Published online Jan 27, 2019. doi: 10.4254/wjh.v11.i1.65
Peer-review started: September 27, 2018
First decision: October 16, 2018
Revised: November 14, 2018
Accepted: December 31, 2018
Article in press: January 1, 2019
Published online: January 27, 2019
Processing time: 122 Days and 9.4 Hours
Hepatitis B virus (HBV) is one of the most significant hepatocarcinogens. The ultimate goal of anti-HBV treatment is to prevent the development of hepatocellular carcinoma (HCC). During the last two decades, with the use of currently available anti-HBV therapies (lamivudine, entecavir and tenofovir disoproxil fumatate), there has been a decrease in the incidence of HBV-associated HCC (HBV-HCC). Furthermore, several studies have demonstrated a reduction in recurrent or new HCC development after initial HCC tumor ablation. However, during an observation period spanning 10 to 20 years, several case reports have demonstrated the development of new, subsequent new and recurrent HCC even in patients with undetectable serum HBV DNA. The persistent risk for HCC is attributed to the presence of covalently closed circular DNA (cccDNA) in the hepatocyte nucleus which continues to work as a template for HBV replication. While a functional cure (loss of hepatitis B surface antigen and undetectable viral DNA) can be attained with nucleos(t)ide analogues, these therapies do not eliminate cccDNA. Of utmost importance is successful eradication of the transcriptionally active HBV cccDNA from hepatocyte nuclei which would be considered a complete cure. The unpredictable nature of HCC development in patients with chronic HBV infection shows the need for a complete cure. Continued support and encouragement for research efforts aimed at developing curative therapies is imperative. The aims of this minireview are to highlight these observations and emphasize the need for a cure for HBV.
Core tip: Despite the advances in the management of hepatitis B virus (HBV) infection and HBV-associated hepatocellular carcinoma (HCC), the risk for new, subsequent new and recurrent HCC persists even after over a decade of antiviral therapy and initial tumor ablation. This is due to the inability of the current antiviral therapy to eliminate the covalently closed circular DNA from the hepatocyte nucleus. There is a great need for an HBV cure drug.
- Citation: Shinn BJ, Martin A, Coben RM, Conn MI, Prieto J, Kroop H, DiMarino AJ, Hann HW. Persistent risk for new, subsequent new and recurrent hepatocellular carcinoma despite successful anti-hepatitis B virus therapy and tumor ablation: The need for hepatitis B virus cure. World J Hepatol 2019; 11(1): 65-73
- URL: https://www.wjgnet.com/1948-5182/full/v11/i1/65.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i1.65
Since the discovery of hepatitis B virus (HBV) by Blumberg et al[1] in 1965 and the development of the first HBV vaccine in 1983, much knowledge has been obtained regarding the pathogenesis, hepatocarcinogenesis, epidemiology and molecular biology of HBV as well as the antiviral treatment.
With vaccination, there has been a decrease in the number of infected people globally especially in the younger generations. There has also been a significant increase in survival of HBV infected individuals due to antiviral treatment and earlier detection of hepatocellular carcinoma (HCC) with simultaneous antiviral treatment.
However HBV is still responsible for 786000 deaths in 2010 with 341000 from HCC and 312000 attributed to cirrhosis[2].
HBV is a major public health issue with more than 257 million carriers living today, and up to one million who die annually from HBV-related liver disease including HCC[3,4]. HCC is the sixth most common cancer and the third leading cause of cancer deaths worldwide. Chronic infection with HBV is responsible for 50% of HCC cases worldwide[5,6]. Current therapies available to treat chronic hepatitis B (CHB) include interferon and the nucleos(t)ide analogues (NAs): Lamivudine, adefovir, entecavir, telbivudine, tenofovir disoproxil fumarate and the recently FDA-approved tenofovir alafenamide. Several studies have been conducted in the past to assess the impact of antiviral therapies, specifically lamivudine, entecavir and tenofovir, on disease progression and HCC development[7-10]. Multiple studies have demonstrated that for patients diagnosed with HBV-related HCC, improved survival can be attained with adjuvant antiviral therapy following curative liver resection and/or local tumor ablation[11,12].
