Revised: April 10, 2009
Accepted: April 17, 2009
Published online: October 31, 2009
AIM: To evaluate the ability of 18F-fluorodeoxyglucose positron emission and computed tomography (18F-FDG PET/CT) in restaging of hepatocellular carcinoma (HCC) after treatment.
METHODS: We reviewed a database of the diagnostic performance of 18F-FDG PET/CT scan for patients with HCC following local or regional treatment. The database consisted of 18F-FDG PET/CT information of 21 male and 4 female (age range, 27-81 years; mean age, 51.6 years) patients who had received surgical resection and/or interventional treatments and then underwent 18F-FDG PET/CT scan. All patients had received enhanced CT scan of the liver two weeks before or after the 18F-FDG PET/CT scan. Intrahepatic recurrence and/or extrahepatic metastases were confirmed by histological analysis or clinical and imaging follow-up. The accuracy of 18F-FDG PET/CT study was determined by histopathological results or by clinical and imaging follow-up.
RESULTS: 18F-FDG PET/CT was abnormal in 19 of the 25 (76.0%) patients. In detecting HCC recurrence, 18F-FDG PET/CT scored 17 true positives, 5 true negatives, 2 false positives and 1 false negative. The sensitivity, specificity and accuracy of 18F-FDG PET/CT in detecting HCC recurrence was 89.5%, 83.3% and 88%, respectively. 18F-FDG PET/CT had an impact on management of these patients by settling the problem of an unexplained increase in alpha-fetoprotein after treatment (14 patients), by monitoring response to the treatment and guiding additional regional therapy (12 patients), by identifying extrahepatic metastases (10 patients), by identifying tumor growth or thrombosis in the portal vein (6 patients), or by guiding surgical resection of extrahepatic metastases (2 patients).
CONCLUSION: Our results suggest that whole body 18F-FDG PET/CT may be useful in the early evaluation of residual, intrahepatic recurrent or extrahepatic metastatic lesions and able to provide valuable information for the management of HCC recurrence.
- Citation: Sun L, Guan YS, Pan WM, Luo ZM, Wei JH, Zhao L, Wu H. Metabolic restaging of hepatocellular carcinoma using whole-body 18F-FDG PET/CT. World J Hepatol 2009; 1(1): 90-97
- URL: https://www.wjgnet.com/1948-5182/full/v1/i1/90.htm
- DOI: https://dx.doi.org/10.4254/wjh.v1.i1.90
Hepatocellular carcinoma (HCC) is one of the most common solid malignancies worldwide, with up to 1 million new cases per year. Its mortality is second to lung cancer in urban and to gastric carcinoma in rural regions of China[1,2]. Surgical treatments, including hepatic resection and liver transplantation, are considered as the most effective treatment of HCC. However, less than 20% of HCC patients can be treated surgically[3]. Interventional treatments have been applied to patients with inoperable HCC[4,5]. Despite initial remission of HCC, after surgical and interventional treatments, recurrence is common. Since patients with recurrent HCC may be amenable to potentially curative resection, early detection of intrahepatic recurrence and/or extrahepatic metastases is extremely important and can facilitate successful retreatment at an early stage. Late diagnosis makes retreatment difficult[6,7].
Conventional computed tomography (CT) and magnetic resonance imaging (MRI) are the main techniques that are used during the follow-up of HCC. However, these techniques may not be reliable enough in detecting residual, recurrent or metastatic lesions[8,9]. The reported increase in sensitivity of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) over CT and magnetic resonance imaging (MRI) has been attributed to the ability of 18F-FDG PET/CT to detect metabolic abnormalities that precede the morphological changes seen by CT[10]. This study was undertaken to further define the usefulness of 18F-FDG PET/CT imaging in evaluating residual, intrahepatic recurrent or extrahepatic metastatic lesions of HCC after primary treatment.
During the period from January 2007 to Oct July 2008, thirty 18F-FDG PET/CT scans were performed in 25 patients (age range, 27-81; 21 men and 4 women), who had undergone both/either surgical resection and/or interventional therapy for HCC. These patients, with/without suspicious intrahepatic recurrence and/or extrahepatic metastases in conventional imaging or according to clinical findings, were retrospectively enrolled in our study. All patients had received enhanced CT scan of the liver during the two weeks preceding or following the 18F-FDG PET/CT scan. The standard for the ultimate diagnosis of tumor recurrence consisted of histopathological confirmation or of clinical and imaging follow-up information for at least 12 mo after the PET/CT examination.
Patients were asked to fast for at least 4 h before undergoing 18F-FDG PET/CT examination. Their blood glucose level had to be within the normal range (70-120 mg/dL) prior to the intravenous injection of 18F-FDG with a radiation dosage within 370 and 666 MBq (10-18 mCi). Data acquisition was performed within 60 min after the injection with an integrated PET/CT system (Discovery STE; GE Medical Systems, Milwaukee, WI, USA). The procedure of data acquisition was as follows: the CT scan was performed covering the patient from the head to the pelvic floor, with 110 kV, 110 mA, tube rotation time of 0.5 s, and 3.3-mm section thickness, which was matched to the PET section thickness. Immediately after CT scanning, a PET emission scan, covering the same transverse field of CT view, was obtained. Acquisition time was 3 min per table position. PET image data sets were reconstructed iteratively by applying the CT data for attenuation correction. Finally, coregistered images were displayed on a workstation.
