Published online Dec 26, 2017. doi: 10.4252/wjsc.v9.i12.235
Peer-review started: October 12, 2017
First decision: November 8, 2017
Revised: November 15, 2017
Accepted: December 3, 2017
Article in press: December 3, 2017
Published online: December 26, 2017
Processing time: 75 Days and 22.4 Hours
Clinical and laboratory results document psoriatic arthritis in a 56-year old patient. The symptoms did not resolve with standard treatments (nonsteroidal anti-inflammatory drugs, steroids and methotrexate). TNF-alpha inhibitors (certolizumab pegol and adalimumab) were added to the treatment regime, with some adverse effects. A trial of human umbilical cord stem cell therapy was then initiated. The stem cells were enriched and concentrated from whole cord blood, by removal of erythrocytes and centrifugation. The patient received several infusions of cord blood stem cells, through intravenous and intra-articular injections. These stem cell treatments correlated with remission of symptoms (joint pain and psoriatic plaques) and normalized serologic results for the inflammatory markers C-reactive protein and erythrocyte sedimentation rate. These improvements were noted within the first thirty days post-treatment, and were sustained for more than one year. The results of this trial suggest that cord blood stem cells may have important therapeutic value for patients with psoriatic arthritis, particularly for those who cannot tolerate standard treatments.
Core tip: Rheumatic diseases are common and often disabling. Standard drug treatments can control inflammation, but many patients do not find relief. Potent biologic drugs (tumor necrosis factor inhibitors) are not tolerated by some. This patient report describes treatment with umbilical cord blood stem cells (CBSC). Clinical observations and serology results document prolonged improvement of psoriatic arthritis. This type of report is important since it documents a dosage, time course, and a beneficial outcome, using an under-employed type of stem cell. These results can help guide clinicians in future trials using CBSC.
- Citation: Coutts M, Soriano R, Naidoo R, Torfi H. Umbilical cord blood stem cell treatment for a patient with psoriatic arthritis. World J Stem Cells 2017; 9(12): 235-240
- URL: https://www.wjgnet.com/1948-0210/full/v9/i12/235.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i12.235
Psoriatic arthritis (PsA) is a chronic inflammatory disease that can result in significant disability. It is characterized by skin rashes, fatigue, and swollen, painful joints. The disease can be progressive; about 20% of the patients develop a severe form of the disease with multiple joint deformities and bone erosion[1,2]. The inflammation associated with PsA can lead to damage in a variety of tissues: the GI tract, heart, lungs, liver and kidney. Remission of symptoms and a better prognosis are more likely when treatment begins early[3,4].
PsA and rheumatoid arthritis (RA) can be difficult to distinguish. Both are autoimmune syndromes where the joints are attacked. The same sets of joints can be affected with tenderness and swelling, though certain patterns tend to be indicative of PsA or RA. There can be similar serology (elevated levels of C-reactive protein and increased erythroid sedimentation rate). These tests measure systemic inflammation, and are not specific for PsA nor RA. To further complicate diagnosis, the onset of psoriatic plaques in PsA can vary. For example, psoriasis precedes PsA in 70%-80% of the patients, while arthritis precedes psoriasis in 15%-20% of the patients. In the remainder of the patients, onset of both symptoms is within a year[5]. In the pre-symptomatic stages of these diseases, inflammation starts at the enthesis in PsA, while inflammation starts in the synovium in RA. However, both diseases can progress to synovitis and bone erosion[5,6]. PsA and RA can often be differentiated by MRI; PsA also has a distinct synovial membrane vascularity and an over-representation of the Th17 subset of T cells[7]. However, these more definitive tests would not be used in an initial diagnosis.
Despite clinical and pathogenic differences, PsA and RA are often treated with the same drugs. Commonly prescribed medications include: Nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, antirheumatic drugs such as methotrexate, and biologic anti-inflammatory drugs. This latter group includes adalimumab (Humira), and certolizumab pegol (Cimzia). Both biologics are antibodies that block the activity of tumor necrosis factor alpha (TNF). All of these medications can control symptoms and prevent joint damage. However, in some patients, these drugs are ineffective or poorly-tolerated. The TNF inhibitors also have significant safety considerations, such as increased risk of opportunistic infections.
In this study, a subset of human umbilical cord blood cells was tested as an experimental treatment for PsA. Cord blood is readily available; its regenerative capabilities are being actively investigated for a number of clinical applications[8,9]. Historically, cord blood and cord blood stem cells have been used for the treatment of hematopoietic disorders; therapies have been expanded to include immune modulation[10]. Umbilical cord blood contains different types of stem cells, which may exert therapeutic and regenerative effects through a variety of mechanisms[10,11]. Given the reports of regenerative and immunomodulatory effects, it is a logical step to test CBSC for inflammatory conditions like PsA.
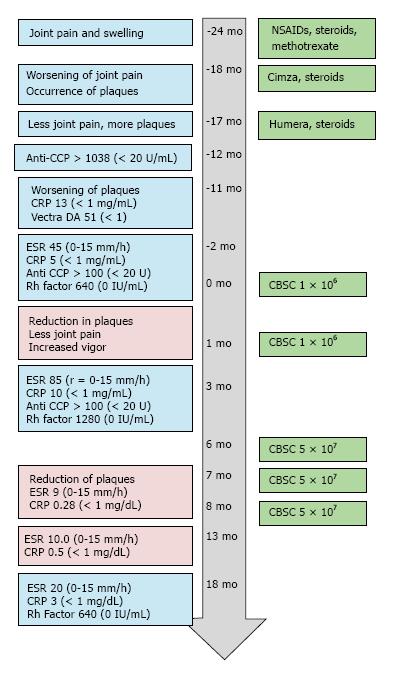
The patient was a 56-year-old male with a two-year history of arthritic symptoms. He presented with joint stiffness and pain, particularly in the metacarpal and proximal phalangeal joints. The joint stiffness and pain was more pronounced upon waking. Six months after initial presentation, the pain and stiffness progressed to wrists, shoulders and jaw. Notably, the pain did not affect all areas simultaneously. The patient described the pain as “jumping” from region to region. Joint swelling was noted, particularly in the proximal interphalangeal and metacarpal joints. Some asymmetry was noted. The fingers of both hands had some degree of dactylitis; the fingernails were pitted and discolored. Tendons were inflamed, but structural deformities of the joints were not noted. Given the absence of psoriatic plaques, laboratory tests were ordered to evaluate the possibility of rheumatoid arthritis and gauge the level of inflammation. The Vectra DA test evaluates 12 serum proteins linked to RA disease activity; the test was scored with a “high level of RA disease activity” (score of 51, with < 1 as a reference value). Both anti-cyclic citrullinated peptide values and C-reactive protein levels were abnormally high (Anti- CCP > 1000, reference ≤ 20 U/mL, C-reactive protein (CRP) 5 to 15, reference range of < 1 mg/mL). The erythroid sedimentation rate (ESR) and values for Rheumatoid Factor were also elevated (ESR, 45, reference = 0-15 mm/h, RhF = 630, reference value 0 IU/mL). A time course of the patient’s lab results, symptoms and treatments are presented in Figure 1.
The patient was initially treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and methotrexate, but experienced minimal improvement. A synthetic corticosteroid (prednisone) was prescribed on an increasing dosing schedule. After one and a half years of these treatments, there were no significant improvements in arthritic symptoms. The patient was counseled to try certolizumab pegol, a TNF-α inhibitor. Since patients are more susceptible to opportunistic infections during anti-TNF therapy, the patient was pre-tested for hepatitis, HIV, and tuberculosis and was found to be negative.
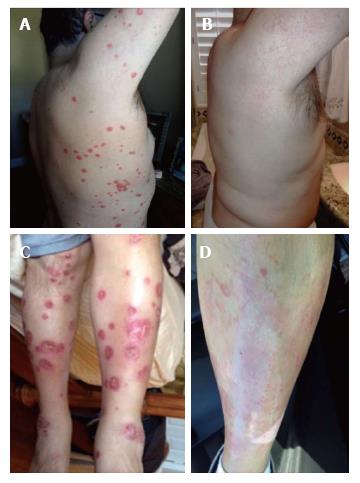
Within two weeks of certolizumab pegol injection, joint symptoms worsened and dermal psoriatic plaques occurred on the trunk and legs. The psoriatic plaques became open weeping wounds on the upper extremities, the trunk, and the lower extremities (Figure 2). Certolizumab was discontinued and another TNF-α inhibitor (adalimumab, brand name Humira) was used, instead. Prednisone treatment was continued. Adalimumab and prednisone improved joint symptoms, but psoriatic plaques worsened over the next 4-6 mo.
The patient sought out alternative therapies and underwent two series of intravenous injections of umbilical cord blood-derived cells in a Republic of Panama-based stem cell clinic. The injections took place one month apart; 200000 cells were administered on four or five consecutive days (for a total of 8 × 105 or 1 × 106 cells). After one week, the patient noted some improvement of the psoriatic plaques, and reduction in joint pain. Two months after these initial cord blood stem cell injections, laboratory tests were repeated. Markers of inflammation increased: Erythrocyte sedimentation rate (ESR) was 85, CRP levels had doubled to 10.3 mg/L. Rheumatoid factor had also doubled (to 1280). Anti-CCP remained the same (> 100 U/mL, compared to a normal value of < 20 U).
Notwithstanding the lack of improvement in laboratory data, the patient was encouraged by the reduction in the number and size of psoriatic plaques and his improved sense of vitality. He sought out treatments of cord blood stem cells in the Unites States and had three rounds of injections, six months after the initial stem cell injections in Panama. The physician in the US-based clinic chose a method of treatment that included a fifty-fold greater number of nucleated umbilical cord blood stem cells. About 5 × 107 CBSC were injected intravenously and intra-articularly, once a month for three months (Figures 1 and 3). The patient reported gradual improvement of associated joint pain and swelling, “higher energy levels” and increased physical capabilities.
Psoriatic plaques were greatly resolved after the third treatment with CBSC (Figures 1 and 2). The psoriatic plaques were almost completely absent from the trunk and limbs. The few that remained were dramatically reduced in size, less raised and less red, and no longer scaly. Significantly, the inflammatory markers of ESR and CRP were within normal range after the fourth round of CBSC injections. The erythroid sedimentation rate (ESR) was measured as 9 mm/h, and the CRP value was 0.28 mg/dL). Five months after the last CBSC injection, ESR and CRP levels remained normal. Eighteen months after the initial CBSC injection, ESR and CRP levels had increased slightly out of normal range, but were far below levels reported prior to CBSC treatment. A reduced dosage of prednisone was continued, during and after CBSC treatment. The patient did not report any significant lifestyle changes over the course of the study (no recreational drugs, smoking, very limited use of alcohol). There were no serious complications related to CBSC treatment. There was mild sequela 48 h after the fifth and final injection; the patient had flu-like symptoms for two days (a mild febrile response and general soreness in muscles and joints). Lymphocyte counts were normal throughout the study. There was increased tolerance to exercise, as inflammatory arthritic symptoms decreased.
This patient report demonstrates several important aspects in treating inflammatory arthritis: Difficulties in diagnosis, the failure of some patients to achieve relief, and the need for new treatment modalities. This patient was initially diagnosed with RA. Definitive diagnosis of PsA of RA can be difficult given the heterogeneity of the diseases and the lack of definitive serology markers. Certain autoantibodies are considered diagnostic of RA: Rheumatoid factor (Rh factor) and cyclic citrullinated peptide antibody (Anti-CCP). However, 5%-13% of PsA patients are seropositive for these, and about 20% of RA patients are negative. ESR and CRP are widely used to guide anti-inflammatory treatments in RA, but are elevated in only about half of the patients with PsA. It should be noted that elevated ESR (> 15 mm/h) can be a very useful marker in treating PsA, since high levels are associated with a more severe disease state and increased mortality[12]. Ultimately, the patient received a diagnosis of PsA since the skin lesions, nail pitting, asymmetric joint pain and general dactylitis (vs joint-specific swelling) are consistent with PsA, and the serology report is indeterminant for a differential diagnosis.
Both PsA and RA treatment regimens usually follow a “step-up” approach. Treatments are tailored to the specific patient, different classes of drugs assessed, evaluations repeated over time and therapies altered, as appropriate. The main principals of treating both rheumatic illnesses are the same: Controlling symptoms and preventing damage, improving the quality of life for the patient, and minimizing side effects[13,14].
This patient’s symptoms were refractory to standard treatments of steroids and methotrexate. Adding a biologic to the treatment regime is standard of care; the TNF inhibitors certolizumab and adalimumab have similar beneficial results (52%-58% of PsA patients, depending on dosage)[4]. However, in this case, administration of certolizumab correlated with increased joint pain and the appearance of psoriatic plaques. Joint symptoms improved somewhat with the substitution of adalimumab, but the psoriatic plaques worsened. Switching from one TNF inhibitor to another is helpful for 30%-74% of the PsA primary non-responders (percentage depending on initial and secondary drug), but this leaves a significant cohort that respond to neither. Further, 20%-71% of patients administered TNF inhibitors reported treatment-emergent adverse events[15]. In addition to the risk of serious side effects, TNF inhibitors may lose efficacy or fail, due to the patients developing antibodies against them[16]. This patient did not have satisfactory improvement after treatment with NSAIDs, steroids, methotrexate and TNF inhibitors; in addition to experiencing adverse effects that correlated with treatment.
This case may have an unusual presentation for PsA, but it highlights a problem common to all inflammatory arthropathies. Some patients will progress to severe joint damage and experience significant morbidity or even loss of life. Others will be only mildly affected, and not progress to irreversible joint damage. A prognostic test or biomarker has not been developed, so we can’t identify the patients who would benefit from early and aggressive treatments. At the same time, it is important not to over-treat patients. Many, left untreated, would not progress to joint destruction. The potential side effects and high costs of anti-TNF biologicals make this point especially salient.
Hence, there is a need to seek out new therapies for inflammatory arthritic diseases. Stem cells, and specifically cord blood stem cells, have been used for regenerative therapies and immune modulation[10,11]. The mesenchymal stem cells (MSC) or MSC progenitors, present in umbilical cord blood are of particular interest as effectors, since they release a variety of regenerative growth factors such as VEGF, FGF, and PDGF[17]. Interestingly, MSC can migrate to sites of injury and inflammation and have the potential to “calm down” an overactive immune system[18]. This observation is the basis of experimental MSC treatments for a variety of autoimmune diseases (e.g., type I diabetes, Crohn’s disease, lupus, and rheumatoid arthritis). Preclinical data suggests that umbilical cord MSC may attenuate arthritic diseases by increasing regulatory T cells while decreasing T follicular helper cells, in addition to decreasing the pro-inflammatory Th17 cells[19]. Numerous reports conclude that the stem cells present in cord blood can support tissue repair and reduce excessive inflammatory responses through a variety of mechanisms[8].
We cannot assign causality between the CBSC injections and this patient’s clinical improvements, but the correlations are intriguing. The patient reported small, gradual improvements almost immediately after initial, small doses of CBSC injections (approximately 1 × 106 cells). After several months and higher numbers of CBSC (approximately 5 × 107), serological markers of inflammation fell into normal range and there were dramatic improvements in the number, size, color and consistency of the psoriatic plaques. In part, we cannot draw conclusions because we can’t substantiate the quality and quantity of the cells administered in a foreign clinic. Another part is there is little or no published evidence from clinical trials using CBSC for inflammatory arthritis. As more studies are published, we will gain an understanding of dosage, an expected time course and anticipated results. The overall results from this patient suggest further clinical trials with CBSC should be pursued. He experienced a remarkable reduction in symptoms over an extended period of time (> 12 mo after the initial CBSC injections). It is hoped that this patient report will lead to additional studies using CBSC and ultimately give new treatment options to other RA and PsA patients.
A 56-year-old male patient presented with joint stiffness and pain, skin lesions and fatigue.
Swelling, pain and reduced range of motion in the metacarpal and proximal phalangeal joints; joint symptoms progressed to include wrist and shoulder joints. Stiffness and pain were more pronounced on waking. Two years of arthritic symptoms preceded red, scaly skin lesions.
Rheumatoid arthritis, osteoarthritis, rheumatic fever, systemic lupus erythematosus, gout, secondary syphilis.
Values for C-reactive protein (CRP) and red blood cell sedimentation rate (ESR) became normal after cord blood stem cell (CBSC) treatment.
Anatomical pathology indicating psoriatic arthritis (PsA) resolved after treatment with CBSC.
Nonsteroidal anti-inflammatory drugs (NSAIDs), prednisone (steroid), methotrexate, TNF blockers (certolizumab and adalimumab), and umbilical cord blood stem cells.
Mesenchymal stem cells (from bone marrow or fat) have been used to treat rheumatoid arthritis.
Human umbilical cord blood was obtained from CorCell Cord Blood. Erythrocytes were removed using an ammonium chloride lysis buffer, used according to manufacturer’s directions (eBioScience). Flow analysis of similar preparations revealed about 3% of the population had the MSC marker, CD90+. Live, nucleated cells were concentrated by centrifugation, enumerated, and resuspended in cryopreservative (CryoGold Serum-Free Freezing Media, purchased from Stemgent, Inc.) The resulting samples were aliquoted into 1.8 mL cryogenic vials and gradually chilled to -160 ˚C. Immediately before use, cells were thawed and assessed for viability. Post thaw viability was 80%, as determined by an automated cell counter (Bio-Rad TC-20). Figure 3 shows one of the preparations administered to the patient.
This patient had persistent PsA symptoms, and did not find relief with standard therapies. Remission of symptoms correlated with injections of umbilical cord blood stem cells. Serological tests for inflammation (ESR and CRP) were normal for over a year after the initial CBSC injections.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kaliyadan F, Tanabe S, Tsuchiya A S- Editor: Cui LJ L- Editor: A E- Editor: Lu YJ
| 1. | Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64 Suppl 2:ii14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 654] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 2. | Coates LC, FitzGerald O, Helliwell PS, Paul C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Semin Arthritis Rheum. 2016;46:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Bond SJ, Farewell VT, Schentag CT, Gladman DD. Predictors for radiological damage in psoriatic arthritis: Results from a single centre. Ann Rheum Dis. 2007;66:370-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | 4 Mantravadi S, Ogdie A, Kraft WK. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev Clin Pharmacol. 2017;10:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Kerschbaumer A, Fenzl KH, Erlacher L, Aletaha D. An overview of psoriatic arthritis - epidemiology, clinical features, pathophysiology and novel treatment targets. Wien Klin Wochenschr. 2016;128:791-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 320] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Veale DJ, Fearon U. What makes psoriatic and rheumatoid arthritis so different? RMD Open. 2015;1:e000025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Rizk M, Aziz J, Shorr R, Allan DS. Cell-based therapy using umbilical cord blood for novel indications in regenerative therapy and immune modulation: An updated systematic scoping review of the literature. Biol Blood Marrow Transplant. 2017;23:1607-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Franceschetti T, De Bari C. The potential role of adult stem cells in the management of the rheumatic diseases. Ther Adv Musculoskelet Dis. 2017;9:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Damien P, Allan DS. Regenerative therapy and immune modulation using umbilical cord blood-derived cells. Biol Blood Marrow Transplant. 2015;21:1545-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Roura S, Pujal JM, Galvez-Monton C, Bayes-Genis A. The role and potential of umbilical cord blood in an era of new therapies: A review. Stem Cell Res Ther. 2015;6:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Punzi L, Podswiadek M, Oliviero F, Lonigro A, Modesti V, Ramonda R, Todesco S. Laboratory findings in psoriatic arthritis. Reumatismo. 2007;59 Suppl 1:52-55. [PubMed] |
| 13. | Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, Bautista-Molano W, Boehncke WH, Campbell W, Cauli A. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. [RCA] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 14. | Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, Emery P, Landewe R, Oliver S, Aletaha D. European league against rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 635] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 15. | Yamauchi PS, Bissonnette R, Teixeira HD, Valdecantos WC. Systematic review of efficacy of anti-tumor necrosis factor (TNF) therapy in patients with psoriasis previously treated with a different anti-TNF agent. J Am Acad Dermatol. 2016;75:612-618 e616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Hsu L, Snodgrass BT, Armstrong AW. Antidrug antibodies in psoriasis: A systematic review. Br J Dermatol. 2014;170:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 437] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 18. | Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 634] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 19. | Sun Y, Kong W, Huang S, Shi B, Zhang H, Chen W, Zhang H, Zhao C, Tang X, Yao G. Comparable therapeutic potential of umbilical cord mesenchymal stem cells in collagen-induced arthritis to TNF inhibitor or anti-CD20 treatment. Clin Exp Rheumatol. 2017;35:288-295. [PubMed] |