Current antiviral therapies used to treat CHB control viral replication through inhibition of reverse transcriptase and subsequent interruption of HBV DNA formation. While treatment can slow and/or prevent the progression of liver disease, it does not eliminate the covalently closed circular DNA (cccDNA) from infected hepatocytes and is therefore unable to cure the infection. As a result, patients usually require lifelong therapy to control viral replication and help prevent hepatocarcinogenesis.
However, it has been reported that despite several years of antiviral therapy, a persistent risk for carcinogenesis still exists[13-15]. Studies have also demonstrated that even after more than a decade of successful HBV suppression, the risk for HBV-related HCC remains[16-18]. The aims of this minireview are to bring light to these observations and emphasize the need for a cure for HBV[19-21].
HBV related hepatocarcinogenesis has been extensively described in the past[22-27]. HBV is an enveloped partially double-stranded relaxed circular DNA virus of the Hepadnaviridae family. The viral replication cycle begins when HBV recognizes highly-sulfated heparin sulfate proteoglycans on the hepatocyte surface and gains entry by binding the liver-specific receptor, sodium taurocholate co-transporting polypeptide (NTCP or SLC10A1)[28,29]. Once in the cell, the virus enters the hepatocyte nucleus where the relaxed circular DNA is converted to cccDNA. While little is known about the formation and regulation of cccDNA, it is thought that most of the steps needed for this conversion are provided by the host cell[19-21,30]. Viral cccDNA remains in the nucleus of the infected host cell and is used as the template for transcription of four viral mRNA intermediates. These mRNA intermediates eventually undergo translation to produce seven viral proteins including DNA polymerase and the core protein. One of these mRNA intermediates, called pregenomic RNA, is critical for the viral replication. It undergoes reverse transcription and serves as the template for new viral DNA. The newly formed viral DNA and viral proteins form viral nucleocapsids that obtain HBV envelope proteins prior to being released from the hepatocyte as mature enveloped virions[19-21,30]. These virions then go on to infect other hepatocytes.
As to HBV associated hepatocarcinogenesis, inside the hepatocyte nucleus, HBV DNA integration with the host genome takes place during the acute phase of infection[31,32]. This integration is thought to be one of several mechanisms that leads to carcinogenesis and HCC. Activation of cellular oncogenes, inactivation of tumor suppressor genes, chronic liver injury, inflammation and regeneration, activation of cellular proto-oncogenes, suppression of growth regulating genes and increased HBx protein have all been implicated in the development of HCC[33] (Figure 1).
Current therapies available to treat CHB include interferon and NAs: lamivudine, adefovir, entecavir, telbivudine, tenofovir disoproxil fumarate and the recently FDA-approved tenofovir alafenamide. While interferon works through immune modulation and has a weak antiviral effect, the NAs inhibit viral replication through direct inhibition of viral reverse transcriptase. The goal of these antiviral medications is to improve quality of life and survival by preventing the progression of CHB and development of cirrhosis and HCC. Currently the treatment objectives are categorized as shown in Table 1. While a “functional cure” is defined as the loss of hepatitis B surface antigen (HbsAg) and/or seroconversion to antibody to hepatitis B surface antigen with undetectable serum HBV DNA, it is important to remember that this is not a complete cure[19]. This complete cure is what is desperately needed to end the persistent risk for HCC.
| HBsAg | Anti-HBs | Viremia | cccDNA | |
| Functional cure | - | + | - | + |
| Complete cure | - | + | - | - |
While a complete cure is not yet available, several studies have assessed the impact of NAs on disease progression and HCC development. A randomized controlled trial by Liaw et al[7] randomized patients with advanced fibrosis or cirrhosis secondary to CHB to receive daily Lamivudine or placebo for up to 5 years. HCC occurred in 17/436 (3.9%) of patients treated with Lamivudine and 16/215 (7.4%) of patients who received placebo (P = 0.047). After a median treatment duration of 32.4 mo, the incidence of HCC was significantly reduced in the Lamivudine group and the study was stopped[7].
A retrospective study by Eun et al[8] conducted from March 1997 to February 2005 also showed a decreased incidence of HCC with use of lamivudine in patients with chronic HBV and compensated cirrhosis. HCC occurred in 4.9% of patients in the group treated with Lamivudine with sustained viral suppression compared to 25% of patients in the untreated group.
Similar results have also been shown with newer antivirals such as entecavir and tenofovir. Hosaka et al[9] assessed the risk of HCC in patients with CHB treated with entecavir compared to a control group of treatment-naïve patients. After 5 years of treatment, patients treated with entecavir had a cumulative HCC incidence of 3.7% compared to 13.7% in the treatment-naïve group (P < 0.001). Similarly, Kim et al[10] examined the incidence of HCC in patients treated with tenofovir disoproxil fumerate (TDF). Patients with CHB, including those with cirrhosis, were treated with TDF and assessed for incidence of HCC. The investigators found that there was a decreased incidence of HCC noted by the third year of treatment with TDF compared to the predicted incidence.
Since the first antiviral drug for HBV became available, antiviral therapy has been used in patients following surgical resection or local ablative therapy for HBV-associated HCC. Longer survival with concomitant antiviral therapy following tumor ablation has been observed in a number of studies conducted in Japan, Hong Kong, Taiwan and China since 2007. Similar observations of improved survival were first reported in the United States in 2011[34]. A meta-analysis conducted by Yuan et al[11] reviewed sixteen of these studies in an effort to investigate the effect of antiviral therapy after curative therapy in patients with HBV-associated HCC. The papers in the analysis included four studies from China, three from Hong Kong, one from Taiwan, six from Japan and one from the United States. The meta-analysis looked at the impact of NAs on the one and three year recurrence rates, as well as one, three and five year overall survival and disease-free survival rates. The results revealed that NAs significantly reduced the recurrence of HBV-related HCC after curative therapy and improved both overall survival and disease-free survival[11].
Liu et al[12] also conducted a meta-analysis to assess the effect of adjuvant antiviral therapy following hepatectomy and/or percutaneous ablation for HBV-related HCC. They looked at twenty-one studies that compared antiviral therapy with placebo or no treatment. The primary end-points assessed were recurrence-free survival and overall survival. While this meta-analysis did have some limitations including significant between-study heterogeneity and most of the studies being observational in nature, NAs did show a significant improvement in prognosis after curative treatment in patients with HBV-associated HCC[12].
While overall incidence of HCC has reduced with antiviral therapy, HCC can still develop despite successful viral suppression, most commonly in patients with cirrhosis. The most commonly reported cases of HBV-associated HCC developed within five to ten years despite antiviral therapy[13-15]. However, recent observations at the Liver Disease Prevention Center, Division of Gastroenterology and Hepatology of Thomas Jefferson University Hospital have demonstrated development of HBV-associated HCC even after a decade of successful viral suppression[16,17]. Patients from these observations were maintained on antiviral treatment anywhere from 5.2-18.4 years with undetectable serum HBV DNA for up to 12.4 years before HCC development. Due to close monitoring and follow up, most of the tumors were discovered early and were ablated successfully (Table 2)[17].
| Patient | Date start Tx | Chang in child class on Tx | Date HCC Dx | Years on anti-HBV Tx at HCC DX | Years with HBV DNA (-) | Age (yr) at HCC Dx | Size (cm) and site of HCC | HBV DNA at HCC Dx | Anti-HBV Tx | Status |
| 1 | 4/1998 | B→A | 7/2007 | 9.3 | 3.41 | 53 | 1.1 Junction | UD | LAM + TDF | Alive |
| 2 | 6/2002 | A→A | 8/2007 | 5.2 | 4.7 | 70 | 1.0 Rt | UD | LAM + TDF | Alive |
| 3 | 1/1998 | B→A | 3/2008 | 10.2 | 8.2 | 68 | 2.8 × 2.5 | UD | LAM + TDF | Dead |
| 4 | 5/1998 | A→A | 2/2008 | 9.8 | 6.7 | 76 | 1.8 × 0.9 Lt | UD | LAM + TDF | Alive |
| 5 | 7/2004 | B→B | 9/2009 | 5.2 | 4.7 | 52 | 3.9 Rt | UD | LAM + TDF | Alive |
| 6 | 7/2001 | B→B | 9/2010 | 9.2 | 4.1 | 54 | 2.8 Rt | UD | LAM + TDF | Dead |
| 7 | 2/2004 | A→A | 6/2013 | 9. 3 | 7.7 | 57 | 2.5 Lt med | UD | TDF | Dead |
| 8 | 2/1996 | A→A | 7/2013 | 17.4 | 9.7 | 73 | 1.6 × 1.4 Rt | UD | TDF | Dead |
| 9 | 8/1997 | A→A | 6/2014 | 16.8 | 5.9 | 54 | 2.2 × 1.9 Lt lat | UD | ETV | Alive |
| 10 | 5/1996 | A→A | 10/2014 | 18.4 | 10.4 | 74 | 3.4 Rt | UD | LAM + TDF | Dead |
| 11 | 2/2000 | A→A | 4/2015 | 15.2 | 12.4 | 62 | 3.4 × 3.4 Rt | UD | TDF | Alive |
| 12 | 2/2000 | B→A | 5/2015 | 15.3 | 12.2 | 65 | 3.8 Rt | UD | TDF | Alive |
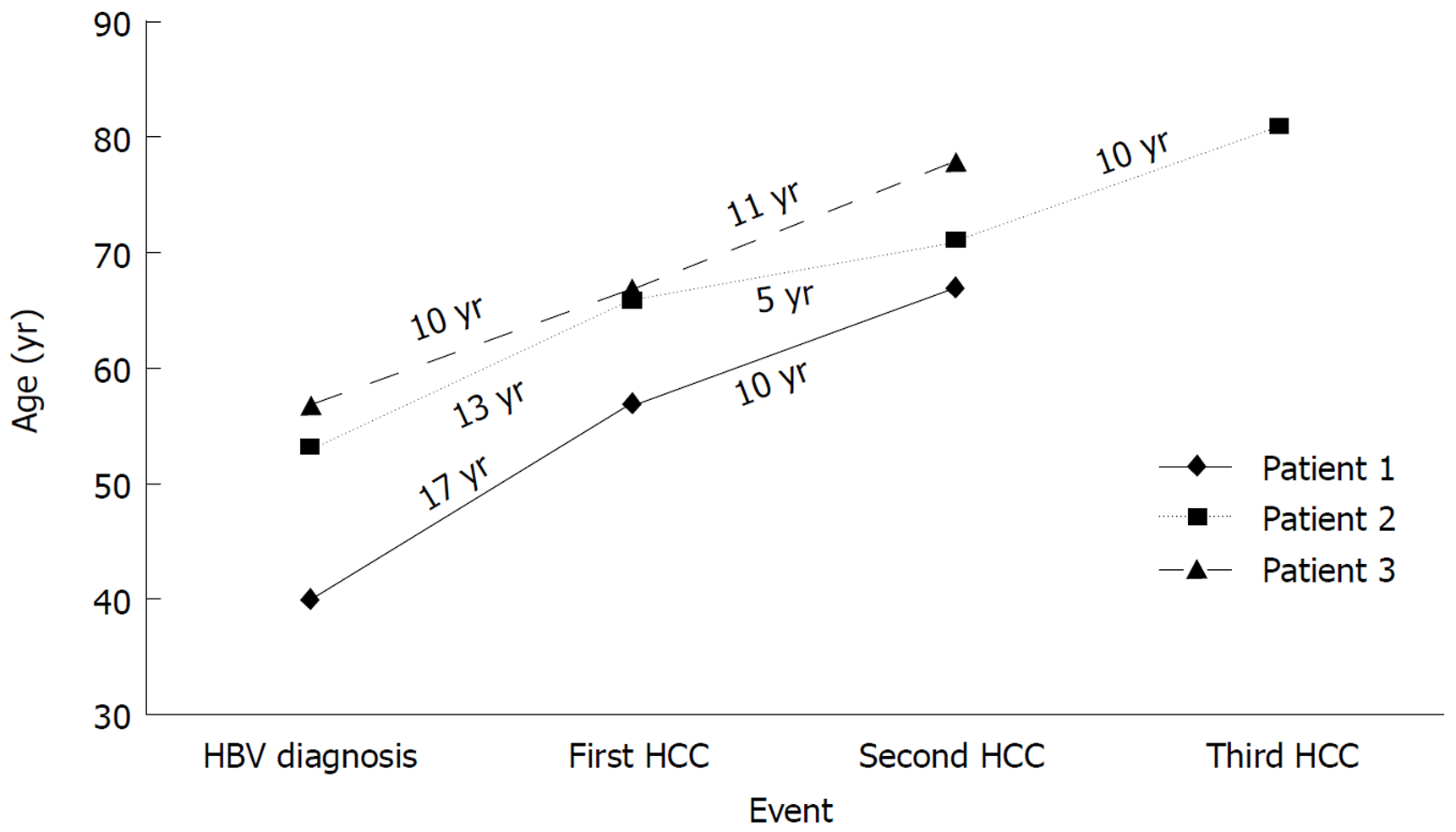
After resection or ablation of the initial HCC, the patients were continued on antiviral therapy and maintained successful viral suppression. However, the persistent risk for subsequent new and recurrent HCC in this patient population has been observed. We recently described three cases of subsequent new and recurrent HCC in patients who underwent successful initial tumor ablation and had suppression of serum HBV DNA for years. Figure 2 depicts these three patients and shows the time from their HBV diagnosis to the initial HCC and eventual development of subsequent new and recurrent HCC. One of these patients described in Figure 3 was diagnosed as HbsAg (+) in 1987. She did not receive antiviral therapy as it was not available until after 1998. In 2000, thirteen years later, she was diagnosed with HCC. Following successful transarterial chemoembolization (TACE) and radiofrequency ablation she was maintained on antiviral therapy for five years at which point she was found to have a 1.1 cm × 0.8 cm arterially enhancing lesion with washout appearance near the previously treatment site on abdominal magnetic resonance imaging consistent with recurrent HCC. She underwent TACE and was maintained on antiviral therapy for another ten years at which point she developed another 0.8 cm recurrent HCC despite an undetectable serum HBV DNA[18].
Without eradication of cccDNA from infected hepatocytes, HBV continues to carry the risk of development of HCC despite functional cure being achieved as described above. As we become more aware of the persistent risk for new, subsequent new and recurrent HCC, we become more aware of our need for a cure for HBV infection. Antiviral therapy is able to control viral replication and slow the progression of liver disease in the majority of treated patients, but the persistence of cccDNA and continual suppression of the innate and adaptive host immune response causes a persistent risk of hepatocarcinogenesis. Several steps in the HBV replication cycle have been identified as potential targets for future therapies. These therapies can be separated into four categories based on their target of action: (1) complete inhibition of HBV replication; (2) restoration of host innate immunity and adaptive anti-HBV T and B cell responses; (3) selective sensitization of HBV infected hepatocytes to immune elimination; and (4) direct targeting of cccDNA[21].
Complete inhibition of HBV replication: The first category, complete inhibition of HBV replication, should have in theory already been achieved with existing NAs. However, complete inhibition of HBV replication is not achieved with NAs alone since inhibition of DNA polymerase is only one step of the entire HBV replication cycle. If this had been achieved, we would see elimination of HBV DNA and even cccDNA as infected hepatocytes are replaced. In addition to direct inhibition of HBV DNA polymerase, other steps in the replication cycle are being targeted. Entry inhibitors are being investigated and work through targeting the NTCP receptor preventing de novo infection of hepatocytes as well as the spread of the virus from infected hepatocytes to non-infected hepatocytes. Capsid inhibitors are also being investigated and have the potential to work at several different steps in the HBV replication cycle where the capsid is essential including genome packaging, reverse transcription, intracellular trafficking and re-importation into the nucleus. These therapies have the potential to augment the action of NAs and lead to complete inhibition of HBV DNA[19].
Restoration of host innate and adaptive immunity: In regards to restoration of host innate and adaptive immune responses, several targets have been identified. However, the challenge of stimulating an antiviral immune-mediated response without triggering detrimental anti-HBV flares is difficult. Inhibiting HBV gene expression has been investigated with RNA interference and nucleic acid polymers. Checkpoint inhibitors such as anti-PD-1 monoclonal antibodies currently used in oncology are also being investigated. In HBV, the HBV-specific CD8+ T cells express high levels of inhibitory molecules so inhibition of these checkpoint molecules can rescue HBV-specific CD8+ T cells. Engineering HBV-specific T cells through transfer of HBV-specific T cell receptors and therapeutic vaccines aimed at stimulating HBV-specific T cell immunity in patients chronically infected with HBV are also under investigation. Finally, toll-like receptor agonists are being developed to help recognize the virus and subsequently stimulate production of interferon-alpha and other cytokines in an effort to activate natural killer cells and other lymphocytes[21].
Immune elimination of HBV infected hepatocytes: The third category of therapies under investigation includes medications that target cellular inhibitor of apoptosis proteins (cIAPs). These proteins have been found to attenuate TNF signaling during HBV infection which leads to restricted death of infected hepatocytes. This lack of infected hepatocyte death leads to viral persistence. Birinapant, a specific therapy currently being investigated in preclinical models, antagonizes cIAP and has shown anti-HBV activity[21].
Direct Targeting of cccDNA: The final category of potentially curative HBV therapies under investigation involves direct targeting of cccDNA. There are several strategies to target cccDNA which include inhibition of cccDNA formation, destruction of cccDNA and functional silencing of cccDNA through targeting proteins involved in cccDNA transcription and/or stability. Given the knowledge we have regarding persistent cccDNA and impaired immune responses in patients with CHB, it is likely that a combination of these newly investigated therapies will need to be used in order to achieve complete cure[21].
The ultimate goal of HBV treatment is to prevent the development of HCC and death from the virus. Of utmost importance is successful eradication of the transcriptionally active HBV cccDNA from hepatocyte nuclei. While we currently aim for a functional cure with loss of HBsAg and undetectable viral DNA, a complete cure can only be attained with eradication of cccDNA. The several concerns regarding the unpredictable nature of HCC development in patients with CHB shows the need for a cure and we should continue to support and encourage research efforts aimed at developing curative therapies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Ponti F, Pellicano R S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
| 1. | Blumberg BS, Alter HJ, Visnich S. A "new" antigen in leukemia sera. JAMA. 1965;191:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 732] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9582] [Article Influence: 737.1] [Reference Citation Analysis (0)] |
| 3. | MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Hepatitis B, 2016. Geneva: World Health Organization 2016; . |
| 5. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2507] [Article Influence: 192.8] [Reference Citation Analysis (2)] |
| 6. | Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15 Suppl 4:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J; Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 8. | Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kobayashi M, Kumada H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 542] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 10. | Kim WR, Berg T, Loomba R. Long Term Tenofovir Disproxil Fumarate (TDF) Therapy and the Risk of Hepatocellular Carcinoma. J Hepatol. 2013;58:S19. |
| 11. | Yuan P, Chen P, Qian Y. Evaluation of Antiviral Therapy Performed after Curative Therapy in Patients with HBV-Related Hepatocellular Carcinoma: An Updated Meta-Analysis. Can J Gastroenterol Hepatol. 2016;2016:5234969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Liu GM, Huang XY, Shen SL, Hu WJ, Peng BG. Adjuvant antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after curative treatment: A systematic review and meta-analysis. Hepatol Res. 2016;46:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, Vasiliadis T, Mimidis K, Gogos C, Ketikoglou I, Manesis EK; HEPNET. Greece Cohort Study Group. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 15. | Vlachogiannakos J, Papatheodoridis G. Hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. World J Gastroenterol. 2013;19:8822-8830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Dargan A, Wong SY, Coben R, Conn M, Dimarino AJ, Hann HW. Persistent risk for hepatocellular carcinoma after more than a decade of successful hepatitis B virus suppression. Minerva Gastroenterol Dietol. 2017;63:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Yoo J, Hann HW, Coben R, Conn M, DiMarino AJ. Update Treatment for HBV Infection and Persistent Risk for Hepatocellular Carcinoma: Prospect for an HBV Cure. Diseases. 2018;6:E27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Shinn BJ, Kistler C, Roth C, Hann HW. Need for HBV Cure: persistent Risk for Subsequent New and Recurrent HCC Even After a Decade of Successful Anti-HBV Therapy and Initial Tumor Ablation. Arch Cancer Res. 2018;6:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, Michel ML, Autran B, Cosset FL, Strick-Marchand H, Trépo C, Kao JH, Carrat F, Lacombe K, Schinazi RF, Barré-Sinoussi F, Delfraissy JF, Zoulim F. Towards an HBV cure: state-of-the-art and unresolved questions--report of the ANRS workshop on HBV cure. Gut. 2015;64:1314-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Zoulim F, Lebossé F, Levrero M. Current treatments for chronic hepatitis B virus infections. Curr Opin Virol. 2016;18:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Levrero M, Testoni B, Zoulim F. HBV cure: why, how, when? Curr Opin Virol. 2016;18:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Gurtsevitch VE. Human oncogenic viruses: hepatitis B and hepatitis C viruses and their role in hepatocarcinogenesis. Biochemistry (Mosc). 2008;73:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Franceschi S, Montella M, Polesel J, La Vecchia C, Crispo A, Dal Maso L, Casarin P, Izzo F, Tommasi LG, Chemin I, Trépo C, Crovatto M, Talamini R. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Matsubara K, Tokino T. Integration of hepatitis B virus DNA and its implications for hepatocarcinogenesis. Mol Biol Med. 1990;7:243-260. [PubMed] |
| 25. | Fourel G, Trepo C, Bougueleret L, Henglein B, Ponzetto A, Tiollais P, Buendia MA. Frequent activation of N-myc genes by hepadnavirus insertion in woodchuck liver tumours. Nature. 1990;347:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 184] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Popper H, Roth L, Purcell RH, Tennant BC, Gerin JL. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1987;84:866-870. [PubMed] |
| 27. | Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 868] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1600] [Article Influence: 123.1] [Reference Citation Analysis (1)] |
| 29. | Yan H, Peng B, Liu Y, Xu G, He W, Ren B, Jing Z, Sui J, Li W. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J Virol. 2014;88:3273-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1700] [Cited by in RCA: 1712] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 31. | Murakami Y, Saigo K, Takashima H, Minami M, Okanoue T, Bréchot C, Paterlini-Bréchot P. Large scaled analysis of hepatitis B virus (HBV) DNA integration in HBV related hepatocellular carcinomas. Gut. 2005;54:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013;33:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Xu HZ, Liu YP, Guleng B, Ren JL. Hepatitis B Virus-Related Hepatocellular Carcinoma: Pathogenic Mechanisms and Novel Therapeutic Interventions. Gastrointest Tumors. 2014;1:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 34. | Hann HW, Bergin D, Coben R, DiMarino AJ. Prevention of new hepatocellular carcinoma with concomitant antiviral therapy in chronic hepatitis B patients whose initial tumor was successfully ablated. Int J Cancer. 2011;128:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |