Copyright
©The Author(s) 2020.
World J Hepatol. Oct 27, 2020; 12(10): 754-765
Published online Oct 27, 2020. doi: 10.4254/wjh.v12.i10.754
Published online Oct 27, 2020. doi: 10.4254/wjh.v12.i10.754
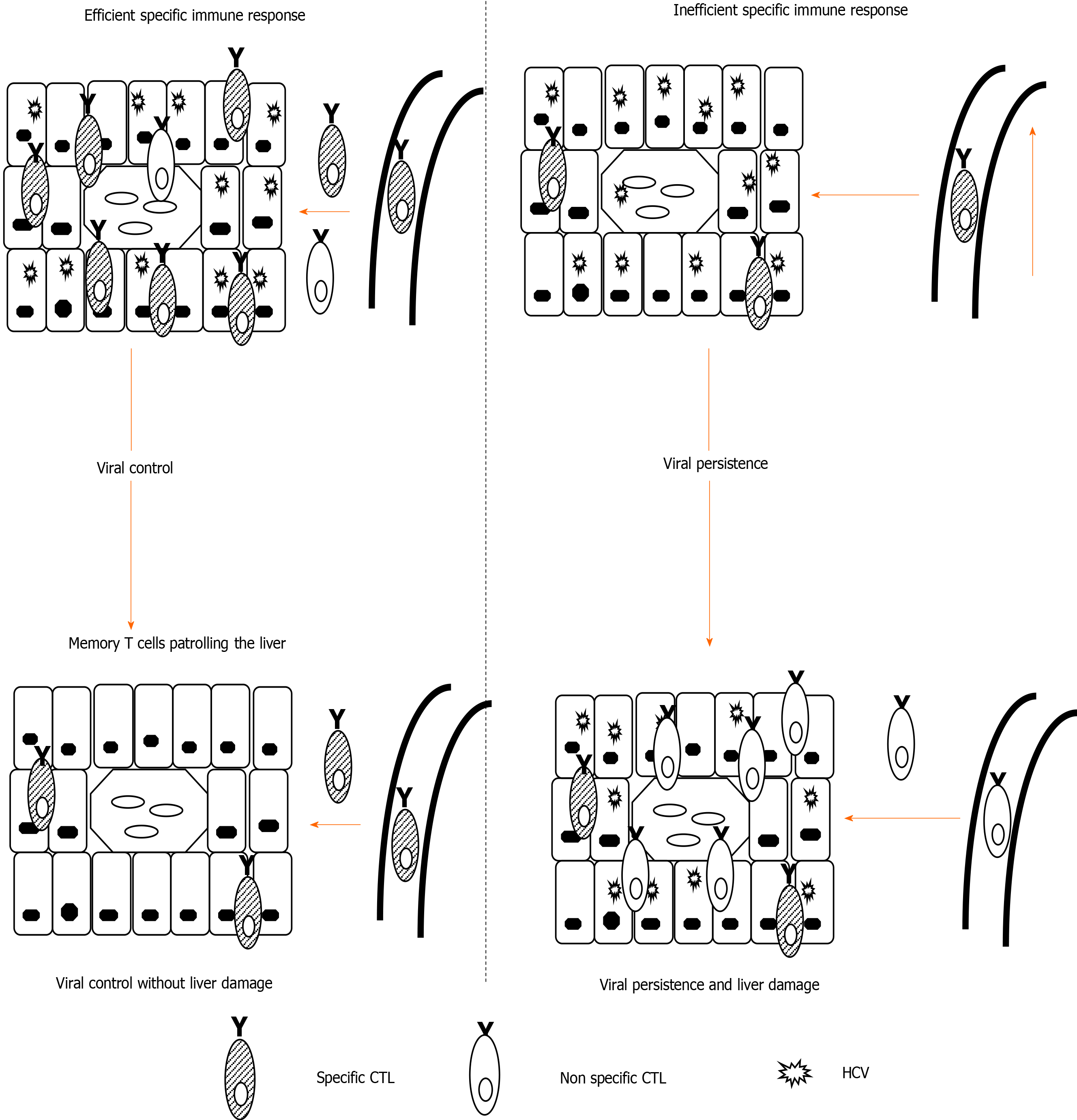
Figure 1 Theoretical model of liver damage during chronic viral hepatitis due to non-specific inflammatory infiltrate.
