Copyright
©2014 Baishideng Publishing Group Inc.
World J Hepatol. Oct 27, 2014; 6(10): 716-737
Published online Oct 27, 2014. doi: 10.4254/wjh.v6.i10.716
Published online Oct 27, 2014. doi: 10.4254/wjh.v6.i10.716
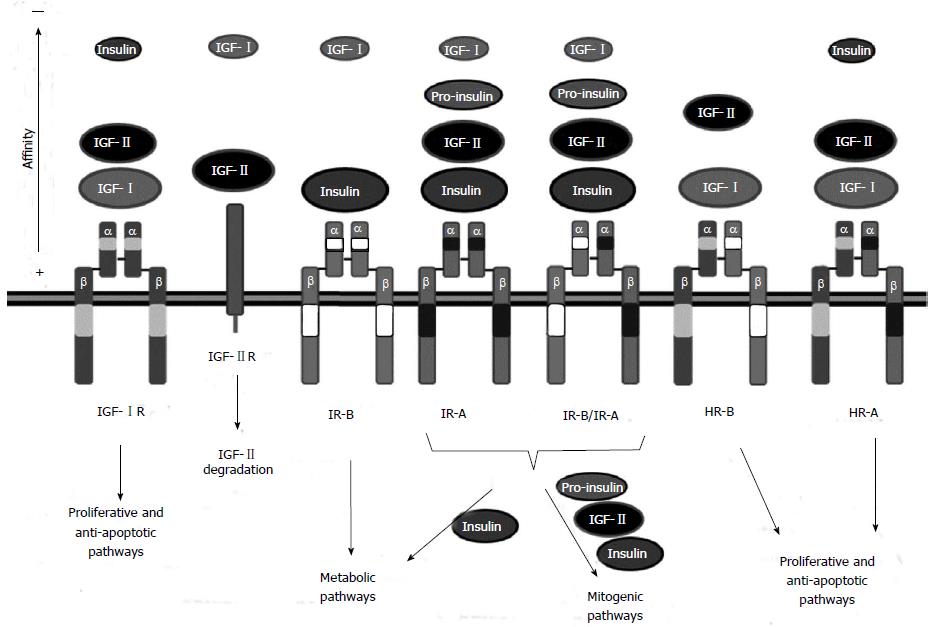
Figure 1 Insulin-like growth factor receptors and their ligands.
The insulin-like growth factor (IGF) system is composed of three receptors: IGF-IR, IGF-IIR and insulin receptor (IR). IGF-IR is the main receptor of the IGF system. It can bind IGF-I and IGF-II with high affinity. IGF-IIR is a negative regulator of the pathway that binds IGF-II and promotes IGF-II degradation. Finally, IR mediates insulin signaling. Two IR isoforms exist: the adult IR-B isoform that binds insulin and the fetal IR-A isoform, that can bind IGF-II in addition to insulin promoting mitogenic signaling. The IR-A isoform is commonly overexpressed in hepatocellular carcinoma (HCC). IR isoforms can form IR-A/IR-B hybrids which behave as IR-A receptors. Moreover, HR-A or HR-B hybrid receptors can be formed between IGF-IR and IR-A or IR-B respectively. These hybrid receptors lack high affinity binding to insulin and act, similar to the IGF-IR receptor, promoting proliferation and survival.
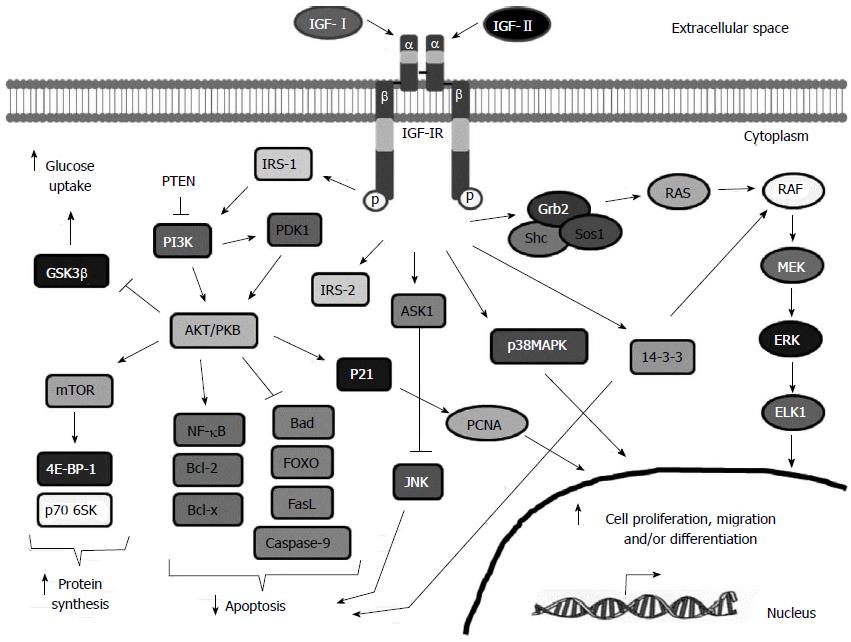
Figure 2 Insulin-like growth factor-I receptor signaling pathway.
Insulin-like growth factor-I (IGF-I), IGF-II and to a lesser extent, insulin, can bind to IGF-IR and promote a conformational change that leads to the autophosphorylation and activation of the IGF-IR. Phosphorylated receptor recruits specific docking proteins including IRS1-4, Shc and 14-3-3 which trigger mainly the PI3K/AKT/mTOR and the RAF/MEK/ERK signaling pathways. Activation of the IGF-IR induces protein and glycogen synthesis, glucose uptake, cell proliferation, migration and survival and cell differentiation depending on the cell type. See text for more details. GSK-3β: Glycogen synthase kinase-3; mTOR: Mammalian target of rapamycin; PTEN: Phosphatase and tensin homolog; PI3K: Phosphatidylinositide 3-kinase; AKT/PKB: Protein kinase B; NFKB: Nuclear factor kappa-light-chain-enhancer of activated B cells; Bcl-2: B-cell lymphoma 2; IRS-2: Insulin receptor substrate 2; FOXO: Forkhead box protein O1; PCNA: Proliferating cell nuclear antigen; RAF: Rapidly Accelerated Fibrosarcoma; MEK: Mitogen-Activated Protein Kinase; ERK: Extracellular-signal-regulated kinase; ELK1: ETS-Like kinase; ELK1: ETS-Like kinase.
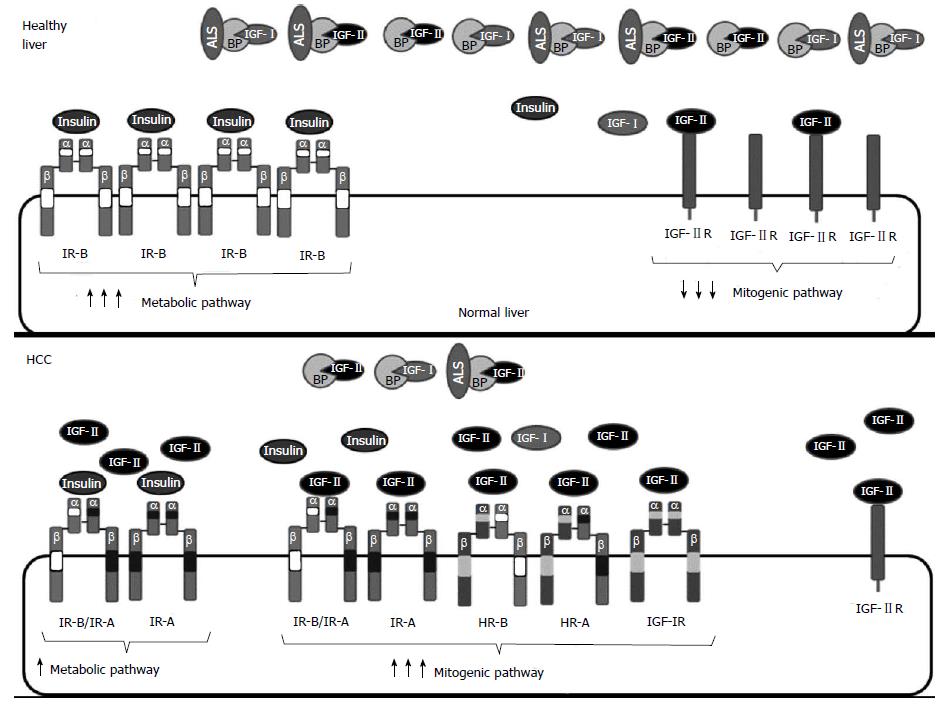
Figure 3 Insulin growth factor system alterations in hepatocellular carcinoma.
Healthy hepatocytes secrete high amounts of insulin growth factor (IGF)-I and express IGF-IIR and IR-B, but do not express IGF-IR. Many HCC are characterized by increased IGF signaling. Such IGF system overactivation is mediated by higher levels of ligands and/or receptors. Levels of functional IGF-II increase in many hepatocellular carcinoma (HCC): (1) by reactivation of the fetal promoter of IGF-II that leads to IGF-II overexpression; (2) by a decrease in circulating IGFBPs that chelate IGF-II; or (3) by decreased degradation through IGF-IIR, which is expressed at lower levels due to aberrant imprinting, loss of heterozygosity or gene deletions. Furthermore, IGF-IR and/or IR-A may be overexpressed in HCC. Overexpression of IGF-IR and IR-A leads to an increase in the formation of homodimeric HR-A or heterodimeric IR-A/IR-B receptors that bind IGF-II with high affinity resulting in decreased metabolic signaling and increased proliferation.
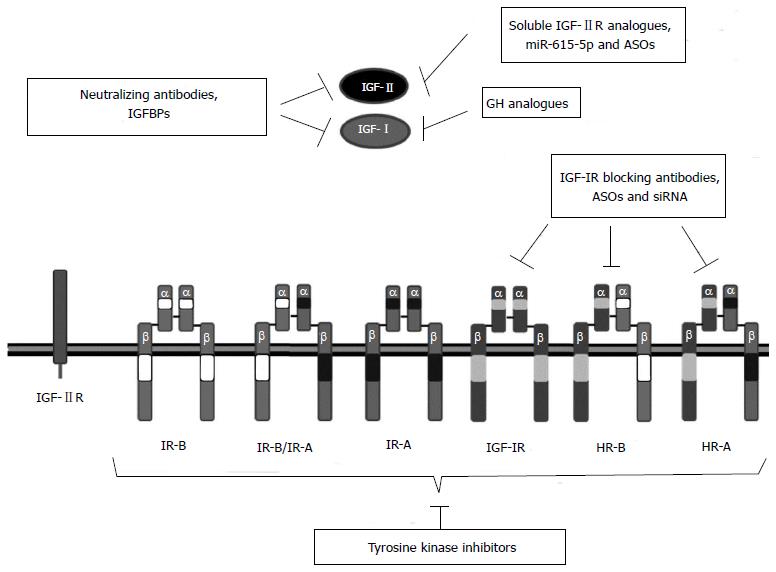
Figure 4 Insulin growth factor targeting strategies in hepatocellular carcinoma treatment.
Different strategies can be used to inhibit insulin growth factor (IGF) signaling. They can be ligand based or receptor based therapies. Ligand based therapies affect signaling through all IGF receptors, but these strategies should not be therapeutic in tumors with activating mutations in IGF-IR receptor or downstream proteins. Growth hormone (GH) analogues reduce IGF-I expression by blocking GH-GHR interaction. Specific ASOs and miR-615-5p decrease IGF-II expression. Soluble forms of IGF-IIR and IGFBPs can decrease IGF-II bioavailability. Ligand directed neutralizing antibodies impede binding to receptors. Receptor based therapies focus mainly on the inhibition of IGF-IR signaling. Antisense oligonucleotides (ASOs) and siRNAs decrease IGF-IR expression and IGF-IR blocking antibodies impede ligand mediated activation. These strategies do not affect IR-A and IR-A/IR-B, which could be crucial in the progression of some hepatocellular carcinoma. Tyrosine kinase inhibitors inhibit insulin receptor (IR)-A, IGF-IR and hybrid receptor autophosphorylation and subsequent activation but commonly they also inhibit IR-B receptor which is essential for the metabolism of normal cells. IGFBPs: IGF high affinity binding proteins.
- Citation: Enguita-Germán M, Fortes P. Targeting the insulin-like growth factor pathway in hepatocellular carcinoma. World J Hepatol 2014; 6(10): 716-737
- URL: https://www.wjgnet.com/1948-5182/full/v6/i10/716.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i10.716