Copyright
©The Author(s) 2021.
World J Hepatol. Apr 27, 2021; 13(4): 483-503
Published online Apr 27, 2021. doi: 10.4254/wjh.v13.i4.483
Published online Apr 27, 2021. doi: 10.4254/wjh.v13.i4.483
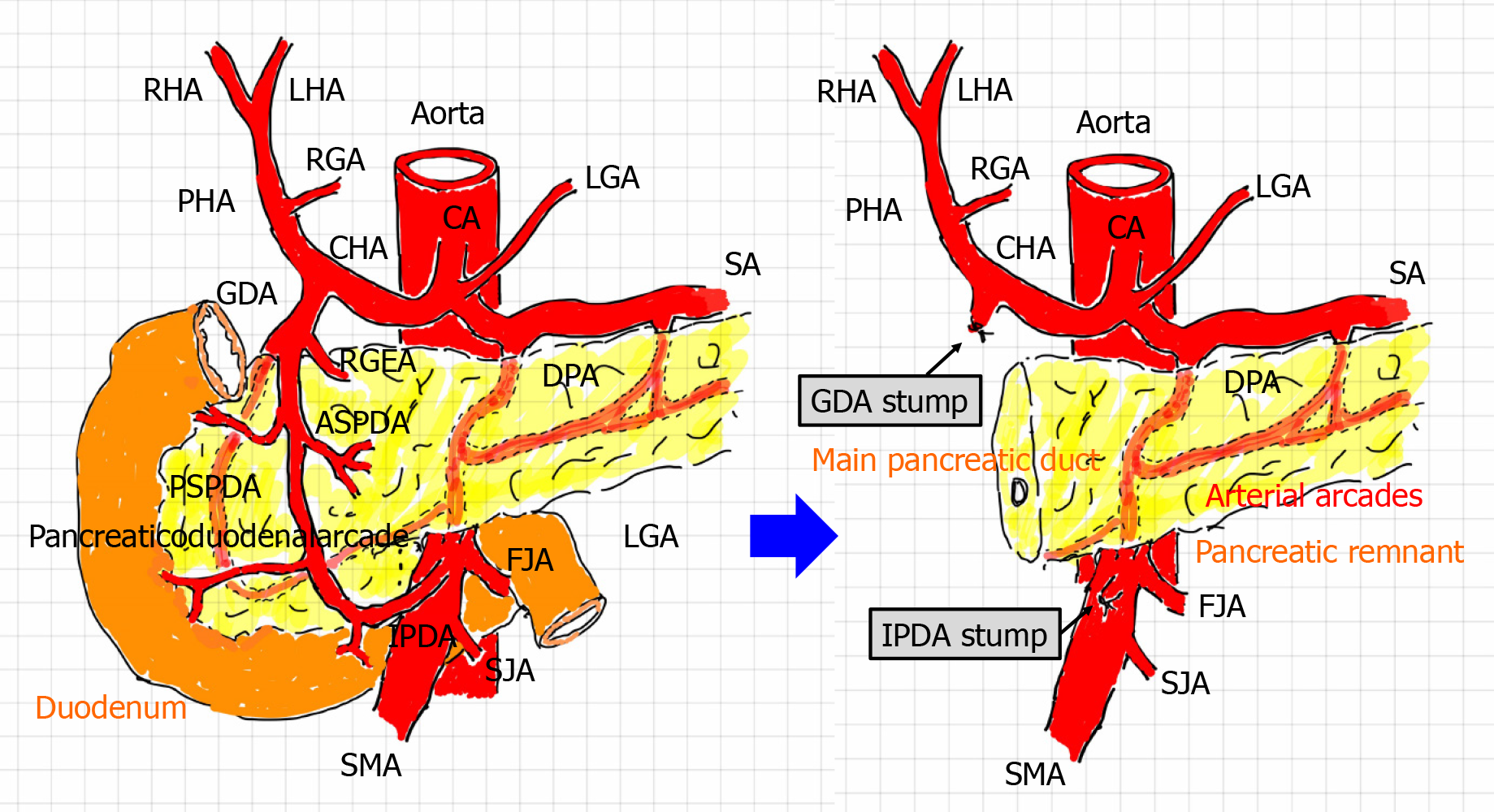
Figure 1 Arterial flow before and after pancreaticoduodenectomy.
