Published online Aug 26, 2016. doi: 10.4252/wjsc.v8.i8.231
Peer-review started: May 28, 2016
First decision: July 6, 2016
Revised: July 21, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: August 26, 2016
Processing time: 84 Days and 4.7 Hours
Therapy-related myeloid neoplasms are neoplastic processes arising as a result of chemotherapy, radiation therapy, or a combination of these modalities given for a primary condition. The disease biology varies based on the etiology and treatment modalities patients receive for their primary condition. Topoisomerase II inhibitor therapy results in balanced translocations. Alkylating agents, characteristically, give rise to more complex karyotypes and mutations in p53. Other etiologies include radiation therapy, high-dose chemotherapy with autologous stem cell transplantation and telomere dysfunction. Poor-risk cytogenetic abnormalities are more prevalent than they are in de novo leukemias and the prognosis of these patients is uniformly dismal. Outcome varies according to cytogenetic risk group. Treatment recommendations should be based on performance status and karyotype. An in-depth understanding of risk factors that lead to the development of therapy-related myeloid neoplasms would help developing risk-adapted treatment protocols and monitoring patients after treatment for the primary condition, translating into reduced incidence, early detection and timely treatment.
Core tip: Therapy-related myeloid neoplasms are becoming an increasing problem as the survival of cancer patients lengthens. The etiology has an important influence on the biological characteristics, time to onset and prognosis of the resultant disease. Although treatment of therapy-related myeloid neoplasms represents a substantial challenge due to prior treatment and comorbidities, cure is possible, especially with allogeneic stem cell transplantation, particularly in those with good-risk karyotype. Ultimately, individual assessment of risk factors may lead to developing risk-adapted therapies to reduce the incidence of this serious complication without affecting therapy for the underlying disorders.
- Citation: Zahid MF, Parnes A, Savani BN, Litzow MR, Hashmi SK. Therapy-related myeloid neoplasms - what have we learned so far? World J Stem Cells 2016; 8(8): 231-242
- URL: https://www.wjgnet.com/1948-0210/full/v8/i8/231.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i8.231
Therapy-related myeloid neoplasms, which include both therapy-related myelodysplastic syndromes (t-MDS) and therapy-related acute myeloid leukemia (t-AML), are well-known sequelae of conventional anticancer chemotherapy and radiotherapy for solid tumors, such as ovarian cancer[1], breast cancer[2], testicular cancer[3] and various sarcomas[4], as well as hematologic malignancies[5-7]. Therapy-related myeloid neoplasms constitute approximately 10%-20% of all cases of AML and MDS[8], with incidence varying depending upon the underlying malignancy, type of cytotoxic agents and/or radiotherapy, and timing of administration and dosage of treatment modalities[9]. Therapy-related myeloid neoplasms can present at any age, but the median age at diagnosis is reported to be approximately 61 years in adults[10,11].
After conventional-dose anticancer chemoradiotherapy, the incidence of t-MDS/AML has been reported between 0.8%-6.3% at 20 years post-treatment, with a median time of 3-5 years from treatment to development of t-MDS/AML[12]. In contrast, the incidence of t-MDS/AML after high-dose chemotherapy and autologous hematopoietic stem cell transplant (auto-HSCT) ranges from 1.1%-24.3% at 5 years post-transplant with a median time to development of only 1-2 years post-transplant[12-16]. Use of etoposide (a topoisomerase II inhibitor) priming for stem-cell mobilization and total-body-irradiation (TBI) based conditioning regimens are particularly associated with t-MDS/AML after auto-HSCT[16,17].
According to the World Health Organization classification, therapy-related myeloid neoplasms are broadly categorized into two subtypes: (1) an alkylating agent/radiotherapy-related type; and (2) a topoisomerase II inhibitor-related type[18]. The development of t-MDS/AML after alkylating agents/radiotherapy usually occurs after a median latency of 4-7 years, with two-thirds of patients presenting with MDS and one-third presenting with AML[12,19]. There is prominence of peripheral cytopenias and dysplasia of multiple myeloid lineages with frequently observed abnormalities of chromosome 5 [-5/del(5q)] and chromosome 7 [-7/del(7q)][19,20]. Conversely, topoisomerase II inhibitor-related t-MDS/AML has a relatively shorter latency between exposure to drugs and onset (median of 2-3 years)[21]. Patients with this subtype often present with overt AML without features of preceding MDS. AML in this subtype shows monocytic predominance[21,22] with a high incidence of balanced translocations involving chromosomal segments 11q23, 17q21 and/or 21q22[21]. While the risk of developing t-MDS/AML after alkylating agents/radiotherapy rises with increasing age, the risk of the same after topoisomerase II inhibitors appears to remain constant across all age groups[18,23].
Therapy-related myeloid neoplasms are clonal hemato–poietic stem cell disorders that arise due to iatrogenic somatic mutations after treatment with cytotoxic chemotherapy/radiotherapy. These somatic mutations impart increased proliferative capacity and survival advantage in the affected hematopoietic progenitors[12].
Alkylating agents have established significant clinical applications in virtually all cancer types and were the first chemotherapeutic drugs to be associated with therapy-related myeloid neoplasms[24]. These drugs work by transferring alkyl groups to oxygen and nitrogen atoms on DNA bases, resulting in the formation of highly mutagenic DNA base lesions (such as O6-methylguanine and N3-methylcytosine) and inducing DNA damage[25]. Alkylated DNA-based lesions, specifically O6-methylguanine, cause mispairing during DNA replication, and while this replication error is efficiently repaired by mismatch-repair enzymes, alkylated bases cannot be cleaved by mismatch-repair enzymes, leading to mutagenicity, secondary DNA double-stranded breaks and eventual cytotoxicity[26,27]. Mono-functional alkylating agents, such as nitrosoureas, dacarbazine and temozolomide, have one active moiety and are able to induce such lesions. In contrast, bi-functional alkylators, such as cyclophosphamide, melphalan and chlorambucil, have two active moieties and are able to form crosslinks within and between DNA strands in addition to forming alkylated base lesions[28]. Inter-strand DNA crosslinks halt replication forks during DNA replication, resulting in the formation of double-stranded DNA breaks. These breaks can give rise to chromosomal translocations, insertions, inversions and loss-of-heterozygosity involving several vital cellular genes[29,30].
Drugs targeting DNA topoisomerases are also well-known to cause t-MDS/AML[31]. DNA topoisomerase enzymes mediate the unknotting and relaxing of DNA supercoils, thereby allowing DNA replication to occur. These enzymes accomplish this by creating transient single-stranded (DNA topoisomerase I) and double-stranded (DNA topoisomerase II) DNA breaks. The release of topoisomerases from the DNA strands is followed by the re-ligating of these transient DNA breaks[32]. Topoisomerase II inhibitors, such as epipodophyllotoxins (etoposide and teniposide) and anthracyclines (daunorubicin, doxorubicin, etc.) prevent the release of topoisomerase II from cleaved DNA, preventing the re-ligation of strands and persistence of double-stranded breaks[26]. These DNA breaks are highly mutagenic and frequently result in translocations involving the genes MLL at 11q23, RUNX1 at 21q22 and RARA at 17q21[33-35].
The substantial incidence of various leukemias and myeloid disorders in the survivors of the Hiroshima and Nagasaki nuclear attacks has established a firm causal relationship between ionizing radiation and hematologic malignancies[36-38]. Epidemiological data from several studies involving individuals receiving therapeutic radiation has corroborated its leukemogenicity[3,39-41]. Cellular exposure to ionizing radiations has multiple mechanisms of causing DNA damage and mutations. Energy in each individual photon of radiation is able to disrupt the sugar-phosphate backbone of the DNA molecule, leading to single- and double-strand breaks[28]. In addition to this direct effect, cellular exposure to ionizing radiations results in radiolysis of water molecules leading to the formation of reactive oxygen species (most notably hydrogen peroxide, superoxide and hydroxyl radicals)[42]. These highly reactive molecules are capable of oxidizing and deaminating DNA bases and disruption of the sugar-phosphate backbone. As discussed with alkylating agents and topoisomerase II inhibitors earlier in this section, double-stranded breaks are highly mutagenic and contribute to leukemogenesis in therapy-related myeloid neoplasms.
In the context of auto-HSCT, DNA damage is multifactorial, arising as a result of treatment with cytotoxic agents used in induction therapy prior to auto-HSCT, possibly from the transplant process itself (stem cell mobilization, stem cell collection and storage) and from the stress of engraftment and hematopoietic recovery during the post-transplant period[43-46], apart from the chemotherapy agents and TBI used in the conditioning regimen. It is probable that some progenitor cells persist within the patients despite pre-transplant conditioning and acquire mutations overtime, for example from injury caused by the conditioning regimen, leading to t-MDS/AML after auto-HSCT[16]. To scientifically ascertain this hypothesis, future studies may focus on genetically marking the autograft and performing assays of t-MDS/AML clones in patients who develop this complication post-transplant to ascertain whether progenitor cells persisting in the patient after pre-transplant conditioning give rise to t-MDS/AML or is it the rescuing hematopoietic progenitors that give rise to t-MDS/AML. Currently, the ongoing Center for International Blood and Marrow Transplant Research study LE14-01 is the largest retrospective study to date (to the best of our knowledge) on t-MDS/AML after auto-HSCT[47]. The results of this study may provide deeper insight into t-MDS/AML in patients receiving auto-HSCT.
The p53 gene plays a crucial role in DNA damage response pathways, DNA repair mechanisms, cell cycle control and apoptosis. Abnormalities affecting p53 hinder the cell’s ability to repair damaged DNA and results in genomic instability and accumulation of various genetic lesions that contribute to leukemogenesis[12]. It is noteworthy that less than 10% of patients with de novo MDS and AML harbor p53 mutations, whereas 27%-50% of patients with t-MDS/AML demonstrate p53 mutations[48-50]. These are non-germline mutations that are often seen as a late adverse effect of therapy with alkylating agents and often occur simultaneously with chromosome 5 [-5/del(5q)] and chromosome 7 [-7/del(7q)] losses[12,50].
Telomeres are repeat sequences of non-coding DNA that flank the 3’ ends of linear chromosomes, permitting the replication of 3’ chromosomal ends and are vital for preventing dicentric fusion and chromosomal abnormalities[51]. Each mitotic division results in fractional loss of telomeric DNA, with cumulative telomeric loss leading to cellular senescence, a process by which normal cells lose their ability to divide after a specific number of cell divisions. In addition, loss of telomeric DNA also leads to genomic instability and somatic mutations[52,53]. Exposure to chemotherapeutic agents places proliferative stress on the bone marrow to allow for hematopoietic recovery after/in between cycles of chemotherapy[54]. The increased proliferative rates accelerate the loss of telomeric DNA, which would otherwise be conserved by the telomerase enzyme under physiologic conditions[52]. It is evident that telomere shortening is associated with the development of myeloid malignancies, such as MDS and AML, in both de novo[55] and therapy-related settings[43,56,57]. The nested case-control study by Chakraborty et al[57] showed that after auto-HSCT, those patients who developed t-MDS/AML showed a substantial increase in the rate of telomeric shortening after day +100 in comparison to the control group who did not develop t-MDS/AML. Other studies[43,56] also demonstrated similar observations. These findings corroborate that increased telomeric loss and telomere dysfunction contributes to leukemogenesis and likely precedes the development of t-MDS/AML in premalignant cells.
Intensive chemotherapy is one of the established therapeutic approaches to t-MDS/AML and its role has been investigated in earlier studies. In a retrospective study of 122 patients with t-MDS/AML at the MD Anderson Cancer Center, intensive chemotherapy with cytarabine yielded a complete remission (CR) rate of 37%[58]. In the same study, pooled data of 496 patients from 13 different studies revealed a cumulative CR rate of 27%[58]. No doubt, CRs have been achieved in this and other early studies on t-MDS/AML, but these rates are lower and short-lived in comparison to de novo MDS/AML[11,59,60]. The fatal course of t-MDS/AML is due to profound and persistent cytopenias due to ineffective hematopoiesis regardless of the fraction of immature blasts accumulating in the bone marrow[61]. In contrast, a subsequent study reported a surprisingly high CR rate of 82% for t-MDS/AML treated with high-dose cytarabine + mitoxantrone[62].
For therapy-related acute promyelocytic leukemia (t-APL) and t-AML with good-risk cytogenetics, specifically inv(16) and t(8;21), induction chemotherapy is recommended, similar to the treatment guidelines for their de novo counterparts[28]. For t-APL, outcomes are encouraging with regimens containing all-trans retinoic acid, as evidenced by two large European studies[63,64]. One study reported a CR rate of 87%[64]. The other study reported a CR rate of 80% with actuarial survival of 59% at 8 years[63]. Since outcomes with non-transplant strategies are encouraging in t-APL, this allows patients to be spared from the toxicities associated with allogeneic hematopoietic stem cell transplant (allo-HSCT). However, recent evidence does not favor the same recommendations for t-AML with inv(16) and t(8;21) as these patients have shown shorter event-free and overall survival in comparison to patients with de novo AML exhibiting inv(16) and t(8;21)[65-67]. This suggests that these patients may also require allo-HSCT for a durable cure, as is the case with t-MDS/AML with intermediate- and poor-risk cytogenetics[12,61,68]. The general conclusion drawn from literature on the subject is that outcomes of t-MDS/AML treated with conventional chemotherapy are generally poor, with median survival as low as only 6 mo[12].
With suboptimal survival rates for t-MDS/AML after allo-HSCT and even lower with conventional chemotherapy, exploration of alternative treatments and novel therapies is highly warranted to improve survival in this subset of patients. Azacitidine has shown promising efficacy in the treatment of high-risk MDS and AML[69,70] with a limited side effect profile and impressive tolerability, especially in patients with poor performance status and comorbidities[71]. Several recent retrospective studies suggested notable activity of azacitidine against t-MDS/AML, with overall response rates ranging from 39%-43% and median overall survival from 14.5-21 mo[72-74]. Azacitidine yielded the most benefit and better overall survival when used as first-line therapy[74] and detailed analysis of these studies showed similar outcomes between patients with de novo MDS/AML and those with t-MDS/AML[72,73]. A recent retrospective account of patients treated with azacitidine at the Memorial Sloan-Kettering Cancer Center and patients treated with decitabine in two industry-sponsored clinical trials (D0007[75] and DACO-020[76]) was published by Klimek et al[77]. In a cohort of 42 patients with t-MDS, this account reported an overall response rate (CR + marrow CR + hematologic response) of 38%[77]. However, a multicenter retrospective case series published in 2015 reported relatively inferior outcomes compared to the aforementioned studies (overall survival: 9.6 mo; overall response rate: 35.7%)[78].
Prebet et al[79] recently reported results of the E1905 study, a phase II randomized trial comparing the effects of combination therapy with azacitidine and the histone deacetylase inhibitor, entinostat, against monotherapy with azacitidine. The results showed lower hematologic normalization rates (17% vs 46% in the monotherapy arm), shorter overall survival (6 mo vs 13 mo in the monotherapy arm) and increased toxicity in the combination arm, recommending against the use of the azacitidine + entinostat combination for t-MDS/AML[79]. A predecessor of the same study demonstrated pharmacologic antagonism of entinostat when added to azacitidine[80]. However, the same study showed that prolonged administration of azacitidine alone increased the rate of hematologic responses when compared to standard dosing, representing an area of future research interest[80].
The standard approach for most patients with t-MDS/AML is allo-HSCT, which has consistently been shown to be a potential curative option for t-MDS/AML[12,61,68]. Outcomes of patients with t-MDS/AML after allo-HSCT, albeit limited and mostly based on retrospective studies, are still uniformly poor due to the high-intensity and transplant-related complications associated with the procedure and the refractory nature of the disease. For example, an account of 13 patients receiving allo-HSCT for t-MDS/AML after auto-HSCT reported that all patients died of either transplant-related complications (11 patients) or relapse (2 patients) with a median overall survival of only 1.8 mo[81]. One study reporting outcomes of 461 patients estimated a 35% overall survival 3 years after allo-HSCT[82]. Another large study involving 306 patients reported a median survival of only 8-10 mo and a 5 year overall survival of less than 10%[35]. Other studies have also reported poor outcomes[68,83-86], with non-relapse mortality ranging between 54%-58%[86-88]. Since most clinical trials in the AML or MDS arena have usually excluded t-AML/MDS, to our knowledge, prospective phase III randomized data evaluating the role of allo-HSCT in t-MDS/AML is lacking.
Some studies have described notable influences of conditioning regimens on survival rates. In a large study by Witherspoon et al[88], the 5-year disease-free survival for patients receiving conditioning with busulfan (BU) targeted to 600-900 ng/mL steady-state plasma concentration with cyclophosphamide (CY) [(t-BU/CY)] was 30%, the highest in the patient cohort. Survival rates were significantly lower for other regimens (standard BU/CY: 19%; chemotherapy/TBI: 8%) in comparison to t-BU/CY (P = 0.006). In the same report, the 5-year cumulative non-relapse mortality was lowest for t-BU/CY (42%) vs that for standard BU/CY and chemotherapy/TBI regimens (52% and 58%, respectively); (P = 0.02)[88]. Subsequently, an even larger study (including 251 patients) also showed a greater 5-year disease-free survival for patients conditioned with t-BU/CY (BU targeted to 800-900 ng/mL steady-state plasma concentration) of 43% vs that for standard BU/CY, fludarabine (Flu)/BU, Flu/TBI and high-dose TBI/CY (28%, 24%, 23%, 18%, respectively); (P = 0.001)[87]. This study also showed the lowest 5-year cumulative non-relapse mortality for the t-BU/CY regimen (28%) vs high-dose TBI/CY, Flu/TBI and standard BU/CY (53%, 54% and 61%, respectively); (P < 0.001)[87].
The dismal outlook of these patients is likely multifactorial, resulting from relapse-related and/or non-relapse-related mortality. The likelihood of relapse significantly correlates with disease stage. For example, a report from the Fred Hutchinson Cancer Research Center showed varying rates of relapse among their patient cohort (no relapses in the refractory anemia/refractory anemia with ringed sideroblasts group; 22% relapse in the refractory anemia with excess blasts group; and 36% relapse in the refractory anemia with excess blasts in transformation/AML group)[85]. Another study reported similar findings[88]. Likewise, disease karyotype also correlates with relapse rate. The impact of karyotype on outcomes in both de novo and t-MDS/AML were compared in large prospective studies which showed disease karyotype to be an independent prognostic factor in both groups, with poor-risk cytogenetic abnormalities more common in the t-MDS/AML group[84,89]. An optimized, 3-group cytogenetic classification proposed by Armand et al[90] was found to be the strongest predictor of overall survival in t-MDS/AML by its impact on relapse risk after allo-HSCT. Through this classification, cytogenetic abnormalities in these patients were divided into good-risk [normal, -5, (del)20q or -Y], poor-risk (chromosome 7 abnormalities, complex karyotype) and intermediate-risk (all others)[90]. Also, relapses are less likely with unrelated donor transplants, likely due to a more potent graft vs leukemia effect[12,91] and lower peripheral blood blast count (correlating with early-stage disease and low disease burden)[92].
Other outcome parameters after allo-HSCT have been scrutinized. Patient performance status strongly influences survival[79]. Treatment for the primary malignancy causes injury to various organ systems and depletion of normal hematopoietic progenitors, diminishing the patients’ ability to withstand the intensive nature and toxicities associated with allo-HSCT. In addition, damage to bone marrow stromal elements from prior therapy (especially radiotherapy) alters the bone marrow microenvironment, making hematopoietic regeneration more difficult[61]. Younger patients (children, adolescents, young adults) have a better bone marrow reserve and better ability to withstand the toxicities associated with multiple treatments (both for the primary disease and allo-HSCT)[4], hence it would be expected that survival is better in this group in contrast to elderly. Since therapy-related myeloid neoplasms are relatively uncommon in young age groups[8,9], there is paucity of literature concerning the prognostic factors and survival in younger patients. This is a potential area of research interest. Future studies are warranted to ascertain if different prognostic factors confer survival advantage in younger patients with therapy-related myeloid neoplasms, or if the dismal outcomes in elderly are just a result of sheer fact of age.
Patients are also immunocompromised from prior treatment regimens and hence often acquire life-threatening infections, a well-known and feared cause of mortality after allo-HSCT. Additionally, relapse of the primary malignancy, especially metastatic cancer or disseminated lymphoma, carries its own risks of morbidity and mortality[61]. Also, the timing of allo-HSCT affects the outlook of patients, as a recent study demonstrated that those who received allo-HSCT later than 6 mo after diagnosis have inferior survival rates[93]. Thus it is imperative to refer a newly diagnosed case of t-MDS/AML to a transplant center early.
In addition to disease stage and karyotype, somatic mutations of specific genes may also have implications on prognostication. For example, frame-shift mutations of the nucleophosmin gene, internal tandem duplications of the fms-like tyrosine kinase 3 gene and double mutations in the CEBPA gene are now routinely assessed in the workup of AML patients and incorporated into therapeutic algorithms[94]. They have also been observed in t-MDS/AML[95,96]. While these (and perhaps other specific gene mutations) may have impact on t-MDS/AML prognosis, these mutations usually occur and have prognostic value in cases with normal cytogenetics[94], a karyotype which is relatively rare in t-MDS/AML, making their prognostic utility uncertain in cases of t-MDS/AML.
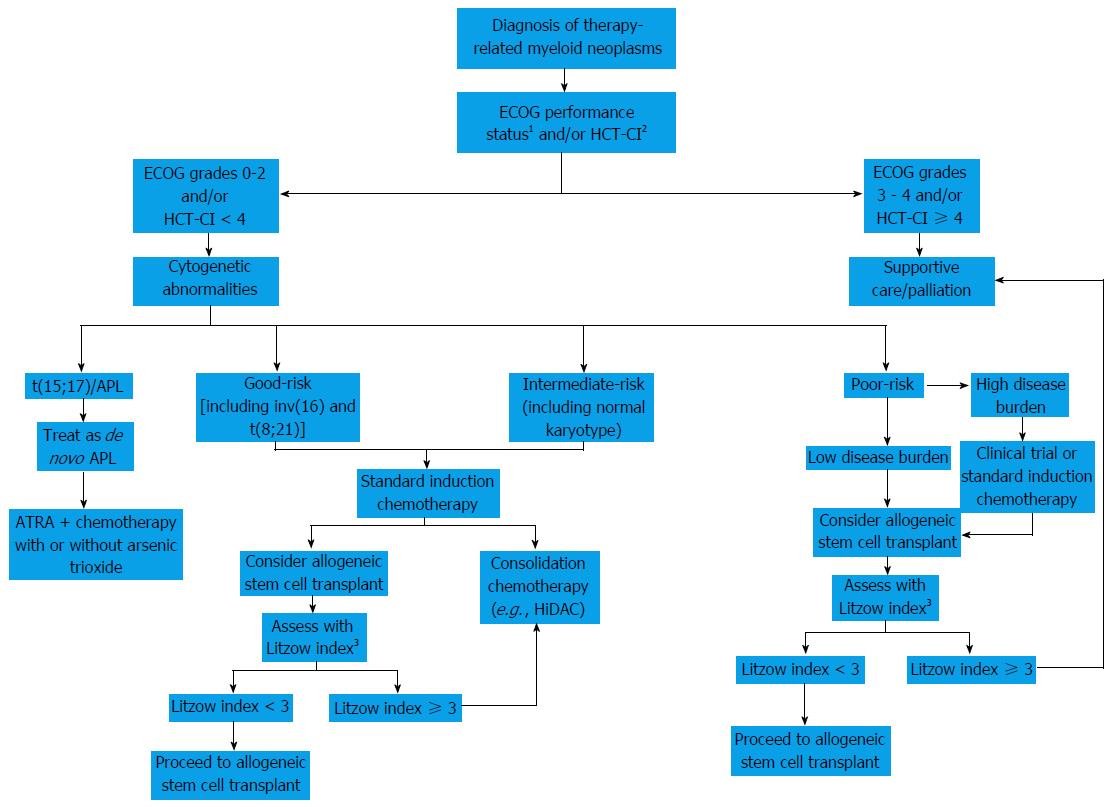
When taking only t-MDS into account, the International Prognostic Scoring System, a cornerstone in the prognostication of patients with MDS, has shown unsatisfactory ability to predict the outcome of patients after treatment[81]. Instead, an alternative prediction model utilizes the following four factors to gauge survival for patients with t-MDS and t-AML after allo-HSCT: (1) age greater than 35 years; (2) poor-risk cytogenetics; (3) advanced-stage t-MDS or t-AML not in CR after allo-HSCT; and (4) donor other than an HLA-identical sibling or a matched or partially-matched unrelated donor[68]. Five-year overall survival varies with the number of these factors present: None (50%), 1 (26%), 2 (21%), 3 (10%) and 4 (4%)[68]. Male sex has also been indicative of poor outcomes[86]. A proposed algorithmic approach to patients with therapy-related myeloid neoplasms is elaborated in Figure 1.
Keeping in mind the poor outcomes of t-MDS/AML, measures for early detection of this disorder would allow for timely and pre-emptive treatment approaches, such as reduced intensity conditioning allo-HSCT. This approach would yield substantial advantages as opposed to waiting for the development of overt t-MDS/AML, when disease burden is higher and requires more intensive therapy which can have its own risks of morbidity and mortality[28]. In this section we will outline some methods for prediction and/or early detection of t-MDS/AML in patients at risk.
Metaphase cytogenetics and karyotyping analyze actively dividing cells, though the number of cells analyzed is limited (20-30 cells)[44]. It is worthy of note that patients developing t-MDS/AML, for example after auto-HSCT, may not show karyotypic abnormalities before the procedure. Conventional cytogenetics may lack sufficient sensitivity and specificity to efficiently recognize patients with increased predisposition to t-MDS/AML[16,44].
Interphase fluorescence in situ hybridization (FISH) offers several advantages over conventional cytogenetics, mainly the lack of need for cells to be actively dividing and the ability to analyze a greater number of cells (several hundreds)[44]. FISH is also able to detect abnormal clones prior to auto-HSCT. For example, in one report, FISH was able to detect clonal abnormalities in 9 out of 12 patients (75%) who later developed t-MDS/AML after auto-HSCT[97]. In another study, FISH identified abnormal cell clones in 20 out of 20 patients who went on to develop t-MDS/AML[98]. Identification of clonal abnormalities in a high percentage of cells may indicate proliferative and survival advantages and foreshadows development of t-MDS/AML[44]. However, the locus specificity of FISH requires prior selection of multiple markers for adequate analysis and its labor- and time-intensive methodology are notable limitations[44].
Loss of heterozygosity (LOH) employs a polymerase chain reaction (PCR) analysis of a selected sample to detect loss of one allele at a specific locus and large chromosomal deletions. This technique is also labor- and time-intensive and is a population-based assay that requires prior selection of loci to be analyzed. In addition, its sensitivity is poor, unable to detect less than 20% cells for LOH of a selected locus[44]. Nevertheless, it may have impressive specificity, as a positive result suggests an abnormal cell clone. Thus, LOH may prove to be a viable “rule-in” test in this context and may be followed by more sensitive techniques, such as high-throughput analysis and next-generation sequencing (NGS)[44,99]. However, prospective studies with large numbers of patient samples are needed to ascertain its validity as a predictor of t-MDS/AML.
Clonality assay based on X chromosome-inactivation at the human androgen receptor gene is another useful method. This is a PCR-based technique that does not require information about loci prior to analysis and detects abnormal clones with survival/proliferative advantage over normal polyclonal cells[44]. In a single center study by Mach-Pascual et al[100], monoclonal hematopoiesis, as indicated by X-inactivation-based clonality at the human androgen receptor locus, prior to auto-HSCT was predictive of the development of t-MDS/AML. Four out of 10 patients (40%) demonstrating monoclonal hematopoiesis before transplant subsequently developed t-MDS/AML vs only 2 out of 53 patients with polyclonal hematopoiesis (P = 0.004)[100]. However, this method is limited by the need for high numbers of monoclonal cells to be present for diagnosis (low sensitivity) and its applicability only to female patients[44]. Altered gene expression in CD34+ progenitors may also be used. A large study by Li et al[101] showed that a 38-gene panel analyzing gene expression in peripheral blood CD34+ progenitors showed remarkable ability to distinguish patients who would eventually develop t-MDS/AML from those who would not develop the complication after auto-HSCT. The implication of this study is that development of t-MDS/AML requires the acquisition of mutations in multiple genes as opposed to just one gene[44]. Additionally, due to different kinds and combinations of mutations, patients with this disorder show significant heterogeneity with multiple subtypes. Therefore, characterization of single gene mutations may not have a satisfactory predictive value in identifying patients prone to developing t-MDS/AML[12,28,44].
Significant advances have happened for identification of unique biomarkers associated with leukemias which is mainly driven by gene expression analysis and NGS, which have the potential to significantly improve the diagnostic and prognostic criteria. The utilization of a signature NGS panel for each disease (e.g., AML, ALL, MDS, etc.) is increasing worldwide[102,103]. In t-MDS/AML, the impact of NGS panel on long term outcomes are awaited. What we do know is some of clonal mutations with known association with leukemogenesis, i.e., TET2, DNMT3A, and ASXL1[104,105], if found in a patient who is at risk of t-MDS/AML may predict a high likelihood of developing t-MDS/AML. Caution must be exercised with such an approach, as some cases of t-MDS/AML may have germline mutations in cancer susceptibility genes[106], thus a careful family history to discover cancer susceptibility is warranted in at-risk patients.
In summary, when a bone marrow biopsy is being obtained for work up for cytopenias in an at-risk patient (e.g., cancer survivor who received chemotherapy or radiation), obtaining an NGS panel specific for MDS and AML should be considered.
Based on our knowledge of the risk factors and pathogenesis of t-MDS/AML, development of risk reduction strategies is a certain possibility. Standardized screening tests, including but not limited to the ones discussed in the previous section, may help identify patients at substantial risk. Accordingly, alterations of chemotherapeutic regimens and treatment modalities may be made under a risk-adapted model, thereby minimizing the risk of t-MDS/AML while providing adequate treatment to the underlying malignancy[12].
In the context of high-dose chemotherapy and auto-HSCT, modifications can be made to stem cell mobilization and harvesting and pre-transplant conditioning regimens, circumventing the use of alkylating agents, topoisomerase inhibitors and radiotherapy, to eliminate as many risk factors as possible. Specific FISH loci, such as 5q-, 7q-, +8, -11 and 20q-, may be screened preemptively to predict outcomes when any specific abnormalities in blood work are being worked up[44]. Alternatively, if the risk of t-MDS/AML is substantial (for example, in the case of hematologic malignancies evidence of cytogenetic or FISH abnormalities prior to transplant and high risk disease), these patients can be offered other therapeutic options, such as pre-emptive work up for allo-HSCT (HLA typing) and non-transplant modalities (emerging novel therapies and targeted agents).
There is much needed effort for further exploration and validation of biomarkers specifically for t-MDS/AML to develop a viable risk assessment tool for this subgroup of patients. When it comes to cancer survivorship, we urge the current professional societies, e.g., National Comprehensive Cancer Network, American Society of Clinical Oncology, and European Society for Medical Oncology to consider screening the at-risk population of cancer survivors for t-MDS/AML, at least with a complete blood count with peripheral smear annually, which is a relatively simple and economically feasible option for screening for t-MDS/AML.
Lastly, most of the large randomized studies in the arena of AML and MDS have traditionally excluded t-MDS/AML and thus prospective phase III data for t-MDS/AML with regards to outcomes is absent. It is imperative that prospective clinical trials be conducted specifically for t-MDS/AML to delineate optimum treatment options. The cancer community has accomplished a lot in the past five decades in alleviating the burden of cancer by improvements in both radiation and chemotherapy fields, and current efforts on personalized or individualized medicine are looking very promising for further improvements in decreasing cancer mortality. However, as the cancer survivors are living longer[107,108], the incidence of t-MDS/AML continues to increase and currently is one of the fastest growing cancers worldwide. Efforts must be made by clinicians and researchers globally for establishment of risk reduction strategies for this fatal cancer.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Guo ZS, Moschovi MA S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Travis LB, Holowaty EJ, Bergfeldt K, Lynch CF, Kohler BA, Wiklund T, Curtis RE, Hall P, Andersson M, Pukkala E. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 247] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Curtis RE, Boice JD, Stovall M, Bernstein L, Greenberg RS, Flannery JT, Schwartz AG, Weyer P, Moloney WC, Hoover RN. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 293] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Travis LB, Andersson M, Gospodarowicz M, van Leeuwen FE, Bergfeldt K, Lynch CF, Curtis RE, Kohler BA, Wiklund T, Storm H. Treatment-associated leukemia following testicular cancer. J Natl Cancer Inst. 2000;92:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Bhatia S, Krailo MD, Chen Z, Burden L, Askin FB, Dickman PS, Grier HE, Link MP, Meyers PA, Perlman EJ. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children’s Oncology Group. Blood. 2007;109:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Boivin JF, Hutchison GB, Zauber AG, Bernstein L, Davis FG, Michel RP, Zanke B, Tan CT, Fuller LM, Mauch P. Incidence of second cancers in patients treated for Hodgkin’s disease. J Natl Cancer Inst. 1995;87:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Neglia JP, Meadows AT, Robison LL, Kim TH, Newton WA, Ruymann FB, Sather HN, Hammond GD. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;325:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 366] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Travis LB, Curtis RE, Glimelius B, Holowaty E, Van Leeuwen FE, Lynch CF, Adami J, Gospodarowicz M, Wacholder S, Inskip P. Second cancers among long-term survivors of non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1993;85:1932-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, Friis LS, Kjeldsen E, Marcher CW, Preiss B. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J Clin Oncol. 2015;33:3641-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 9. | Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, Fraumeni JF, Curtis RE. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 11. | Takeyama K, Seto M, Uike N, Hamajima N, Ino T, Mikuni C, Kobayashi T, Maruta A, Muto Y, Maseki N. Therapy-related leukemia and myelodysplastic syndrome: a large-scale Japanese study of clinical and cytogenetic features as well as prognostic factors. Int J Hematol. 2000;71:144-152. [PubMed] |
| 12. | Bhatia S. Therapy-related myelodysplasia and acute myeloid leukemia. Semin Oncol. 2013;40:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | André M, Henry-Amar M, Blaise D, Colombat P, Fleury J, Milpied N, Cahn JY, Pico JL, Bastion Y, Kuentz M. Treatment-related deaths and second cancer risk after autologous stem-cell transplantation for Hodgkin’s disease. Blood. 1998;92:1933-1940. [PubMed] |
| 14. | Govindarajan R, Jagannath S, Flick JT, Vesole DH, Sawyer J, Barlogie B, Tricot G. Preceding standard therapy is the likely cause of MDS after autotransplants for multiple myeloma. Br J Haematol. 1996;95:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Howe R, Micallef IN, Inwards DJ, Ansell SM, Dewald GW, Dispenzieri A, Gastineau DA, Gertz MA, Geyer SM, Hanson CA. Secondary myelodysplastic syndrome and acute myelogenous leukemia are significant complications following autologous stem cell transplantation for lymphoma. Bone Marrow Transplant. 2003;32:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Krishnan A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, Fung H, Bhatia R, Kashyap A, Molina A. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588-1593. [PubMed] |
| 17. | Milligan DW, Ruiz De Elvira MC, Kolb HJ, Goldstone AH, Meloni G, Rohatiner AZ, Colombat P, Schmitz N. Secondary leukaemia and myelodysplasia after autografting for lymphoma: results from the EBMT. EBMT Lymphoma and Late Effects Working Parties. European Group for Blood and Marrow Transplantation. Br J Haematol. 1999;106:1020-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 1459] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 19. | Karp JE, Sarkodee-Adoo CB. Therapy-related acute leukemia. Clin Lab Med. 2000;20:71-81, ix. [PubMed] |
| 20. | Le Beau MM, Albain KS, Larson RA, Vardiman JW, Davis EM, Blough RR, Golomb HM, Rowley JD. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol. 1986;4:325-345. [PubMed] |
| 21. | Pedersen-Bjergaard J, Philip P. Balanced translocations involving chromosome bands 11q23 and 21q22 are highly characteristic of myelodysplasia and leukemia following therapy with cytostatic agents targeting at DNA-topoisomerase II. Blood. 1991;78:1147-1148. [PubMed] |
| 22. | Pedersen-Bjergaard J, Andersen MK, Christiansen DH, Nerlov C. Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia. Blood. 2002;99:1909-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Swerdlow SH, Campo E, Harris NL. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. IARC Press: Lyon, France, 2008. . |
| 24. | Rowley JD, Golomb HM, Vardiman JW. Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease. Blood. 1981;58:759-767. [PubMed] |
| 25. | Drabløs F, Feyzi E, Aas PA, Vaagbø CB, Kavli B, Bratlie MS, Peña-Diaz J, Otterlei M, Slupphaug G, Krokan HE. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst). 2004;3:1389-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 463] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 26. | Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5:943-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst). 2007;6:1079-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 464] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 28. | Sill H, Olipitz W, Zebisch A, Schulz E, Wölfler A. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol. 2011;162:792-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1248] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 30. | Richardson C, Jasin M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature. 2000;405:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 378] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 31. | Pedersen-Bjergaard J, Daugaard G, Hansen SW, Philip P, Larsen SO, Rørth M. Increased risk of myelodysplasia and leukaemia after etoposide, cisplatin, and bleomycin for germ-cell tumours. Lancet. 1991;338:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 230] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1452] [Cited by in RCA: 1342] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 33. | Andersen MK, Johansson B, Larsen SO, Pedersen-Bjergaard J. Chromosomal abnormalities in secondary MDS and AML. Relationship to drugs and radiation with specific emphasis on the balanced rearrangements. Haematologica. 1998;83:483-488. [PubMed] |
| 34. | Pedersen-Bjergaard J, Pedersen M, Roulston D, Philip P. Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. Blood. 1995;86:3542-3552. [PubMed] |
| 35. | Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 36. | Descatha A, Jenabian A, Conso F, Ameille J. Occupational exposures and haematological malignancies: overview on human recent data. Cancer Causes Control. 2005;16:939-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Little JB. Cellular, molecular, and carcinogenic effects of radiation. Hematol Oncol Clin North Am. 1993;7:337-352. [PubMed] |
| 38. | Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, Kamada N, Dohy H, Matsuo T, Matsui T [corrected to Matsuo T]. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res. 1994;137:S68-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 445] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Haddy N, Le Deley MC, Samand A, Diallo I, Guérin S, Guibout C, Oberlin O, Hawkins M, Zucker JM, de Vathaire F. Role of radiotherapy and chemotherapy in the risk of secondary leukaemia after a solid tumour in childhood. Eur J Cancer. 2006;42:2757-2764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Le Deley MC, Suzan F, Cutuli B, Delaloge S, Shamsaldin A, Linassier C, Clisant S, de Vathaire F, Fenaux P, Hill C. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Ojha RP, Fischbach LA, Zhou Y, Felini MJ, Singh KP, Thertulien R. Acute myeloid leukemia incidence following radiation therapy for localized or locally advanced prostate adenocarcinoma. Cancer Epidemiol. 2010;34:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Rassool FV, Gaymes TJ, Omidvar N, Brady N, Beurlet S, Pla M, Reboul M, Lea N, Chomienne C, Thomas NS. Reactive oxygen species, DNA damage, and error-prone repair: a model for genomic instability with progression in myeloid leukemia? Cancer Res. 2007;67:8762-8771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Bhatia R, Van Heijzen K, Palmer A, Komiya A, Slovak ML, Chang KL, Fung H, Krishnan A, Molina A, Nademanee A. Longitudinal assessment of hematopoietic abnormalities after autologous hematopoietic cell transplantation for lymphoma. J Clin Oncol. 2005;23:6699-6711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Gilliland DG, Gribben JG. Evaluation of the risk of therapy-related MDS/AML after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Pedersen-Bjergaard J, Andersen MK, Christiansen DH. Therapy-related acute myeloid leukemia and myelodysplasia after high-dose chemotherapy and autologous stem cell transplantation. Blood. 2000;95:3273-3279. [PubMed] |
| 46. | Stone RM. Myelodysplastic syndrome after autologous transplantation for lymphoma: the price of progress. Blood. 1994;83:3437-3440. [PubMed] |
| 47. | CIBMTR. Studies in progress: Risks and outcomes of therapy related myeloid neoplasms after autologous hematopoietic cell transplantation. Available from: https://www.cibmtr.org/Studies/Observational/StudyLists/Pages/ObservationalStudy.aspx?OSID=a0JE000000cybOmMAI. |
| 48. | Ben-Yehuda D, Krichevsky S, Caspi O, Rund D, Polliack A, Abeliovich D, Zelig O, Yahalom V, Paltiel O, Or R. Microsatellite instability and p53 mutations in therapy-related leukemia suggest mutator phenotype. Blood. 1996;88:4296-4303. [PubMed] |
| 49. | Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001;19:1405-1413. [PubMed] |
| 50. | Horiike S, Misawa S, Kaneko H, Sasai Y, Kobayashi M, Fujii H, Tanaka S, Yagita M, Abe T, Kashima K. Distinct genetic involvement of the TP53 gene in therapy-related leukemia and myelodysplasia with chromosomal losses of Nos 5 and/or 7 and its possible relationship to replication error phenotype. Leukemia. 1999;13:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2511] [Cited by in RCA: 2633] [Article Influence: 77.4] [Reference Citation Analysis (1)] |
| 52. | Hackett JA, Feldser DM, Greider CW. Telomere dysfunction increases mutation rate and genomic instability. Cell. 2001;106:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 275] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 53. | Lange K, Holm L, Vang Nielsen K, Hahn A, Hofmann W, Kreipe H, Schlegelberger B, Göhring G. Telomere shortening and chromosomal instability in myelodysplastic syndromes. Genes Chromosomes Cancer. 2010;49:260-269. [PubMed] |
| 54. | Hake CR, Graubert TA, Fenske TS. Does autologous transplantation directly increase the risk of secondary leukemia in lymphoma patients? Bone Marrow Transplant. 2007;39:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Ueda Y, Calado RT, Norberg A, Kajigaya S, Roos G, Hellstrom-Lindberg E, Young NS. A mutation in the H/ACA box of telomerase RNA component gene (TERC) in a young patient with myelodysplastic syndrome. BMC Med Genet. 2014;15:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Beauchamp-Nicoud A, Feneux D, Bayle C, Bernheim A, Léonard C, Koscielny S, Tchernia G, Bourhis JH. Therapy-related myelodysplasia and/or acute myeloid leukaemia after autologous haematopoietic progenitor cell transplantation in a prospective single centre cohort of 221 patients. Br J Haematol. 2003;122:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Chakraborty S, Sun CL, Francisco L, Sabado M, Li L, Chang KL, Forman S, Bhatia S, Bhatia R. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Kantarjian HM, Estey EH, Keating MJ. Treatment of therapy-related leukemia and myelodysplastic syndrome. Hematol Oncol Clin North Am. 1993;7:81-107. [PubMed] |
| 59. | Hoyle CF, de Bastos M, Wheatley K, Sherrington PD, Fischer PJ, Rees JK, Gray R, Hayhoe FG. AML associated with previous cytotoxic therapy, MDS or myeloproliferative disorders: results from the MRC’s 9th AML trial. Br J Haematol. 1989;72:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Larson RA, Wernli M, Le Beau MM, Daly KM, Pape LH, Rowley JD, Vardiman JW. Short remission durations in therapy-related leukemia despite cytogenetic complete responses to high-dose cytarabine. Blood. 1988;72:1333-1339. [PubMed] |
| 61. | Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35:418-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 62. | Godley LA, Njiaju UO, Green M, Weiner H, Lin S, Odenike O, Rich ES, Artz A, Van Besien K, Daugherty CK. Treatment of therapy-related myeloid neoplasms with high-dose cytarabine/mitoxantrone followed by hematopoietic stem cell transplant. Leuk Lymphoma. 2010;51:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Beaumont M, Sanz M, Carli PM, Maloisel F, Thomas X, Detourmignies L, Guerci A, Gratecos N, Rayon C, San Miguel J. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21:2123-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 64. | Pagana L, Pulsoni A, Tosti ME, Avvisati G, Mele L, Mele M, Martino B, Visani G, Cerri R, Di Bona E. Clinical and biological features of acute myeloid leukaemia occurring as second malignancy: GIMEMA archive of adult acute leukaemia. Br J Haematol. 2001;112:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Borthakur G, Lin E, Jain N, Estey EE, Cortes JE, O’Brien S, Faderl S, Ravandi F, Pierce S, Kantarjian H. Survival is poorer in patients with secondary core-binding factor acute myelogenous leukemia compared with de novo core-binding factor leukemia. Cancer. 2009;115:3217-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Gustafson SA, Lin P, Chen SS, Chen L, Abruzzo LV, Luthra R, Medeiros LJ, Wang SA. Therapy-related acute myeloid leukemia with t(8; 21) (q22; q22) shares many features with de novo acute myeloid leukemia with t(8; 21)(q22; q22) but does not have a favorable outcome. Am J Clin Pathol. 2009;131:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Schnittger S, Bacher U, Haferlach C, Kern W, Haferlach T. Rare CBFB-MYH11 fusion transcripts in AML with inv(16)/t(16; 16) are associated with therapy-related AML M4eo, atypical cytomorphology, atypical immunophenotype, atypical additional chromosomal rearrangements and low white blood cell count: a study on 162 patients. Leukemia. 2007;21:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Litzow MR, Tarima S, Pérez WS, Bolwell BJ, Cairo MS, Camitta BM, Cutler CS, de Lima M, Dipersio JF, Gale RP. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 764] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 70. | Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1862] [Cited by in RCA: 2043] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 71. | Garcia-Manero G, Huang X, Cabrero M, DiNardo CD, Pemmaraju N, Daver NG, Borthakur G, Wierda WG, Kadia T, Alvarado Y. A bayesian phase II randomized trial of azacitidine versus Azacitidine Vorinostat in patients with newly diagnosed AML or high-risk MDS with poor performance status, organ dysfunction, or other comorbidities. Blood. 2014;124:3277. |
| 72. | Bally C, Thépot S, Quesnel B, Vey N, Dreyfus F, Fadlallah J, Turlure P, de Botton S, Dartigeas C, de Renzis B. Azacitidine in the treatment of therapy related myelodysplastic syndrome and acute myeloid leukemia (tMDS/AML): a report on 54 patients by the Groupe Francophone Des Myelodysplasies (GFM). Leuk Res. 2013;37:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Duong VH, Lancet JE, Alrawi E, Al-Ali NH, Perkins J, Field T, Epling-Burnette PK, Zhang L, List AF, Komrokji RS. Outcome of azacitidine treatment in patients with therapy-related myeloid neoplasms with assessment of prognostic risk stratification models. Leuk Res. 2013;37:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Fianchi L, Criscuolo M, Lunghi M, Gaidano G, Breccia M, Levis A, Finelli C, Santini V, Musto P, Oliva EN. Outcome of therapy-related myeloid neoplasms treated with azacitidine. J Hematol Oncol. 2012;5:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1186] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 76. | Steensma DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G, Albitar M, Larsen JS, Arora S, Cullen MT. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842-3848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 77. | Klimek VM, Dolezal EK, Tees MT, Devlin SM, Stein K, Romero A, Nimer SD. Efficacy of hypomethylating agents in therapy-related myelodysplastic syndromes. Leuk Res. 2012;36:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Minoia C, Sgherza N, Loseto G, Greco G, Buquicchio C, Merchionne F, Toldo C, Galise I, Melpignano A, Tarantini G. Azacitidine in the front-line treatment of therapy-related myeloid neoplasms: a multicenter case series. Anticancer Res. 2015;35:461-466. [PubMed] |
| 79. | Prebet T, Sun Z, Ketterling RP, Zeidan A, Greenberg P, Herman J, Juckett M, Smith MR, Malick L, Paietta E. Azacitidine with or without Entinostat for the treatment of therapy-related myeloid neoplasm: further results of the E1905 North American Leukemia Intergroup study. Br J Haematol. 2016;172:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | Prebet T, Sun Z, Figueroa ME, Ketterling R, Melnick A, Greenberg PL, Herman J, Juckett M, Smith MR, Malick L. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905. J Clin Oncol. 2014;32:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 81. | Friedberg JW, Neuberg D, Stone RM, Alyea E, Jallow H, LaCasce A, Mauch PM, Gribben JG, Ritz J, Nadler LM. Outcome in patients with myelodysplastic syndrome after autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3128-3135. [PubMed] |
| 82. | Kröger N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwieser D, Devergie A, Ruutu T, Cornish J, Ljungman P. Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. 2009;94:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | Mauritzson N, Albin M, Rylander L, Billström R, Ahlgren T, Mikoczy Z, Björk J, Strömberg U, Nilsson PG, Mitelman F. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976-1993 and on 5098 unselected cases reported in the literature 1974-2001. Leukemia. 2002;16:2366-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 85. | Witherspoon RP, Deeg HJ. Allogeneic bone marrow transplantation for secondary leukemia or myelodysplasia. Haematologica. 1999;84:1085-1087. [PubMed] |
| 86. | Yakoub-Agha I, de La Salmonière P, Ribaud P, Sutton L, Wattel E, Kuentz M, Jouet JP, Marit G, Milpied N, Deconinck E. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French society of bone marrow transplantation. J Clin Oncol. 2000;18:963-971. [PubMed] |
| 87. | Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, Sandmaier BM, Witherspoon RP, Nash RA, Sanders JE. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Witherspoon RP, Deeg HJ, Storer B, Anasetti C, Storb R, Appelbaum FR. Hematopoietic stem-cell transplantation for treatment-related leukemia or myelodysplasia. J Clin Oncol. 2001;19:2134-2141. [PubMed] |
| 89. | Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22:2510-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 90. | Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM, Ritz J, Alyea EP, Antin JH, Soiffer RJ. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Zahid MF, Rizzieri DA. Haploidentical Hematopoietic Stem Cell Transplantation: Expanding the Horizon for Hematologic Disorders. Adv Hematol. 2016;2016:1423493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Anderson JE, Gooley TA, Schoch G, Anasetti C, Bensinger WI, Clift RA, Hansen JA, Sanders JE, Storb R, Appelbaum FR. Stem cell transplantation for secondary acute myeloid leukemia: evaluation of transplantation as initial therapy or following induction chemotherapy. Blood. 1997;89:2578-2585. [PubMed] |
| 93. | Spina F, Alessandrino PE, Milani R, Bonifazi F, Bernardi M, Luksch R, Fagioli F, Formica C, Farina L. Allogeneic stem cell transplantation in therapy-related acute myeloid leukemia and myelodysplastic syndromes: impact of patient characteristics and timing of transplant. Leuk Lymphoma. 2012;53:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2353] [Cited by in RCA: 2559] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 95. | Andersen MT, Andersen MK, Christiansen DH, Pedersen-Bjergaard J. NPM1 mutations in therapy-related acute myeloid leukemia with uncharacteristic features. Leukemia. 2008;22:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Au WY, Fung AT, Ma ES, Liang RH, Kwong YL. Low frequency of FLT3 gene internal tandem duplication and activating loop mutation in therapy-related acute myelocyticleukemia and myelodysplastic syndrome. Cancer Genet Cytogenet. 2004;149:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 97. | Abruzzese E, Buss D, Rainer R, Pettenati MJ, Rao PN. Progression of a myelodysplastic syndrome to pre-B acute lymphoblastic leukemia: a case report and cell lineage study. Ann Hematol. 1996;73:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Lillington DM, Micallef IN, Carpenter E, Neat MJ, Amess JA, Matthews J, Foot NJ, Young BD, Lister TA, Rohatiner AZ. Detection of chromosome abnormalities pre-high-dose treatment in patients developing therapy-related myelodysplasia and secondary acute myelogenous leukemia after treatment for non-Hodgkin’s lymphoma. J Clin Oncol. 2001;19:2472-2481. [PubMed] |
| 99. | Kohlmann A, Bacher U, Schnittger S, Haferlach T. Perspective on how to approach molecular diagnostics in acute myeloid leukemia and myelodysplastic syndromes in the era of next-generation sequencing. Leuk Lymphoma. 2014;55:1725-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Mach-Pascual S, Legare RD, Lu D, Kroon M, Neuberg D, Tantravahi R, Stone RM, Freedman AS, Nadler LM, Gribben JG. Predictive value of clonality assays in patients with non-Hodgkin’s lymphoma undergoing autologous bone marrow transplant: a single institution study. Blood. 1998;91:4496-4503. [PubMed] |
| 101. | Li L, Li M, Sun C, Francisco L, Chakraborty S, Sabado M, McDonald T, Gyorffy J, Chang K, Wang S. Altered hematopoietic cell gene expression precedes development of therapy-related myelodysplasia/acute myeloid leukemia and identifies patients at risk. Cancer Cell. 2011;20:591-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Braggio E, Egan JB, Fonseca R, Stewart AK. Lessons from next-generation sequencing analysis in hematological malignancies. Blood Cancer J. 2013;3:e127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 103. | Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S, Wartman LD, Christopher M, Lamprecht TL, Helton NM. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA. 2015;314:811-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 104. | Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2574] [Cited by in RCA: 2568] [Article Influence: 233.5] [Reference Citation Analysis (0)] |
| 105. | Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3384] [Cited by in RCA: 3446] [Article Influence: 313.3] [Reference Citation Analysis (0)] |
| 106. | Churpek JE, Marquez R, Neistadt B, Claussen K, Lee MK, Churpek MM, Huo D, Weiner H, Bannerjee M, Godley LA. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer. 2016;122:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 107. | de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, Forsythe L, Scoppa S, Hachey M, Rowland JH. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 517] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 108. | Herrmann C, Cerny T, Savidan A, Vounatsou P, Konzelmann I, Bouchardy C, Frick H, Ess S. Cancer survivors in Switzerland: a rapidly growing population to care for. BMC Cancer. 2013;13:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 110. | Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |