Published online Apr 27, 2019. doi: 10.4254/wjh.v11.i4.379
Peer-review started: January 10, 2019
First decision: March 5, 2019
Revised: March 22, 2019
Accepted: April 8, 2019
Article in press: April 8, 2019
Published online: April 27, 2019
Processing time: 106 Days and 15.1 Hours
Patients with liver disease are concomitantly at increased risk of venous thromboembolism (VTE) and bleeding events due to changes in the balance of pro- and anti-hemostatic substances. As such, recommendations for the use of pharmacological VTE prophylaxis are lacking. Recent studies have found no difference in rates of VTE in those receiving and not receiving pharmacological VTE prophylaxis, though most studies have been small. Thus, our study sought to establish if pharmacological VTE prophylaxis is effective and safe in patients with liver disease.
To determine if there is net clinical benefit to providing pharmacological VTE prophylaxis to cirrhotic patients.
In this retrospective study, 1806 patients were propensity matched to assess if pharmacological VTE prophylaxis is effective and safe in patients with cirrhosis. Patients were divided and evaluated based on receipt of pharmacological VTE prophylaxis.
The composite primary outcome of VTE or major bleeding was more common in the no prophylaxis group than the prophylaxis group (8.7% vs 5.1%, P = 0.002), though this outcome was driven by higher rates of major bleeding (6.9% vs 2.9%, P < 0.001) rather than VTE (1.9% vs 2.2%, P = 0.62). There was no difference in length of stay or in-hospital mortality between groups. Pharmacological VTE prophylaxis was independently associated with lower rates of major bleeding (OR = 0.42, 95%CI: 0.25-0.68, P = 0.0005), but was not protective against VTE on multivariable analysis.
Pharmacological VTE prophylaxis was not associated with a significant reduction in the rate of VTE in patients with liver disease, though no increase in major bleeding events was observed.
Core tip: While patients with cirrhosis have historically been considered to be coagulopathic, recent data suggests that these patients may be both hypo- and hypercoagulable. Recommendation for provision of chemoprophylaxis to prevent venous thromboembolism (VTE) in this group of patients is lacking. In our study, pharmacological VTE prophylaxis decreased composite rates of major bleeding and VTE but was not protective against VTE, further demonstrating the uncertain utility of chemoprophylaxis in this population.
- Citation: Yerke J, Bauer SR, Bass S, Torbic H, Militello M, Roach E, Hanouneh I, Welch S. Effectiveness of venous thromboembolism prophylaxis in patients with liver disease. World J Hepatol 2019; 11(4): 379-390
- URL: https://www.wjgnet.com/1948-5182/full/v11/i4/379.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i4.379
It is widely recognized that patients with liver disease, particularly end stage, have acquired bleeding disorders resulting from a reduction of procoagulant factors, thrombocytopenia, and abnormalities in fibrinolysis[1]. More recently, however, the risk of venous thromboembolism (VTE) is being recognized and is likely due to elevations in factor VIII and von Willebrand factor along with a reduction of the endogenous anticoagulant protein C[1]. Several studies have evaluated the risk of VTE in patients with end-stage liver disease with varying results ranging from an incidence of 0.5% to 6.3%[2-9]. Factors that have been implicated in a higher rate of VTE occurrence include albumin levels < 3 g/dL and concomitant comorbidities, particularly chronic kidney disease, heart failure, and malignancy[3-5].
These variable findings are likely due to several factors including differences in severity of liver disease, etiology of liver disease, concomitant comorbidities, and potentially the receipt of pharmacological VTE prophylaxis. The use of pharmacological VTE prophylaxis is commonly omitted in patients with end stage liver disease due to the widely held belief that the risk of bleeding outweighs the benefit of prophylaxis[6,7,10,11]. Additionally, it is unclear if pharmacological prophylaxis is effective in preventing thrombosis as it has been shown to be in other patient populations.
Few studies have evaluated the effectiveness and risk of VTE prophylaxis in patients with liver disease. Those that have suggest no reduction in rate of VTE events, but suggest potential increases in the rate of major bleeding[6,7,9,12]. However, these studies have significant limitations, most notably the lack of defined prophylactic therapy, defined VTE and bleeding events, heterogeneity among patients, and small sample size. The net benefit of pharmacological VTE prophylaxis is of particular interest because VTE events have been shown to confer a higher 30-d mortality when they occur in a patient with cirrhosis compared to the general population[13]. Therefore, our study seeks to compare differences in the rate of VTE and major bleeding between patients with liver disease receiving and not receiving pharmacological VTE prophylaxis.
This retrospective cohort study was performed at a large, tertiary-care academic medical center and approved by the Cleveland Clinic Institutional Review Board (Cleveland, OH, United States). Adult patients admitted for 48 h or more from November 2008 through July 2015 with discharge International Classification of Diseases, 9th edition (ICD-9) diagnosis codes corresponding to cirrhotic liver disease were included in the study. Patients were excluded if they developed an incident VTE within 48 h of admission, if they had a diagnosis of congenital or acquired thrombophilia (defined as factor V Leiden, anti-phospholipid syndrome, prothrombin G20210A, protein C or S deficiency, prothrombin mutation, or anti-thrombin deficiency) or hemophilia, or if they received treatment dose anticoagulation for any indication other than an incident VTE. For patients with multiple admissions, the most recent admission was included for analysis. Patients admitted for liver transplantation were included up until their transplant.