Reviewer 1 and reviewer 2, who were aware of the clinical and other imaging data, read the 18F-FDG PET/CT images on a high-resolution computer screen. The reviewers reached a consensus in cases of discrepancy. Reviewer 1 had 21 years of experience in both nuclear medicine and radiology, and reviewer 2 had 6 years of experience in both expertises. If a focus within the liver had hypermetabolic activity greater than that of the adjacent normal liver tissue, it was considered intrahepatic recurrence. 18F-FDG PET/CT scan was considered positive or suspiciously positive for metastases whenever abnormal non-physiologic metabolic activity was identified at extrahepatic sites. Diffuse mild activity in the intestinal tract was considered normal physiologic uptake. Quantification of tumor metabolic activity was obtained using the Standardized Uptake Value (SUV) normalized to body weight. mean ± SD of maximum-pixel SUV (SUVmax) of the lesions were calculated.
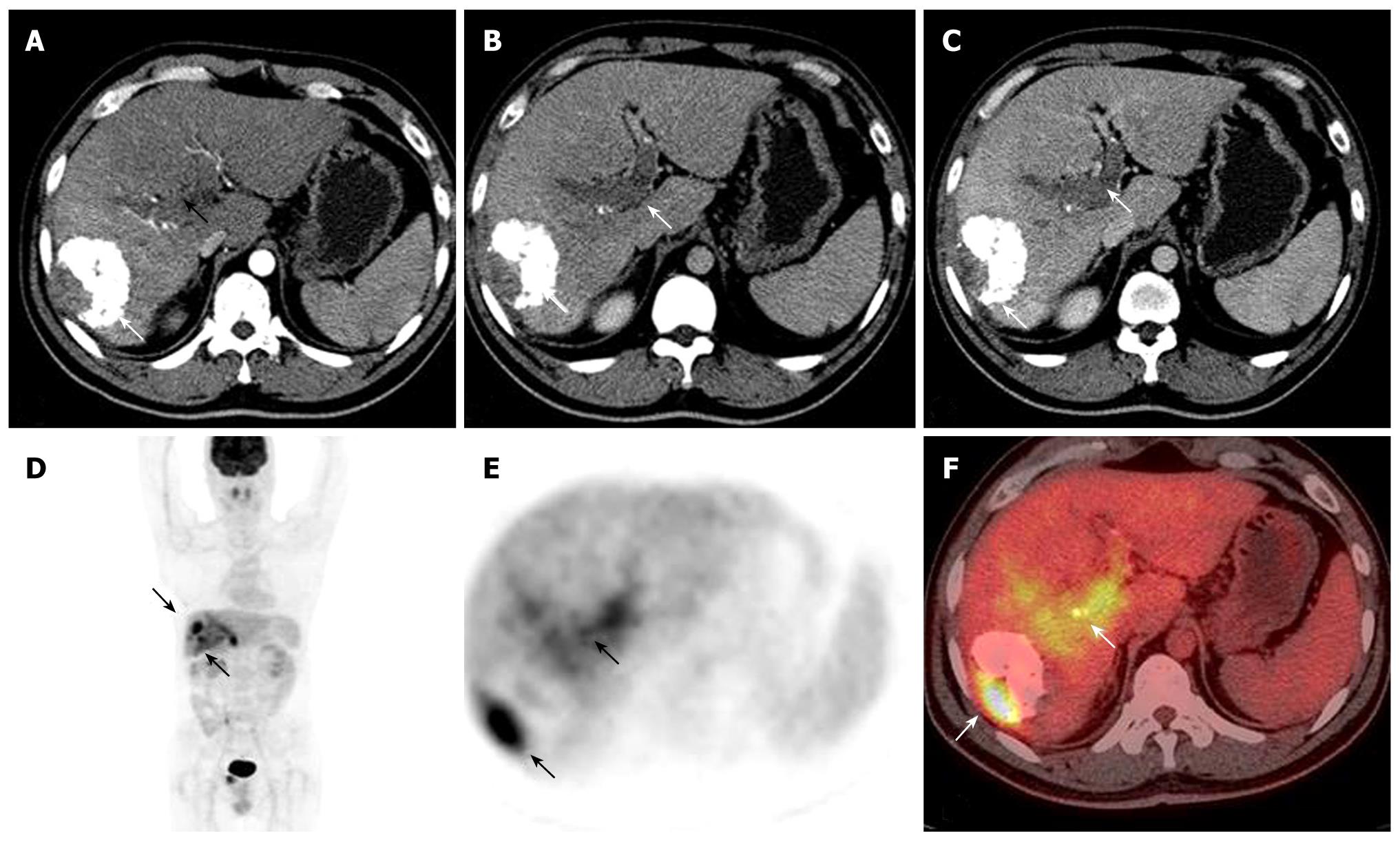
The characteristics of the patients are shown in Table 1. Mean time after treatment to PET/CT exam was 13 mo (10 d-12 year) and mean follow up time after PET/CT exam was 12 mo. At the time of intrahepatic recurrence and extrahepatic metastases being suspected, the mean patients’ age was 51.6 years with a tendency to a preponderant male gender distribution (84%). The suspicion of intrahepatic recurrence and/or extrahepatic metastases leading to a 18F-FDG PET/CT was based on an unexplained increase in alpha-fetoprotein (AFP) (n = 14) (Figure 1), suspected intrahepatic recurrence routine follow-up after TACE (n = 8), or suspected extrahepatic metastases at CT or MRI (n = 7). The features of recurrent HCC after treatment are summarized in Table 2.
| Clinical characteristics | Data |
| Mean age (yr) | 51.6 (range 27-81 yr) |
| Gender | |
| Males | 21 |
| Females | 4 |
| Prior therapy | |
| TACE | 6 |
| Liver resection | 9 |
| TACE after surgery | 8 |
| TACE + RFA or PEI | 2 |
| Unexplained AFP increase after treatment | 14 |
| Mean time from treatment to recurrence | 11.5 mo |
| Mean time after treatment to PET/CT exam | 10 d - 12 yr; mean 13 mo |
| Mean follow up time after PET/CT exam | 3 mo - 21 mo; mean 12 mo |
| Characteristics | n |
| Intrahepatic recurrence | 8 |
| Intrahepatic recurrence and extrahepatic metastases | 5 |
| Extrahepatic metastases without intrahepatic recurrence | 4 |
| Single intrahepatic recurrence | 7 |
| Multiple intrahepatic recurrence | 8 |
| Lung | 4 |
| Bone | 1 |
| Omentum and mesentery | 3 |
| Retroperitoneal lymph nodes | 2 |
| Adrenal | 1 |
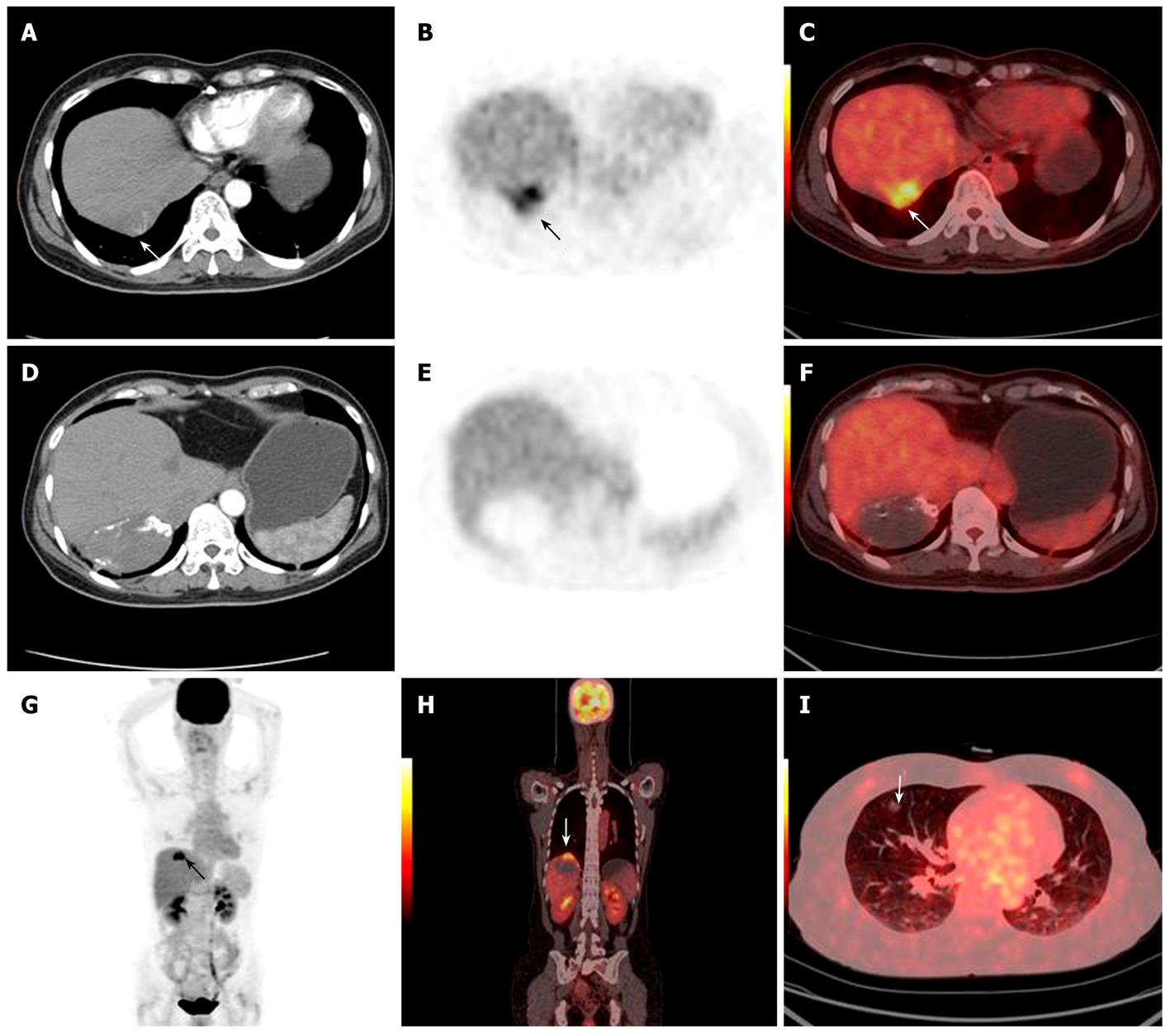
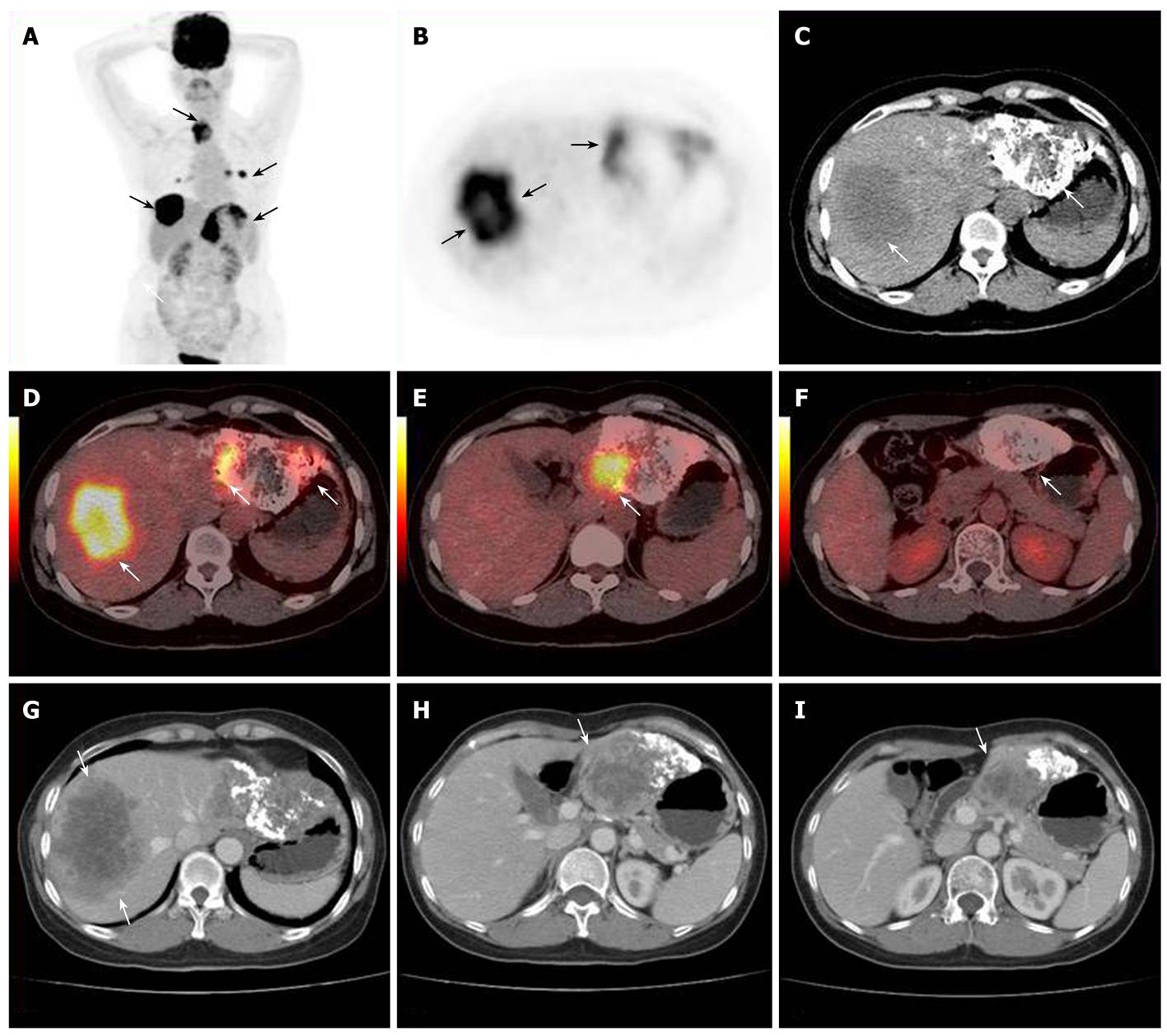
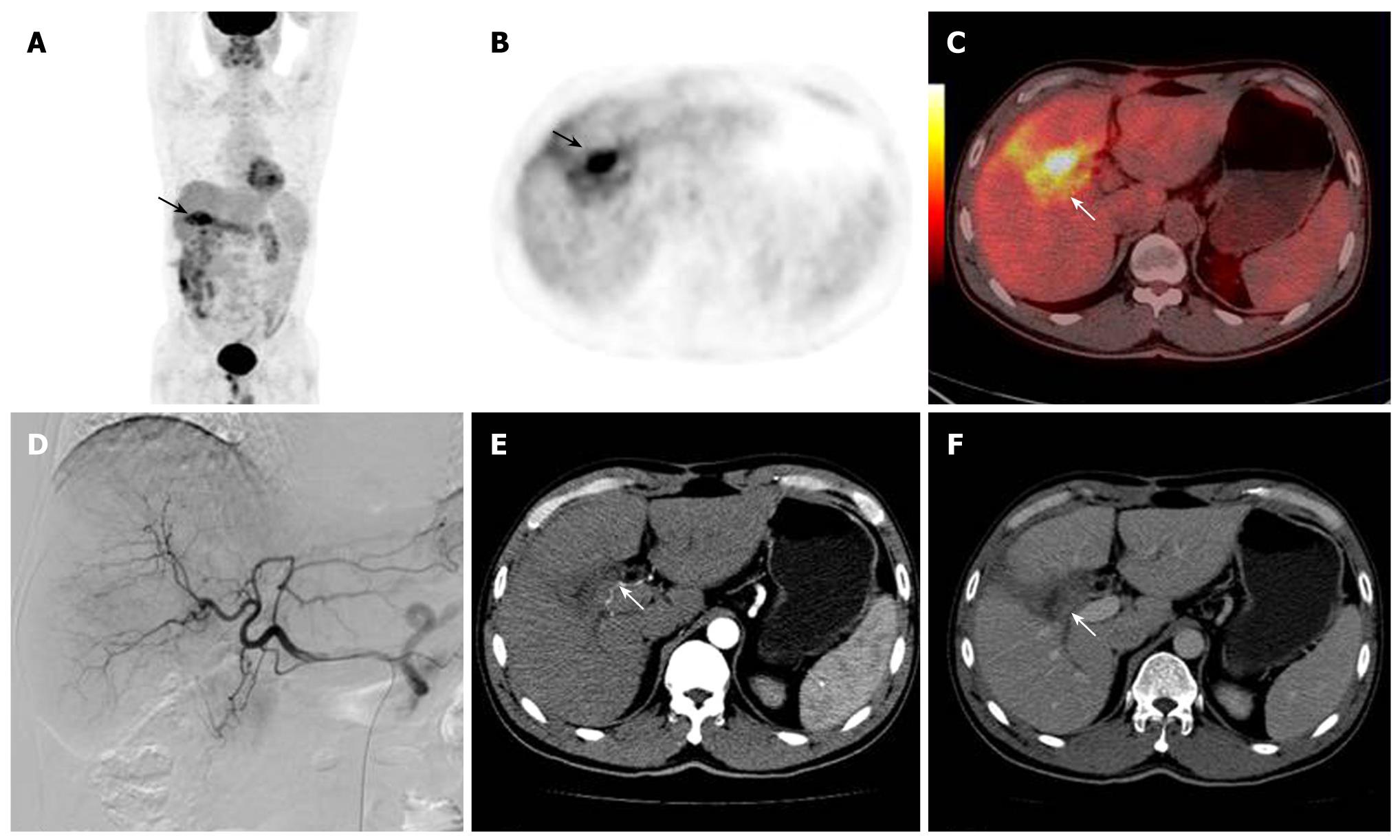
The accuracy of 18F-FDG PET/CT detection was determined by histopathological examination or based on other clinical evidence. The 18F-FDG PET/CT of the 25 cases of HCC with a post-treatment follow-up lead to 17 true positive (Figures 2 and 3), 1 false negative, 5 true negative and 2 false positive results. The 2 false positive PET/CT findings were later verified as post-operative inflammation (Figure 4). Regarding the single patient with a false negative PET/CT, the final diagnosis was low-level metabolism metastasis of the left adrenal gland.
The sensitivity, specificity and accuracy levels of the 18F-FDG PET/CT in detecting post-treatment HCC recurrence were 89.5%, 83.3% and 88%, respectively. 18F-FDG PET/CT imaging had an impact on management of these patients, by solving the problem of an unexplained increase in AFP after treatment (14 patients), by monitoring the response to treatment and guiding additional regional therapy (12 patients), by identifying extrahepatic metastases (10 patients), by identifying tumor growth or thrombosis in the portal vein (6 patients), or by guiding surgical resection of extrahepatic metastases (2 patients).
Of the cases with positive post-treatment findings, 90% occurred within the first 1 year, while less than 9% occurred after 2 years. Positive findings were frequently detected as both intrahepatic lesions and extrahepatic metastases.
18F-FDG PET/CT study was performed in 14 patients, who had undergone either surgical resection or interventional therapy for HCC, but who had subsequently developed increasing serum levels of AFP during routine follow-up. In 5 of these patients, 18F-FDG PET/CT was carried out due to suspected disease recurrence upon liver enhanced CT scan. The remaining 9 had high post-treatment AFP serum levels during routine follow-up, but liver enhanced CT scan and physical examinations were normal. 18F-FDG PET/CT findings were abnormal in 11 of the 14 patients with unexplained increase in AFP after treatment. Intrahepatic lesions were detected in 8 and extrahepatic metastases were found in 3 patients with abnormal PET/CT findings.
Mortality due to HCC ranks as the second highest in cancer-related deaths nationwide and its morbidity is increasing among the male population[11]. In the past decades, numerous non-surgical interventional therapies have been continuously introduced as a result of the technical developments of locoregional approaches for HCC. The therapy consisting in surgical resection combined with interventional treatment has played an important role in the treatment of HCC[12]. However, intrahepatic recurrence and extrahepatic metastases are still the major obstacles to improve further the prognosis of HCC. The recurrence rate of intrahepatic carcinomas varies from 36.8% to 82%, while 30% of the recurrences are extrahepatic[13]. The 5-year recurrence rates are, respectively, 38% and 61.5%, while the 5-year disease-free survival are 16% or 38.6% after curative resection; overall, recurrent HCC is the single most important cause of death after treatment[14].
Despite the high recurrence rate of HCC, long-term survival can be achieved after treatment by early detection. This is critical, because recurrences confined to the liver may be amenable to treatment with an additional survival benefit. Surgical resection for isolated extrahepatic recurrence of HCC is also recommended to increase long-term survival[15,16]. So far, contrast enhanced CT or MRI are considered as the most sensitive test for assessing therapeutic efficacy. These imaging examinations play a crucial role in the follow-up of HCC treated by surgical resection and interventional procedures, since local treatment efficacy, recurrent disease and some of therapy-induced complications can be evaluated[17,18]. However, CT and MRI have both advantages and disadvantages in the evaluation of local treatment efficacy, recurrent disease and some of the therapy-induced complications, while local CT or MRI have also limitations in the detection of extrahepatic HCC[19,20].
Major advantages of whole body 18F-FDG PET/CT are the capability to perform full-body examinations, the potential to detect intrahepatic recurrence and extrahepatic metastases in one single examination and the possibility of distinguishing new active disease from scar or necrotic tissue[21]. The whole-body nature of the 18F-FDG PET/CT study also contributes to the increased sensitivity through detection of distant metastatic lesions[22]. Tumors with increased 18F-FDG uptake are more metabolically active and biologically aggressive. The degree of 18F-FDG uptake and its distribution within the tumor and the surrounding hepatic parenchyma could provide useful information for specifying the degree of tumor necrosis and working out strategies for subsequent additional locoregional therapy[23,24].
AFP is a useful marker for monitoring treatment effects on HCC patients. It is considered a relatively specific test for HCC among high-risk patients, particularly those with advanced disease and with levels tending to increase in follow-up examinations. However, measurements of AFP have been demonstrated to have limited value for detecting recurrence at an early stage. In addition, elevated AFP level may also be transiently present in patients with cirrhosis and/or hepatitis[25,26]. In some patients with elevated serum level of AFP, no tumors can be detected by conventional enhanced CT and MRI scanning. Our results suggest that 18F-FDG PET/CT is a valuable imaging tool in patients who have rising AFP levels after HCC treatment, even when conventional examinations are normal. Whole-body 18F-FDG PET/CT scan provides also an important and valuable imaging study for detecting extrahepatic metastases.
Iodized oil is widely used in transcatheter arterial chemoembolization (TACE). It is difficult to completely kill the tumor cells with TACE in only one session, and it is very important to objectively assess the viability and necrosis of the tumors after TACE, in order to assess the indication to additional treatment sessions, with the aim to improve the general therapeutic effects and the survival rate[27]. Generally, the CT follow-up of patients treated with TACE alone could be affected by artifacts produced by high local concentrations of lipiodol, making it difficult to evaluate the characteristics of the lesion. On the other hand, the homogeneous and complete deposition of lipiodol within the lesion would indicate a high degree of necrosis of the tumors: however, it is difficult to estimate correctly the viability and necrosis of the tumors in case of inhomogeneous deposition, because lipiodol-negative areas do not correspond to viable areas of the tumors[28,29]. On the contrary, whole-body 18F-FDG PET/CT scan may represent an important and valuable imaging tool to detect residual, intrahepatic metastatic or extrahepatic metastatic lesions. In particular, the capability of detecting residual lesions is not affected by high concentrations of lipiodol.
The same diagnostic criteria described above for lesions treated with TACE can be applied to assess the therapeutic responses to percutaneous ethanol injection (PEI) and radio-frequency ablation (RFA). The imaging appearances of the lesions after these two treatments are very similar. However, the absence of contrast enhancement within the ablated lesion at CT and MRI performed during follow-up after treatment cannot always indicate a successful treatment, as later follow-up studies may demonstrate tumor regrowth at the periphery of the ablated lesion[30]. 18F-FDG PET/CT is useful to assess residual HCC after treatment with RFA and to guide its further local treatment.
HCC carries a high risk of invasion of the portal vein. The detection and etiologic characterization of these thrombi are essential for treatment planning, particularly in patients with hepatic tumors, because malignant thrombosis is a negative prognostic factor. The management of HCC with portal vein tumor thrombosis is complicated and controversial. In our group, five consecutive patients with biopsy-proven HCC, and thrombosis of the main portal vein and/or left/right portal vein on ultrasound (US), CT or MRI were identified by 18F-FDG PET/CT. 18F-FDG PET/CT showed a highly metabolically active thrombus in 4 of the 5 patients. 18F-FDG PET/CT scored a true positive result in all 4 patients, and a true negative result in the other patient. No false positive results were observed using 18F-FDG PET/CT[31,32].
Resection of isolated extrahepatic HCC metastases has been advocated to obtain long-term survival. Accurate re-staging plays the most important role in making a decision on extrahepatic metastases resection. In our study, in a 60-year old woman with increasing AFP levels after HCC resection, conventional chest and abdominal CT did not find any evidence of intrahepatic recurrence or extrahepatic metastases. 18F-FDG PET/CT detected a lymph node with high metabolic activity at the level of the omentum. After excision of this lymph node, histopathological examination revealed a metastatic HCC and AFP level returned to normal. This case shows that 18F-FDG PET/CT may provide a highly significant, additional information regarding the accurate detection of extrahepatic spread of tumors and additional important information in patients with presumably resectable extrahepatic metastases[33]. This test should be used for patients at high risk of extrahepatic disease and should be evaluated prospectively in all patients considered for resection of intrahepatic recurrence and extrahepatic metastases[34].
The diagnostic contrast-enhanced multiphase CT, as part of the combined 18F-FDG PET/CT protocol, has the potential to provide considerable added value in specific clinical conditions leading, as a result, to a change in the management of a substantial proportion of patients. The greatest benefit of the diagnostic CT lies in the possibility of localizing a pathological FDG uptake and of correctly delineating the tumor boundaries, leading to changes in the interpretation of 18F-FDG PET/CT, at least in some patients.
Our study has some limitations. First, it was a retrospective analysis. Second, we recognize that not all of the extrahepatic lesions had evidence for metastatic HCC at biopsy. However, in a patient with a known HCC and with no other primary tumor, the development of a new lesion (e.g. a new bone lesion) or the increase over time of previously diagnosed extrahepatic lesions strongly suggest metastatic HCC. These criteria for metastatic disease are used by oncologists and surgeons in planning therapy.
In conclusion, High recurrence rates after treatment of HCC indicate that there is substantial room for improvement of current imaging strategies for the early detection of recurrent lesions. Whole body 18F-FDG PET/CT scan may provide valuable information for the early detection and guide salvage treatment for recurrent HCC.
Despite initial remission of hepatocellular carcinoma (HCC) after surgical and interventional treatments, recurrence is common. Since patients with recurrent HCC may be amenable to potentially curative resection, early detection of intrahepatic recurrence and/or extrahepatic metastases is extremely important and can facilitate successful retreatment at an early stage. Late diagnosis makes retreatment difficult. This study was undertaken to further define the usefulness of 18F-fluorodeoxyglucose positron emission and computed tomography (18F-FDG PET/CT) imaging in evaluating residual, intrahepatic recurrent or extrahepatic metastatic lesions of HCC after primary treatment.
18F-FDG PET and, particularly, 18F-FDG PET/CT are widely accepted imaging methods in the management of a wide variety of cancers. The reported increase in sensitivity of 18F-FDG PET/CT over CT and MRI has been attributed to the ability of 18F-FDG PET/CT to detect metabolic abnormalities that precede the morphologic changes seen by CT. However, the usefulness and limitations of 18F-FDG PET/CT in evaluating residual, intrahepatic recurrent or extrahepatic metastatic lesions of HCC after primary treatment still need further clinical evaluation.
Whole body 18F-FDG PET/CT was effective in detecting relapse in evaluating residual, intrahepatic recurrent or extrahepatic metastatic lesions of HCC after primary treatment and also had important clinical impacts on the management of recurrent HCC.
High recurrence rates after treatment of HCC indicate that there is substantial room for improvement of current imaging strategies for the early detection of recurrent lesions. Whole body 18F-FDG PET/CT scan could provide valuable information for early detection and might guide salvage treatment for recurrent HCC.
This is an interesting report of PET and PET/CT for HCC. Whereas it is of clinical significance, the presentation of the paper needs some modification. It deserves some comments for further consideration.
Peer reviewer: Bin Wang, Professor, PhD, MD, Deputy President of Weifang Medical University; Dean of School of Radiology, Director of Centre of Molecular Imaging, Chairman Medical Imaging Center, the Affiliated Hospital, Weifang Medical University, 7166 Baotong West Street, Weifang 261053, Shandong Province, China
S- Editor Zhang HN L- Editor Negro F E- Editor Ma WH
| 2. | Kudo M. Hepatocellular carcinoma 2009 and beyond: from the surveillance to molecular targeted therapy. Oncology. 2008;75 Suppl 1:1-12. |
| 3. | Alsowmely AM, Hodgson HJ. Non-surgical treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2002;16:1-15. |
| 4. | Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67-76. |
| 5. | Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Soll R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. |
| 6. | Zangos S, Eichler K, Balzer JO, Straub R, Hammerstingl R, Herzog C, Lehnert T, Heller M, Thalhammer A, Mack MG. Large-sized hepatocellular carcinoma (HCC): a neoadjuvant treatment protocol with repetitive transarterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT). Eur Radiol. 2007;17:553-563. |
| 7. | Vogl TJ, Eichler K, Zangos S, Mack M, Hammerstingl R. [Hepatocellular carcinoma: Role of imaging diagnostics in detection, intervention and follow-up]. Rofo. 2002;174:1358-1368. |
| 8. | Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, Neuhaus P, Felix R. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349-357. |
| 9. | Kamel IR, Bluemke DA, Eng J, Liapi E, Messersmith W, Reyes DK, Geschwind JF. The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2006;17:505-512. |
| 10. | Sun L, Wu H, Guan YS. Positron emission tomography/computer tomography: challenge to conventional imaging modalities in evaluating primary and metastatic liver malignancies. World J Gastroenterol. 2007;13:2775-2783. |
| 11. | Tang ZY. Hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15 Suppl:G1-G7. |
| 12. | Herber S, Schneider J, Brecher B, Hohler T, Thelen M, Otto G, Pitton MB. [TACE: therapy of the HCC before liver transplantation--experiences]. Rofo. 2005;177:681-690. |
| 13. | Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg. 2006;244:265-273. |
| 14. | Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Improved long-term survival after liver resection for hepatocellular carcinoma in the modern era: retrospective study from HCV-endemic areas. World J Surg. 2006;30:1567-1578. |
| 15. | Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330-339. |
| 16. | Verhoef C, Holman FA, Hussain SM, de Man RA, de Wilt JH, IJzermans JN. Resection of extrahepatic hepatocellular carcinoma metastasis can result in long-term survival. Acta Chir Belg. 2005;105:533-536. |
| 17. | Guan YS, Sun L, Zhou XP, Li X, Zheng XH. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol. 2004;10:3543-3548. |
| 18. | Guan YS, Hu Y, Liu Y. Multidetector-row computed tomography in the management of hepatocellular carcinoma with transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2006;21:941-946. |
| 19. | Iwazawa J, Ohue S, Mitani T, Abe H, Hashimoto N, Hamuro M, Nakamura K. Identifying feeding arteries during TACE of hepatic tumors: comparison of C-arm CT and digital subtraction angiography. AJR Am J Roentgenol. 2009;192:1057-1063. |
| 20. | Herber S, Biesterfeld S, Franz U, Schneider J, Thies J, Schuchmann M, Duber C, Pitton MB, Otto G. Correlation of multislice CT and histomorphology in HCC following TACE: predictors of outcome. Cardiovasc Intervent Radiol. 2008;31:768-777. |
| 21. | Langenhoff BS, Oyen WJ, Jager GJ, Strijk SP, Wobbes T, Corstens FH, Ruers TJ. Efficacy of fluorine-18-deoxyglucose positron emission tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol. 2002;20:4453-4458. |
| 22. | Ruf J, Lopez Hanninen E, Oettle H, Plotkin M, Pelzer U, Stroszczynski C, Felix R, Amthauer H. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266-272. |
| 23. | Barker DW, Zagoria RJ, Morton KA, Kavanagh PV, Shen P. Evaluation of liver metastases after radiofrequency ablation: utility of 18F-FDG PET and PET/CT. AJR Am J Roentgenol. 2005;184:1096-1102. |
| 24. | Chen YK, Hsieh DS, Liao CS, Bai CH, Su CT, Shen YY, Hsieh JF, Liao AC, Kao CH. Utility of FDG-PET for investigating unexplained serum AFP elevation in patients with suspected hepatocellular carcinoma recurrence. Anticancer Res. 2005;25:4719-4725. |
| 25. | Wudel LJ Jr, Delbeke D, Morris D, Rice M, Washington MK, Shyr Y, Pinson CW, Chapman WC. The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am Surg. 2003;69:117-124; discussion 124-126. |
| 26. | Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, Sato M, Yamanaka N, Shimamura Y, Ohto M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699-704. |
| 27. | Meyer BC, Frericks BB, Voges M, Borchert M, Martus P, Justiz J, Wolf KJ, Wacker FK. Visualization of hypervascular liver lesions During TACE: comparison of angiographic C-arm CT and MDCT. AJR Am J Roentgenol. 2008;190:W263-W269. |
| 28. | Herber S, Biesterfeld S, Franz U, Schneider J, Thies J, Schuchmann M, Duber C, Pitton MB, Otto G. Correlation of multislice CT and histomorphology in HCC following TACE: predictors of outcome. Cardiovasc Intervent Radiol. 2008;31:768-777. |
| 29. | Fujita T, Ito K, Tanabe M, Yamatogi S, Sasai H, Matsunaga N. Iodized oil accumulation in hypervascular hepatocellular carcinoma after transcatheter arterial chemoembolization: comparison of imaging findings with CT during hepatic arteriography. J Vasc Interv Radiol. 2008;19:333-341. |
| 30. | Gervais DA, Kalva S, Thabet A. Percutaneous image-guided therapy of intra-abdominal malignancy: imaging evaluation of treatment response. Abdom Imaging. 2009;34:593-609. |
| 31. | Sun L, Guan YS, Pan WM, Chen GB, Luo ZM, Wei JH, Wu H. Highly metabolic thrombus of the portal vein: 18F fluorodeoxyglucose positron emission tomography/computer tomography demonstration and clinical significance in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1212-1217. |
| 32. | Sun L, Wu H, Pan WM, Guan YS. Positron emission tomography/computed tomography with (18)F-fluorodeoxyglucose identifies tumor growth or thrombosis in the portal vein with hepatocellular carcinoma. World J Gastroenterol. 2007;13:4529-4532. |
| 33. | Sun L, Guan YS, Pan WM, Chen GB, Luo ZM, Wu H. Positron emission tomography/computer tomography in guidance of extrahepatic hepatocellular carcinoma metastasis management. World J Gastroenterol. 2007;13:5413-5415. |