Copyright
©The Author(s) 2015.
World J Hepatol. Dec 8, 2015; 7(28): 2792-2810
Published online Dec 8, 2015. doi: 10.4254/wjh.v7.i28.2792
Published online Dec 8, 2015. doi: 10.4254/wjh.v7.i28.2792
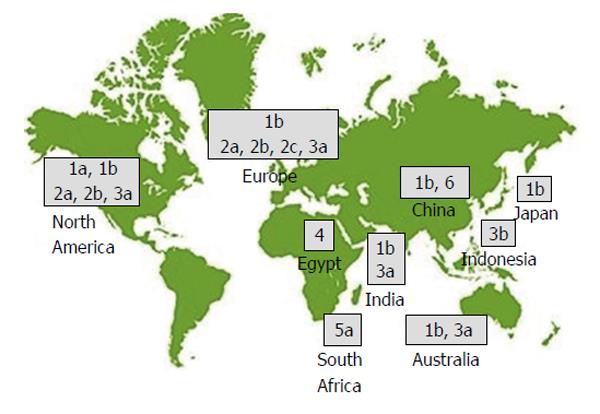
Figure 1 Global hepatitis C virus genotype distribution.
Johns Hopkins Hospital Division of Gastroenterology and Hepatology. Available from: URL: https://gi.jhsps.org/Upload/200710291122_15908_000.jpg.
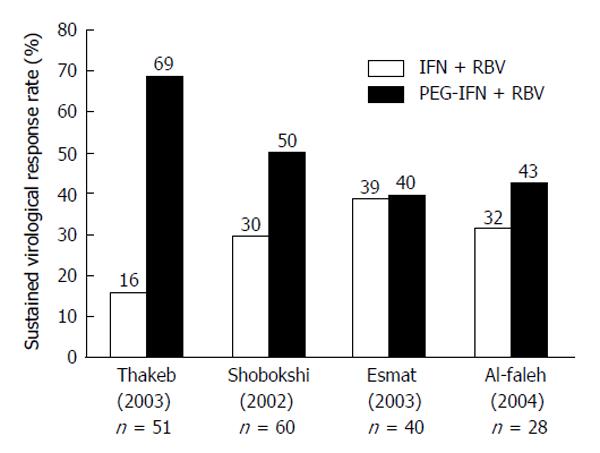
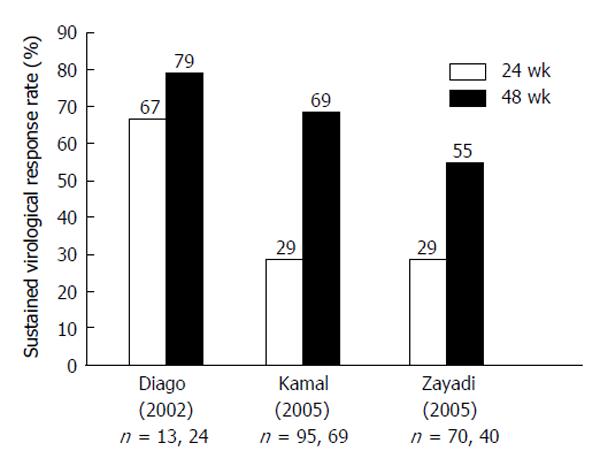
Figure 2 Sustained virologic response to 48 wk of combination therapy in patients with hepatitis C virus genotype-4 using standard-dose pegylated-interferon and ribavirin (pegylated-interferon-α2a 180 μg or pegylated-interferon-α2b 1.
5 mg/kg and RBV 1-1.2 g/d). All studies were randomized control trials with intention-to-treat analysis: (P < 0.001) (P < 0.01) (P = not supplied) (P = 0.43)[128-131]. PEG-IFN: Pegylated-interferon; RBV: Ribavirin.
- Citation: Abdel-Ghaffar TY, Sira MM, El Naghi S. Hepatitis C genotype 4: The past, present, and future. World J Hepatol 2015; 7(28): 2792-2810
- URL: https://www.wjgnet.com/1948-5182/full/v7/i28/2792.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i28.2792