Left-side: Depiction of an efficient hepatitis C virus (HCV)-specific cytotoxic T cell (CTL) controlling HCV in the liver; Right-side: Depiction of HCV-specific exhausted CTLs unable to control HCV replication. Hepatocytes steadily secrete chemokines that attract specific and non-specific infiltrate, the latter of which is responsible for liver damage. CTL: Cytotoxic T cell; HCV: Hepatitis C virus.
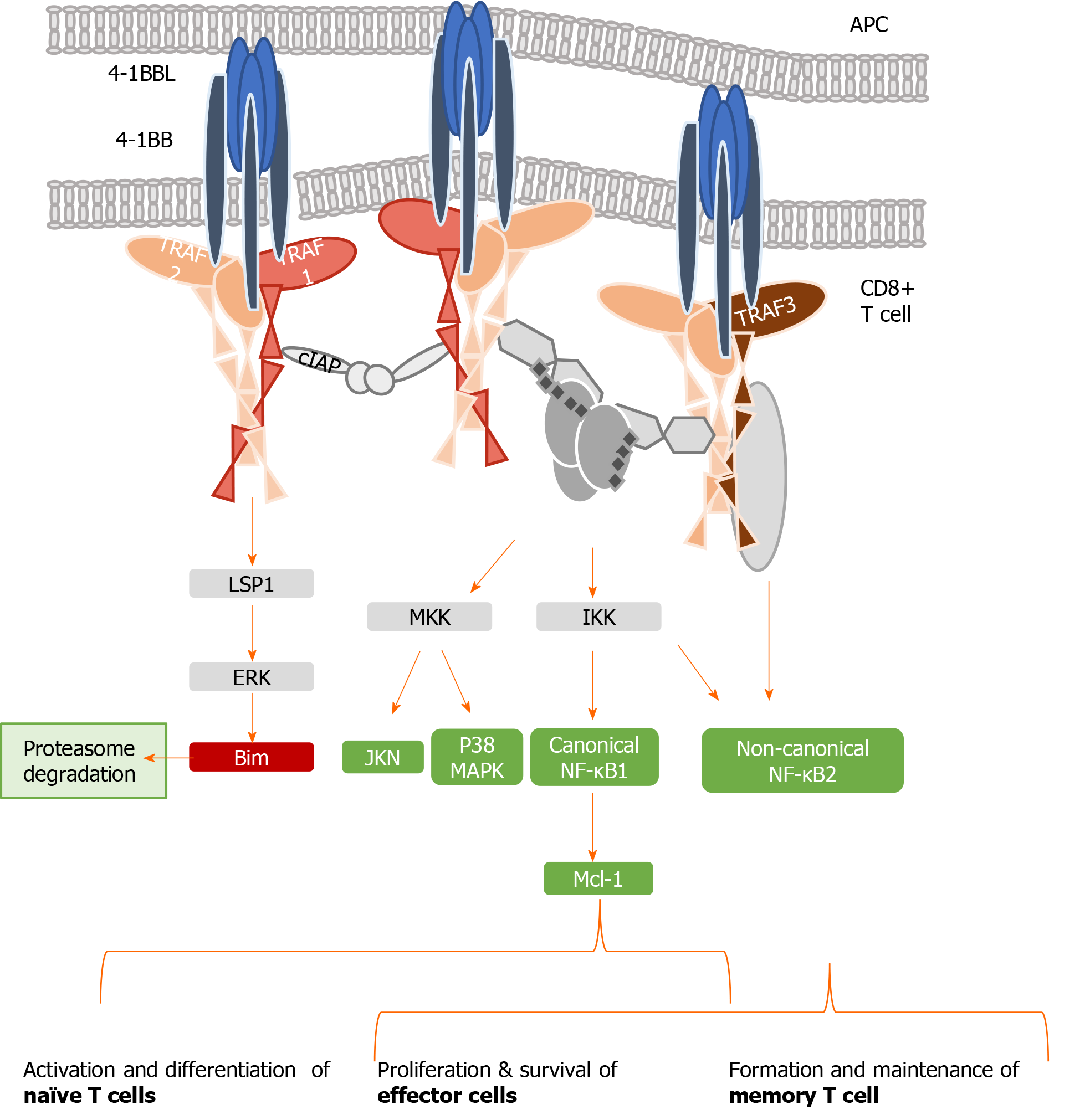
Figure 2 Tumor necrosis family receptor superfamily member 9/tumor necrosis factor receptor-associated factor 1 signaling complex.
Schematic representation of tumor necrosis family receptor (TNFR) superfamily member 9 (4-1BB) signaling pathways, indicating the interaction between the trimeric 4-1BB ligand presented by the antigen presenting cell and the three molecules of the receptor 4-1BB. The signal transduction occurs through tumor necrosis factor receptor-associated factor (TRAF) 1. Representative combinations of TRAF1, 2, and 3 and their interactions with adaptor proteins are presented. Canonical activation of nuclear factor kappa B (NF-κB) leads to the activation of naïve T cells, which differentiate into effector cells and proliferate after antigen encounter. Non-canonical NF-κB bestows proliferation and survival of effector cells and also drives the generation and maintenance of memory T cells in a delayed manner. APC: Antigen-presenting cell; 4-1BB: Tumor necrosis family receptor superfamily member 9; 4-1BBL: 4-1BB-ligand; TRAF: Tumor necrosis factor receptor-associated factor; cIAP: Cellular inhibitor of apoptosis protein; ERK: Extracellular signal-regulated kinase; MKK: Mitogen-activated protein kinase kinase; IKK: Inhibitory kappa B kinase; MAPK: Mitogen-activated protein kinases; NF-κB: Nuclear factor kappa B; Mcl-1: Myeloid leukemia cell differentiation protein.
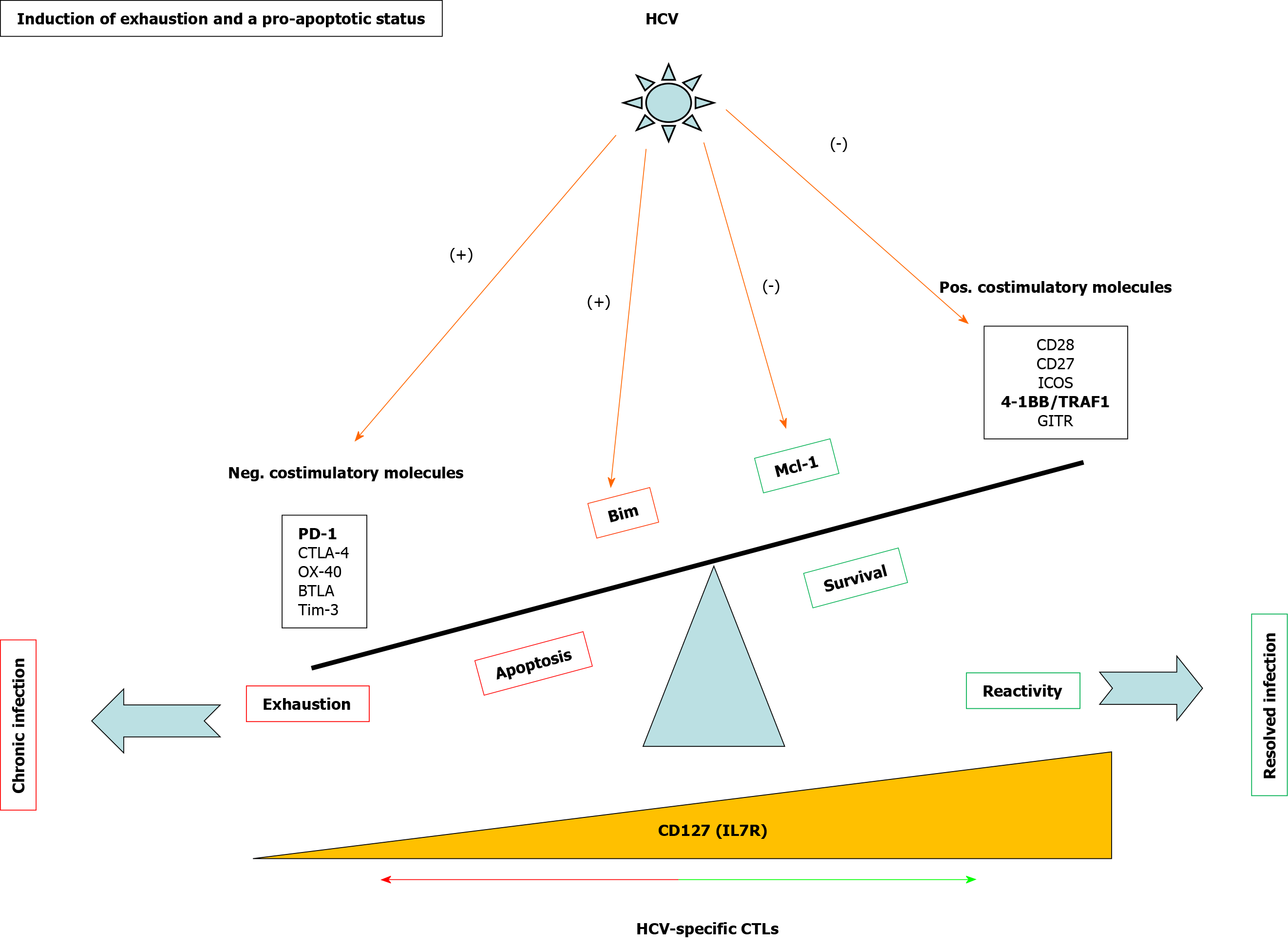
Figure 3 Mechanisms involved in T cell exhaustion and apoptosis during persistent hepatitis C virus infection.
Scheme showing positive and negative checkpoints and proteins involved in CD8 T cell reactivity and apoptosis during hepatitis C virus infection. In bold are highlighted the pathways discussed in the current review. HCV: Hepatitis C virus; 4-1BB: Tumor necrosis family receptor superfamily member 9; TRAF: Tumor necrosis factor receptor-associated factor; GITR: Glucocorticoid-induced tumor necrosis factor receptor-related protein; CTL: Cytotoxic T lymphocyte; Neg: Negative; Pos: Positive; PD-1: Programmed cell death protein-1; Mcl-1: Myeloid leukemia cell differentiation protein; IL: Interleukin.
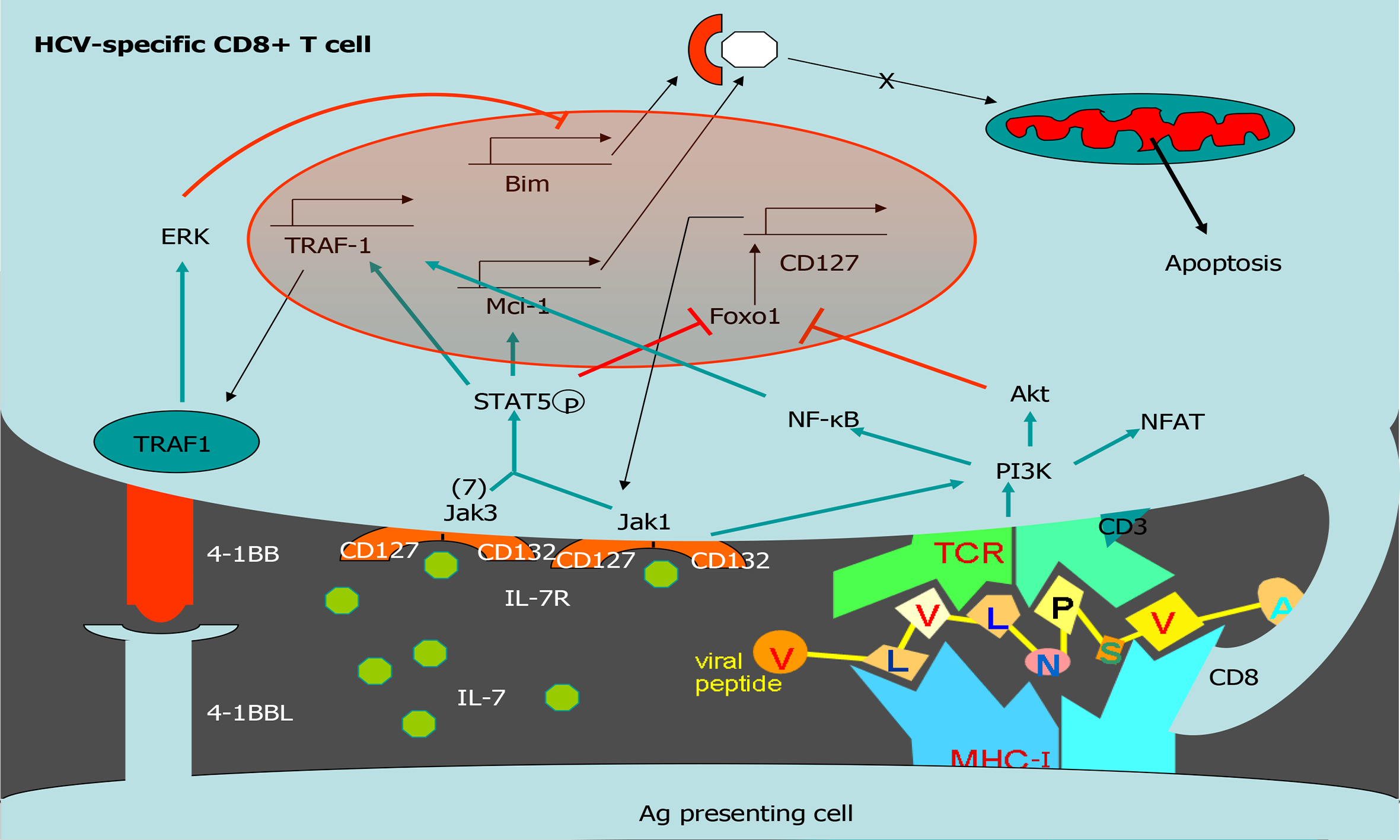
Figure 4 Tumor necrosis factor receptor-associated factor 1 pathways involved in T cell survival.
Scheme of T cell survival pathways. Interleukin (IL)-7/IL-7 receptor (CD127) increases the level of the anti-apoptotic molecule myeloid leukemia cell differentiation protein (Mcl-1) via signal transducer and activator of transcription 5. After T cell receptor activation, tumor necrosis factor receptor (TNFR)-associated factor 1 (TRAF1) level is upregulated via nuclear factor-kappa B. TRAF1 is the signal transducer of the positive checkpoint TNFR superfamily member 9 (4-1BB). 4-1BB stimulation downregulates Bim via extracellular signal-related kinase. IL-7 induces TRAF1 expression, increasing its anti-apoptotic effect by improving 4-1BB signaling. Together, 4-1BB and CD127 balance Bim and Mcl-1. HCV: Hepatitis C virus; 4-1BB: Tumor necrosis family receptor superfamily member 9; ERK: Extracellular signal-regulated kinase; TRAF1: Tumor necrosis factor receptor-associated factor 1; Mcl-1: Myeloid leukemia cell differentiation protein; IL: Interleukin; NF-Κb: Nuclear factor kappa B; MHC: Major histocompatibility complex; TCR: T cell receptor.
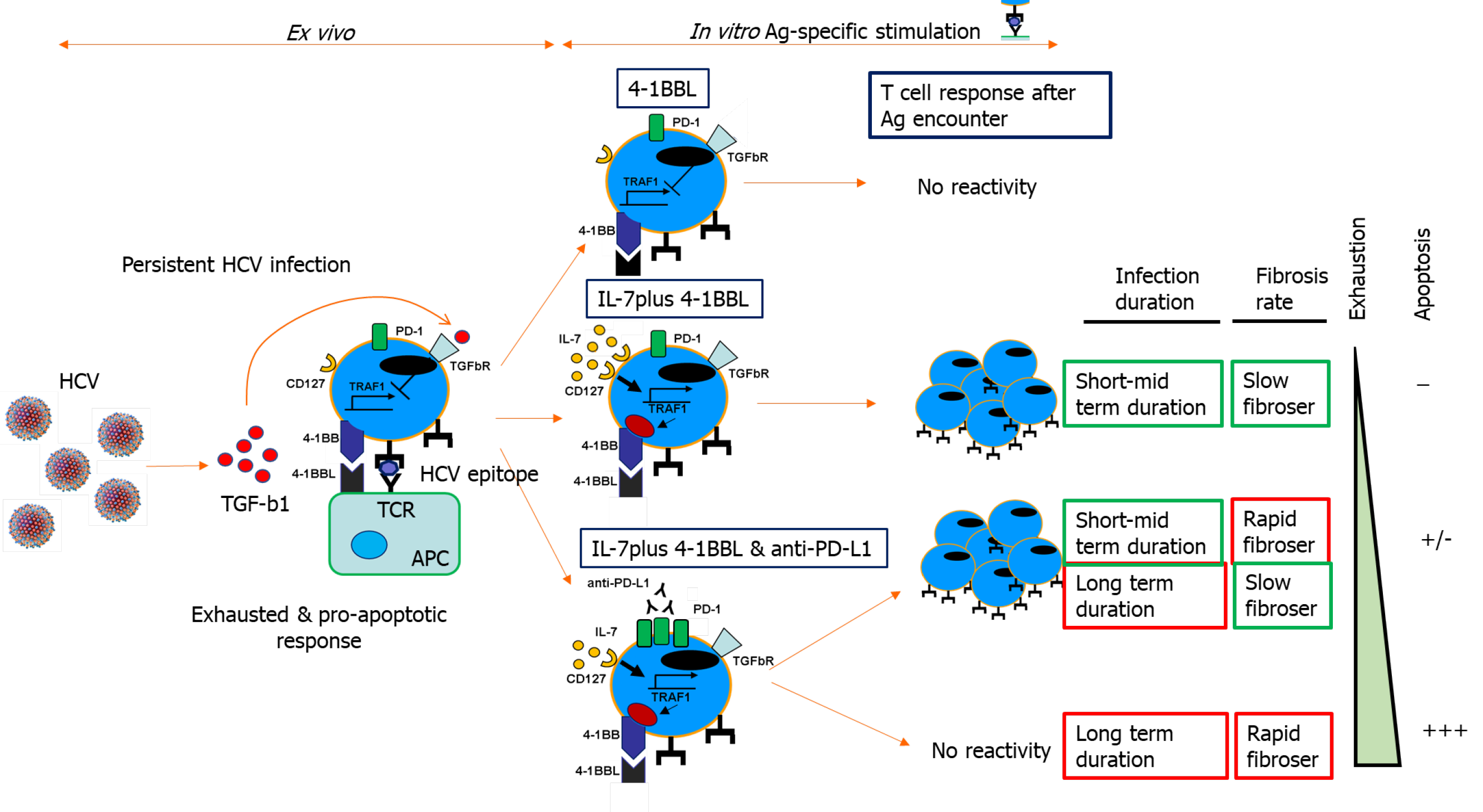
Figure 5 Tumor necrosis factor receptor-associated factor 1-related pathogenic mechanism involved in T cell exhaustion and liver fibrosis progression during persistent hepatitis C virus infection.
Scheme showing transforming growth factor beta 1-mediated CD8 T cell impairment during chronic hepatitis C virus infection due to tumor necrosis factor receptor-associated factor 1 (TRAF1). In patients with mild clinical progression, T cell reactivity can be restored by TRAF1 upregulation with interleukin (IL)-7 treatment. Those with rapid fibrosis or with long-term infection need IL-7 treatment combined with programmed cell death protein 1 blockade. Cases with rapid fibrosis and long infection duration cannot be restored, probably due to T cell deletion. Ag: Antigen; 4-1BB: Tumor necrosis family receptor superfamily member 9; 4-1BBL: 4-1BB-ligand; PD-1: Programmed cell death protein-1; HCV: Hepatitis C virus; TRAF1: Tumor necrosis factor receptor-associated factor 1; TGF: Transforming growth factor; IL: Interleukin; APC: Antigen-presenting cell; TCR: T cell receptor.
- Citation: Peña-Asensio J, Sanz-de-Villalobos E, Miquel J, Larrubia JR. Tumor necrosis family receptor superfamily member 9/tumor necrosis factor receptor-associated factor 1 pathway on hepatitis C viral persistence and natural history. World J Hepatol 2020; 12(10): 754-765
- URL: https://www.wjgnet.com/1948-5182/full/v12/i10/754.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i10.754