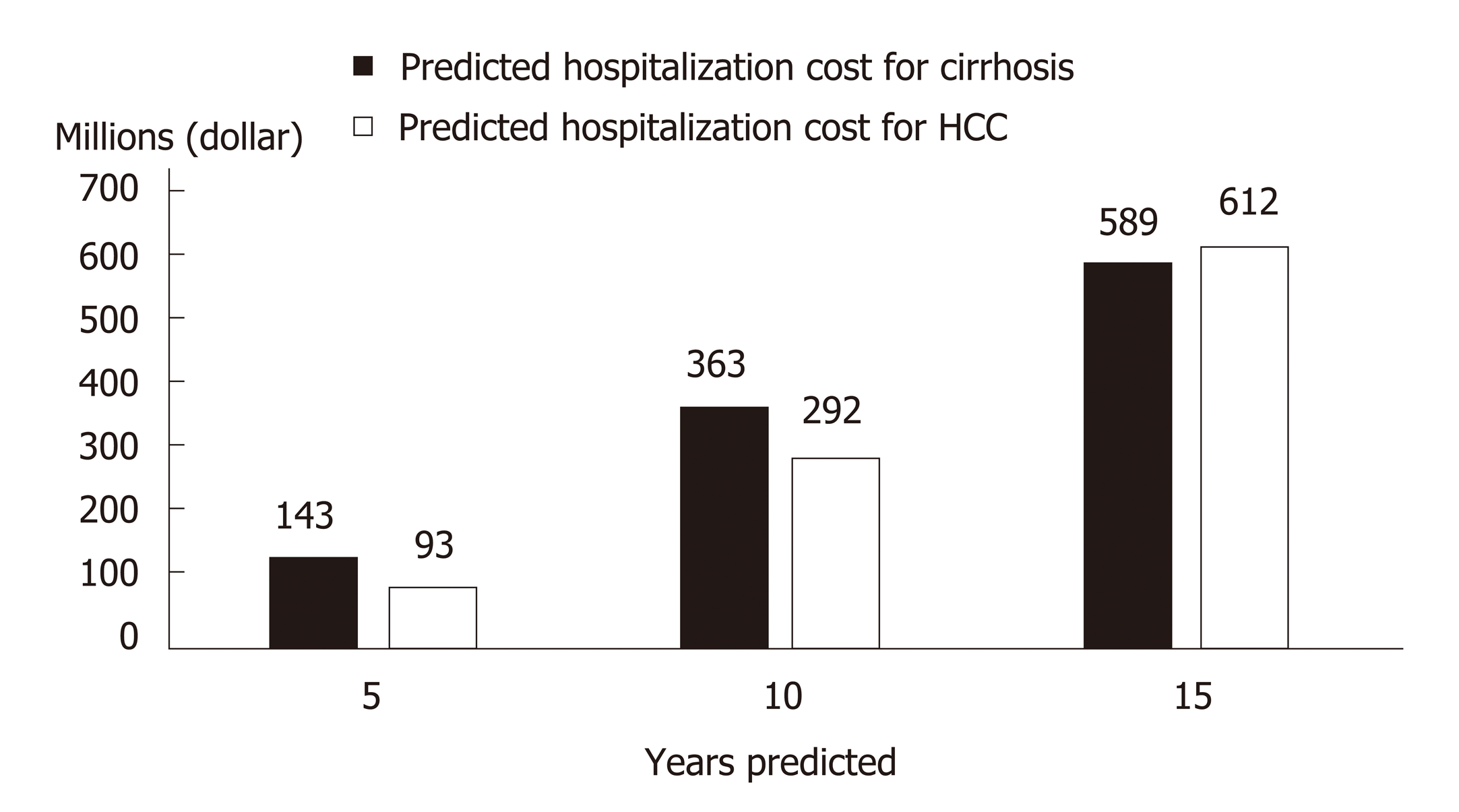
Costing studies on the management of CHC generally found that more advanced disease stages are associated with higher medical costs, which constitute a financial burden to patients, healthcare systems, and society[83,84]. Within the CHC population in China, nearly a quarter are hospitalized at least once per year with a median duration of 2 wk[85]. Since later disease stages are costlier, treatment delay would lead to increased future costs associated with disease progression. A modelling study in China captured such a scenario and highlighted the impact of treatment delay on younger patients who, with a longer life expectancy, would incur higher life-time disease management costs than older patients with a similar initial disease state[86]. With a 3-year treatment deferment, the projected cost for managing future ESLD in non-cirrhotic patients aged 40 increased from RMB 4407359 to RMB 7997253, while the corresponding cost increment for non-cirrhotic patients aged 70 was from RMB 2091499 to RMB 5565547[86]. At the population level, more cases of cirrhosis and HCC would occur as CHC patients age, leading to higher healthcare costs in the future[87]. China faces both an increasing population of younger, incident patients and an aging population of prevalent patients[9-11]. Without effective treatment, it was predicted that over the next 15 years, 420000 new cases of HCV-related cirrhosis and 254000 new cases of HCV-related HCC would occur, leading to future treatment costs of 589 million and 611 million dollars respectively (Figure 3)[8,88].
Besides the cost of managing liver-related morbidity of CHC, economic burden also arises from HCV-associated EHMs. In a meta-analysis of 102 studies conducted between 1996 and 2014, the annual medical costs of managing EHMs, in 2014 dollars, amounted to approximately $1.5 billion[89]. Therefore, curing HCV would also be expected to reduce the cost of managing EHMs as well as preventing expensive long-term liver morbidities.
In addition to direct costs related to CHC management, the growing involvement of younger, work age HCV patients in China also poses a broader societal issue[11,84]. HCV patients may suffer from fatigue, low energy, and impaired general health; the resultant impairment of work productivity would have repercussions on the financial and psychological well-being of the HCV-infected young individuals and their families[89]. Employers of HCV-infected individuals would also face reduced output and earnings due to loss of worker productivity, in the forms of absenteeism and presenteeism[90]. Based on a modelling study in HCV GT1-infected Chinese patients, the monetized productivity losses resulting from non-treatment amount to RMB 37.78 billion per year[91].
By effectively curing HCV infection, HCV therapeutic innovation would help alleviate the heavy economic and societal burdens caused by CHC, yet such innovation would require upfront investments. To determine which treatment strategy offers the best “value for money” (cost-effectiveness), health economic models are used to weigh the costs of different HCV treatment options against their long-term cost savings.
Health economic analysis in HCV predominantly focuses on the direct medical costs of managing HCV. As discussed previously, more advanced disease stages are associated with higher medical costs, and patients who achieve SVR have lower probabilities of progressing to the later, costlier disease stages. Thus, in theory, more effective HCV treatments should save more on long-term medical costs by avoiding disease progression in more patients. Health economic models evaluate the costs thus saved, along with the benefits of life extension and improved quality of life, against the upfront investments needed for implementing certain treatment methods. Currently, health economic research on HCV treatment in China is expanding rapidly. Emerging health economic analysis results from China will be introduced, together with analyses from countries with more experience using DAAs, to evaluate the potential costs and benefits associated with upfront investments in HCV treatment innovation.
In health economic analysis, the gain in patients’ quantity and quality of life achieved using a certain treatment is measured in quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER) measures the cost needed to achieve a unit gain in QALYs; if the ICER of a treatment is below the willingness-to-pay threshold, it is considered cost-effective. In a systematic review of health economic studies in the United States, Europe, and Australia, second-generation DAAs, compared to first-generation DAAs, PR therapy, or non-treatment, were shown to be either cost-saving or cost-effective in the majority of the analyses as judged by the ICERs calculated[92]. Furthermore, a modelling analysis in the United States compared various second-generation DAA regimens for treating GT1 patients; the results predicted that a treatment strategy of 8-wk LDV/SOF for GT1, treatment-naïve (TN), non-cirrhotic patients with a viral load less than 6 million copies, and SOF/VEL for all other GT1 patients was the most cost-effective strategy, resulting in 35% fewer cases of advanced liver disease events, with up to 57% reduction in cost per SVR relative to the other comparator regimens[93].
Moving beyond the conventional methodology using ICERs, recent health economic studies devised other indicators to better elucidate the economic value of HCV therapies, by monetizing QALYs gained so that they can be directly compared against the costs of treatment. A United States economic model study in HCV GT1 patients predicted that all-oral therapies, in relation to PR therapy, improved health by 1.622 QALYs per patient, thereby leading to an overall decrease of 32730–500599 dollar in quality-adjusted cost of care, which was defined as the increase in the price of treatment minus the increase in the value of the patient’s expected QALYs when valued at 50000–300000 dollar per QALY[94].
Unlike the abovementioned counties and regions, China is still early in its transition from IFN to DAA-based regimens. Health economic studies in China are fast emerging, mostly focused on comparing DAA regimens with IFN-based treatments, reflecting an acute need for cost-effectiveness data to inform potential health technology assessment decisions at this stage. The DCV + ASV regimen was predicted to be more or comparably cost-effective relative to IFN- or RBV-containing regimens in GT1b patients in two analyses[95,96]; an SMV-containing regimen was also predicted to be more cost-effective than PEG-IFN-based therapy in GT1 patients[97]. Another study predicted that compared to PR, O/P/r + D would be cost-saving in GT1b patients, and SOF + RBV cost-effective in GT2/3 patients and cost-saving in GT6 patients, respectively[98]. One of the aforementioned studies simulating 10000 Chinese CHC patients with various HCV genotypes predicted that all the then-available second-generation DAA regimens (DCV + ASV, O/P/r + D, SOF/VEL, and EBR/GZR) would have cost-effectiveness advantages over PR therapy, with the pan-genotypic SOF/VEL conferring the greatest gain in QALYs (by 17%) and reduction in lifetime cost (by 49%) relative to PR[56]. Among genotype-specific DAA regimens, one study predicted that for GT1b Chinese patients stratified by cirrhosis status and treatment history, EBR/GZR would be more cost-effective than DCV + ASV[99].
Cost-effectiveness analyses derived from a real-world prospective cohort predicted that for Chinese GT1b cirrhotic, PR-experienced patients, 12-wk LDV/SOF and 12-wk SOF+DCV would be cost-saving and cost-effective, respectively, compared to repeated PR treatment for 72 wk[53]. Analyzing real-world data from the PR era, a Taiwanese study reported that the costs per SVR for PR treatment were the highest in GT1/6 patients co-infected with HIV, due to low SVR rates in these populations; EBR/GZR, though more expensive than PR, would theoretically offer similar costs per SVR thanks to significantly higher SVR rates[100]. These two studies highlight the value of DAAs for traditional difficult-to-treat patient populations, and illustrate how the benefit of short treatment durations and high efficacy can offset the impact of high drug price for DAAs to maintain favorable cost-effectiveness profiles. Furthermore, two budget impact studies, from Hong Kong and three cities in Mainland China respectively, both predicted that although subsidizing DAAs would incur additional short-term drug costs, the resultant gain in patients’ health benefit and the avoidance of long-term disease management costs would be desirable[101,102].
Besides the factor of avoiding disease progression-associated long-term costs of HCV sequalae, health economic models take into account additional factors contributing to cost of care[94]. For PR therapy, such additional factors include the costs of monitoring and managing adverse events, and of re-treating patients who failed or discontinued the treatment[4]. With a high incidence of adverse events and the need for frequent monitoring, PR treatment can negatively impact patients’ quality of life (which will be discussed in a later section), resulting in lower QALY gains compared to DAA treatment. DAA therapy with its better safety and efficacy profiles can avoid such costs altogether if used as a first-line treatment. Indeed, one study predicted that for treatment-naïve Chinese GT1 patients, treatment with all-oral DAA regimens, either immediate or with a 1-year delay, would generate positive net monetary benefits of 6832 dollar and 3115 dollar, respectively, compared to immediate PEG-IFN-based treatment, at a willingness-to-pay threshold of 21209 dollar per SVR[103]. On the other hand, certain DAA therapies may incur costs associated with genotype/subtype or baseline resistance testing; such costs could be avoided with the application of pan-genotypic DAA regimens and regimens with high resistance barrier. Further studies would be required to fully elucidate the costs and savings associated with different DAA regimens in this respect.
In summary, modelling analyses conducted in the United States, Europe, and high-income Asian countries thus far have suggested that DAAs are likely to be cost effective compared to conventional IFN-based therapies; emerging health economic evidence in China is in line with these international findings. DAA regimens are easy to use, of shorter treatment duration, and requiring less monitoring than IFN-based therapy; the management cost thus saved, together with the long-term saving in CHC-related medical cost, would likely outweigh the upfront investment in DAAs. This is consistent with the WHO’s recommendation, whereby treatment regimens with better tolerability and safety profiles that simplify the care pathway would be preferred by patients and policy makers, which may also facilitate care coverage expansion and equity in treatment access[5].
The health economic analyses discussed above focus mostly on medical costs and do not capture the societal value of curing HCV. As will be discussed here, the additional potential benefits of curing HCV, such as improved productivity in Chinese workers and reduced HCV transmission, would further offset the upfront investment in HCV treatment.
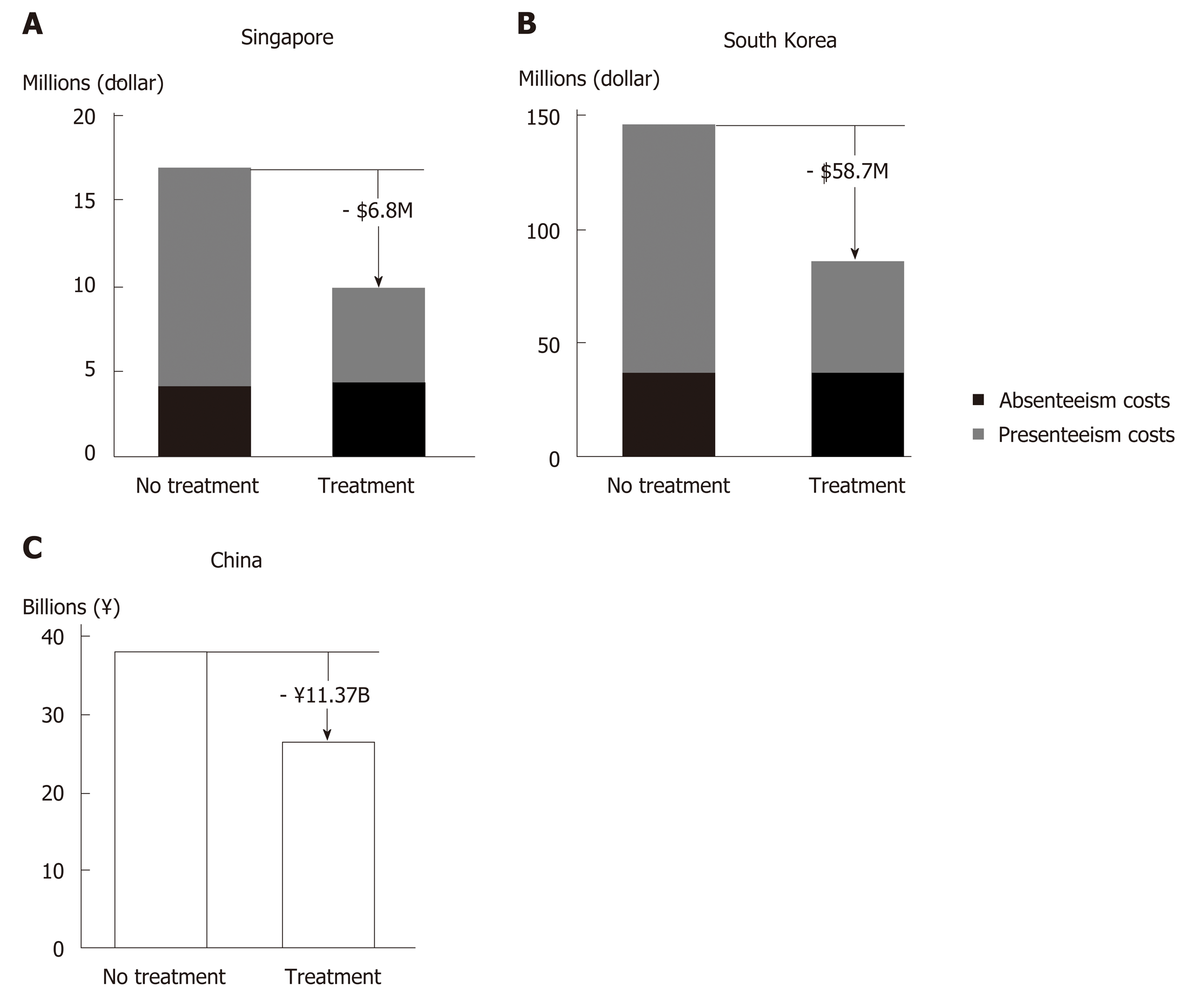
Reduced productivity impairment has been predicted in Asian CHC patients with successful HCV treatment (Figure 4)[104]. Hence, introduction of therapies with high SVR rates in China would likely improve work productivity among Chinese CHC patients, and in turn ameliorate the financial burden of HCV infection at an individual and family level. The resulting economic stability within a family unit owing to attainment of HCV cure could be of great benefit to China’s societal fabric.
From the employer standpoint, improved worker productivity increases revenue generation[104]. A modelling study in HCV GT1-infected Chinese patients showed that treatment with SOF/VEL would generate annual productivity gains equivalent to RMB 11.37 billion, mainly driven by reduced presenteeism (Figure 4C)[91]. The consideration of presenteeism in addition to absenteeism in the model is a more accurate representation of the actual reduction in productivity impairment in the context of Asian culture that values stoic industriousness.
Besides work productivity, another aspect typically not captured in health economic analyses is the benefits of stopping onward transmission through effective HCV treatment in key populations. Based on projections from a modelling study in the UK, at 10%–100% treatment uptake among IDUs and cost of GBP 20000 per QALY, reduced HCV transmission with DAA therapy led to an additional net monetary benefit of GBP 24304–90559 per patient[105]. One modelling study in China estimated the cost associated with HCV management in IDUs at RMB 21900 per patient per year[82]. With a predicted 20-year cumulative HCV incidence in 48.28% of IDUs, effective TasP in IDUs in China would conceivably translate into considerable societal and economic values[82].
In summary, effective HCV treatment can benefit society through improving working productivity and reducing HCV transmission. As reflected in the modelling studies above, such benefits would translate into tremendous value in addition to the economic benefit of reducing medical costs.
While SVR is the main clinical indicator of HCV treatment success, a patient’s overall experience with the disease and the treatment process can be captured by patient reported outcomes (PROs). PROs are directly reported by patients without interpretation by HCPs and are used as proxy indicators of patients’ overall wellbeing. As mentioned earlier, HCV patients often experience debilitating fatigue and impairment to work productivity and non-work activities[89]. HCV infection also causes neuropsychiatric manifestations such as “brain fog”, whereby patients suffer from difficulty in attention and memory, alongside other cognitive impairments[106]. Clearly, HCV infection and its systemic manifestations negatively impact patients’ health-related quality of life (HRQoL)[89,106]. As such, the importance of assessing PROs in HCV management has gained considerable attention internationally over the past decade[107,108]. PRO data can also contribute to informing healthcare policy making[109]. Patients’ demographic characteristics and cultural background may influence how they perceive and report their conditions; thus country-specific PRO data would be important for an accurate understanding of the impact of the disease[4].
Multiple PRO instruments can be used to assess HCV patients’ HRQoL, which typically assess patients’ conditions in several aspects, or domains; the outcomes are reported using domain and summary scores (Supplementary Table 1).
HCV infection negatively impacts patients’ health and quality of life throughout the disease stages. Evidence indicates that there is already measurable damage to HRQoL in asymptomatic or undiagnosed patients with HCV infection[110]. PRO measurements deteriorate further as the disease progresses to more severe and advanced stages, as reported by studies from Thailand and Japan[111,112].
In China, studies on PROs in HCV patients have been scarce. Nevertheless, existing data showed that impairment in quality of life contributes to the disease burden of HCV infection. As part of the nationwide CCgenos study, cross-sectional data collected in 2011 from 997 untreated patients with chronic HCV infection reported a mean Euro-QoL 5 Dimensions descriptive score of 0.780/1[113]. The percentage of patients reporting moderate or severe problems was about 34% for both the domains of pain/discomfort and anxiety/depression, and 7%–8% for the domains of mobility and usual activities[113]. Similarly, results from local studies in rural Liaoning and Beijing using the Short Form-36 (SF-36) and/or the Chronic Liver Disease Questionnaire (CLDQ) scales revealed low quality of life among CHC patients[114,115]. In a community-based survey of CHB and CHC patients in Shanghai using the Quality of Life Instruments for Chronic Disease-Chronic Hepatitis and the Family Burden Interview Schedule, multivariable analyses identified HCV infection and elevated serum alanine aminotransferase level as direct risk factors negatively impacting both the patients’ quality of life and the burden on their caregivers[116]. Clearly, the wellbeing and quality of life of Chinese HCV patients and caregivers are adversely affected by the disease, calling for closer attention to patient experience in HCV management.
To understand how treating and curing HCV infection can affect patients’ experience and wellbeing, PROs are typically measured pre-treatment, at different time points during treatment, at the end of treatment (EoT), and 12 or 24 wk post-treatment.
Impact of PR therapy on PROs: PR therapy is known to cause on-treatment deterioration in PROs due to side effects of and intolerance to the regime. A cross-sectional study in Taiwan reported that CHC patients on PR treatment (n = 108) scored significantly lower than untreated CHC patients on some of the SF-36 and CLDQ scales[117]. Illustrating the on-treatment PRO impairment more clearly, another Taiwanese study involving 47 PR-treated CHC patients showed that by treatment week 12, the patients’ mean scores for all 8 SF-36 domains decreased significantly from baseline[118].
Upon PR treatment completion, PRO parameters would return to pre-treatment levels, or improve further upon treatment success. In the afore-mentioned study of 47 PR-treated patients, the SF-36 domain scores of those who achieved SVR (n = 21) improved significantly over baseline by week 24 post-treatment. In contrast, the domain scores of non-SVR patients (n = 26), though recovered from treatment week 12 to pre-treatment level by EoT, did not improve further post-treatment[118]. A study in Guangzhou involving 72 CHC patients treated with PR reported that by EoT and similarly at week 24 post-treatment, patients’ quality of life as measured by the Generic Quality of Life Inventory-74 questionnaire improved significantly over baseline, being significantly better than that of 30 untreated CHC patients at the same timepoints. This study did not report on-treatment QoL measurements or patients’ SVR status[119].
Impact of DAA regimens on PROs: Throughout the development of DAAs, various combination therapies have been studied and used, including DAA + IFN + RBV, DAA + RBV, and DAA-only regimens. For SOF-based regimens, PRO data collected from pivotal clinical studies in Western countries, Japan and other Asian regions (China, Hong Kong, Taiwan, South Korea and Vietnam) consistently demonstrated that for all three types of DAA combination therapy, achieving SVR12 was associated with post-treatment PRO improvement, although compared to PR-containing or IFN-free, RBV-containing regimens, DAA-only treatment offered better on-treatment patient experience[120-124].
Specifically for HCV patients in China, pooled analysis of two phase 3 studies on SOF-based regimens showed that all three types of DAA combination therapy achieved high SVR12 rates (94.6%–100%)[125]. Patients treated with SOF + IFN + RBV regimen showed marked HRQoL decrease from treatment week 2, and those treated with SOF + RBV experienced modest on-treatment HRQoL decline. For both groups, the HRQoL scores remained at trough level until EoT, before improving to and beyond pre-treatment levels[125]. In contrast, the HRQoL scores of patients receiving DAA-only treatment (LDV/SOF) started to improve from treatment week 4, and continued improving during and after the treatment period[125]. By week 12 post-treatment, the HRQoL scores of the LDV/SOF-treated group were significantly higher than those of the other two treatment groups[125]. Considering the good safety profile and tolerability documented for SOF/VEL in clinical and real-world studies, SOF/VEL is likely to have beneficial effects on PROs, similar to LDV/SOF. Other non-SOF-based, DAA-only regimens have also generally been associated with stable on-treatment PRO profiles and PRO improvements at EoT or post-treatment[126-130].
In summary, curing HCV infection is generally associated with improved patient experience and quality of life, irrespective of the therapy used. Again, the benefits availed by PR therapy are likely to be limited in the light of its low treatment success rate. In fact, poor adherence due to severe on-treatment HRQoL impairment is considered an important factor contributing to the low real-life SVR rates with PR therapy[4]. IFN-containing DAA combination regimens can achieve high SVR rates, but like PR therapy, are associated with severe PRO impairment during treatment. IFN-free, RBV-containing DAA regimens lead to mild on-treatment PRO impairment. In contrast, DAA-only regimens can avoid such negative impact on patients, and can confer rapid, sustained improvements in PROs during and after treatment. In resource-limited settings or in difficult-to-treat patients, the use of IFN and/or RBV may be a pragmatic necessity. Nevertheless, the need to minimize on-treatment life quality deterioration and to optimize patient experience should be taken into consideration when choosing the appropriate treatment regimens for HCV patients, and DAA-only regimens have demonstrated added value in this respect.
In the DAA era, the goal of HCV elimination has become more realistic than ever. Nevertheless, there remain some challenges and important issues that deserve more attention in the management of HCV.
With existing pan-genotypic regimens, GT3 HCV tends to be more difficult to treat than the other genotypes. Both GLE/PIB and SOF + DCV require treatment duration extensions for certain subpopulations of GT3 patients, and SOF/VEL’s drug label in China suggests the addition of RBV for GT3, cirrhotic patients[5,131]. More research would be needed to optimize the treatment strategy for GT3 patients, especially in China where the proportion of GT3b subtype and the prevalence of baseline NS5A RASs are higher than in Western countries[51,132].
Special attention needs to be paid to patients coinfected with hepatitis B virus (HBV), which has a prevalence of 4.11% among HCV patients in China[133]. HBV/HCV coinfected patients not on active anti-HBV treatment should be monitored for potential HBV reactivation during and after DAA treatment[5].
Patients who fail certain DAA regimens may develop treatment-emergent RASs, the transmission and accumulation of which could potentially cause public health issues. A study on HCV resistance in China by Huang et al[134]. reported a significantly higher overall frequency of NS5A RASs in treatment-naïve GT1b patients in 2016 than in 2008 (42.0% vs 18.4%; P = 0.002). To minimize the risk of treatment-emergence RASs, it is important to select for initial treatment regimens with high resistance barriers (such as NS5B inhibitors), or to diligently conduct baseline RAS testing if planning to use regimens known to be prone to clinical resistance. SOF/VEL/VOX, the regimen reserved for rescue treatment of patients with DAA failures, is not yet available in China, but would likely be a valuable tool in the future as more and more Chinese patients undergo DAA treatments.
While DAAs offer high rates of virologic cure, the issue of HCV reinfection is coming increasingly into attention. High reinfection rates associated with high risk behaviors may hamper HCV elimination in key populations, such as IDUs and MSM. For patients prone to high-risk behavior, reinfection risk counseling and linkage to harm reduction services should be provided before and after HCV treatment, such as referring actively injecting IDUs to methadone substitution treatment or needle and syringe exchange programs, linking MSM to condom distribution programs, and other behavioral interventions where necessary[135].
Besides curative therapies, another approach explored for facilitating HCV elimination is the development of prophylactic HCV vaccines. Faced with challenges ranging from the high genetic variability of HCV to a lack of appropriate animal model systems for efficacy evaluation, research in this area thus far has not met with success (for research progress on vaccine candidates, please refer to reviews by Ghasemi et al[136] and Yan et al[137]).
In addition to the value aspects discussed in this article, there are other factors pertaining to curing HCV infection that, though not yet incorporated into value assessment models, obviously carry considerable importance for patients and society. For example, the hope of being cured and the removal of societal stigmatization would be valuable from individual patients’ perspectives. Often, HCV patients are ostracized by the community and discriminated in the workplace. Individuals diagnosed with HCV infection may suffer from anxiety and fear, and some may feel hopeless and give up on seeking treatment. However, with the availability of highly effective DAA therapies, the possibility of achieving a complete cure with relatively short and well tolerated treatments would help alleviate the fear in many patients and contribute towards destigmatizing HCV infection. Another such example is the scientific “spill-over” effect, whereby introducing and investing in innovative treatment technology may stimulate future research for better understanding of HCV and advancement in HCV prevention and control. It could be worthwhile for Chinese researchers to explore how these aspects can be incorporated into novel value assessment models to better inform health technology assessment and public health policy making.
With the first DAA regimens approved in 2017 and the registration process expedited for innovative HCV medicines, China is undoubtedly shifting from the PR era towards that of DAAs for HCV treatment. In light of the principles set out in the 2017–2020 National Viral Hepatitis Action Plan and the goals of the Healthy China 2030 Plan, we would like to suggest that Chinese policy makers take further measures to improve the availability of, and enable large-scale access to, innovative HCV treatment, so as to capitalize fully on the value of effective HCV cure.
In order to maximize the benefits of highly effective HCV treatment, it would be essential to have as many HCV-infected patients as possible diagnosed and treated. The targets set out in the WHO Global Health Sector Strategy on viral hepatitis are that, by 2030, 90% of HCV-infected patients are diagnosed and 80% of those diagnosed receive HCV treatment[5]. In this respect, efforts would be needed from Chinese policy makers and healthcare professionals to improve the public awareness of HCV through continued education. Public health resources would also be needed to support the service coverage of HCV screening, diagnosis, and linkage to care. Specifically, targeted efforts and aids may be needed to ensure that the diagnosis and treatment needs are met in rural and less developed areas of China, and that HCV management capabilities can be enhanced in lower-tier hospitals and healthcare facilities.
The value of curing HCV infection extends far beyond the clinical endpoint of SVR. At patient level, achieving virologic cure improves the long-term health outcomes and quality of life. At society level, providing prompt and effective treatment can help avoid future HCV-related disease and financial burdens. As China stands on the threshold of the DAA era, it would be important for stakeholders and policy makers to consider, that when evaluated holistically, the long-term benefits associated with curing HCV infection would outweigh the initial investment needed for implementing effective HCV therapies.
The authors would like to thank Mei Kwan Chan, MSc and Bo Lyu, PhD (employees of Costello Medical, Singapore, funded by Gilead Sciences, Shanghai, China), for writing assistance and editorial support for the development of the manuscript. The authors are entirely responsible for the scientific content of this paper.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Campollo O, Farshadpour F, Hu KQ, Lin ZY, Shimizu Y, Tajiri K S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. 2017. Available from: https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_September_21_2017_e.pdf. |
| 2. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66:153-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 805] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 3. | Chinese Society of Hepatology, Chinese Medical Association. Wei L; Chinese Society of Infectious Diseases, Chinese Medical Association, Hou JL. [The guideline of prevention and treatment for hepatitis C: a 2015 update]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:906-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Tanaka A, Eguchi Y, Lim YS, Yu ML, Kawada N, Dan YY, Brooks-Rooney C, Negro F, Mondelli MU. The impact of hepatitis C virus outside the liver: Evidence from Asia. Liver Int. 2017;37:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. 2018. Available from: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/. |
| 6. | World Health Organization. WHO model list of essential medicines. 2017; Available from: http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017_FINAL_amendedAug2017.pdf?ua=1. |
| 7. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 8. | Chinese Foundation for Hepatitis Prevention and Control. Report on the current status and prevention and treatment strategies for hepatitis C in China. Beijing: People's Medical Publishing House Co 2017; 3-5. |
| 9. | Duan Z, Jia JD, Hou J, Lou L, Tobias H, Xu XY, Wei L, Zhuang H, Pan CQ. Current challenges and the management of chronic hepatitis C in mainland China. J Clin Gastroenterol. 2014;48:679-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Rao H, Wei L, Lopez-Talavera JC, Shang J, Chen H, Li J, Xie Q, Gao Z, Wang L, Wei J, Jiang J, Sun Y, Yang R, Li H, Zhang H, Gong Z, Zhang L, Zhao L, Dou X, Niu J, You H, Chen Z, Ning Q, Gong G, Wu S, Ji W, Mao Q, Tang H, Li S, Wei S, Sun J, Jiang J, Lu L, Jia J, Zhuang H. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2014;29:545-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Nie B, Zhang K, Liu J, Guo Y, Tu Z. Prevalence of hepatitis C virus genotypes in China patients: a systematic review and meta-analysis. Jianyan Yixue Yu Linchuang. 2016;13:2876-2881. [DOI] [Full Text] |
| 12. | National Health Commission of the People's Republic of China. National essential drug list (2018). 2018; Available from: http://www.gov.cn/fuwu/2018-10/30/5335721/files/e7473e46d9b24aadad3eb25127ffd986.pdf. |
| 13. | Lazarus JV, Wiktor S, Colombo M, Thursz M; EASL International Liver Foundation. Micro-elimination - A path to global elimination of hepatitis C. J Hepatol. 2017;67:665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 14. | National Health Commission of the People's Republic of China. Action plan for the prevention and treatment of viral hepatitis in China (2017-2020). 2017; Available from: http://www.nhc.gov.cn/ewebeditor/uploadfile/2017/11/20171113134002475.pdf. |
| 15. | CPC Central Committee, State Council of the People's Republic of China. Healthy China 2030 blueprint. 2016; Available from: http://www.nhc.gov.cn/zhuz/mtbd/201610/21d120c917284007ad9c7aa8e9634bb4.shtml. |
| 16. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. [PubMed] |
| 17. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, Nishiguchi S, Kuroki T, Imazeki F, Yokosuka O, Kinoyama S, Yamada G, Omata M. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [PubMed] |
| 18. | Bennett H, Waser N, Johnston K, Kao JH, Lim YS, Duan ZP, Lee YJ, Wei L, Chen CJ, Sievert W, Yuan Y, Li H. A review of the burden of hepatitis C virus infection in China, Japan, South Korea and Taiwan. Hepatol Int. 2015;9:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 20. | Fan JH, Wang JB, Jiang Y, Xiang W, Liang H, Wei WQ, Qiao YL, Boffetta P. Attributable causes of liver cancer mortality and incidence in china. Asian Pac J Cancer Prev. 2013;14:7251-7256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Pan YF, Zheng Y, Qin T, Feng L, Zhang Q, Ping XG, Pan YT, Wang XP, Bai L, Li HH. Disease progression in Chinese patients with hepatitis C virus RNA-positive infection via blood transfusion. Exp Ther Med. 2016;12:3476-3484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Lu J, Xiang X, Cao Z, Wang W, Zhao G, Tang W, Chen L, Guo S, Zhuang Y, Shi D, Chen L, Bao S, Cai W, Wang H, Zhou H, Xie Q. Younger trend of cirrhosis incidence in genotype 3 HCV infected patients in Eastern China. J Med Virol. 2017;89:1973-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2019;69:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 24. | Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: Impact on mortality in patients without advanced liver disease. Hepatology. 2018;68:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 25. | Carrat F. First prospective evidence of decreased mortality after direct acting antivirals in the French ANRS CO22 HEPATHER cohort. Hepatology. 2017;66:1271A. [DOI] [Full Text] |
| 26. | Mangia A, Lawitz E, Gane E, Conway B, Ruane P, Abergel A, Mcnabb B, Osinusi A, Chen F, Dvory-Sobol H, Brainard D, Subramanian M, Leggett B, Panero JLC, Agarwal K, Younes ZH, Muir A. Long-term follow-up of patients with chronic HCV infection and compensated or decompensated cirrhosis following treatment with sofosbuvir-based regimens. J Hepatol. 2018;68 Suppl 1:S67-S68. [DOI] [Full Text] |
| 27. | Ogata F, Kobayashi M, Akuta N, Osawa M, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Kobayashi M, Saitoh S, Suzuki Y, Suzuki F, Arase Y, Ikeda K, Kumada H. Outcome of All-Oral Direct-Acting Antiviral Regimens on the Rate of Development of Hepatocellular Carcinoma in Patients with Hepatitis C Virus Genotype 1-Related Chronic Liver Disease. Oncology. 2017;93:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Zhang CH, Xu GL, Jia WD, Li JS, Ma JL, Ge YS. Effects of interferon treatment on development and progression of hepatocellular carcinoma in patients with chronic virus infection: a meta-analysis of randomized controlled trials. Int J Cancer. 2011;129:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Ji F, Dang S, Cai Z, Xue H, Huang N, Liu L, Zhang S, Guo Y, Jia X, Wang Y, Li Z, Deng H. Antiviral treatment and long-term clinical outcome of decompensated cirrhotic patients with hepatitis C virus infection. Zhonghua Gan Zang Bing Za Zhi. 2015;23:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Xie ZW, Li JP, Guan YJ, Zhang XY, Guo FX, Chen BB, Pan CQ. A clinical study of antiviral therapy for patients with compensated hepatitis C cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2017;25:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Lee MH, Huang CF, Lai HC, Lin CY, Dai CY, Liu CJ, Wang JH, Huang JF, Su WP, Yang HC, Kee KM, Yeh ML, Chuang PH, Hsu SJ, Huang CI, Kao JT, Chen CC, Chen SH, Jeng WJ, Yang HI, Yuan Y, Lu SN, Sheen IS, Liu CH, Peng CY, Kao JH, Yu ML, Chuang WL, Chen CJ. Clinical Efficacy and Post-Treatment Seromarkers Associated with the Risk of Hepatocellular Carcinoma among Chronic Hepatitis C Patients. Sci Rep. 2017;7:3718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Lee SH, Jin YJ, Shin JY, Lee JW. Assessment of hepatocellular carcinoma risk based on peg-interferon plus ribavirin treatment experience in this new era of highly effective oral antiviral drugs. Medicine (Baltimore). 2017;96:e5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Ji D, Wang C, Shao Q, Li F, Li B, Wu V, Wong A, Wang YD, Chen J, Chen GF, Lau G. No increase in the occurrence rate of hepatocellular carcinoma in Chinese treated by direct-acting antivirals compared to interferon after eradication of hepatitis C virus: a long-term follow-up. J Hepatol. 2017;66 Suppl 1:S23. [DOI] [Full Text] |
| 34. | Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis. 2015;61:730-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 35. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 377] [Article Influence: 47.1] [Reference Citation Analysis (1)] |
| 36. | Wei L, Lok AS. Impact of new hepatitis C treatments in different regions of the world. Gastroenterology. 2014;146:1145-1150; e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 653] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 38. | Pegasys® Prescribing Information. South San Francisco, CA: Genentech United States, Inc., 2017. |
| 39. | Rebetol® Prescribing Information. Whitehouse Station, NJ: Merck Sharp Cohme Corp, 2017. |
| 40. | Rao HY, Li H, Chen H, Shang J, Xie Q, Gao ZL, Li J, Sun Y, Jiang J, Wang L, Zhao L, Zhang L, Yang W, Niu J, Gong Z, Gong G, Yang R, Lee MH, Wei L. Real-world treatment patterns and clinical outcomes of HCV treatment-naive patients in China: an interim analysis from the CCgenos study. J Gastroenterol Hepatol. 2017;32:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Hu P, Ren H. Current status of treatment of chronic hepatitis C and related challenges in the "Pre-DAA Era" in China. Zhonghua Gan Zang Bing Za Zhi. 2016;24:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Nuño Solinís R, Arratibel Ugarte P, Rojo A, Sanchez Gonzalez Y. Value of Treating All Stages of Chronic Hepatitis C: A Comprehensive Review of Clinical and Economic Evidence. Infect Dis Ther. 2016;5:491-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Public Health England. Hepatitis C in England: 2017 report. 2017. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/599738/hepatitis_c_in_england_2017_report.pdf. |
| 44. | Action Hepatitis Canada. Treatment access update: Alberta, Saskatchewan, Manitoba, Yukon, and NIHB formularies all lift eligibility restrictions for hepatitis C treatment. 2018. Available from: http://www.actionhepatitiscanada.ca/news.html. |
| 45. | Wei L, Wang FS, Zhang MX, Jia JD, Yakovlev AA, Xie W, Burnevich E, Niu JQ, Jung YJ, Jiang XJ, Xu M, Chen XY, Xie Q, Li J, Hou JL, Tang H, Dou XG, Gandhi Y, Hu WH, McPhee F, Noviello S, Treitel M, Mo L, Deng J. Daclatasvir plus asunaprevir in treatment-naïve patients with hepatitis C virus genotype 1b infection. World J Gastroenterol. 2018;24:1361-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 46. | Wei L, Xie Q, Hou JL, Tang H, Ning Q, Cheng J, Nan Y, Zhang L, Li J, Jiang J, McNabb B, Zhang F, Camus G, Mo H, Osinusi A, Brainard DM, Gong G, Mou Z, Wu S, Wang G, Hu P, Gao Y, Jia J, Duan Z. Ledipasvir/sofosbuvir for treatment-naive and treatment-experienced Chinese patients with genotype 1 HCV: an open-label, phase 3b study. Hepatol Int. 2018;12:126-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Wei L, Cheng J, Luo J, Li ZP, Duan JL, Hou JD, Jia MX, Zhang Y, Huang Q, Xie GJ, Wang DL, Yang W, Zhao CY, Zhao G, Tang SM, Lin GZ, Gong JJ, Niu ZL, Gao JF, Sarah KB, Linda F, Niloufar M, Wang Y, Wang J. Efficacy and safety of paritaprevir/ritonavir/ombitasvir combined with dasabuvir in non-cirrhotic Asian adult patients with newly diagnosed and treated chronic HCV genotype 1b infection: a randomized, double-blind, placebo-controlled study - China data. Zhonghua Gan Zang Bing Za Zhi. 2018;26:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 48. | Wei L, Jia JD, Wang FS, Niu JQ, Zhao XM, Mu S, Liang LW, Wang Z, Hwang P, Robertson MN, Ingravallo P, Asante-Appiah E, Wei B, Evans B, Hanna GJ, Talwani R, Duan ZP, Zhdanov K, Cheng PN, Tanwandee T, Nguyen VK, Heo J, Isakov V, George J; C-CORAL Investigators. Efficacy and safety of elbasvir/grazoprevir in participants with hepatitis C virus genotype 1, 4, or 6 infection from the Asia-Pacific region and Russia: Final results from the randomized C-CORAL study. J Gastroenterol Hepatol. 2019;34:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Wei L, Xie Q, Hou JL, Jia J, Li W, Xu M, Li J, Wu S, Cheng J, Jiang J, Wang G, Yang Y, Mou Z, Gao ZL, Gong G, Niu JQ, Hu P, Tang H, Lin F, Dou X, Li L, Zhang LL, Nan Y, Massetto B, Yang JC, Knox SJ, Kersey K, German P, Mo H, Jiang D, Brainard DM, Jiang J, Ning Q, Duan Z. Sofosbuvir plus ribavirin with or without peginterferon for the treatment of hepatitis C virus: Results from a phase 3b study in China. J Gastroenterol Hepatol. 2018;33:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Wei L, Xie Q, Huang Y, Wu S, Xu M, Tang H, Cheng J, Gao Y, Mou Z, Dou X, Nan Y, Ning Q, Mao Y, Stamm L, Lu S, Dvory-Sobol H, Mo H, Brainard D, Yang Y, Wang G, Hu P, Zhang L, Gao Z, Lin F, Shang J, Gong G, Li J, Su M, Duan Z, Hou J, Jia J. Safety and Efficacy of sofosbuvir/velpatasvir in genotype 1-6 HCV-infected patients in China: Results from a phase 3 clinical trial. Hepatology. 2018;68 Suppl 1:379A. [DOI] [Full Text] |
| 51. | Wei L, Lim SG, Xie Q, Văn KN, Piratvisuth T, Huang Y, Wu S, Xu M, Tang H, Cheng J, Le Manh H, Gao Y, Mou Z, Sobhonslidsuk A, Dou X, Thongsawat S, Nan Y, Tan CK, Ning Q, Tee HP, Mao Y, Stamm LM, Lu S, Dvory-Sobol H, Mo H, Brainard DM, Yang YF, Dao L, Wang GQ, Tanwandee T, Hu P, Tangkijvanich P, Zhang L, Gao ZL, Lin F, Le TTP, Shang J, Gong G, Li J, Su M, Duan Z, Mohamed R, Hou JL, Jia J. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 52. | An Z, Ding Y, Dou X. Selection and evaluation of treatment regimens with direct-acting antiviral agents for patients with chronic hepatitis C in the real world in China. Linchuang Gan Dan Bing Za Zhi. 2018;34:233-237. [DOI] [Full Text] |
| 53. | Ji D, Chen GF, Wang C, Wang YD, Shao Q, Li B, Zhao J, You SL, Hu JH, Liu JL, Niu XX, Chen J, Lu L, Wu V, Lau G. Twelve-week ribavirin-free direct-acting antivirals for treatment-experienced Chinese with HCV genotype 1b infection including cirrhotic patients. Hepatol Int. 2016;10:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Ji F, Wang W, Dang S, Wang S, Li B, Bai D, Zhao W, Deng H, Tian C, Li Z. Outcomes after sofosbuvir-containing regimens for hepatitis C virus in patients with decompensated cirrhosis: a real-world study. Infect Agent Cancer. 2017;12:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Wu J, Zhou Y, Fu X, Deng M, Zheng Y, Tian G, Li Y, Wang C, Ding C, Ruan B, Yang S, Li L. The Burden of Chronic Hepatitis C in China From 2004 to 2050: An Individual-Based Modeling Study. Hepatology. 2019;69:1442-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Tang H, Wu J, Wu B, Xu X, Ye X, Zhang W. Long-term outcomes associated with sofosbuvir-based regimens for treatment of chronic hepatitis C in China. Value Health. 2018;21 Suppl 2:S65. [DOI] [Full Text] |
| 57. | Wu J, Wu B, Xu X, Ye X, Zhang W. Long-term health outcomes of pan-genotypic direct-acting antiviral treatment of chronic hepatitis C in China. Hepatol Int. 2019;13 Suppl 1:S93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46 Suppl 5:S165-S173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 59. | van der Meer AJ. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ; R. E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 61. | Wang W, Yang Y, Dang S. Current status of research in extrahepatic manifestations of HCV infection. Gan Zang. 2018;23:6-8. |
| 62. | Cacoub P, Desbois AC, Comarmond C, Saadoun D. Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis. Gut. 2018;67:2025-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 63. | Zhang JJ, Li XH, Su ZY, Cao CR. Changes of quality of life after antiviral therapy in patients with chronic hepatitis C and cryoglobulinemia. Zhongguo Linchuang Yanjiu. 2016;29:440-443. [DOI] [Full Text] |
| 64. | Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, Ikeda K, Kumada H. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 65. | Calvaruso V, Craxì A. Why do I treat my patients with mild hepatitis C? Liver Int. 2016;36 Suppl 1:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Zignego AL, Ramos-Casals M, Ferri C, Saadoun D, Arcaini L, Roccatello D, Antonelli A, Desbois AC, Comarmond C, Gragnani L, Casato M, Lamprecht P, Mangia A, Tzioufas AG, Younossi ZM, Cacoub P; ISG-EHCV. International therapeutic guidelines for patients with HCV-related extrahepatic disorders. A multidisciplinary expert statement. Autoimmun Rev. 2017;16:523-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 67. | Polo ML, Laufer N. Extrahepatic manifestations of HCV: the role of direct acting antivirals. Expert Rev Anti Infect Ther. 2017;15:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Gragnani L, Visentini M, Fognani E, Urraro T, De Santis A, Petraccia L, Perez M, Ceccotti G, Colantuono S, Mitrevski M, Stasi C, Del Padre M, Monti M, Gianni E, Pulsoni A, Fiorilli M, Casato M, Zignego AL. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology. 2016;64:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 69. | Saadoun D, Pol S, Ferfar Y, Alric L, Hezode C, Si Ahmed SN, de Saint Martin L, Comarmond C, Bouyer AS, Musset L, Poynard T, Resche Rigon M, Cacoub P. Efficacy and Safety of Sofosbuvir Plus Daclatasvir for Treatment of HCV-Associated Cryoglobulinemia Vasculitis. Gastroenterology. 2017;153:49-52; e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 70. | Arcaini L, Besson C, Frigeni M, Fontaine H, Goldaniga M, Casato M, Visentini M, Torres HA, Loustaud-Ratti V, Peveling-Oberhag J, Fabris P, Rossotti R, Zaja F, Rigacci L, Rattotti S, Bruno R, Merli M, Dorival C, Alric L, Jaccard A, Pol S, Carrat F, Ferretti VV, Visco C, Hermine O. Interferon-free antiviral treatment in B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood. 2016;128:2527-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 71. | Nagao Y, Kimura K, Kawahigashi Y, Sata M. Successful Treatment of Hepatitis C Virus-associated Oral Lichen Planus by Interferon-free Therapy with Direct-acting Antivirals. Clin Transl Gastroenterol. 2016;7:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver Int. 2013;33 Suppl 1:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 73. | Li Y, Pang L. The epidemiology, prevention, and treatment of hepatitis C among drug users. Zhongguo Aizibing Xingbing. 2015;21:652-652. [DOI] [Full Text] |
| 74. | Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 75. | Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, Foster GR, Dillon JF, Goldberg DJ, Dore GJ, Hickman M. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 76. | UNSW Medicine-Kirby Institute. Surveillance and treatment of prisoners with hepatitis C (SToP-C). 2018. Available from: https://kirby.unsw.edu.au/project/stop-c. |
| 77. | Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, Amin J, Bruneau J, Hellard M, Litwin AH, Marks P, Quiene S, Siriragavan S, Applegate TL, Swan T, Byrne J, Lacalamita M, Dunlop A, Matthews GV, Powis J, Shaw D, Thurnheer MC, Weltman M, Kronborg I, Cooper C, Feld JJ, Fraser C, Dillon JF, Read P, Gane E, Dore GJ. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 78. | Olafsson S, Tyrfingsson T, Runarsdottir V, Bergmann OM, Hansdottir I, Björnsson ES, Johannsson B, Sigurdardottir B, Fridriksdottir RH, Löve A, Hellard M, Löve TJ, Gudnason T, Heimisdottir M, Gottfredsson M. Treatment as Prevention for Hepatitis C (TraP Hep C) - a nationwide elimination programme in Iceland using direct-acting antiviral agents. J Intern Med. 2018;283:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 79. | World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. 2016. Available from: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/. |
| 80. | Zhu B, Liu J, Fu Y, Zhang B, Mao Y. Spatio-Temporal Epidemiology of Viral Hepatitis in China (2003-2015): Implications for Prevention and Control Policies. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 81. | Wang H, Yang J, Deng X, Xu K, Wang J, Zhang Y. HIV/HBV/HCV infection among drug users: a meta-analysis of data collected in Chinese mainland. Zhonghua Jibing Kongzhi Zazhi. 2010;14:300-304. |
| 82. | Jin Y, Li M, Liu T, Jin Y, Lu Z, Jia Z. Evaluation of burden of infectious diseases among drug abusers. Zhonghua Yiyuan Ganranxue Zazhi. 2017;27:2125-2128. [DOI] [Full Text] |
| 83. | Kieran JA, Norris S, O'Leary A, Walsh C, Merriman R, Houlihan D, McCormick PA, McKiernan S, Bergin C, Barry M. Hepatitis C in the era of direct-acting antivirals: real-world costs of untreated chronic hepatitis C; a cross-sectional study. BMC Infect Dis. 2015;15:471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Duan ZP, Zhou HY, Duan C, Wang Z, Chen Y, Zheng SJ, Liu S, Tang A, Li H. Survey of Treatment Costs to Hepatitis C in China. Value Health. 2014;17:A805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Sun YT, Zhang YX, Tang H, Mao Q, Wang XZ, Zhang LY, Chen H, Zhong YN, Lin SM, Zhang DZ. Clinical characteristics and current management of hepatitis B and C in China. World J Gastroenterol. 2014;20:13582-13590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Webster S, Ward T, Bennett H, Wygant G, Wang F, Yan J, McEwan P. Chronic hepatitis C and the emergence of direct-acting antiviral regimens in China: treat now or later? Hepatol Int. 2017;11 Suppl 1:S1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164-2170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 360] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 88. | Wei L, Kamae I, Lee MH, Li H. Hepatitis C viral infection disease burden in Asia. Value and Outcomes Spotlight. 2015;1:16-18. |
| 89. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 90. | Gosselin E, Lemyre L, Corneil W. Presenteeism and absenteeism: differentiated understanding of related phenomena. J Occup Health Psychol. 2013;18:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 91. | Ye X, Xu X, Xie Q, Tang H, Xuan J. Impact of sofosbuvir-based regimens for chronic hepatitis C infection: A work productivity model from mainland China. Value Health. 2018;21 Suppl 2:S6. [DOI] [Full Text] |
| 92. | Chhatwal J, He T, Hur C, Lopez-Olivo MA. Direct-Acting Antiviral Agents for Patients With Hepatitis C Virus Genotype 1 Infection Are Cost-Saving. Clin Gastroenterol Hepatol. 2017;15:827-837.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 93. | Younossi ZM, Gordon SC, Dieterich DT, Wong R, Brown KA, Kugelmas M, Saab S, Ahmed A. A decision analytic Markov model to evaluate the health outcomes of sofosbuvir/velpatasvir for patients with chronic hepatitis C virus genotype 1 infection in the US. Hepatology. 2016;64 Suppl 1:421A-422A. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Younossi ZM, Park H, Dieterich D, Saab S, Ahmed A, Gordon SC. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: The quality-adjusted cost of care. Medicine (Baltimore). 2016;95:e5048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Liu Y, Wang Z, Tobe RG, Lin H, Wu B. Cost Effectiveness of Daclatasvir Plus Asunaprevir Therapy for Chinese Patients with Chronic Hepatitis C Virus Genotype 1b. Clin Drug Investig. 2018;38:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Lu Y, Jin X, Duan CA, Chang F. Cost-effectiveness of daclatasvir plus asunaprevir for chronic hepatitis C genotype 1b treatment-naïve patients in China. PLoS One. 2018;13:e0195117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 97. | Chen H, Chen J, Lu Z, Yu H, Chen F. Establish pharmacoeconomics model for treatment of chronic hepatitis C with Markov model. Zhongguo Weisheng Tongji. 2016;33:370-373. |
| 98. | Wu B, Wang Z, Xie Q. Cost-effectiveness of novel regimens for Chinese patients with chronic hepatitis C. Curr Med Res Opin. 2019;35:847-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Chen P, Ma A, Liu Q. Cost-Effectiveness of Elbasvir/Grazoprevir Versus Daclatasvir Plus Asunaprevir in Patients with Chronic Hepatitis C Virus Genotype 1b Infection in China. Clin Drug Investig. 2018;38:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Chen CP, Cheng CY, Zou H, Cheng CH, Cheng SH, Chen CK, Chen CH, Bair MJ. Evaluation of cost-effectiveness of peginterferon plus ribavirin for chronic hepatitis C treatment and direct-acting antiviral agents among HIV-infected patients in the prison and community settings. J Microbiol Immunol Infect. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Li X, Chan NS, Tam AW, Hung IFN, Chan EW. Budget impact and cost-effectiveness analyses of direct-acting antivirals for chronic hepatitis C virus infection in Hong Kong. Eur J Clin Microbiol Infect Dis. 2017;36:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 102. | Cheng WD, Zhang LY, Chen W. Budget impact analysis of direct-acting antiviral regimens for the treatment of hepatitis C virus infection. Zhongguo Wei Sheng Zi Yuan. 2018;21:500-505. [DOI] [Full Text] |
| 103. | Chen H, Chen L. Estimating cost-effectiveness associated with all-oral regimen for chronic hepatitis C in China. PLoS One. 2017;12:e0175189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Younossi ZM, Chan HLY, Dan YY, Lee MH, Lim YS, Kruger E, Tan SC. Impact of ledipasvir/sofosbuvir on the work productivity of genotype 1 chronic hepatitis C patients in Asia. J Viral Hepat. 2018;25:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Bennett H, Gordon J, Jones B, Ward T, Webster S, Kalsekar A, Yuan Y, Brenner M, McEwan P. Hepatitis C disease transmission and treatment uptake: impact on the cost-effectiveness of new direct-acting antiviral therapies. Eur J Health Econ. 2017;18:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Iriana S, Curry MP, Afdhal NH. Neurologic Manifestations of Hepatitis C Virus Infection. Clin Liver Dis. 2017;21:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Kallman J, O'Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci. 2007;52:2531-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 108. | Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 109. | Liu YY, Chen RN, Yao JJ, Yuan CR. Research process in patient-reported outcomes at home and abroad. Xiandai Yufang Yixue. 2013;40:2268-2271. |
| 110. | Strauss E, Porto-Ferreira FA, de Almeida-Neto C, Teixeira MC. Altered quality of life in the early stages of chronic hepatitis C is due to the virus itself. Clin Res Hepatol Gastroenterol. 2014;38:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Matsushita H, Ikeda F, Iwasaki Y, Seki H, Nanba S, Takeuchi Y, Moritou Y, Yasunaka T, Onishi H, Miyake Y, Takaki A, Nouso K, Yamamoto K. Assessment of health-related quality of life and how it predicts the outcome of pegylated interferon and ribavirin therapy for chronic hepatitis C. J Gastroenterol Hepatol. 2014;29:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 112. | Sobhonslidsuk A, Silpakit C, Kongsakon R, Satitpornkul P, Sripetch C, Khanthavit A. Factors influencing health-related quality of life in chronic liver disease. World J Gastroenterol. 2006;12:7786-7791. [PubMed] |
| 113. | Huang R, Rao H, Shang J, Chen H, Li J, Xie Q, Gao Z, Wang L, Wei J, Jiang J, Sun J, Jiang J, Wei L. A cross-sectional assessment of health-related quality of life in Chinese patients with chronic hepatitis c virus infection with EQ-5D. Health Qual Life Outcomes. 2018;16:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Zhang HR, Pan GW, Xu XG, Liu L, Ning Y, Ma L, Ren R. [Study on quality of life and its related factors among patients with chronic hepatitis C in rural area]. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 115. | Jia Y, Li X, Li L, Liang J, Kang Y, Lin F, Deng Z, Wang W, Zhang B, Xu J. Quality of life and its influential factors in outpatients with chronic hepatitis B or C. Linchuang Gandanbing Zazhi. 2015;31:1695-1698. [DOI] [Full Text] |
| 116. | Ren H, Shi Y, Meng W, Hu JY, Chen YH, Pan QC. Study of disease burden of chronic hepatitis B and C patients in Shanghai based on Bronfenbrenner' s ecological systems theory: a community-based survey. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 117. | Chang SC, Yang SS, Chang CC, Lin CC, Chung YC, Li TC. Assessment of health-related quality of life in antiviral-treated Taiwanese chronic hepatitis C patients using SF-36 and CLDQ. Health Qual Life Outcomes. 2014;12:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Kang SC, Hwang SJ, Lee SH, Chang FY, Lee SD. Health-related quality of life and impact of antiviral treatment in Chinese patients with chronic hepatitis C in Taiwan. World J Gastroenterol. 2005;11:7494-7498. [PubMed] |
| 119. | Liu J, Lin CS, Hu SH, Liang ML, Zhao ZX, Gao ZL. [Quality of life in patients with chronic hepatitis C after PEG-Interferon a-2a therapy]. Zhonghua Gan Zang Bing Za Zhi. 2011;19:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Younossi ZM, Stepanova M, Henry L, Han KH, Ahn SH, Lim YS, Chuang WL, Kao JH, Nguyen KV, Lai CL, Chan HL, Wei L. Sofosbuvir and ledipasvir are associated with high sustained virologic response and improvement of health-related quality of life in East Asian patients with hepatitis C virus infection. J Viral Hepat. 2018;25:1429-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Younossi ZM, Stepanova M, Henry L, Han KH, Ahn SH, Lim YS, Chuang WL, Kao JH, Kinh N, Lai CL, Yuen MF, Chan HL, Lai W. The effect of interferon-free regimens on health-related quality of life in East Asian patients with chronic hepatitis C. Liver Int. 2018;38:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Younossi Z, Stepanova M, Omata M, Mizokami M, Nader F, Hunt S. Fatigue in Japanese patients with chronic hepatitis C treated with ledipasvir and sofosbuvir with or without ribavirin. Value Health. 2016;19:A316-A317. [DOI] [Full Text] |
| 123. | Younossi ZM, Stepanova M, Omata M, Mizokami M, Walters M, Hunt S. Quality of life of Japanese patients with chronic hepatitis C treated with ledipasvir and sofosbuvir. Medicine (Baltimore). 2016;95:e4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Younossi ZM, Stepanova M, Henry L, Nader F, Hunt S. An In-Depth Analysis of Patient-Reported Outcomes in Patients With Chronic Hepatitis C Treated With Different Anti-Viral Regimens. Am J Gastroenterol. 2016;111:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 125. | Younossi Z, Stepanova M, Henry L, Duan ZP, Xie Q, Hou JL, Jia J, Wei L. High efficacy and substantial improvement of health-related quality of life (HRQL) in Chinese patients with hepatitis C virus (HCV) infection treated with ledipasvir and sofosbuvir (LDV/SOF). Hepatol Int. 2018;12 Suppl 2:S238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 126. | Behl A, Kumada H, Toyata J, Chayama K, Ishikawa H, Kalsekar A. Comparison of patient reported outcomes in a phase 3 study of all oral dual combination of daclatasvir plus asunaprevir (DCV + ASV) versus telaprevir plus peginterferon alfa ribavirin (TVR) in treatment naive Japanese patients chronically infected with HCV genotype 1b. Hepatol Int. 2015;9 Suppl 1:S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Wygant G, Mo L, Treitel M, Zhao Y. Patient reported outcomes in IFN/ribavirin intolerant/ineligible Asian patients from Mainland China receiving daclatasvir +asunaprevir for the treatment of HCV genotype 1b infection. Hepatol Int. 2017;11 Suppl 1:S1001-S1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | Wygant G, Mo L, Treitel M, Zhao Y. Fatigue severity scores (FSS) and SF-36 patient-reported outcomes (PROs) in Asian interferon/ribavirin intolerant/ineligible patients from Mainland China who received daclatasvir + asunaprevir for the treatment of HCV genotype 1b infection. Hepatol Int. 2017;11 Suppl 1:S1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Cheng W, Sobhonslidsuk A, Jia J, Lindore P, Liang L, Evans B, Ginanni J, Talwani R, Arduino JM, Thuy thi TP, Wang F-s, Lee Y-J, Chu C-j. Impact of a 12-week oral regimen of elbasvir/grazoprevir (EBR/GZR) on health-related quality of life (HRQOL) and fatigue in treatment-naïve patients with chronic hepatitis C virus (HCV) genotype (GT) 1, 4, or 6 infection: data from the C-CORAL study. Hepatol Int. 2017;11 Suppl 1:S298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 130. | Kumada H, Chayama K, Mehta D, Pinsky B. Impact of hepatitis C treatment with glecaprevir + pibrentasvir on patient's health related quality of life: Results from phase 3 CERTAIN trials. Hepatology. 2017;66 Suppl 1:639A-640A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Epclusa® Prescribing Information. Shanghai, China: Gilead Sciences, Shanghai, 2018. |
| 132. | Wei L, Omata M, Lim YS, Chuang WL, Long D, Xie Q, Hou J, Jia J, Svarovskaia E, Martin R, Doehle B, Yang J, De-Oertel S, Massetto B, Kersey K, Brainard D, Mo H, Kinh N, Kao JH, Han KH, Mizokami M, Duan Z. Prevalence of pre-treatment NS5A and NS5B NI resistance associated substitutions in HCV infected patients enrolled in clinical trials in Asia. Hepatol Int. 2017;11 Suppl 1:S189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Yan LB, Rao HY, Ma YJ, Bai L, Chen EQ, Du LY, Yang RF, Wei L, Tang H; CCgenos Study Group. Hepatitis B virus infection in Chinese patients with hepatitis C virus infection: prevalence, clinical characteristics, viral interactions and host genotypes: a nationwide cross-sectional study. BMJ Open. 2016;6:e012016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 134. | Huang W, Wang M, Gong Q, Yu D, Chen P, Lin J, Han Y, Su Y, Qu L, Zhang X. Comparison of Naturally Occurring Resistance-Associated Substitutions Between 2008 and 2016 in Chinese Patients with Chronic Hepatitis C Virus Infection. Microb Drug Resist. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | Falade-Nwulia O, Sulkowski MS, Merkow A, Latkin C, Mehta SH. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J Viral Hepat. 2018;25:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 136. | Ghasemi F, Rostami S, Meshkat Z. Progress in the development of vaccines for hepatitis C virus infection. World J Gastroenterol. 2015;21:11984-12002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Yan Y, Wang X, Huang Z, Zhong J. Demands, challenges and progresses of prophylactic hepatitis C virus vaccine development. Zhongguo Xi Bao Sheng Wu Xue Xue Bao. 2018;40:303-308. [DOI] [Full Text] |