During pancreaticoduodenectomy (PD), the gastroduodenal artery (GDA) from the celiac artery and inferior pancreaticoduodenal artery (IPDA) from the superior mesenteric artery are ligated and then cut. Additionally, the pancreaticoduodenal arcade is resected. Hence, arterial flow to the liver is modified after PD. A hepatopetal blood supply from the GDA and IPDA via the pancreaticoduodenal arcade can no longer be expected. The hepatic artery flow depends on the celiac artery. Lymphadenectomy and nerve dissection for treatment of malignancies might render visceral arteries vulnerable to postoperative wall injuries. Arterial arcades still remain in the pancreatic remnant. ASPDA: Anterior superior pancreaticoduodenal artery; CA: Celiac artery; CHA: Common hepatic artery; DPA: Dorsal pancreatic artery; FJA: First jejunal artery; GDA: Gastroduodenal artery; HA: Hepatic artery; IPDA: Inferior pancreaticoduodenal artery; LGA: Left gastric artery; LHA: Left hepatic artery; PHA: Proper hepatic artery; RGA: Right gastric artery; RGEA: Right gastroepiploic artery; RHA: Right hepatic artery; PSPDA: Posterior superior pancreaticoduodenal artery; SA: Splenic artery; SJA: Second jejunal artery; SMA: Superior mesenteric artery.
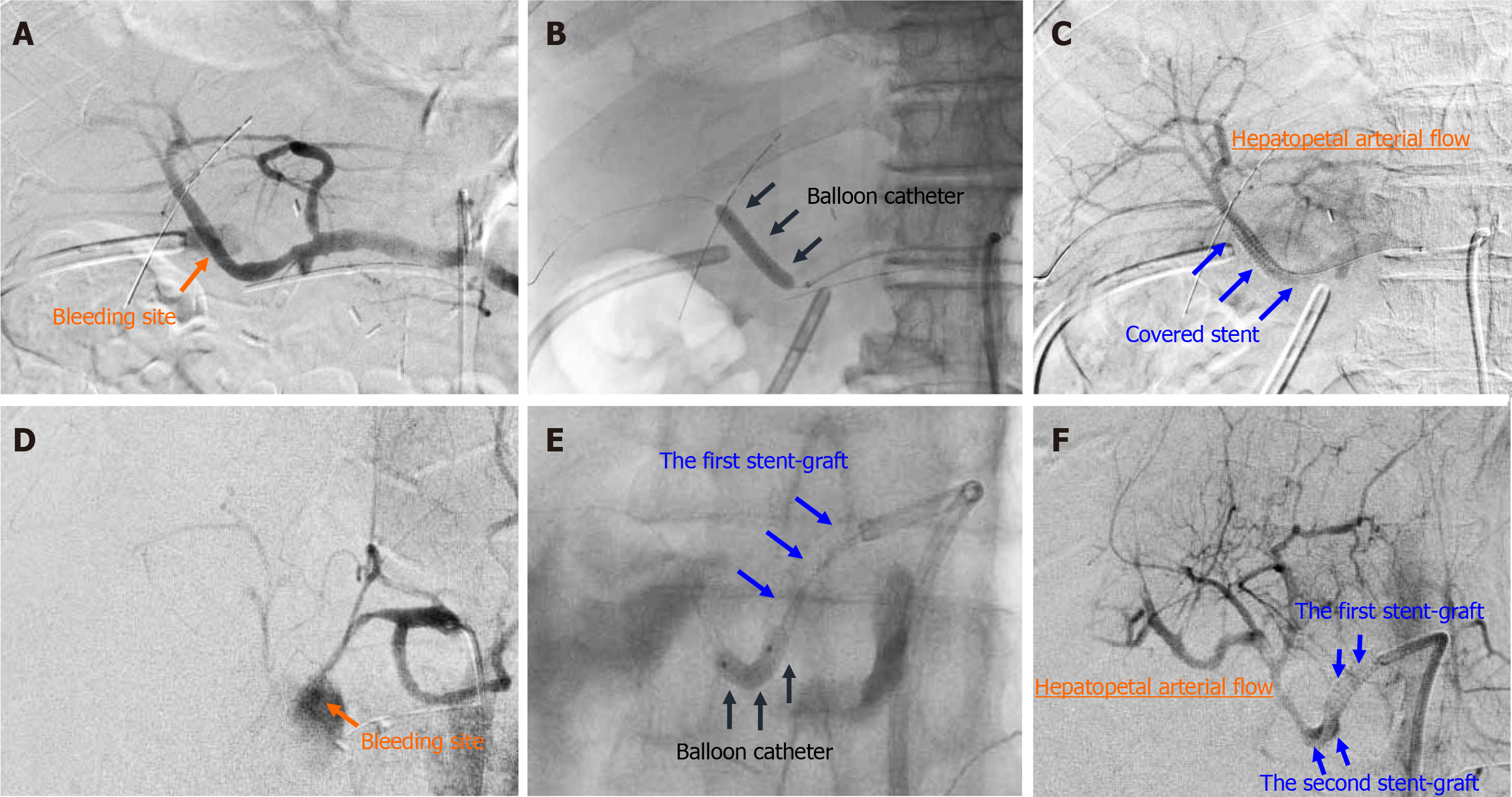
Figure 2 Actual procedures of transcatheter placement of covered stent.
Actual procedures in patient 7 and patient 16 are shown. A: Patient 7, diagnostic angiography clearly detected the bleeding sites; B: Patient 7, the target artery was dilated by a balloon catheter; C: Patient 7, a covered stent was placed at the culprit artery; D: Patient 16, diagnostic angiography clearly detected the bleeding sites; E: Patient 16, the target artery was dilated by a balloon catheter; F: Patient 16, the second overlapping stent-graft was implanted in an overlapping fashion. The hepatopetal arterial flow resumed (C and F). Hence, complete hemostasis and preservation of hepatic artery flow were simultaneously obtained.
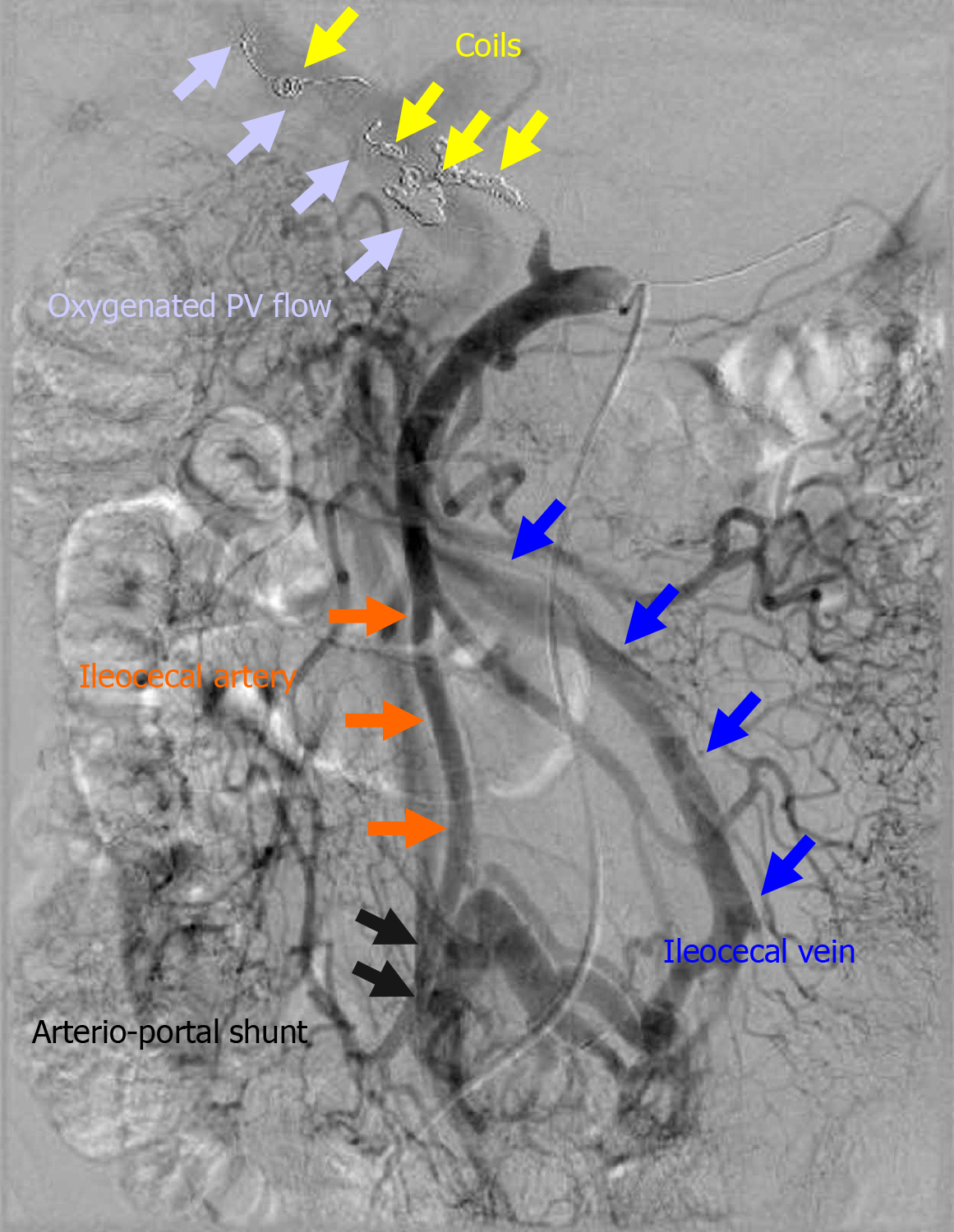
Figure 3 Actual finding of arterioportal shunting.
The ileocecal vein and artery were anastomosed in a side-to-side fashion. In a patient in whom the initial endovascular treatment failed (patient 14), hemostasis was completed by additional transcatheter arterial embolization, and liver infarction subsequently occurred. Therefore, an arterioportal shunt was surgically created to oxygenate the portal vein flow. In this case, arterioportal shunting minimized progression to fatal liver infarction due to hepatic ischemia and refractory liver abscess due to biliary ischemia. PV: Portal vein.
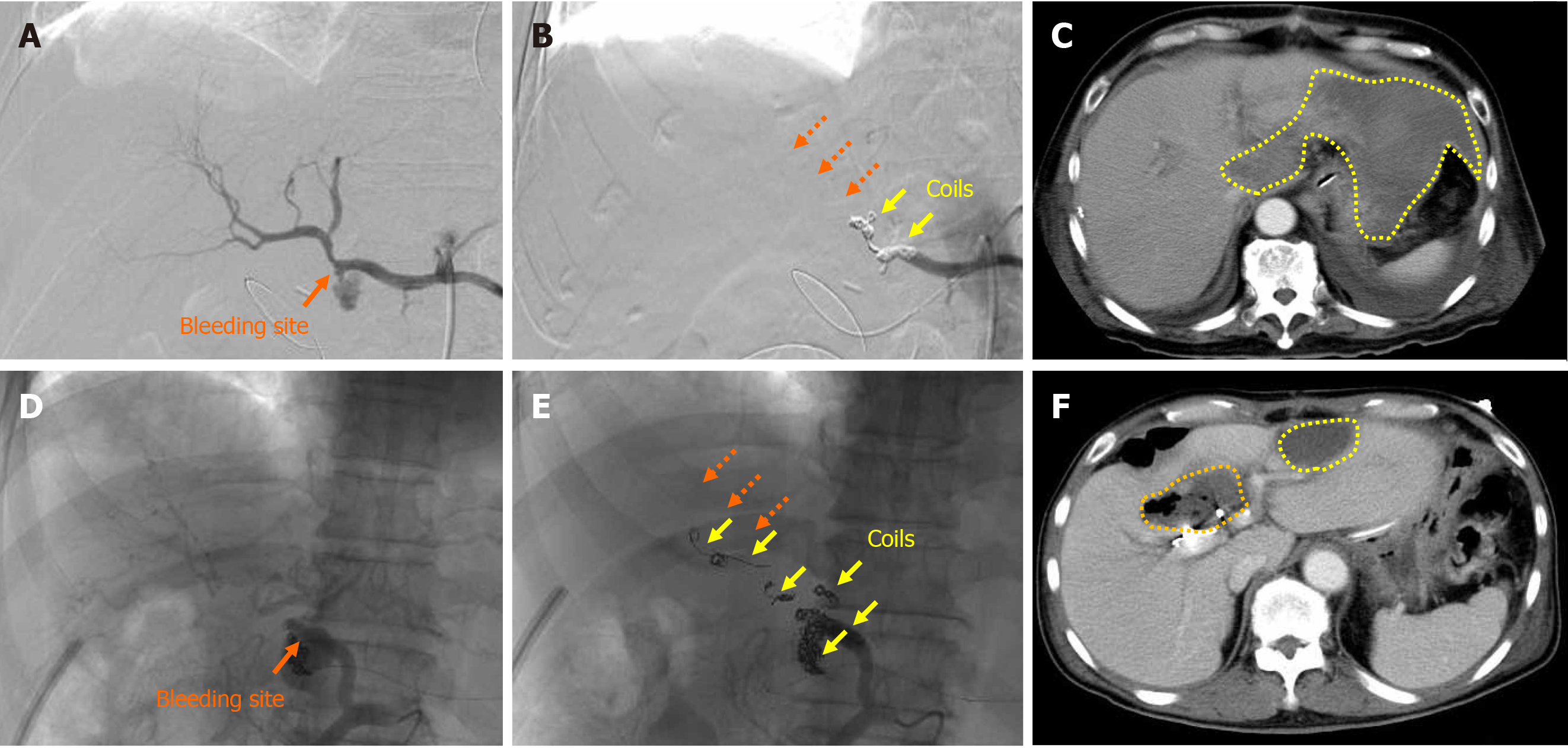
Figure 4 Liver infarction and abscess after transcatheter arterial embolization.
Actual findings in patient 13 and patient 14 are shown. A: Patient 13, the bleeding sites were detected; B: Patient 13, complete hemostasis was obtained by transcatheter arterial embolization, but the hepatopetal arterial flow was completely lost (dotted orange arrows); C: Patient 13, the patient subsequently developed liver infarction due to hepatic ischemia (dotted yellow circles); D: Patient 14, the bleeding sites were detected; E: Patient 14, complete hemostasis was obtained by TAE, but the hepatopetal arterial flow was completely lost (dotted orange arrows); F: Patient 14, the patient subsequently developed liver infarction due to hepatic ischemia (dotted yellow circles) and a liver abscess due to biliary ischemia (dotted orange circle).
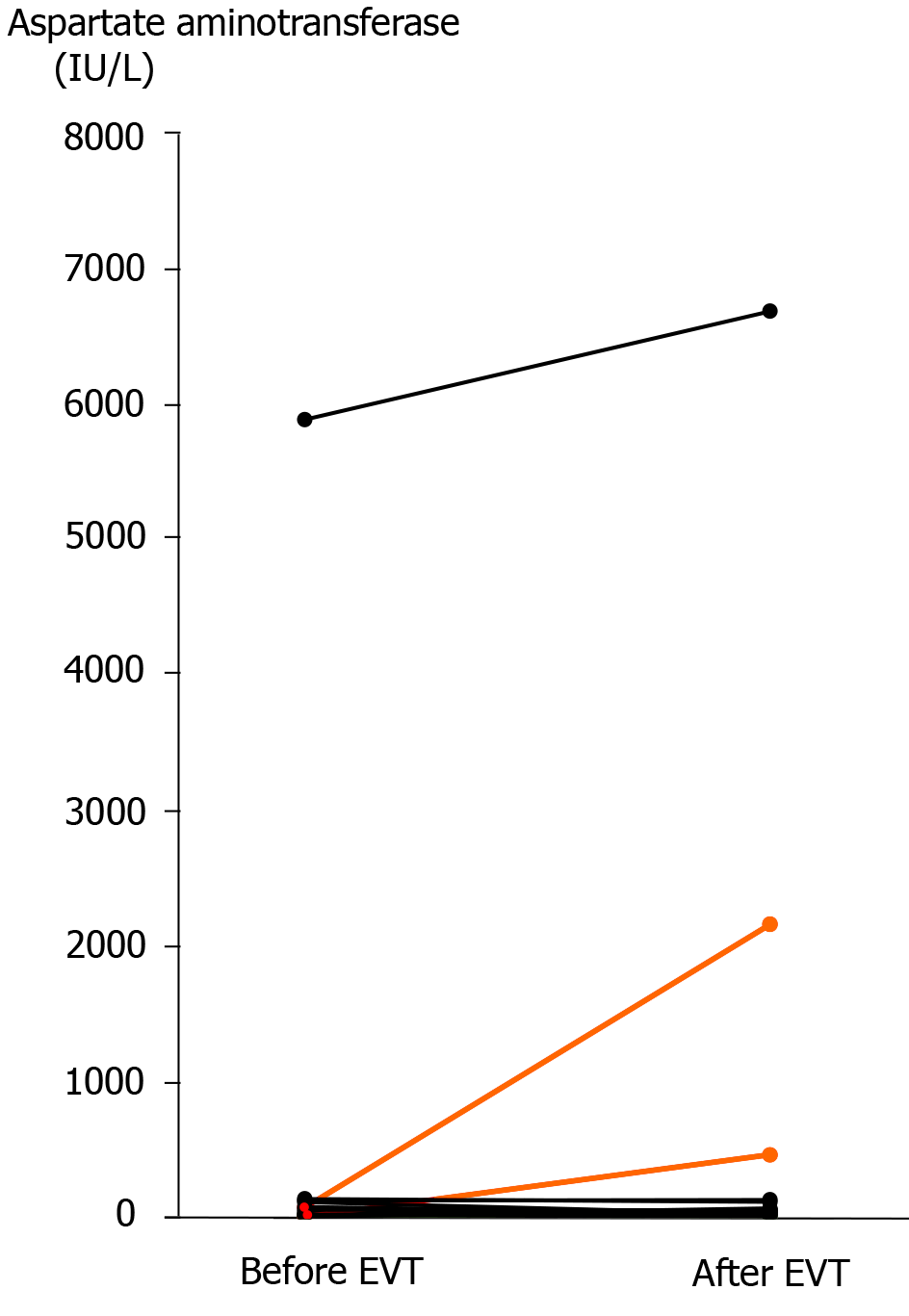
Figure 5 Serum aspartate aminotransferase concentration before and after endovascular treatment.
Actual changes before and after endovascular treatment (EVT) are shown. In two patients who developed liver infarction after transcatheter arterial embolization (patients 13 and 14), the serum aspartate aminotransferase concentration was clearly elevated after EVT (orange lines). A high serum aspartate aminotransferase concentration was observed in a patient who had liver infarction before EVT (patient 16), and this patient finally died of liver failure even after successful stent-graft placement. EVT: Endovascular treatment.
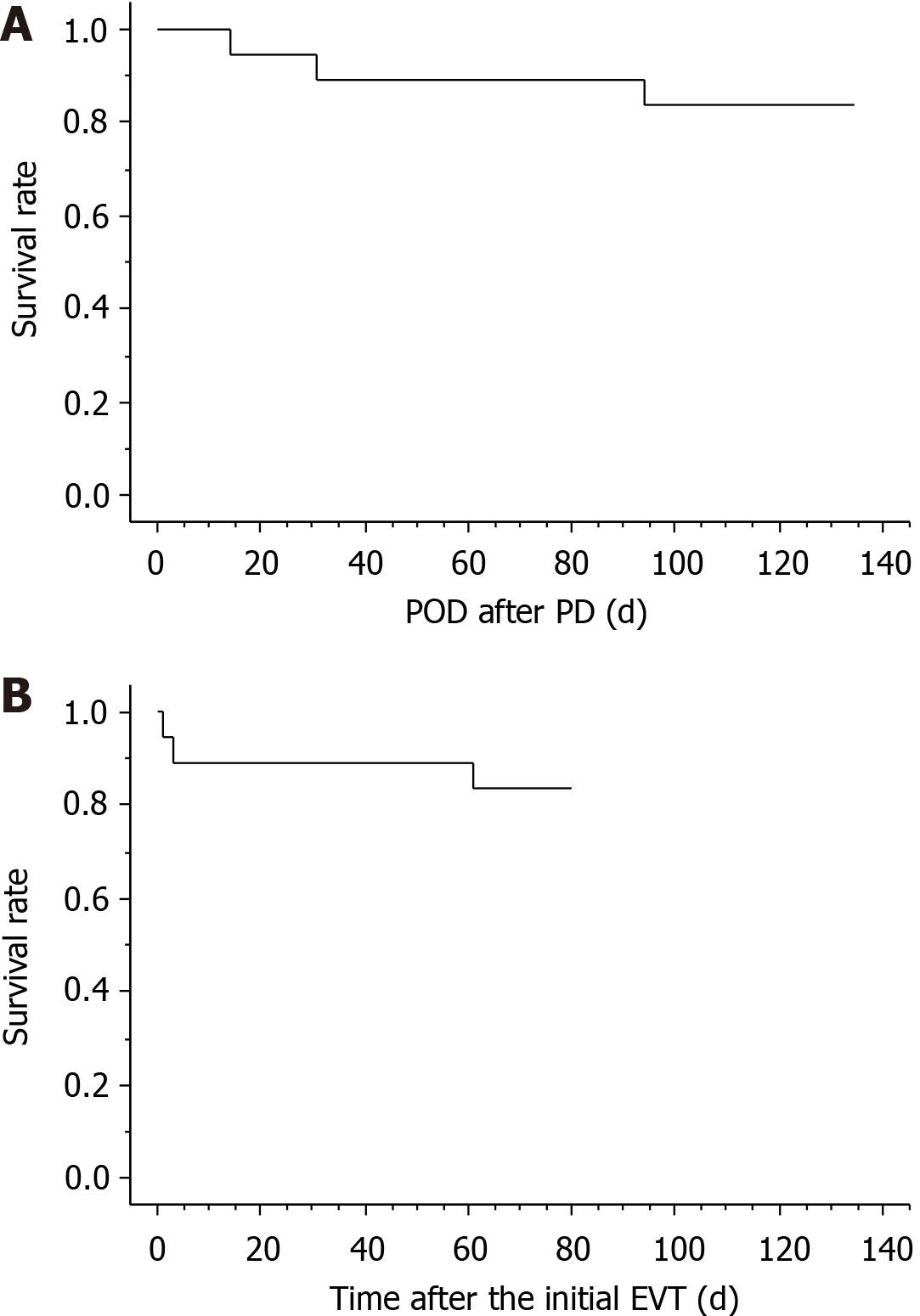
Figure 6 Short-term survival after pancreaticoduodenectomy and endovascular treatment.
Three patients (patients 2, 15, and 16) died during hospitalization, and the actual survival curves after pancreaticoduodenectomy (PD) and endovascular treatment (EVT) are shown. Fourteen (87.5%) patients were successfully treated because the cause of death in one patient (patient 2) was unrelated to arterial hemorrhage (cancer-related death). A: PD; B: EVT. EVT: Endovascular treatment; PD: Pancreaticoduodenectomy; POD: Postoperative day.
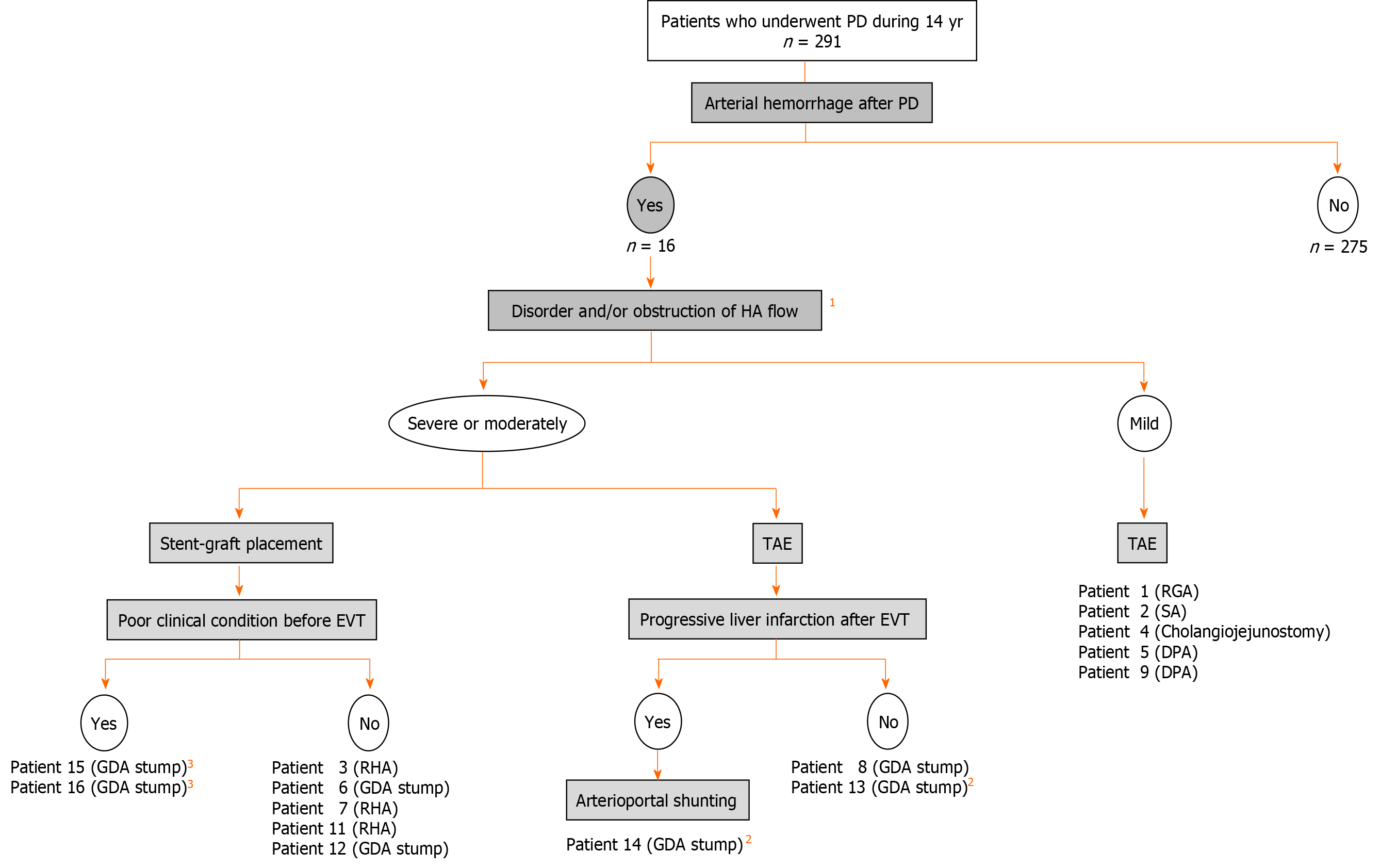
Figure 7 Flowchart of endovascular treatments and arterioportal shunting.
Actual flowchart of our patients who caused arterial hemorrhage after pancreaticoduodenectomy was shown. 1Image findings of computed tomography angiography and/or diagnostic angiography. 2Patients with liver infarction after transcatheter arterial embolization. 3Patients with poor outcome even after successful stent-graft placement. PD: Pancreaticoduodenectomy; HA: Hepatic artery; TAE: Transcatheter arterial embolization; EVT: Endovascular treatment; GDA: Gastroduodenal artery; RHA: Right hepatic artery; SA: Splenic artery; DPA: Dorsal pancreatic artery.
- Citation: Kamada Y, Hori T, Yamamoto H, Harada H, Yamamoto M, Yamada M, Yazawa T, Sasaki B, Tani M, Sato A, Katsura H, Tani R, Aoyama R, Sasaki Y, Okada M, Zaima M. Fatal arterial hemorrhage after pancreaticoduodenectomy: How do we simultaneously accomplish complete hemostasis and hepatic arterial flow? World J Hepatol 2021; 13(4): 483-503
- URL: https://www.wjgnet.com/1948-5182/full/v13/i4/483.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i4.483