The primary outcome was the composite rate of incident VTE and major bleeding. Secondary outcomes included the rate of incident VTE, rate of major bleeding, length of hospital stay, and rate of in-hospital mortality. Incident VTE was defined as a new thrombosis occurring 48 h or more after admission, extension of a VTE in a patient with an untreated prevalent VTE, or additional VTE formation in a patient with an untreated prevalent VTE. An incident VTE was required to be demonstrated by unequivocal radiographic imaging by compression ultrasonography, venography, computed tomography angiography, or ventilation-perfusion scanning[12]. Prevalent VTE was defined as a documented VTE at admission that was not being treated with anticoagulation. An incident bleeding event was considered any new-onset major bleeding event or any major bleeding event that occurred 24 h or more following hemostasis of a previous bleeding event[12]. For example, if a patient was admitted for variceal hemorrhage and did not have further bleeding the patient was not regarded as having incident bleeding, but if the patient developed bleeding more than 24 h after initial hemostasis the patient was regarded as having incident bleeding. Major bleeding was defined as bleeding that was symptomatic and at a critical site (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or that required transfusion of at least 2 units of whole red blood or red cells within 24 h[14].
Patients were allocated to the pharmacological VTE prophylaxis group if they received pharmacological prophylaxis for at least 50% of their hospital stay. Patients receiving prophylaxis less than 50% of their stay were allocated to the no prophylaxis group. Those experiencing a VTE event were grouped according to receipt or no receipt of prophylaxis within 48 h prior to the event and those experiencing a bleeding event were grouped according to receipt or no receipt of prophylaxis in the 24 h prior to the event. All major bleeding and VTE events were identified by the use of ICD-9 codes and manually verified in the electronic medical record.
Appropriate dosing of prophylactic anticoagulants was considered to be two or three doses per day of subcutaneous unfractionated heparin 5000 units, one or two doses per day of subcutaneous enoxaparin 40mg or two doses per day of enoxaparin 30 mg (or renally adjusted equivalent), one dose per day of subcutaneous fondaparinux 2.5 mg (or renally adjusted equivalent), or aspirin 160mg or more per day in orthopedic surgery patients.
The statistical methods of this study were reviewed by R. Samuel Butler from the Cleveland Clinic Department of Biostatistics. Assuming a rate of 7.7% for occurrence of the primary outcome in the no pharmacological VTE prophylaxis group and a rate of 4.4% in the pharmacological VTE prophylaxis group, a sample of 513 patients would provide 80% power to detect a 3.3% difference with a two-sided α = 0.05. This estimate assumes an incidence of VTE of 6.3% in the no prophylaxis group as found by Dabbagh et al[7] as it was thought that this patient population most closely mirrored our study population. No study with a similar patient population to our own that compared incidence of major bleeding in patients with liver disease receiving or not receiving pharmacological prophylaxis was found, so a rate of 1.4% was chosen for this portion of the composite primary outcome. Patients were matched in a 1:1 fashion based on propensity score. Variables included in the propensity score were history of VTE, baseline platelet count, use of mechanical VTE prophylaxis, baseline model for end-stage liver disease (MELD) score, age, the presence of heart failure, the presence of chronic kidney disease, the presence of lung disease (chronic obstructive pulmonary disease, asthma, or idiopathic pulmonary fibrosis), and the etiology of liver disease. Missing data required to calculate the MELD score was considered to be normal. Univariate analyses were completed using Pearson’s Chi-square test for categorical variables and analysis of variance for continuous variables. Multivariable logistic regression was performed to identify independent risk factors for VTE, major bleeding, and in-hospital mortality.
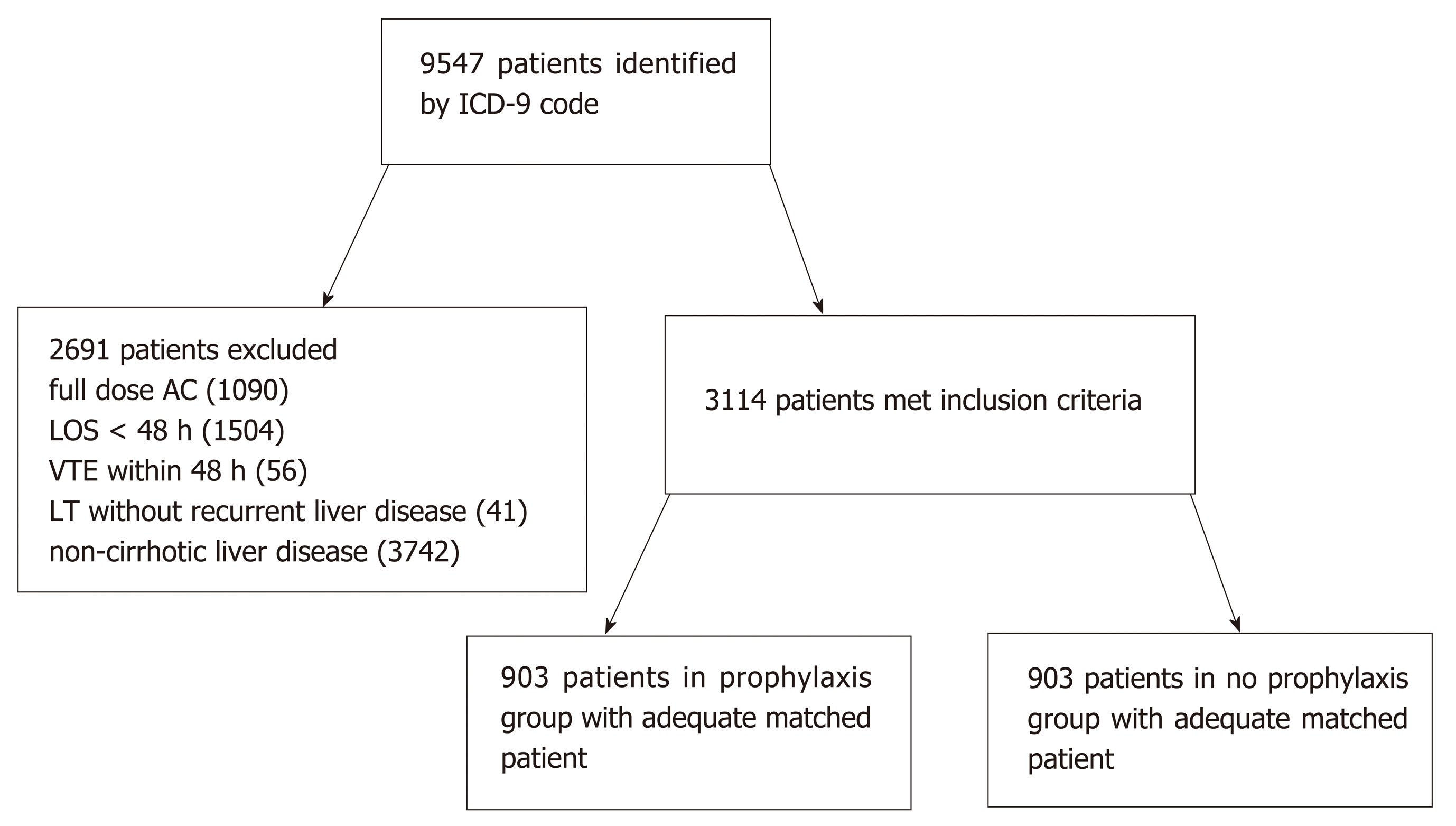
A total of 9547 patients were identified with ICD-9 codes for liver disease, of which 3114 patients met inclusion criteria. The most common reasons for exclusion were those already receiving full dose anticoagulation (n = 1090), hospital length of stay less than 48 h (n = 1504), and liver disease without cirrhotic morphology (n = 3742). Of the 3114 patients, 903 patients in the prophylaxis group were matched by propensity score to 903 patients in the no prophylaxis group (Figure 1) and were included in the analyses. Baseline characteristics according to group are summarized in Table 1. Patients in the no pharmacological prophylaxis group were less likely to require renal replacement therapy (24.5% vs 29.6%, P = 0.015) and had a different distribution of MELD scores (fewer patients with MELD 10-39, more patients with MELD < 10 or ≥ 40). Differences in VTE risk score were noted; however, patients in both groups were predominately categorized as medium (65.4% vs 75.3%) and high risk (20.3% vs 14.2%) in the no prophylaxis and prophylaxis groups respectively. No difference was noted in the primary etiology of hepatic disease. Statistically significant differences were also noted in baseline INR and hemoglobin, but these were considered to be of negligible clinical significance. All other baseline characteristics were similar between groups.
| No prophylaxis(n = 903) | Prophylaxis(n = 903) | |
| Mean age (yr) | 60.1 ± 11.9 | 60.2 ± 11.8 |
| Male | 572 (63.3) | 535 (59.2) |
| RRTa | 221 (24.5) | 267 (29.6) |
| CKD | 226 (25.0) | 238 (26.4) |
| Lung disease | 218 (24.1) | 218 (24.1) |
| Heart failure | 192 (21.3) | 196 (21.7) |
| MVP | 291 (32.2) | 286 (31.7) |
| VTE risk scorec | ||
| Unknown risk score | 103 (11.4) | 78 (8.6) |
| Low risk | 26 (2.9) | 17 (1.9) |
| Medium risk | 591 (65.4) | 680 (75.3) |
| High risk | 183 (20.3) | 128 (14.2) |
| BMI (kg/m2) | 24.4 ± 0.20 | 24.4 ± 0.20 |
| INRc | 1.4 ± 0.57 | 1.3 ± 0.32 |
| Platelet count (k/µL) | 144.2 ± 92.5 | 151.0 ± 97.0 |
| aPTT (s) | 34.8 ± 12.0 | 34.4 ± 10.2 |
| Albumin (g/dL) | 2.9 ± 0.70 | 2.9 ± 0.67 |
| Tbili (mg/dL) | 4.0 ± 7.1 | 3.8 ± 6.6 |
| SCr (mg/dL) | 1.5 ± 1.3 a | 1.6 ± 1.5 |
| Hemoglobin (g/dL) | 10.4 ± 2.3 b | 10.7 ± 2.1 |
| Liver disease etiology | ||
| Alcoholic | 278 (30.8) | 286 (31.7) |
| NASH | 27 (3.0) | 22 (2.4) |
| Acute Hepatitis | 35 (3.9) | 38 (4.2) |
| Other1 | 563 (62.3) | 557 (61.7) |
| Mean MELD score | 17.0 ± 9.1 | 17.4 ± 8.3 |
| MELD categoryc | ||
| MELD > 40 | 20 (2.2) | 8 (0.89) |
| MELD 30-39.9 | 71 (7.9) | 74 (8.2) |
| MELD 20-29.9 | 205 (22.7) | 233 (25.8) |
| MELD 10-19.9 | 338 (37.4) | 386 (42.7) |
| MELD < 10 | 269 (29.8) | 202 (22.4) |
Patients in the no prophylaxis group were more likely to experience the composite endpoint of VTE or major bleeding compared to those in the prophylaxis group (8.7% vs 5.1%, P = 0.002), although this was driven by an increased rate of major bleeding events (6.9% vs 2.9%, P < 0.0001) with no difference observed in the rate of VTE events (1.9% vs 2.2%, P = 0.61). There was no difference in in-hospital mortality (12.1% vs 11.5%, P = 0.72) or hospital length of stay (10.5 ± 12.6 d vs 10.8 ± 14.8 d, P = 0.67) between groups (Table 2).
Multivariable logistic regression for VTE events (Table 3), bleeding events (Table 4), and in-hospital mortality (Table 5) was performed. Lower baseline serum albumin (OR = 0.23, 95%CI: 0.13-0.42, P < 0.0001) was independently associated with development of VTE events, while decreasing baseline hemoglobin (OR = 0.76, 95%CI: 0.68-0.87, P < 0.0001) and albumin (OR = 0.61, 95%CI: 0.42-0.90) were independently associated with development of a major bleed. Pharmacological VTE prophylaxis was independently associated with lower rates of major bleeding (OR = 0.42, 95%CI: 0.25-0.68, P = 0.0005), but was not significantly associated with a difference in rate of incident VTE (OR = 0.99, 95%CI: 0.48-2.06, P = 0.97).
| OR | 95%CI | |
| Prophylaxis group | 0.99 | 0.48-2.06 |
| BMI (kg/m2) | 2.33 | 0.38-14.13 |
| Baseline albumin (g/dL) | 0.23 | 0.13-0.42 |
| Baseline hemoglobin (g/dL) | 1.18 | 0.99-1.41 |
| Male sex | 1.57 | 0.68-3.61 |
| OR | 95%CI | |
| Prophylaxis group | 0.42 | 0.25-0.68 |
| Baseline albumin (g/dL) | 0.61 | 0.42-0.90 |
| Baseline hemoglobin (g/dL) | 0.77 | 0.68-0.87 |
| BMI (kg/m2) | 0.49 | 0.16-1.53 |
| Male sex | 0.87 | 0.54-1.39 |
| MELD < 10 | 0.07 | 0.02-0.21 |
| MELD 10-19 | 0.07 | 0.03-0.18 |
| MELD 20-29 | 0.19 | 0.07-0.49 |
| MELD 30-39 | 0.43 | 0.16-1.15 |
| All MELD categories compared to MELD score > 40 | ||
| OR | 95%CI | |
| Prophylaxis group | 1.03 | 0.73-1.43 |
| Baseline albumin (g/dL) | 0.68 | 0.52-0.88 |
| Baseline hemoglobin (g/dL) | 1.00 | 0.92-1.08 |
| Male sex | 1.00 | 0.71-1.42 |
| Occurrence of primary endpoint | 2.30 | 1.44-3.68 |
| MELD < 10 | 0.05 | 0.02-0.14 |
| MELD 10-19 | 0.09 | 0.04-0.21 |
| MELD 20-29 | 0.31 | 0.13-0.70 |
| MELD 30-39 | 0.93 | 0.39-2.20 |
| All MELD categories compared to MELD score > 40 | ||
Risk factors independently associated with in-hospital mortality included occurrence of the primary endpoint (OR = 2.30, 95%CI: 1.44-3.70, P = 0.0005), decreasing baseline albumin (OR = 0.68, 95%CI: 0.52-0.88, P = 0.004), and increasing MELD category (OR = 0.31, 95%CI: 0.13-0.70, P = 0.0005 for comparison of MELD 20-29 with MELD > 40).
While antihemostatic changes of cirrhosis have been well characterized, prohemostatic changes have also been more recently recognized[1,15-17]. Although liver disease associated coagulopathy results in elevated laboratory tests for coagulation, thrombin generation is not proportionately reduced, leaving some subsets of patients with a hypercoagulable thrombin generation profile[18-21]. However, the propensity of a patient to be hypo- or hypercoagulable is challenging to predict, particularly when using standard laboratory tests of coagulation, such as INR or activated partial thromboplastin time, that have not been validated in this patient population[7,18,22].
While the incidence of VTE has been well established in patients with cirrhosis, whether pharmacological VTE prophylaxis should be provided in an attempt to decrease this incidence is not well known. Major VTE prophylaxis guidelines are silent on this topic[23]. Therefore, this study was conducted to evaluate whether patients with cirrhosis experienced net benefit or harm when prophylactic anticoagulation was provided. We found that those who received pharmacological prophylaxis experienced the primary outcome of incident VTE or incident major bleeding less frequently, although this difference was driven by decreased rates of major bleeding (Table 2).
The overall rate of VTE in our study (2.0%) closely correlates with the incidence seen in several other studies of patients with liver disease, but was significantly lower than the VTE rate used in our power analysis[3,5,7,12,24]. Dabbagh et al[7] was chosen to inform the power analysis as this study contained a large proportion of patients with Child-Pugh Class C liver disease, which more closely mirrors the liver disease population seen at our institution. The differences in incidence of VTE may partially be explained by higher rates of mechanical prophylaxis (31.9% vs 16.3%) in the current study. Similar to previous data, there was no difference in the incidence of VTE between the no prophylaxis group and the prophylaxis group (1.9% vs 2.2%, P = 0.62)[6,9,12,25]. While the matched analysis was likely still susceptible to bias due to an imbalance in baseline characteristics, further correction for between-group differences by multivariable logistic regression also revealed no difference (OR for VTE in the prophylaxis group 0.99, 95%CI: 0.48-2.06). This lack of difference when correcting for other factors may indicate that only minimal, if any, protection from VTE is provided by pharmacological prophylaxis in patients with cirrhosis.
As noted in previous literature, low baseline serum albumin was independently associated with VTE development in this population[4,5]. Low baseline serum albumin was also independently associated with increased odds of major bleeding and in-hospital mortality, as has been observed widely throughout the literature[26-30]. These findings complicate the use of serum albumin as an indicator of appropriateness of pharmacological prophylaxis. These data may also suggest that patients with severe liver disease could concomitantly be at elevated risk of bleeding and thrombosis, with the type of event experienced influenced by acute physiologic insults.
While several different types of liver disease have been associated with increased risk for thrombosis, little is known about how different types of liver disease compare to each other in regards to thrombotic risk[31-37]. In addition, very few studies have evaluated how risk factors for thrombosis have translated to actual thrombotic events. One large study evaluating nearly 5 million patients with liver disease found an increased rate of VTE in patients with non-alcoholic liver disease compared to those with alcoholic liver disease (0.9% vs 0.6%, P < 0.0001)[34]. However, significant differences in baseline characteristics between the non-alcoholic and alcoholic groups were present, including age (60 vs 52 years, P < 0.0001), which is a known risk factor for VTE[34]. Patients with cholestatic cirrhosis have also been shown to be more hypercoagulable on evaluation by thromboelastography than a cohort of mainly patients with alcoholic cirrhosis[33]. However, no difference in etiology of cirrhosis was noted in our study.
Our study found an overall rate of major bleeding of 4.9%, with significantly more major bleeding events occurring in the no prophylaxis group than in the prophylaxis group (6.9% vs 2.9%). Overall bleeding rates in a previous study found no significant difference in rates of any bleeding between those who did not receive prophylaxis versus those that received prophylaxis (8.1% vs 5.5%, P = 0.258) as well as in rates of gastrointestinal hemorrhage (3.2% vs 3.0%, P = 0.52)[12]. However, on multivariable analysis the use of pharmacological prophylaxis was significantly associated with in-hospital bleeding (OR = 2.355, 95%CI: 1.116-4.971)[12]. This result contrasts sharply with our own multivariable analysis, which found that prophylaxis was associated with a decreased incidence of major bleeding (OR = 0.42, 95%CI: 0.25-0.68). Importantly, the bleeding definitions used in the respective studies differed, with Shatzel et al[12] evaluating all bleeding events compared to major bleeding in the current evaluation. However, these discrepant findings are unlikely explained by bleeding definitions[12]. Our study found that lower baseline serum albumin (OR = 0.676, 95%CI: 0.484-0.943) was independently associated with major bleeding, a result that likely highlights the increased bleeding risk that occurs as cirrhosis severity progresses. However, this does not explain the difference in major bleeding observed in the propensity score matched analysis as the mean albumin was not different between groups. Notably, it does not seem that prophylactic anticoagulation imparted any additional bleeding risk within our patient population.
There was no difference in in-hospital mortality between those who did not receive prophylaxis versus those that did (12.1% vs 11.5%, P = 0.72). Factors found to increase the risk of in-hospital mortality include occurrence of the primary endpoint (OR = 2.30, 95%CI: 1.44-3.70, P = 0.0005), decreasing baseline albumin (OR = 0.68, 95%CI: 0.52-0.88, P = 0.004), and increasing MELD category. A higher incidence of mortality in patients with hypoalbuminemia has been consistently observed throughout the literature, a finding that is corroborated by our study[26-30]. Overall, these findings seem to suggest that progression of cirrhosis leads to worsened outcomes in regards to VTE and bleeding events as well as in-hospital mortality.
The results of our study can be applied clinically in many ways. First, decreased serum albumin has consistently been shown to be an independent risk factor for VTE within this population, and was also associated with increased odds of major bleeding and in-hospital mortality. While these results may not be useful in stratifying patients that should receive pharmacological prophylaxis from those that should not, they can help provide insight into patients that require mechanical prophylaxis, as well as heighten the clinician’s suspicion of VTE if signs and symptoms meet this clinical picture. Second, these data suggest that pharmacological VTE prophylaxis is safe in patients with cirrhosis, as patients that received prophylaxis did not experience increased risk of major bleeding. However, efficacy of pharmacological prophylaxis within this population was not established by this study. Finally, our findings suggest that a validated risk tool, such as the Padua Predictive Score, may be more useful in stratifying liver disease patients at risk for VTE[9,12,38].
Our study has several limitations. First, this retrospective review is subject to inherent flaws of the study design; we were reliant on the accuracy of the medication administration record for group allocation. Second, although selection bias was minimized through propensity matching, baseline differences between groups remained. Despite efforts to collect a comprehensive list of baseline characteristics, there may be additional unaccounted differences that influenced the clinician’s decision to administer prophylaxis. Additionally, while absolute standardized differences of baseline characteristics between groups decreased following propensity score matching, some differences remained (Table 6). While we made every effort to make the prophylaxis and no prophylaxis groups as similar as possible, the chance remains that there is a fundamental difference in the patient populations for which we could not account. Thirdly, few patients in this study received low molecular weight heparin. A previous study, primarily evaluating bleeding risk associated with pharmacological prophylaxis, found that patients receiving unfractionated heparin, but not low molecular weight heparin, were at an increased risk of in-hospital bleeding events[12]. This finding may in part be explained by a greater effect on thrombin generation with unfractionated heparin when compared to low molecular weight heparin, suggesting a more potent anticoagulant effect for unfractionated heparin in cirrhotic patients[20]. Therefore, our results should only be applied to patient’s receiving unfractionated heparin. A VTE risk scoring tool that includes risk factors similar to those included in the Caprini score and Padua predictive score was used to evaluate patients within our study[38,39]. This tool was developed, implemented, and validated at the study site, but has not been evaluated within a population of patients with liver disease. Because a validated VTE risk score for this population was not collected and analyzed, it is possible that there was a difference in baseline VTE risk for which we could not account. However, baseline characteristics that were collected that are risk factors for VTE (such as hospital length of stay and comorbidities) were balanced between groups. Finally, our study relied on ICD-9 codes to identify all patient diagnoses, including liver disease, VTE and bleeding events, and comorbid conditions. Although this is consistent with other studies on this topic, confirmation of clinical conditions aside from VTE and bleeding events was not manually performed. Additionally, validation of these events was only completed if events had appropriate ICD-9 codes, leaving us unable to account for events that were not documented appropriately.
| Unmatched | Matched | |||||
| No prophylaxis | Prophylaxis | ASD | No prophylaxis | Prophylaxis | ASD | |
| (n = 2166) | (n = 948) | (n = 903) | (n = 903) | |||
| Age (yr) | 58.1 ± 11.6 | 60.3 ± 11.8 | 0.153 | 60.1 ± 11.9 | 60.2 ± 11.8 | 0.00844 |
| Male | 1349 (62.3) | 561(59.2) | 0.0639 | 572 (63.3) | 535 (59.2) | 0.0842 |
| RRT | 819 (37.8) | 270 (28.5) | 0.199 | 221 (24.5) | 267 (29.6) | 0.115 |
| CKD | 577 (26.6) | 247 (26.1) | 0.0133 | 226 (25.0) | 238 (26.4) | 0.0304 |
| Lung disease | 397 (18.3) | 236 (24.9) | 0.160 | 218 (24.1) | 218 (24.1) | 0.000 |
| Heart failure | 360 (16.6) | 210 (22.2) | 0.140 | 192 (21.3) | 196 (21.7) | 0.0108 |
| MVP | 755 (34.9) | 300 (31.6) | 0.0682 | 291 (32.2) | 286 (31.7) | 0.0119 |
| VTE risk score | 0.390 | 0.218 | ||||
| Unknown | 447 (20.6) | 80 (8.4) | 103 (11.4) | 78 (8.6) | ||
| Low | 86 (4.0) | 17 (1.8) | 26 (2.9) | 17 (1.9) | ||
| Medium | 1341 (61.9) | 716 (75.5) | 591 (65.4) | 680 (75.3) | ||
| High | 292 (13.5) | 135 (14.2) | 183 (20.3) | 128 (14.2) | ||
| BMI (kg/m2) | 24.2 ± 0.19 | 24.4 ± 0.20 | 0.0180 | 24.4 ± 0.20 | 24.4 ± 0.20 | 0.00 |
| INR | 1.51 ± 0.67 | 1.27 ± 0.31 | 0.478 | 1.4 ± 0.57 | 1.3 ± 0.32 | 0.216 |
| Platelet count (k/µL) | 108.3 ± 76.2 | 159.8 ± 108.0 | 0.551 | 144.2 ± 92.5 | 151.0 ± 97.0 | 0.0718 |
| aPTT (s) | 37.68 ± 13.6 | 34.3 ± 10.1 | 0.282 | 34.8 ± 12.0 | 34.4 ± 10.2 | 0.0359 |
| Albumin (g/dL) | 2.8 ± 0.70 | 2.9 ± 0.68 | 0.118 | 2.9 ± 0.70 | 2.9 ± 0.67 | 0.00 |
| Tbili (mg/dL) | 5.88 ± 8.57 | 3.68 ± 6.49 | 0.288 | 4.0 ± 7.1 | 3.8 ± 6.6 | 0.0292 |
| SCr (mg/dL) | 1.65 ± 1.41 | 1.59 ± 1.50 | 0.0414 | 1.5 ± 1.3 | 1.6 ± 1.5 | 0.0713 |
| Hemoglobin (g/dL) | 10.0 ± 2.3 | 10.7 ± 2.1 | 0.269 | 10.4 ± 2.3 | 10.7 ± 2.1 | 0.136 |
| Liver disease etiology | 0.0964 | 0.0416 | ||||
| Alcoholic | 745 (34.4) | 296 (31.2) | 278 (30.8) | 286 (31.7) | ||
| NASH | 75 (3.5) | 25 (2.6) | 27 (3.0) | 22 (2.4) | ||
| Other1 | 1239 (57.2) | 585 (61.7) | 563 (62.3) | 557 (61.7) | ||
| Acute hepatitis | 107 (4.9) | 42 (4.4) | 35 (3.9) | 38 (4.2) | ||
| MELD score | 21.1 ± 10.3 | 17.2 ± 8.3 | 0.422 | 17.0 ± 9.1 | 17.4 ± 8.3 | 0.0459 |
| MELD category | 0.431 | 0.210 | ||||
| >40 | 102 (4.7) | 8 (0.8) | 20 (2.2) | 8 (0.89) | ||
| 30-39 | 364 (16.8) | 74 (7.8) | 71 (7.9) | 74 (8.2) | ||
| 20-29 | 637 (29.4) | 238 (25.1) | 205 (22.7) | 233 (25.8) | ||
| 10-19 | 692 (31.9) | 402 (42.4) | 338 (37.4) | 386 (42.7) | ||
| < 10 | 371 (17.1) | 226 (23.8) | 269 (29.8) | 202 (22.4) | ||
Our study does have some notable strengths. Incident VTE and major bleeding were clearly defined and confirmed by manual chart review. Second, our study had clear definitions for what constituted prophylaxis and no prophylaxis. Third, baseline albumin and comorbid conditions, factors known to increase the risk of VTE in liver disease, were well balanced between groups, decreasing the risk that these variables could have confounded the results. Finally, our study included patients with varying degrees and etiologies of liver disease making these results more generalizable.
In conclusion, patients receiving pharmacological VTE prophylaxis experienced a lower rate of the composite endpoint of VTE and major bleeding, though this was driven by a reduction in the rate of major bleeding. Pharmacological VTE prophylaxis was not associated with a significant reduction in the rate of VTE in patients with liver disease, but was also not associated with an increase in rates of major bleeding.
Hepatic cirrhosis has historically been considered a coagulopathic disease, as traditional measurements of coagulation are often deranged. However, more recent literature suggests an altered coagulation cascade that may be tipped toward thrombosis or bleeding based on acute insults. Major venous thromboembolism (VTE) prophylaxis guidelines currently make no recommendation on whether to provide pharmacological prophylaxis to hospitalized cirrhotic patients. This study sought to improve on previously published retrospective data that has studied this topic, and attempted to provide data about whether pharmacological prophylaxis provides net clinical benefit or causes harm in a cirrhotic population.
The main problem that this study attempted to solve is whether pharmacological VTE prophylaxis prevents thrombotic events in patients with cirrhosis without causing a significant additional bleeding burden. Solving this problem could provide clarity in regards to the optimal strategy to prevent thrombotic events in cirrhotic patients.
The main objective of this study was to determine whether pharmacological VTE prophylaxis was beneficial overall to cirrhotic patients. This was assessed using a composite outcome of incident VTE and incident major bleeding, as the authors considered either of the events included in the composite outcome to be similarly detrimental to a patient. We feel that this study evaluated the benefits of pharmacological prophylaxis to the best of the capabilities of a retrospective study, and showed no harm to patients receiving prophylactic anticoagulation. Our findings could be used to demonstrate that pharmacological prophylaxis is likely safe in a population such as ours, which could allow for a future prospective, randomized controlled trial to be completed in an ethical manner.
This study was a retrospective, cohort trial of patients with cirrhosis that received or did not receive pharmacological VTE prophylaxis during a hospitalization for any indication. Cirrhosis and other baseline past medical history that may have contributed to bleeding or thrombosis were identified using ICD-9 codes. Incident major bleeding and incident VTE were identified using ICD-9 codes and verified in the patient’s medical record by reviewing relevant imaging reports and lab values. We attempted to balance the patient groups by performing propensity score matching, and to account for any additional imbalance through multivariable logistic regression.
Baseline characteristics were largely balanced when comparing groups. Our primary outcome (the composite of incident major bleeding or incident VTE) was found to occur significantly less frequently in the prophylaxis group than in the no prophylaxis group (5.1 vs 8.7%, P < 0.05), though this result was driven largely by a higher rate of major bleeding in the no prophylaxis group. This result was confirmed on multivariable analysis, as receipt of pharmacological prophylaxis was significantly associated with a lower odds of major bleeding (though no significant association with pharmacological prophylaxis was noted on multivariable analysis of VTE).
The major finding of this study was that pharmacological VTE prophylaxis did not increase the incidence of major bleeding in a large cohort of hospitalized cirrhotic patients. This challenges the historical idea that pharmacological prophylaxis should be withheld from cirrhotic patients due to an increased bleeding risk, and is more in line with recent findings that while cirrhotic patients have an altered coagulation cascade, they are at risk for both thrombotic and bleeding complications depending on acute insults. This finding could be the impetus for a large, randomized controlled trial in this patient population that could better answer the question of whether prophylactic anticoagulation truly prevents incident thrombotic events in a cirrhotic population.
We feel that the only way to definitively answer the question of whether pharmacological prophylaxis is effective in preventing incident thrombotic events in a cirrhotic population is through a randomized, controlled trial. However, we feel that the lack of an increase in bleeding complications observed in this study is significant, and should allow for the pursuit of such a study without significant concern for harming a cirrhotic population similar to ours by providing pharmacologic VTE prophylaxis.
The authors acknowledge R. Samuel Butler for performing statistical analysis of the data.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iliescu L, Pallav K S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 951] [Article Influence: 67.9] [Reference Citation Analysis (1)] |
| 2. | Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809-815. [PubMed] |
| 3. | Ali M, Ananthakrishnan AN, McGinley EL, Saeian K. Deep vein thrombosis and pulmonary embolism in hospitalized patients with cirrhosis: a nationwide analysis. Dig Dis Sci. 2011;56:2152-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Northup PG, McMahon MM, Ruhl AP, Altschuler SE, Volk-Bednarz A, Caldwell SH, Berg CL. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101:1524-1528; quiz 1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Gulley D, Teal E, Suvannasankha A, Chalasani N, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53:3012-3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Aldawood A, Arabi Y, Aljumah A, Alsaadi A, Rishu A, Aldorzi H, Alqahtani S, Alsultan M, Felemban A. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Søgaard KK, Horváth-Puhó E, Grønbaek H, Jepsen P, Vilstrup H, Sørensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | Bogari H, Patanwala AE, Cosgrove R, Katz M. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014;134:1220-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Smith CB, Hurdle AC, Kemp LO, Sands C, Twilla JD. Evaluation of venous thromboembolism prophylaxis in patients with chronic liver disease. J Hosp Med. 2013;8:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Moorehead KJ, Jeffres MN, Mueller SW. A Retrospective Cohort Analysis of Pharmacologic VTE Prophylaxis and Padua Prediction Score in Hospitalized Patients With Chronic Liver Disease. J Pharm Pract. 2017;30:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Shatzel J, Dulai PS, Harbin D, Cheung H, Reid TN, Kim J, James SL, Khine H, Batman S, Whyman J, Dickson RC, Ornstein DL. Safety and efficacy of pharmacological thromboprophylaxis for hospitalized patients with cirrhosis: a single-center retrospective cohort study. J Thromb Haemost. 2015;13:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Søgaard KK, Horváth-Puhó E, Montomoli J, Vilstrup H, Sørensen HT. Cirrhosis is Associated with an Increased 30-Day Mortality After Venous Thromboembolism. Clin Transl Gastroenterol. 2015;6:e97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3234] [Cited by in RCA: 3920] [Article Influence: 196.0] [Reference Citation Analysis (0)] |
| 15. | Tripodi A, Primignani M, Chantarangkul V, Dell'Era A, Clerici M, de Franchis R, Colombo M, Mannucci PM. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 398] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell'Era A, Fabris F, Salerno F, Mannucci PM. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 18. | Gatt A, Riddell A, Calvaruso V, Tuddenham EG, Makris M, Burroughs AK. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J Thromb Haemost. 2010;8:1994-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Potze W, Arshad F, Adelmeijer J, Blokzijl H, van den Berg AP, Porte RJ, Lisman T. Routine coagulation assays underestimate levels of antithrombin-dependent drugs but not of direct anticoagulant drugs in plasma from patients with cirrhosis. Br J Haematol. 2013;163:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Potze W, Arshad F, Adelmeijer J, Blokzijl H, van den Berg AP, Meijers JC, Porte RJ, Lisman T. Differential in vitro inhibition of thrombin generation by anticoagulant drugs in plasma from patients with cirrhosis. PLoS One. 2014;9:e88390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Northup PG. Hypercoagulation in liver disease. Clin Liver Dis. 2009;13:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, Mannuccio Mannucci P. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 23. | Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S-e496S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2569] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 24. | Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Gómez Cuervo C, Bisbal Pardo O, Pérez-Jacoiste Asín MA. Efficacy and safety of the use of heparin as thromboprophylaxis in patients with liver cirrhosis: a systematic review and meta-analysis. Thromb Res. 2013;132:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Myers RP, Shaheen AA, Faris P, Aspinall AI, Burak KW. Revision of MELD to include serum albumin improves prediction of mortality on the liver transplant waiting list. PLoS One. 2013;8:e51926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Myers RP, Shaheen AA, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol. 2011;54:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Zoli M, Cordiani MR, Marchesini G, Iervese T, Labate AM, Bonazzi C, Bianchi G, Pisi E. Prognostic indicators in compensated cirrhosis. Am J Gastroenterol. 1991;86:1508-1513. [PubMed] |
| 29. | Merkel C, Bolognesi M, Angeli P, Noventa F, Caregaro L, Sacerdoti D, Gatta A. Prognostic indicators of survival in patients with cirrhosis and esophageal varices, without previous bleeding. Am J Gastroenterol. 1989;84:717-722. [PubMed] |
| 30. | Realdi G, Fattovich G, Hadziyannis S, Schalm SW, Almasio P, Sanchez-Tapias J, Christensen E, Giustina G, Noventa F. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21:656-666. [PubMed] |
| 31. | Assy N, Bekirov I, Mejritsky Y, Solomon L, Szvalb S, Hussein O. Association between thrombotic risk factors and extent of fibrosis in patients with non-alcoholic fatty liver diseases. World J Gastroenterol. 2005;11:5834-5839. [PubMed] |
| 32. | Papatheodoridis GV, Chrysanthos N, Cholongitas E, Pavlou E, Apergis G, Tiniakos DG, Andrioti E, Theodossiades G, Archimandritis AJ. Thrombotic risk factors and liver histologic lesions in non-alcoholic fatty liver disease. J Hepatol. 2009;51:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Ben-Ari Z, Panagou M, Patch D, Bates S, Osman E, Pasi J, Burroughs A. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol. 1997;26:554-559. [PubMed] |
| 34. | Saleh T, Matta F, Alali F, Stein PD. Venous thromboembolism with chronic liver disease. Am J Med. 2011;124:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Mangia A, Margaglione M, Cascavilla I, Gentile R, Cappucci G, Facciorusso D, Grandone E, Di Minno G, Rizzetto M, Andriulli A. Anticardiolipin antibodies in patients with liver disease. Am J Gastroenterol. 1999;94:2983-2987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Prieto J, Yuste JR, Beloqui O, Civeira MP, Riezu JI, Aguirre B, Sangro B. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology. 1996;23:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Di Minno MN, Tufano A, Rusolillo A, Di Minno G, Tarantino G. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J Gastroenterol. 2010;16:6119-6122. [PubMed] |
| 38. | Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, De Bon E, Tormene D, Pagnan A, Prandoni P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8:2450-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 807] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 39. | Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38:12-19. [PubMed] |